Effect of Direct Steam Injection and Instantaneous Ultra-High-Temperature (DSI-IUHT) Sterilization on the Physicochemical Quality and Volatile Flavor Components of Milk
Abstract
1. Introduction
2. Results and Discussion
2.1. The Influence of Processing on the Physical Properties of Milk
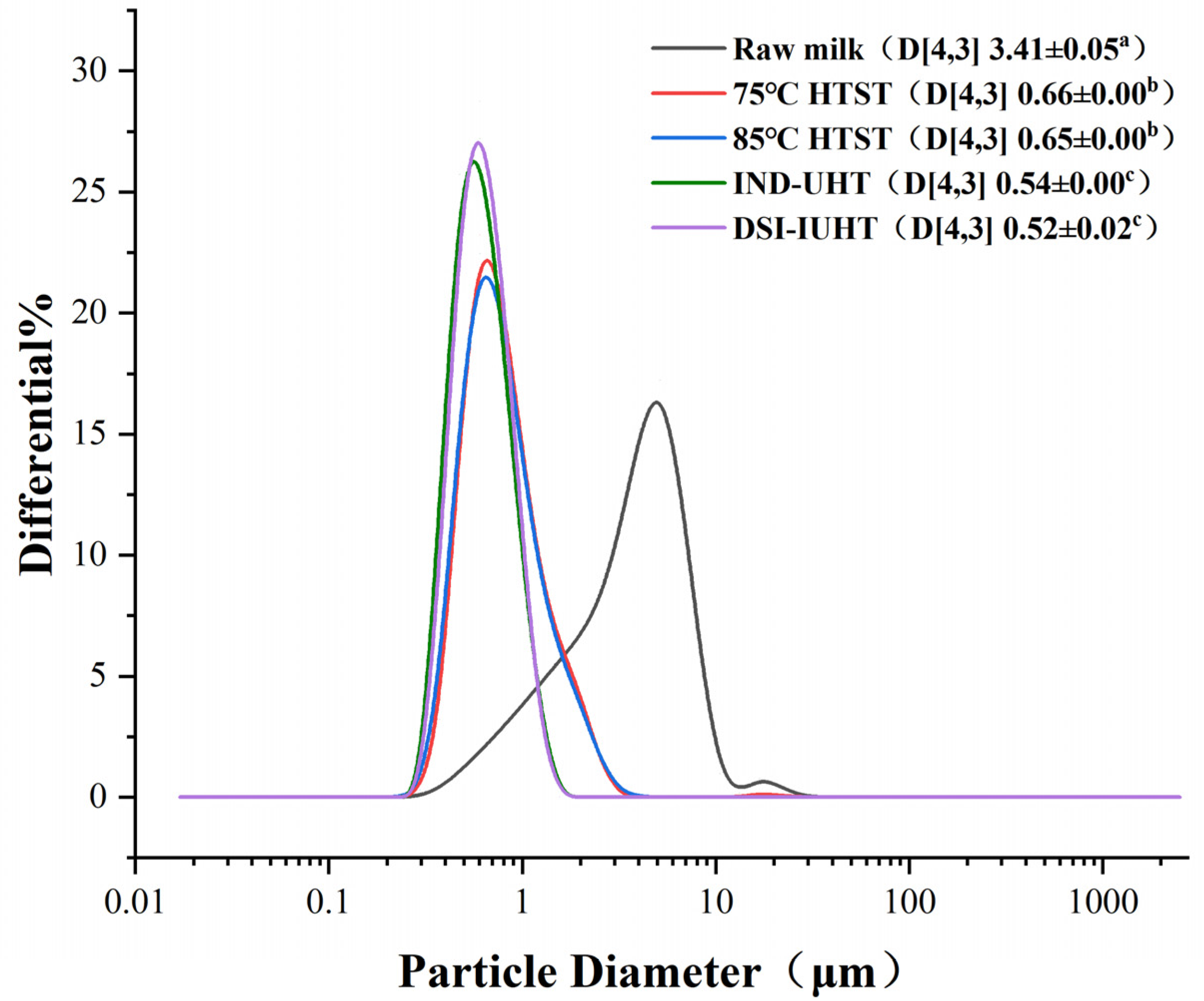
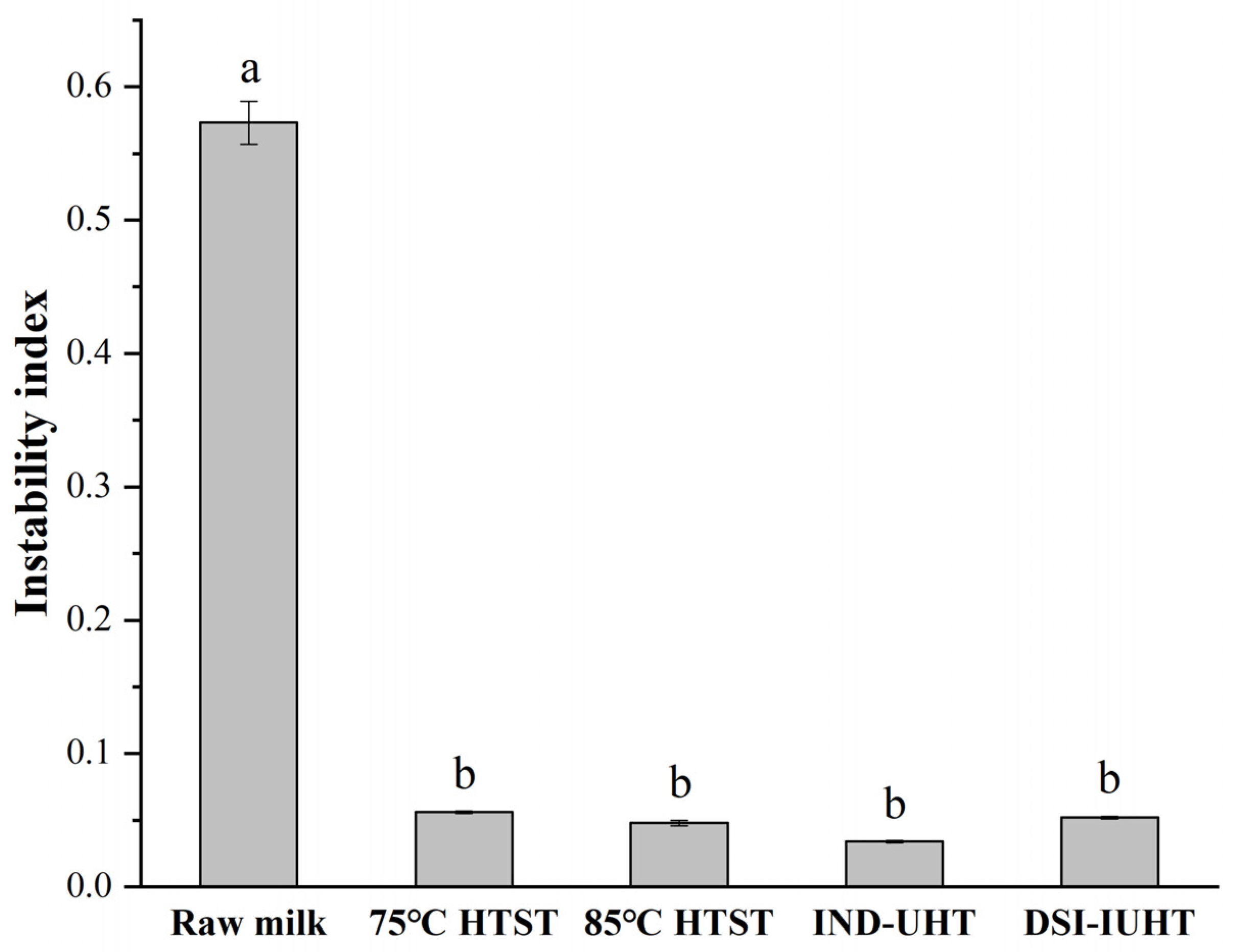
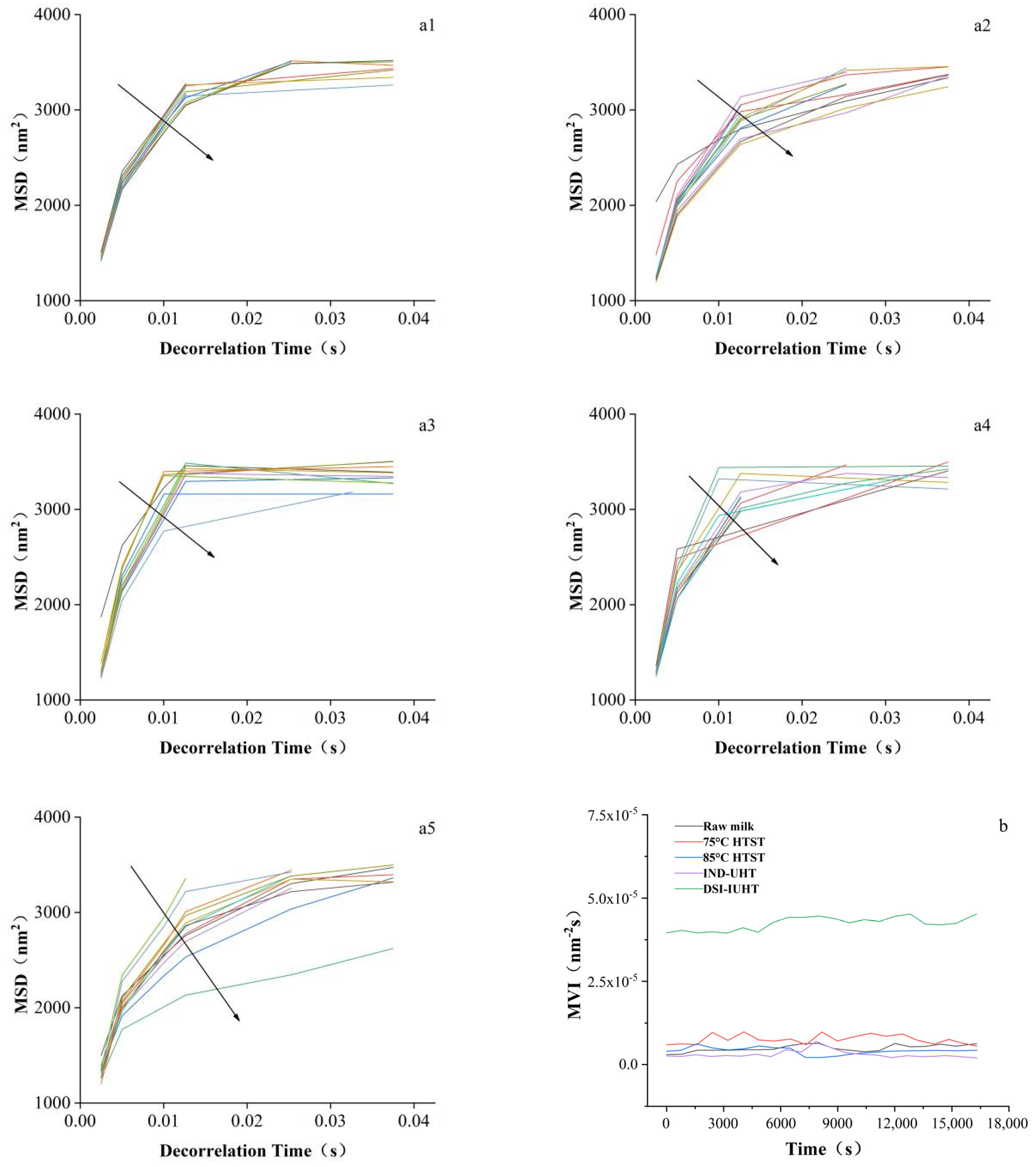
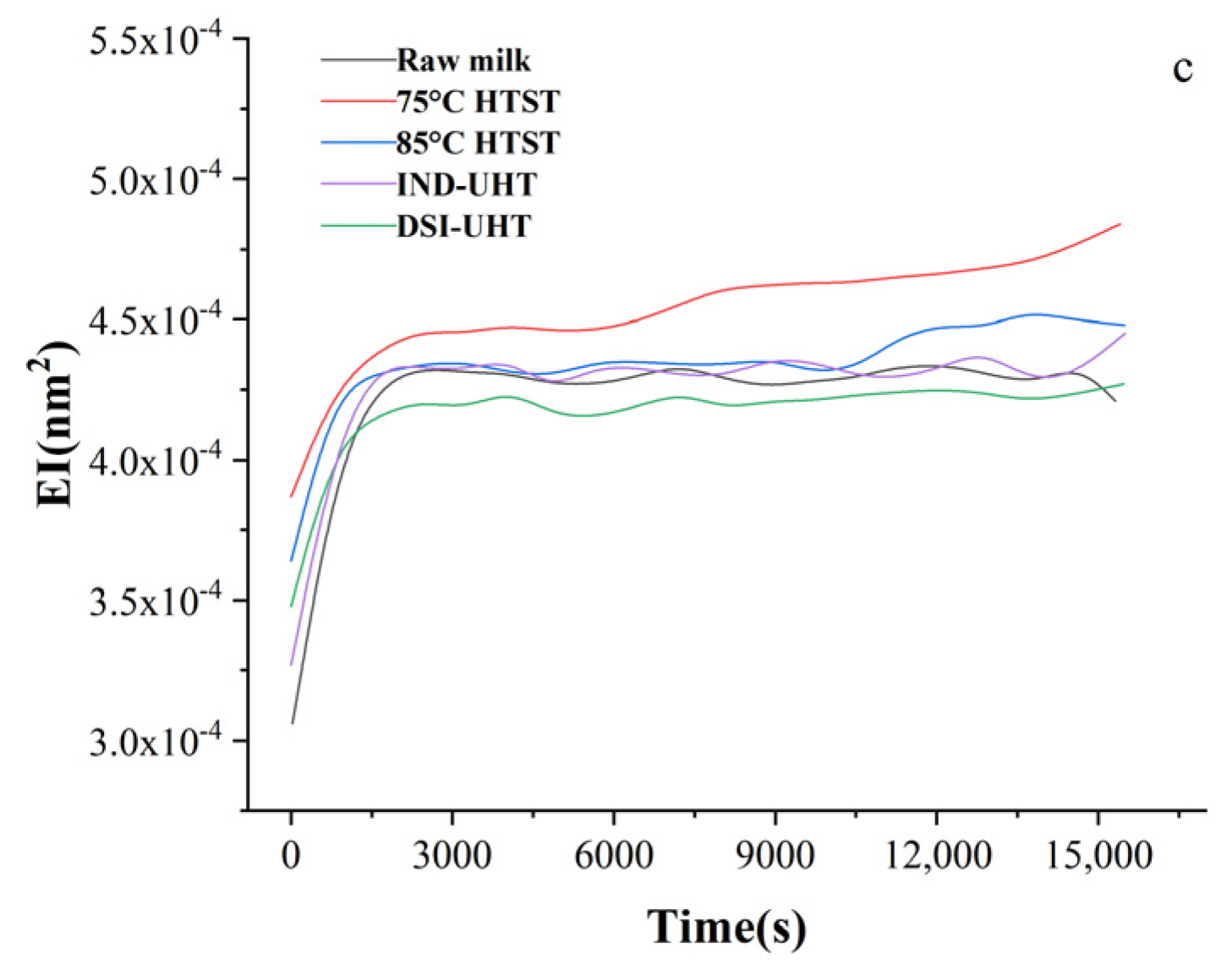
2.1.1. Particle Size and Physical Stability
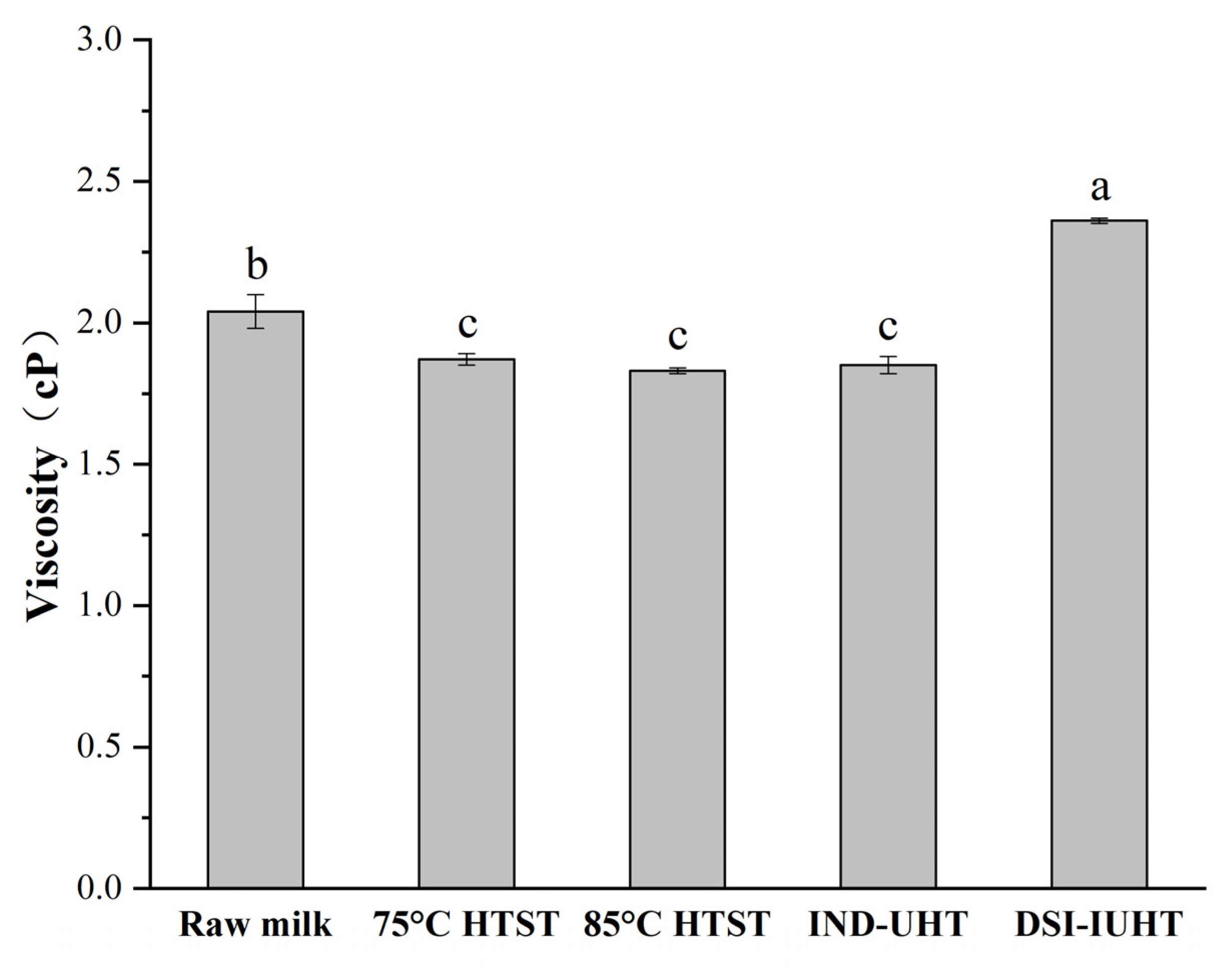
2.1.2. The Rheological and Apparent Viscosity
2.1.3. The WPD Rate
2.2. Analysis of the Odor-Active Compounds in Milk
2.2.1. Quantitation of the Odor-Active Compounds in Milk
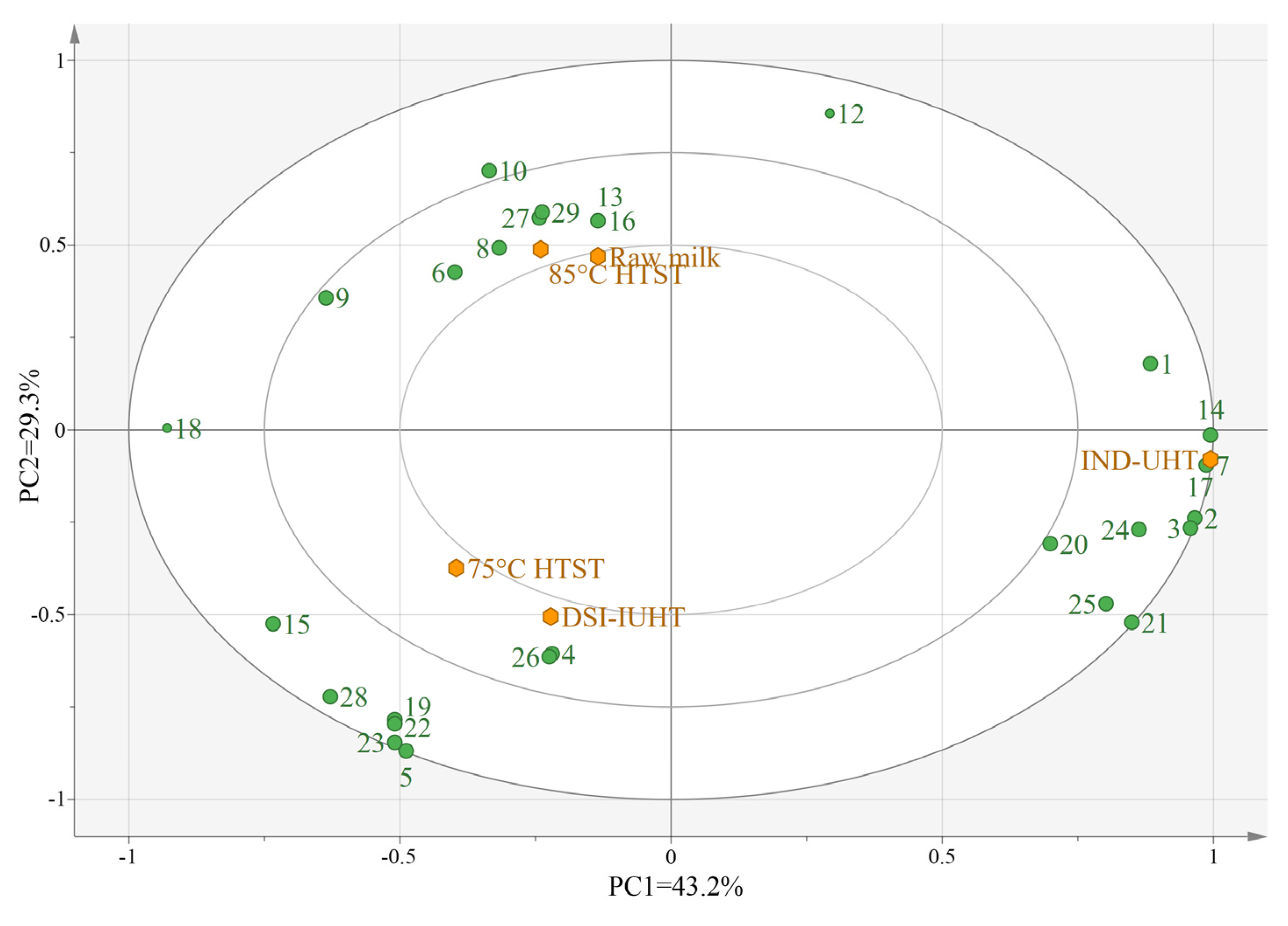
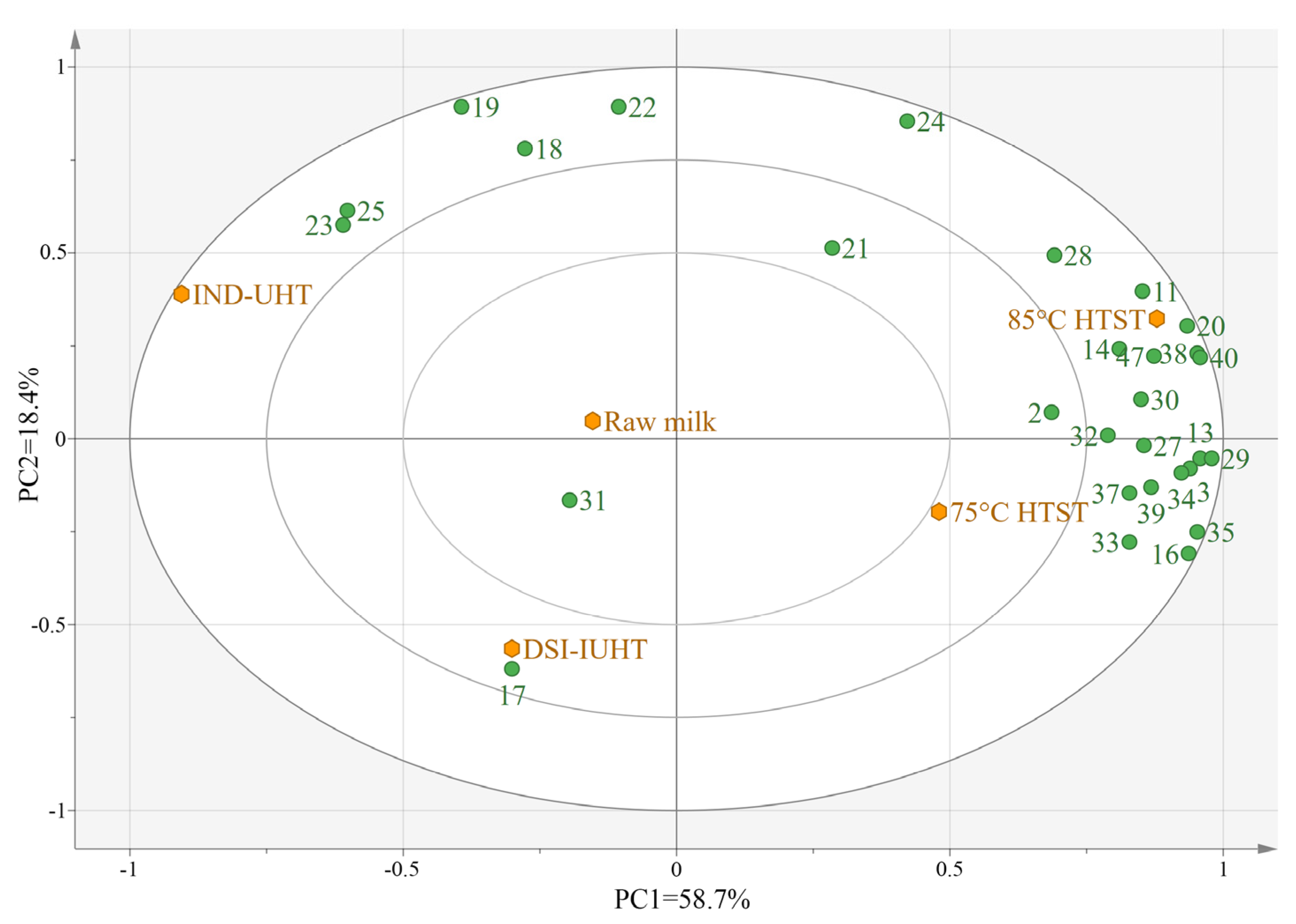
2.2.2. Principal Component Analysis (PCA) of the Milk Samples
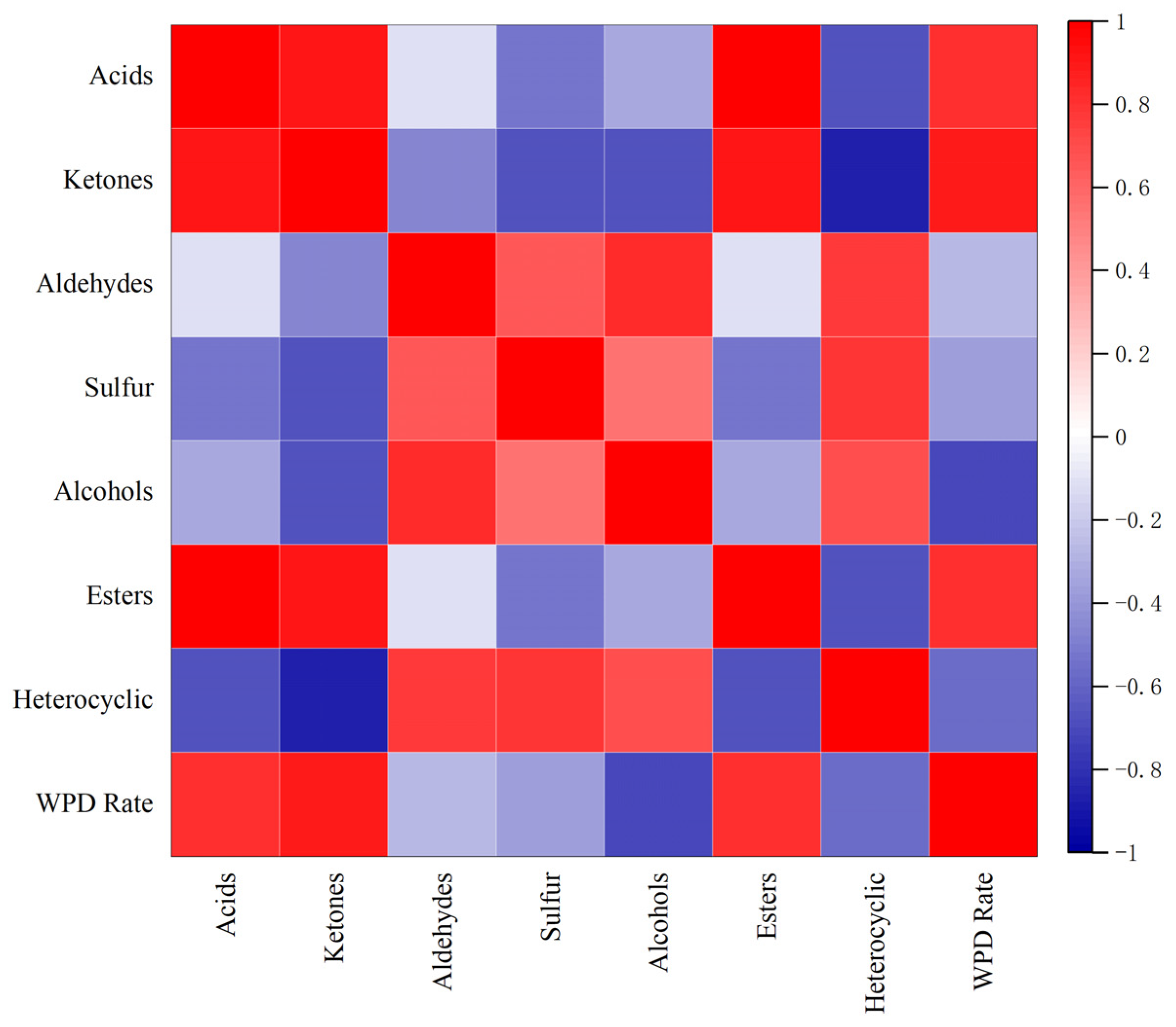
2.2.3. Correlation between WPD and Flavor Substances
3. Materials and Methods
3.1. Samples
3.2. Particle Size Determination
3.3. Physical Stability Determination
3.4. Apparent Viscosity Determination
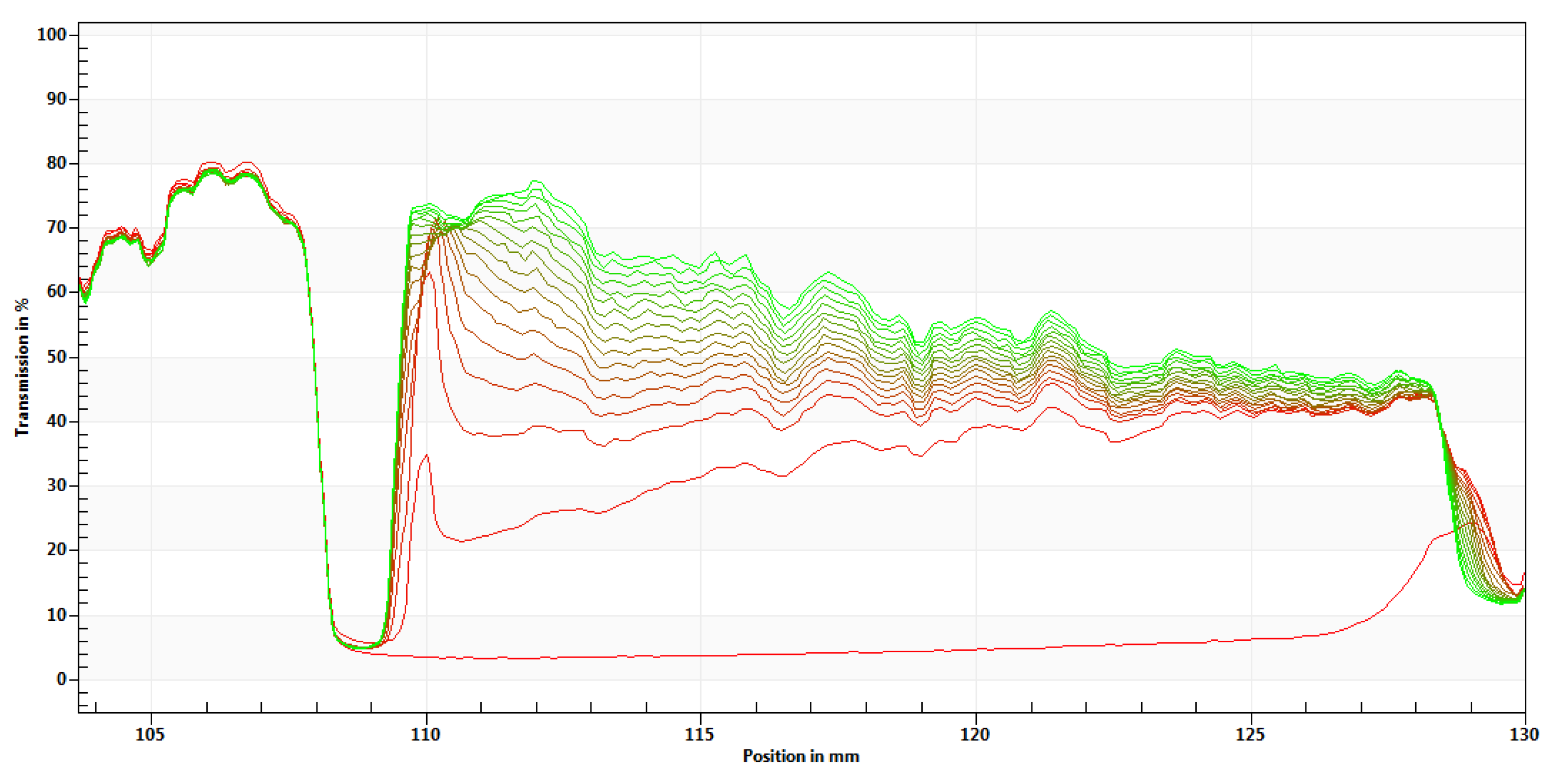
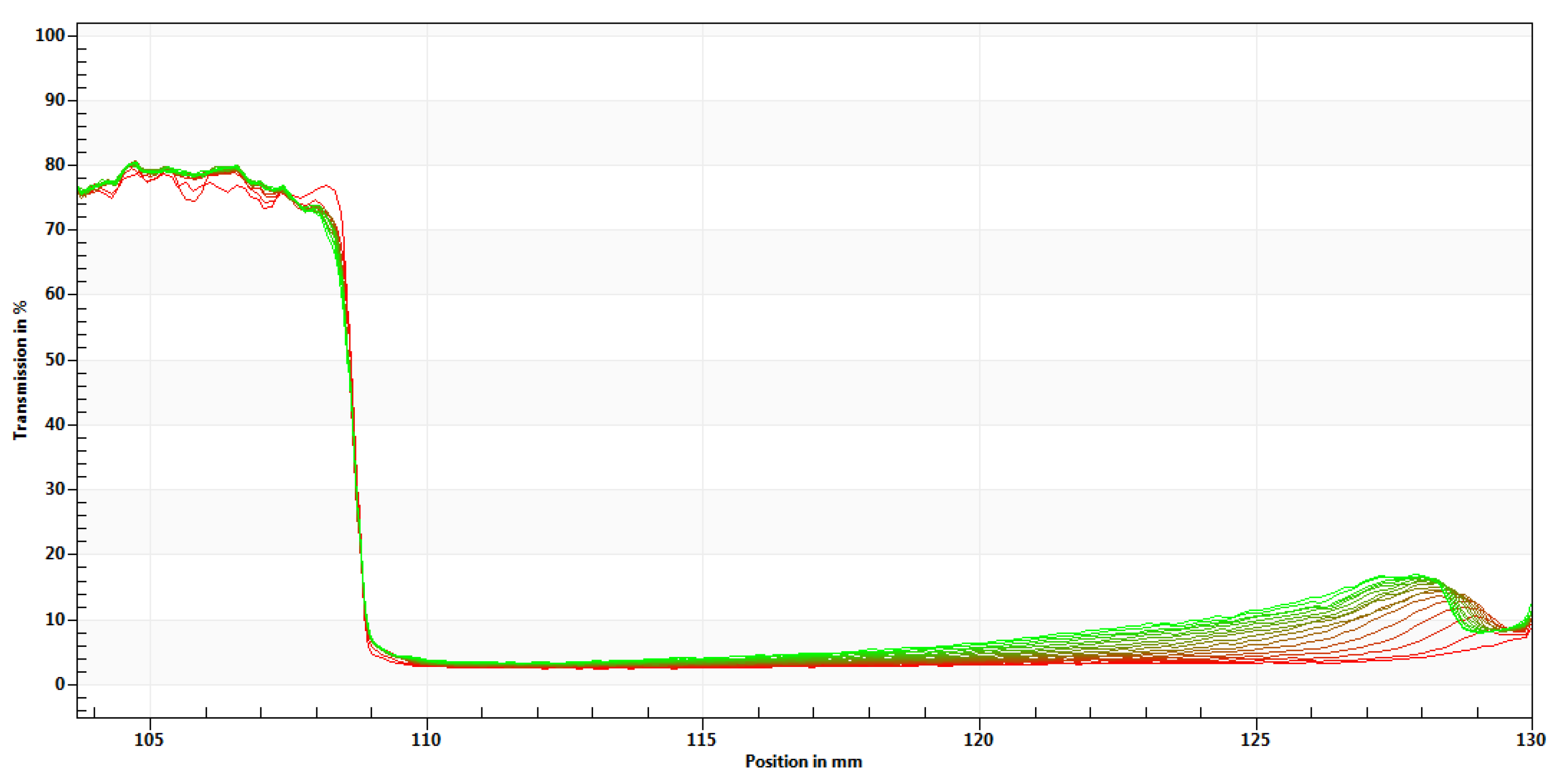
3.5. Microrheological Behavior
3.6. WPD Determination
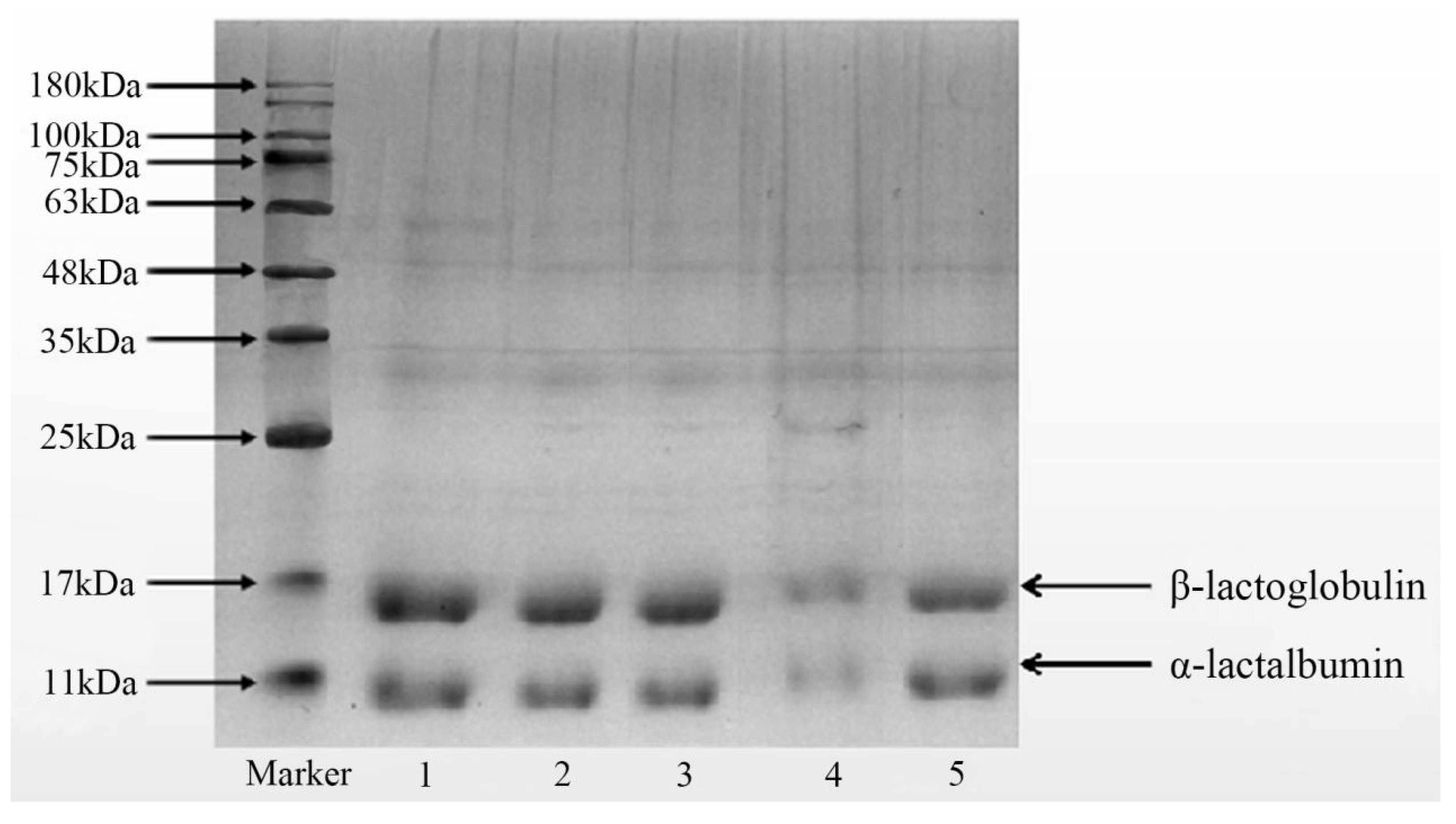
3.6.1. Sample Treatment and Denaturation Determination
3.6.2. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.7. Analysis of VCs
3.7.1. SPME of the Volatile Components
3.7.2. SAFE of the Volatile Components
3.7.3. GC-MS Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
References
- Cole, S.; Goetze, A.; Meunier-Goddik, L. Pasteurized milk. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2020; pp. 444–450. [Google Scholar] [CrossRef]
- Ranvir, S.; Sharma, R.; Gandhi, K.; Nikam, P.; Mann, B. Physico-chemical Changes during Processing and Storage of UHT Milk. Indian J. Dairy Sci. 2021, 74, 39–47. [Google Scholar] [CrossRef]
- Akkerman, M.; Johansen, L.B.; Rauh, V.; Sorensen, J.; Larsen, L.B.; Poulsen, N.A. Relationship between Casein Micelle size, Protein Composition and Stability of UHT Milk. Int. Dairy J. 2021, 112, 104856. [Google Scholar] [CrossRef]
- Reineccius, G.A. Flavor Interactions with Proteins. Curr. Opin. Food Sci. 2022, 47, 100884. [Google Scholar] [CrossRef]
- Barbano, D.M.; Ma, Y.; Santos, M.V. Influence of Raw Milk Quality on Fluid Milk Shelf Life. J. Dairy Sci. 2006, 89, E15–E19. [Google Scholar] [CrossRef]
- Lewis, M. Improving Pasteurised and Extended Shelf-life Milk. In Improving the Safety and Quality of Milk Milk Production and Processing; Woodhead Publishing: Sawston, UK, 2010; pp. 277–301. [Google Scholar]
- Jo, Y.; Benoist, D.M.; Barbano, D.M.; Drake, M.A. Flavor and Flavor Chemistry Differences among Milks Processed by High-temperature, Short-time Pasteurization or Ultra-pasteurization. J. Dairy Sci. 2018, 101, 3812–3828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kang, D.; Zhang, W.; Lorenzo, J.M. Recent Advantage of Interactions of Protein-flavor in Foods: Perspective of Theoretical Models, Protein Properties and Extrinsic Factors. Trends Food Sci. Technol. 2021, 111, 405–425. [Google Scholar] [CrossRef]
- Damodaran, S.; Kinsella, J.E. Interaction of Carbonyls with Soy Protein: Conformational Effects. J. Agric. Food Chem. 1981, 29, 1253–1257. [Google Scholar] [CrossRef]
- Lubbers, S.; Landy, P.; Voilley, A. Retention and Release of Aroma Compounds in Foods Containing Proteins. Food Technol. 1998, 52, 68–74. [Google Scholar]
- Deeth, H.; Bansal, N. Whey Proteins: An Overview—ScienceDirect. In Whey Proteins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–50. [Google Scholar]
