Effect of Acidic Electrolysed Water and Pulsed Light Technology on the Sensory, Morphology and Bioactive Compounds of Pennywort (Centella asiatica L.) Leaves
Abstract
1. Introduction
2. Results and Discussion
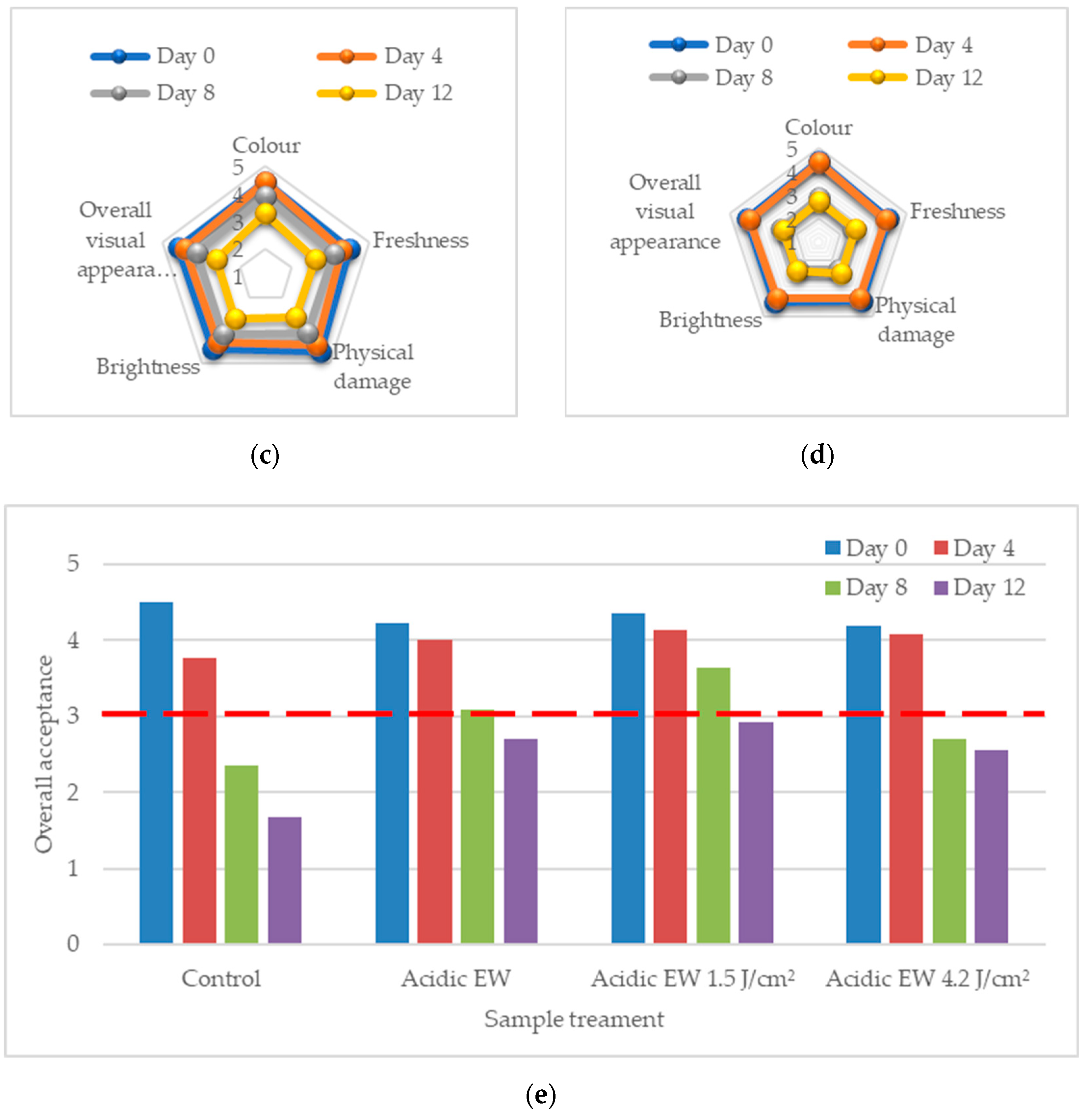