- Kühn, J.; Considine, T.; Singh, H. Interactions of Milk Proteins and Volatile Flavor Compounds: Implications in the Development of Protein Foods. J. Food Sci. 2006, 71, R72–R82. [Google Scholar] [CrossRef]
- Tromelin, A.; Guichard, E. Use of Catalyst in a 3D-QSAR Study of the Interactions between Flavor Compounds and β-Lactoglobulin. J. Agric. Food Chem. 2003, 51, 1977–1983. [Google Scholar] [CrossRef]
- Tromelin, A.; Guichard, E. 2D- and 3D-QSAR Models of Interaction between Flavor Compounds and beta-Lactoglobulin Using Catalyst and Cerius2. QSAR Comb. Sci. 2004, 23, 214–233. [Google Scholar] [CrossRef]
- Anantharamkrishnan, V.; Hoye, T.; Reineccius, G.A. Covalent Adduct Formation Between Flavor Compounds of Various Functional Group Classes and the Model Protein β-Lactoglobulin. J. Agric. Food Chem. 2020, 68, 6395–6402. [Google Scholar] [CrossRef]
- Chung, S.; Villota, R. Binding of Alcohols by Soy Protein in Aqueous Solutions. J. Food Sci. 2010, 54, 1604–1606. [Google Scholar] [CrossRef]
- Wiking, L.; Stagsted, J.; Lennart, B.; Nielsen, J.H. Milk Fat Globule Size is Affected by Fat Production in Dairy Cows. Int. Dairy J. 2004, 14, 909–913. [Google Scholar] [CrossRef]
- Mercan, E.; Sert, D.; Akın, N. Effect of High-pressure Homogenisation on Viscosity, Particle size and Microbiological Characteristics of Skim and Whole Milk Concentrates. Int. Dairy J. 2018, 87, 93–99. [Google Scholar] [CrossRef]
- Kieczewska, K.; Ambroziak, K.; Krzykowska, D.; Aljewicz, M. The Effect of High-pressure Homogenisation on the Size of Milk Fat Globules and MFGM Composition in Sweet Buttermilk and Milk. Int. Dairy J. 2021, 113, 104898. [Google Scholar] [CrossRef]
- Michalski, M.C.; Gassi, J.Y.; Famelart, M.H.; Leconte, N.; Camier, B.; Michel, F.; Briard, V. The Size of Native Milk Fat Globules Affects Physico-chemical and Sensory Properties of Camembert Cheese. Le Lait 2003, 83, 131–143. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Liu, J.; Xu, D.; Cao, Y.; Sun, B. The Effect of Unadsorbed Proteins on the Physiochemical Properties of the Heteroaggregates of Oppositely Charged Lactoferrin Coated Lutein Droplets and Whey Protein Isolate Coated DHA Droplets. Food Funct. 2018, 9, 3956–3964. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Xu, D.; Zhu, Y.; Cao, Y.; Wang, J.; Sun, B. Comparision of Heteroaggregation, Layer-by-layer and Directly Mixing Techniques on the Physical Properties and in Vitro Digestion of Emulsions. Food Hydrocoll. 2019, 95, 228–237. [Google Scholar] [CrossRef]
- Wiking, L.; Gregersen, S.B.; Hansen, S.F.; Hammershøj, M. Heat-induced Changes in Milk Fat and Milk Fat Globules and its Derived Effects on Acid Dairy Gelation—A Review. Int. Dairy J. 2021, 127, 105213. [Google Scholar] [CrossRef]
- Anema, S.G. Effect of pH on the Association of Denatured Whey Proteins with Casein Micelles in Heated Reconstituted Skim Milk. J. Agric. Food Chem. 2007, 55, 3635–3642. [Google Scholar] [CrossRef]
- Huppertz, T. Heat Stability of Milk. In Advanced Dairy Chemistry: Volume 1B: Proteins: Applied Aspects; McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer: New York, NY, USA, 2016; pp. 179–196. [Google Scholar]
- Zhang, G. Evaluating the Viscoelastic Properties of Biological Tissues in a New Way. J. Musculoskelet. Neuronal Interact 2005, 5, 85–90. [Google Scholar] [PubMed]
- Mason, T.G.; Weitz, D.A. Optical Measurements of Frequency-dependent Linear Viscoelastic Moduli of Complex Fluids. Phys. Rev. Lett. 1995, 74, 1250. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T.; Gazi, I.; Luyten, H.; Nieuwenhuijse, H.; Alting, A.; Schokker, E. Hydration of Casein Micelles and Caseinates: Implications for Casein Micelle Structure. Int. Dairy J. 2017, 74, 1–11. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, M.; Zhang, X.; Li, X.; Chen, D.; Qin, Y.; Wang, J.; Wang, C. The Effect of Heat Treatment on the Microstructure and Functional Properties of Whey Protein from Goat Milk. J. Dairy Sci. 2020, 103, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Magan, J.B.; Tobin, J.T.; O’Callaghan, T.F.; Kelly, A.L.; Fenelon, M.A.; Hennessy, D.; Mccarthy, N.A. Physicochemical Properties of Whole Milk Powder Derived from Cows Fed Pasture or Total Mixed Ration Diets. J. Dairy Sci. 2019, 102, 9611–9621. [Google Scholar] [CrossRef]
- Huppertz, T.; Patel, H.G. Heat-induced Changes in Milk Proteins in High-carbohydrate Media. In Proceedings of the 2014 ADSA-ASAS-CSAS Joint Annual Meeting, Kansas City, MO, USA, 20–24 July 2014. [Google Scholar]
- Parmar, P.; Singh, A.K.; Meena, G.S.; Borad, S.; Raju, P. Application of Ohmic Heating for Concentration of Milk. J. Food Sci. Technol. 2018, 55, 4956–4963. [Google Scholar] [CrossRef]
- Wijayanti, H.B.; Brodkorb, A.; Hogan, S.A.; Murphy, E.G. Thermal Denaturation, Aggregation, and Methods of Prevention. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 185–247. [Google Scholar]
- Oldfield, D.J.; Singh, H.; Taylor, M.W.; Pearce, K.N. Kinetics of Denaturation and Aggregation of Whey Proteins in Skim Milk Heated in an Ultra-high Temperature (UHT) Pilot Plant. Int. Dairy J. 1998, 8, 311–318. [Google Scholar] [CrossRef]
- Obeid, S.; Guyomarc’h, F.; Tanguy, G.; Leconte, N.; Rousseau, F.; Dolivet, A.; Leduc, A.; Wu, X.; Cauty, C.; Jan, G.; et al. The Adhesion of Homogenized Fat Globules to Proteins is Increased by Milk Heat Treatment and Acidic pH: Quantitative insights provided by AFM force spectroscopy. Food Res. Int. 2020, 129, 108847. [Google Scholar] [CrossRef]
- Akkerman, M.; Rauh, V.M.; Christensen, M.; Johansen, L.B.; Hammershoj, M.; Larsen, L.B. Effect of Heating Strategies on Whey Protein Denaturation—Revisited by Liquid Chromatography Quadrupole Time-of-flight mass Spectrometry. J. Dairy Sci. 2016, 99, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Solano-Lopez, C.E.; Ji, T.; Alvarez, V.B. Volatile Compounds and Chemical Changes in Ultrapasteurized Milk Packaged in Polyethylene Terephthalate Containers. J. Food Sci. 2010, 70, c407–c412. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M.; Leardi, R.; Toppino, P.M. Influence of Heat Treatment on the Volatile Compounds of Milk. J. Agric. Food Chem. 1997, 45, 3171–3177. [Google Scholar] [CrossRef]
- Fuquay, J.W.; McSweeney, P.L.; Fox, P.F. Encyclopedia of Dairy Sciences, 2nd ed.; Fox, P.F., Mcsweeney, P.L.H., Eds.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Ho, C.T.; Mussinan, C.J.; Shahidi, F.; Contis, E.T. Fat-derived Volatiles of Various Products of Cows’, Goats’ and Ewes’ Milk. In Recent Advances in Food and Flavor Chemistry; Vagenas, G., Roussis, I.G., Eds.; Royal Society of Chemistry: London, UK, 2010; pp. 132–138. [Google Scholar] [CrossRef]
- Davis, J.G. Dairy Science and Technology: Personal Recollections. Int. J. Dairy Technol. 1987, 40, 79–81. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Effect of Heating on Maillard Reactions in Milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Nursten, H.E. Recent Developments in Studies of the Maillard Reaction. Food Chem. 1981, 6, 263–277. [Google Scholar] [CrossRef]
- Havemose, M.S.; Justesen, P.; Bredie, W.L.P.; Nielsen, J.H. Measurement of Volatile Oxidation Products from Milk using Solvent-assisted Flavour Evaporation and Solid Phase Microextraction. Int. Dairy J. 2007, 17, 746–752. [Google Scholar] [CrossRef]