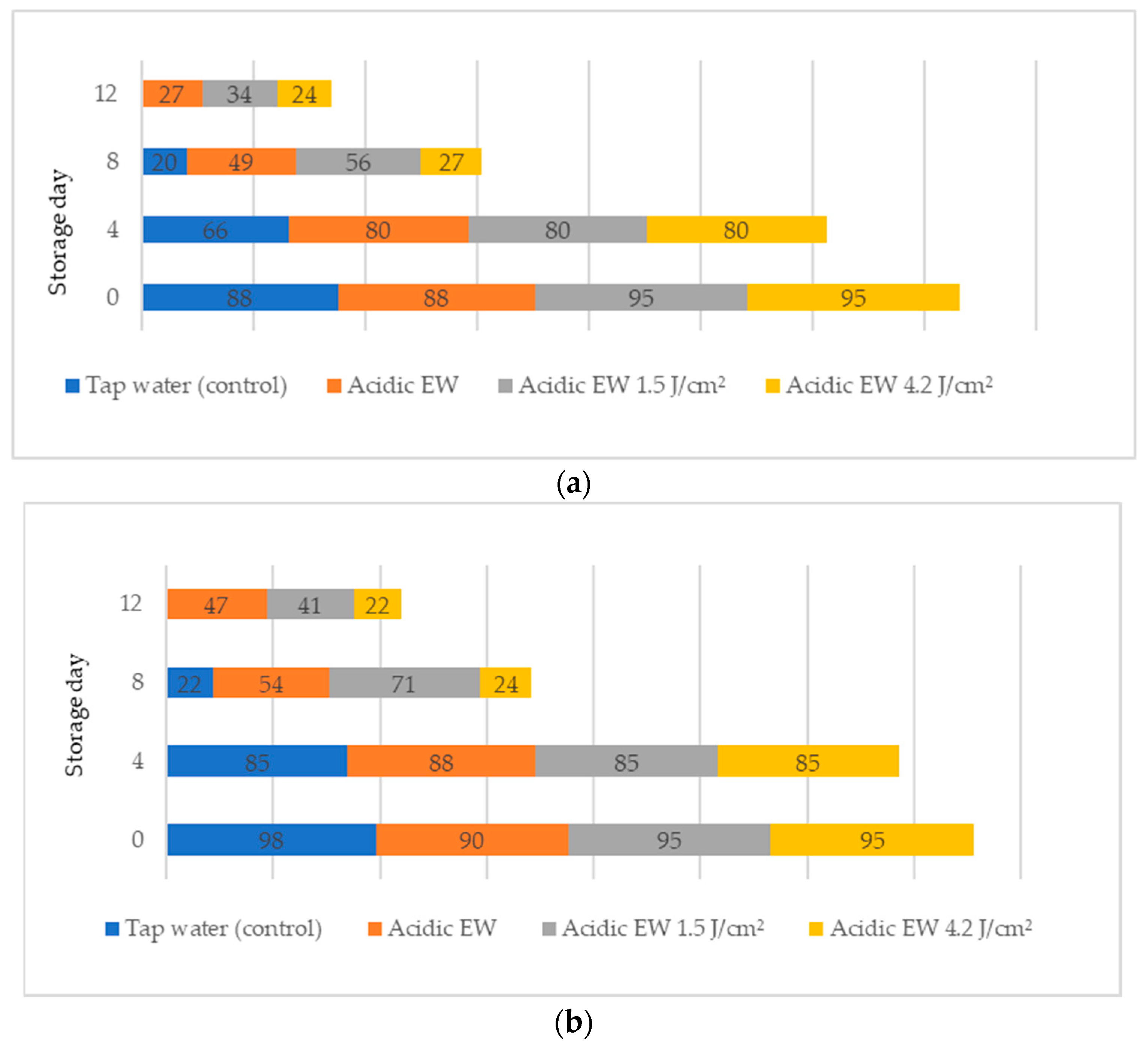
2.1. Sensory Acceptance Quality
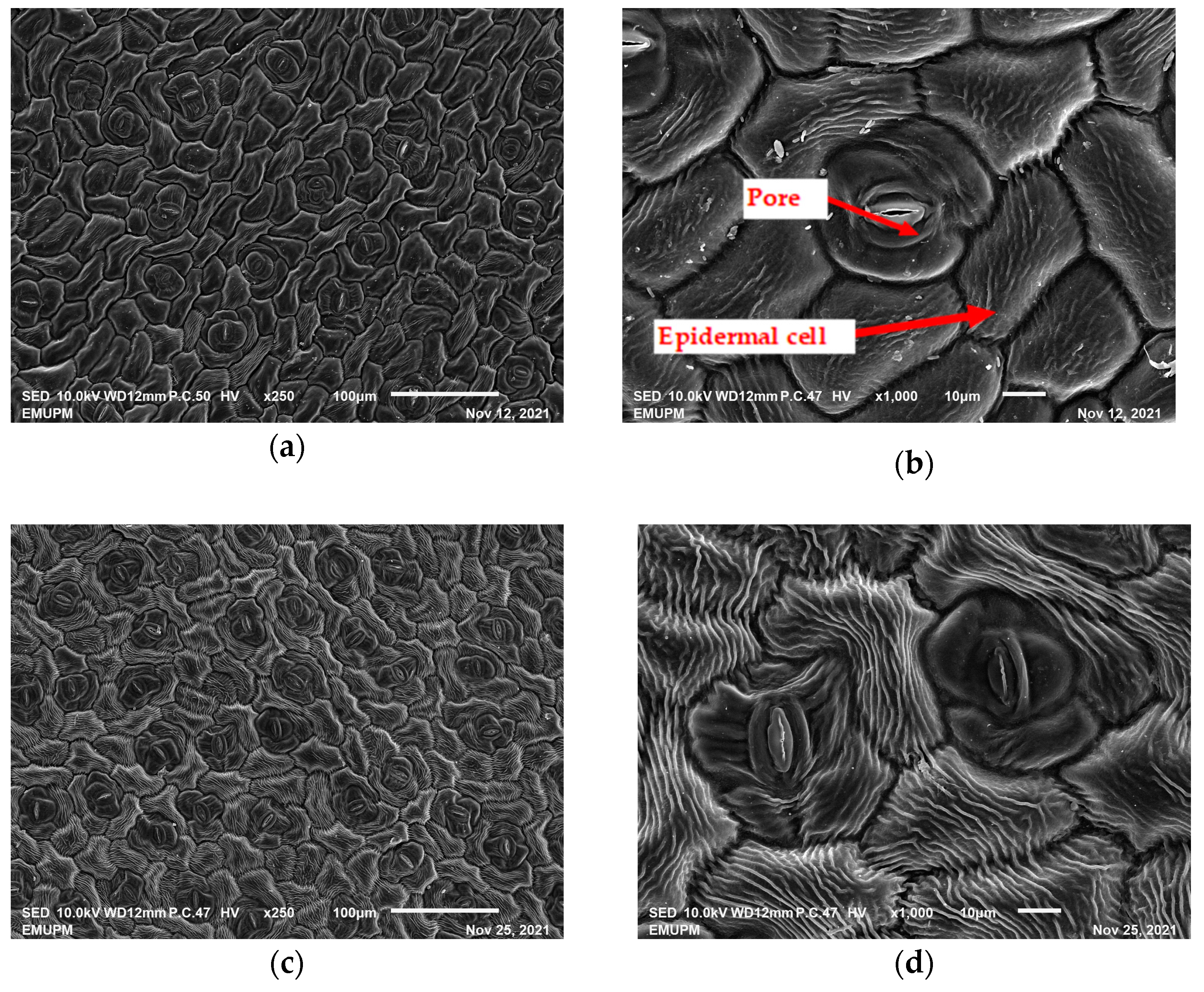