- Shirley, R.L. Nitrogen and Energy Nutrition of Ruminants; Library of Congress Cataloging in Publication Data; Academic Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter Partn. B.V: Utrecht, The Netherlands, 2013. [Google Scholar]
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.A.; Qian, M.C. Quantitative Determination of Thermally Derived Off-Flavor Compounds in Milk Using Solid-Phase Microextraction and Gas Chromatography. J. Dairy Sci. 2005, 88, 3764–3772. [Google Scholar] [CrossRef]
- Sun, B. Handbook of Edible Spices; China Petrochemical Press: Beijing, China, 2004. [Google Scholar]
- Zhang, X.M.; Ai, N.S.; Wang, J.; Tong, L.J.; Sun, B.G. Lipase-catalyzed Modification of the Flavor Profiles in Recombined Skim Milk Products by Enriching the Volatile Components. J. Dairy Sci. 2016, 99, 8665–8679. [Google Scholar] [CrossRef]
- Yue, J.; Zheng, Y.; Liu, Z.; Deng, Y.; Jing, Y.; Luo, Y.; Yu, W.; Zhao, Y. Characterization of Volatile Compounds in Microfiltered Pasteurized Milk Using Solid-Phase Microextraction and GC×GC-TOFMS. Int. J. Food Prop. 2015, 18, 2193–2212. [Google Scholar] [CrossRef]
- Calvo, M.M.; de la Hoz, L. Flavour of Heated Milks. A review. Int. Dairy J. 1992, 2, 69–81. [Google Scholar] [CrossRef]
- Ard, Y. Flavour Formation by Amino acid Catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, A.B.; Vestergaard, J.S.; Varming, C.; Bredie, W.L.P.; Ipsen, R.H. Composition of Volatile Compounds in Bovine Milk Heat Treated by Instant Infusion Pasteurisation and Their Correlation to Sensory Analysis. Int. J. Dairy Technol. 2011, 64, 34–44. [Google Scholar] [CrossRef]
- Valero, E.; Villamiel, M.; Miralles, B.; Sanz, J.; Martınez-Castro, I. Changes in Flavour and Volatile Components during Storage of Whole and Skimmed UHT Milk. Food Chem. 2001, 72, 51–58. [Google Scholar] [CrossRef]
- Licón, C.C.; Mendoza, J.H.D.; Maggi, L.; Berruga, M.I.; Aranda, R.M.M.; Carmona, M. Optimization of Headspace Sorptive Extraction for the Analysis of Volatiles in Pressed Ewes’ Milk Cheese. Int. Dairy J. 2012, 23, 53–61. [Google Scholar] [CrossRef]
- High, R.; Bremer, P.; Kebede, B.; Eyres, G.T. Comparison of Four Extraction Techniques for the Evaluation of Volatile Compounds in Spray-Dried New Zealand Sheep Milk. Molecules 2019, 24, 1917. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Han, Z.; Blank, I.; Meng, F.; Wang, B.; Cao, Y.P.; Huaixiang, T.; Chen, C. Isolation, Identification and Sensory Evaluation of Kokumi Peptides from by-Products of Enzyme-Modified Butter. J. Sci. Food Agric. 2022, 102, 6668–6675. [Google Scholar] [CrossRef]
- Barrefors, P.; Granelli, K.; Appelqvist, L.A.; Bjoerck, L. Chemical Characterization of Raw Milk Samples with and Without Oxidative Off-Flavor. J. Dairy Sci. 1995, 78, 2691–2699. [Google Scholar] [CrossRef]
- Mills, O.E.; Solms, J. Interaction of Selected Flavour Compounds with Whey Proteins. Lebensm.-Wiss.-Technol. 1984, 17, 331–335. [Google Scholar]
- Weel, K.; Boelrijk, A.E.; Burger, J.J.; Gruppen, H.; Smit, G. Effect of Aroma-Protein Interactions on in Vivo Aroma Release. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2003; p. 65. [Google Scholar]
- Dinu, V.; Borah, P.K.; Muleya, M.; Scott, D.J.; Lithgo, R.; Pattem, J.; Harding, S.E.; Yakubov, G.E.; Fisk, I.D. Flavour Compounds Affect Protein Structure: The Effect of Methyl Anthranilate on Bovine Serum Albumin Conformation. Food Chem. 2022, 388, 133013. [Google Scholar] [CrossRef]
- Logan, A.; Day, L.; Pin, A.; Auldist, M.; Leis, A.; Puvanenthiran, A.; Augustin, M.A. Interactive Effects of Milk Fat Globule and Casein Micelle Size on the Renneting Properties of Milk. Food Bioprocess Technol. 2014, 7, 3175–3185. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xu, D.; Cao, Y.; Wang, S.; Wang, B.; Sun, B.; Yuan, F.; Gao, Y. Enhancing Physicochemical Properties of Emulsions by Heteroaggregation of Oppositely Charged Lactoferrin Coated Lutein Droplets and Whey Protein Isolate Coated DHA Droplets. Food Chem. 2018, 239, 75–85. [Google Scholar] [CrossRef]
- Gruppi, A.; Dermiki, M.; Spigno, G.; FitzGerald, R.J. Impact of Enzymatic Hydrolysis and Heat Inactivation on the Physicochemical Properties of Milk Protein Hydrolysates. Foods 2022, 11, 516. [Google Scholar] [CrossRef]
- Li, Y.; Joyner, H.S.; Carter, B.G.; Drake, M.A. Effects of Fat Content, Pasteurization Method, Homogenization Pressure, and Storage Time on the Mechanical and Sensory Properties of Bovine Milk. J. Dairy Sci. 2018, 101, 2941–2955. [Google Scholar] [CrossRef]
- Magan, J.B.; O’Callaghan, T.F.; Zheng, J.M.; Zhang, L.; Mandal, R.; Hennessy, D.; Fenelon, M.A.; Wishart, D.S.; Kelly, A.L.; McCarthy, N.A. Impact of Bovine Diet on Metabolomic Profile of Skim Milk and Whey Protein Ingredients. Metabolites 2019, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Genene, A.; Hansen, E.B.; Eshetu, M.; Hailu, Y.; Ipsen, R. Effect of Heat Treatment on Denaturation of Whey Protein and Resultant Rennetability of Camel Milk. LWT 2019, 101, 404–409. [Google Scholar] [CrossRef]
- Uk, L. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Wang, J.; Yang, Z.J.; Wang, Y.D.; Cao, Y.P.; Wang, B.; Liu, Y. The Key Aroma Compounds and Sensory Characteristics of Commercial Cheddar Cheeses. J. Dairy Sci. 2021, 104, 7555–7571. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Song, S.; Xiao, Z.; Niu, Y.; Zhu, G.; Yu, D. Characterization of Aroma Compounds of Chinese Famous Liquors by Gas Chromatography-Mass Spectrometry and Flash GC Electronic-nose. J. Chromatogr. B 2014, 945, 92–100. [Google Scholar]
| Raw Milk | 75 °C HTST | 85 °C HTST | IND-UHT | DSI-IUHT | |
|---|---|---|---|---|---|
| TP% | 3.465 ± 0.009 a | 3.550 ± 0.164 a | 3.613 ± 0.094 a | 3.618 ± 0.081 a | 3.595 ± 0.023 a |
| UWP% | 0.716 ± 0.005 a | 0.570 ± 0.003 b | 0.540 ± 0.006 c | 0.242 ± 0.005 e | 0.435 ± 0.001 d |
| NPN% | 0.029 ± 0.000 a | 0.028 ± 0.000 a | 0.029 ± 0.000 a | 0.031 ± 0.000 a | 0.029 ± 0.000 a |
| WPD rate% | - | 23.06 ± 2.27 a | 28.64 ± 1.26 b | 70.59 ± 0.98 c | 43.07 ± 0.51 d |
| No | Compound | RI | CAS 1 | Molecule Formula | Content (μg/kg) | Identification 2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Milk | 75 °C HTST | 85 °C HTST | IND-UHT | DSI-IUHT | ||||||
| Aldehyde Compounds | ||||||||||
| 1 | Nonanal | 1382 | 124-19-6 | C9H18O | ND | ND | 2.59 ± 0.23 | 5.58 ± 0.34 | ND | MS, RI |
| Total aldehydes | ND | ND | 2.59 | 5.58 | ND | |||||
| Ketone compounds | ||||||||||
| 2 | 2-Heptanone | 1182 | 110-43-0 | C7H14O | ND | ND | 0.24 ± 0.19 | 54.41 ± 2.80 | 12.40 ± 0.96 | MS, RI |
| 3 | 2-Nonanone | 1390 | 821-55-6 | C9H18O | ND | ND | ND | 25.16 ± 0.22 | 6.62 ± 0.78 | MS, RI |
| Total ketones | ND | ND | 0.24 | 79.57 | 19.02 | |||||
| Alcohol compounds | ||||||||||
| 4 | 3-Hexanol | 1232 | 623-37-0 | C6H14O | ND | ND | ND | ND | 9.59 ± 1.55 | MS, RI |
| 5 | 2-Ethyl-1-hexanol | 1484 | 104-76-7 | C8H18O | ND | 0.88 ± 0.13 | ND | ND | 1.01 ± 0.11 | MS, RI |
| 6 | 1-Octanol | 1606 | 111-87-5 | C8H18O | ND | 0.71 ± 0.05 | 1.84 ± 0.14 | ND | ND | MS, RI |
| 7 | 1-Dodecanol | 1953 | 112-53-8 | C12H26O | ND | ND | ND | 10.62 ± 0.26 | ND | MS, RI |
| Total alcohols | ND | 1.59 | 1.84 | 10.62 | 10.6 | |||||
| Acids compounds | ||||||||||
| 8 | Formic acid | 1438 | 64-18-6 | CH2O2 | ND | 3.07 ± 0.39 | 33.46 ± 2.63 | ND | 4.32 ± 0.27 | MS, RI |
| 9 | Acetic acid | 1449 | 64-19-7 | C2H4O2 | 3.73 ± 0.18 | 4.90 ± 0.61 | 13.50 ± 1.51 | ND | 6.42 ± 1.04 | MS, RI |
| 10 | Butanoic acid | 1637 | 107-92-6 | C4H8O2 | 1.98 ± 0.25 | 0.49 ± 0.04 | 0.63 ± 0.03 | ND | ND | MS, RI |
| 11 | Hexanoic acid | 1846 | 142-62-1 | C6H12O2 | 5..44 ± 0.18 | 0.85 ± 0.08 | 1.97 ± 0.08 | 0.86 ± 0.03 | ND | MS, RI |
| 12 | Octanoic acid | 2050 | 124-07-2 | C8H16O2 | 8.87 ± 0.82 | ND | 4.37 ± 0.23 | 4.74 ± 0.23 | ND | MS, RI |
| 13 | Nonanoic acid | 2171 | 112-05-0 | C9H18O2 | 4.12 ± 0.24 | ND | ND | ND | ND | MS, RI |
| 14 | Decanoic acid | 2279 | 334-48-5 | C10H20O2 | 15.80 ± 1.05 | ND | 3.97 ± 0.35 | 112.83 ± 2.18 | 3.16 ± 0.09 | MS, RI |
| 15 | Tetradecanoic acid | 2716 | 544-63-8 | C14H28O2 | 1.59 ± 0.10 | 3.07 ± 0.36 | 0.54 ± 0.01 | ND | 2.08 ± 0.25 | MS, RI |
| 16 | Hexadecanoic acid | 2928 | 57-10-3 | C16H32O2 | 13.64 ± 2.04 | ND | ND | ND | ND | MS, RI |
| Total acids | 49.73 | 11.53 | 56.47 | 117.57 | 15.98 | |||||
| Esters compounds | ||||||||||
| 17 | Hexadecanoic acid, methyl ester | 2271 | 112-39-0 | C17H34O2 | ND | ND | ND | 9.37 ± 0.16 | ND | MS, RI |
| Total esters | 0 | 0 | 0 | 9.37 | 0 | |||||
| Sulfurics components | ||||||||||
| 18 | Dimethyl sulfide | 1120 | 75-18-3 | C2H6S | 34.93 ± 0.94 | 53.91 ± 5.00 | 64.92 ± 8.19 | ND | 54.85 ± 3.88 | MS, RI |
| 19 | Dimethyl disulfide | 1128 | 624-92-0 | C2H6S2 | ND | 3.64 ± 0.26 | ND | ND | 2.11 ± 0.31 | MS, RI |
| 20 | Dimethyl sulfone | 1887 | 67-71-0 | C2H6O2S | ND | 0.15 ± 0.02 | 0.31 ± 0.02 | 0.52 ± 0.08 | 0.33 ± 0.05 | MS, RI |
| Total sulfurics | 34.93 | 57.7 | 65.23 | 0.52 | 57.29 | |||||
| Aromatic heterocyclic compounds | ||||||||||
| 21 | Methyl-benzene | 1042 | 108-88-3 | C7H8 | 36.34 ± 0.42 | 54.06 ± 8.92 | 37.37 ± 6.12 | 101.47 ± 5.81 | 64.91 ± 7.20 | MS, RI |
| 22 | Styrene | 1254 | 100-42-5 | C8H8 | 0.60 ± 0.04 | 7.21 ± 0.35 | 0.68 ± 0.10 | 0.66 ± 0.12 | 4.62 ± 0.11 | MS, RI |
| 23 | P-xylene | 1119 | 106-42-3 | C8H10 | ND | 3.44 ± 0.56 | ND | ND | 2.83 ± 0.35 | MS, RI |
| 24 | Limonene | 1200 | 138-86-3 | C10H16 | ND | 1.75 ± 0.11 | ND | 4.80 ± 0.35 | ND | MS, RI |
| 25 | Benzaldehyde | 1529 | 100-52-7 | C7H6O | ND | ND | ND | 4.15 ± 0.21 | 2.72 ± 0.30 | MS, RI |
| 26 | 4-Ethyl-benzaldehyde | 1730 | 4748-78-1 | C9H10O | ND | 0.33 ± 0.02 | ND | ND | 25.83 ± 4.01 | MS, RI |
| 27 | 2-Furancarboxaldehyde | 1550 | 98-01-1 | C5H4O2 | ND | 0.95 ± 0.05 | 32.33 ± 2.72 | 0.46 ± 0.02 | 0.43 ± 0.03 | MS, RI |
| 28 | Acetophenone | 1697 | 98-86-2 | C8H8O | ND | 61.16 ± 5.49 | 18.72 ± 1.08 | ND | 45.83 ± 4.39 | MS, RI |
| 29 | Maltol | 2031 | 118-71-8 | C6H6O3 | ND | ND | 10.15 ± 0.77 | ND | ND | MS, RI |
| Total aromatic heterocyclic | 36.94 | 128.9 | 99.25 | 111.54 | 147.17 | |||||
| No | Compound | RI | CAS 1 | Molecule Formula | Content (μg/kg) | Identification 2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Milk | 75 °C HTST | 85 °C HTST | IND-UHT | DSI-IUHT | ||||||
| Aldehyde Compounds | ||||||||||
| 1 | Pentanal | 1037 | 110-62-3 | C5H10O | ND | ND | ND | 79.74 ± 3.22 | ND | MS, RI |
| 2 | Hexanal | 1035 | 66-25-1 | C6H12O | 161.20 ± 1.13 | 139.49 ± 11.35 | 100.95 ± 7.58 | 21.26 ± 2.89 | 61.27 ± 0.73 | MS, RI |
| 3 | Nonanal | 1382 | 124-19-6 | C9H18O | 620.31 ± 33.69 | 901.24 ± 19.01 | 849.72 ± 41.09 | 29.77 ± 4.92 | 329.09 ± 8.06 | MS, RI |
| 4 | Decanal | 1485 | 112-31-2 | C10H20O | ND | 116.38 ± 5.58 | ND | ND | ND | MS, RI |
| 5 | Dodecanal | 1711 | 112-54-9 | C12H24O | ND | ND | 699.85 ± 16.02 | ND | ND | MS, RI |
| 6 | Tetradecanal | 2229 | 124-25-4 | C14H28O | ND | ND | 660.78 ± 94.73 | ND | ND | MS, RI |
| 7 | (Z)-6-Nonenal | 1459 | 2277-19-2 | C9H16O | ND | ND | ND | 421.69 ± 46.15 | ND | MS, RI |
| Total aldehydes | 781.51 | 1118.57 | 2349.84 | 552.4576 | 390.36 | |||||
| Alcohol compounds | ||||||||||
| 8 | 2-Methyl-3-pentanol | 1167 | 565-67-3 | C6H14O | ND | ND | ND | 70.57 ± 9.31 | ND | MS, RI |
| 9 | (Z)-3-Hexen-1-ol | 1340 | 928-96-1 | C6H12O | ND | ND | ND | 474.71 ± 36.64 | ND | MS, RI |
| 10 | (Z)-4-Hexen-1-ol | 1407 | 928-91-6 | C6H12O | ND | ND | ND | 24.83 ± 2.96 | ND | MS, RI |
| 11 | 2-Ethyl-1-hexanol | 1484 | 104-76-7 | C8H18O | ND | 60.55 ± 2.05 | 149.54 ± 15.80 | 8.86 ± 1.02 | ND | MS, RI |
| 12 | 3-Methyl-2-hexanol | 1331 | 2313-65-7 | C7H16O | 101.88 ± 3.92 | ND | ND | ND | ND | MS, RI |
| 13 | 1-Dodecanol | 1953 | 112-53-8 | C12H26O | 451.93 ± 3.93 | 665.08 ± 31.17 | 1050.33 ± 49.34 | 123.79 ± 16.75 | 544.24 ± 2.88 | MS, RI |
| 14 | 1-Tetradecanol | 2200 | 112-72-1 | C14H30O | 660.65 ± 45.34 | 626.46 ± 40.78 | 714.11 ± 86.05 | ND | ND | MS, RI |
| 15 | 1-Hexadecanol | 2400 | 36653-82-4 | C16H34O | 365.00 ± 51.16 | ND | ND | ND | ND | MS, RI |
| Total alcohols | 1579.46 | 1352.09 | 1913.98 | 702.75432 | 544.24 | |||||
| Acids compounds | ||||||||||
| 16 | Acetic acid | 1449 | 64-19-7 | C2H4O2 | 273.94 ± 22.12 | 391.62 ± 20.56 | 479.38 ± 25.45 | ND | 317.44 ± 4.97 | MS, RI |
| 17 | Propanoic acid | 1526 | 79-09-4 | C3H6O2 | 55.18 ± 8.45 | ND | ND | ND | 57.75 ± 5.40 | MS, RI |
| 18 | Butanoic acid | 1637 | 107-92-6 | C4H8O2 | 93.92 ± 9.13 | ND | 92.83 ± 3.31 | 102.03 ± 5.64 | 33.00 ± 1.54 | MS, RI |
| 19 | Hexanoic acid | 1846 | 142-62-1 | C6H12O2 | 770.46 ± 90.07 | 381.85 ± 15.96 | 677.05 ± 38.07 | 957.84 ± 65.42 | 303.89 ± 11.12 | MS, RI |
| 20 | Heptanoic acid | 1918 | 111-14-8 | C7H14O2 | 126.97 ± 14.53 | 183.48 ± 2.55 | 262.58 ± 23.76 | ND | ND | MS, RI |
| 21 | Octanoic acid | 2050 | 124-07-2 | C8H16O2 | 6071.59 ± 372.34 | 2125.93 ± 103.15 | 4496.77 ± 339.10 | 2347.32 ± 169.45 | 1081.68 ± 78.52 | MS, RI |
| 22 | Nonanoic acid | 2171 | 112-05-0 | C9H18O2 | 1041.09 ± 64.46 | 1398.84 ± 59.43 | 1829.44 ± 55.52 | 2410.64 ± 231.67 | 512.19 ± 67.26 | MS, RI |
| 23 | Decanoic acid | 2279 | 334-48-5 | C10H20O2 | 3156.02 ± 70.91 | 1618.49 ± 40.82 | 1456.28 ± 125.20 | 3174.16 ± 162.65 | 1169.86 ± 31.19 | MS, RI |
| 24 | Tetradecanoic acid | 2716 | 544-63-8 | C14H28O2 | 1560.06 ± 29.90 | 2161.64 ± 131.16 | 3006.48 ± 201.21 | 2415.36 ± 91.68 | 584.99 ± 23.58 | MS, RI |
| 25 | Hexadecanoic acid | 2928 | 57-10-3 | C16H32O2 | 15,609.72 ± 896.56 | 16,850.52 ± 4304.50 | 34,828.35 ± 2413.06 | 84,559.69 ± 5620.35 | 25,243.76 ± 1286.06 | MS, RI |
| 26 | 9-Decenoic acid | 2356 | 14436-32-9 | C10H18O2 | 460.54 ± 9.51 | ND | ND | ND | ND | MS, RI |
| Total acids | 29,219.39 | 25,112.35 | 47,129.21 | 95,967.046 | 29,304.6 | |||||
| Esters compounds | ||||||||||
| 27 | Acetic acid, butyl ester | 887 | 123-86-4 | C6H12O2 | ND | 144.03 ± 0.02 | 295.78 ± 18.68 | ND | 125.51 ± 4.43 | MS, RI |
| 28 | Methyl tetradecanoate | 2066 | 124-10-7 | C15H30O2 | 33.03 ± 4.06 | ND | 110.77 ± 20.87 | ND | ND | MS, RI |
| 29 | Isopropyl myristate | 2063 | 110-27-0 | C17H34O2 | 167.62 ± 17.98 | 376.16 ± 27.13 | 384.50 ± 53.44 | 29.31 ± 3.94 | 147.05 ± 9.25 | MS, RI |
| 30 | Hexadecanoic acid, methyl ester | 2281 | 112-39-0 | C17H34O2 | 2570.46 ± 33.78 | 6715.59 ± 404.62 | 5090.39 ± 117.87 | 702.90 ± 44.01 | 989.49 ± 86.48 | MS, RI |
| 31 | Hexadecanoic acid, ethyl ester | 2288 | 628-97-7 | C18H36O2 | 1346.74 ± 10.11 | ND | ND | ND | 377.65 ± 20.43 | MS, RI |
| 32 | Octadecanoic acid, methyl ester | 2445 | 112-61-8 | C19H38O2 | 1277.59 ± 72.60 | 3420.89 ± 66.30 | 1955.53 ± 60.09 | ND | ND | MS, RI |
| 33 | Dibutyl phthalate | 2393 | 84-74-2 | C16H22O4 | ND | 14,894.02 ± 473.02 | 12,460.73 ± 764.53 | ND | 7270.73 ± 114.18 | MS, RI |
| Total esters | 5395.44 | 25,550.67 | 20,297.67 | 732.21 | 8910.429 | |||||
| Sulfurics components | ||||||||||
| 34 | Dimethyl sulfide | 760 | 75-18-3 | C2H6S | 157.11 ± 1.14 | 217.85 ± 3.87 | 409.93 ± 11.77 | ND | 210.18 ± 3.60 | MS, RI |
| 35 | Dimethyl sulfone | 1887 | 67-71-0 | C2H6O2S | 495.56 ± 2.08 | 629.96 ± 38.29 | 760.41 ± 19.10 | 166.31 ± 20.96 | 525.89 ± 4.26 | MS, RI |
| Total sulfurics | 652.67 | 847.81 | 1170.34 | 166.309 | 736.07 | |||||
| Aromatic heterocyclic compounds | ||||||||||
| 36 | Toluene | 1042 | 108-88-3 | C7H8 | ND | ND | ND | 311.89 ± 13.42 | ND | MS, RI |
| 37 | Methyl-benzene | 1105 | 108-88-3 | C7H8 | 1071.28 ± 8.97 | 3029.22 ± 110.98 | 1816.26 ± 52.82 | ND | 557.92 ± 15.13 | MS, RI |
| 38 | p-Xylene | 1119 | 106-42-3 | C8H10 | 288.45 ± 5.27 | 415.01 ± 15.36 | 833.04 ± 37.62 | ND | 152.99 ± 1.62 | MS, RI |
| 39 | Ethylbenzene | 1123 | 100-41-4 | C8H10 | 59.99 ± 6.84 | 108.94 ± 4.14 | 228.55 ± 9.11 | ND | 129.10 ± 15.52 | MS, RI |
| 40 | Limonene | 1200 | 138-86-3 | C10H16 | 535.53 ± 13.46 | 1313.19 ± 21.90 | 1589.56 ± 55.73 | 76.83 ± 5.34 | 132.16 ± 5.30 | MS, RI |
| 41 | 4-Ethyl-benzaldehyde | 1730 | 53951-50-1 | C9H10O | ND | ND | ND | ND | 86.67 ± 7.67 | MS, RI |
| 42 | 2-Furanmethanol | 1711 | 98-00-0 | C5H6O2 | ND | ND | ND | 11.29 ± 0.45 | ND | MS, RI |
| 43 | 2(5H)-Furanone | 1767 | 497-23-4 | C4H4O2 | ND | ND | ND | 161.53 ± 8.62 | ND | MS, RI |
| 44 | Acetophenone | 1699 | 98-86-2 | C8H8O | ND | ND | ND | ND | 50.72 ± 1.07 | MS, RI |
| 45 | Naphthalene | 1779 | 91-20-3 | C10H8 | ND | 56.85 ± 3.87 | ND | ND | ND | MS, RI |
| 46 | 2-Methyl-naphthalene | 1891 | 91-57-6 | C11H10 | ND | 96.41 ± 13.75 | ND | ND | ND | MS, RI |
| 47 | Butylated Hydroxytoluene | 1956 | 128-37-0 | C15H24O | 672.25 ± 2.92 | 480.87 ± 15.08 | 1282.75 ± 72.55 | ND | 327.57 ± 28.19 | MS, RI |
| Total aromatic heterocyclic | 2627.5 | 5500.49 | 5750.16 | 561.5468 | 1437.13 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Han, Z.; Wang, B.; Wang, Y.; Ran, Y.; Zhang, L.; Li, Y.; Lu, C.; Lu, X.; Ma, L. Effect of Direct Steam Injection and Instantaneous Ultra-High-Temperature (DSI-IUHT) Sterilization on the Physicochemical Quality and Volatile Flavor Components of Milk. Molecules 2023, 28, 3543. https://doi.org/10.3390/molecules28083543
Ding H, Han Z, Wang B, Wang Y, Ran Y, Zhang L, Li Y, Lu C, Lu X, Ma L. Effect of Direct Steam Injection and Instantaneous Ultra-High-Temperature (DSI-IUHT) Sterilization on the Physicochemical Quality and Volatile Flavor Components of Milk. Molecules. 2023; 28(8):3543. https://doi.org/10.3390/molecules28083543
Chicago/Turabian StyleDing, Hao, Zhaosheng Han, Bei Wang, Yadong Wang, Yawen Ran, Liebing Zhang, Yan Li, Chun Lu, Xiaoli Lu, and Ligang Ma. 2023. "Effect of Direct Steam Injection and Instantaneous Ultra-High-Temperature (DSI-IUHT) Sterilization on the Physicochemical Quality and Volatile Flavor Components of Milk" Molecules 28, no. 8: 3543. https://doi.org/10.3390/molecules28083543
APA StyleDing, H., Han, Z., Wang, B., Wang, Y., Ran, Y., Zhang, L., Li, Y., Lu, C., Lu, X., & Ma, L. (2023). Effect of Direct Steam Injection and Instantaneous Ultra-High-Temperature (DSI-IUHT) Sterilization on the Physicochemical Quality and Volatile Flavor Components of Milk. Molecules, 28(8), 3543. https://doi.org/10.3390/molecules28083543