2.2. Scanning Electron Microscopic (SEM)
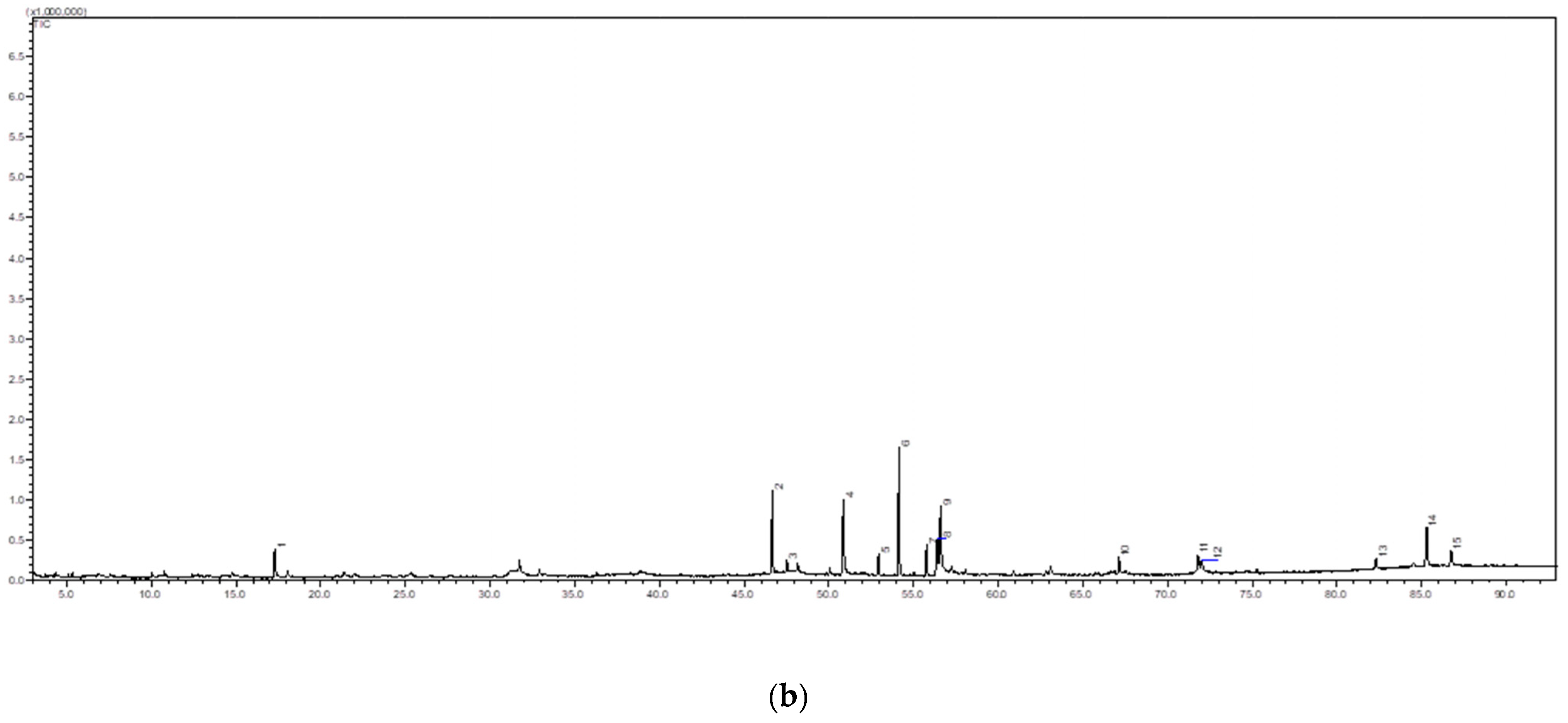
2.3. Transmission Electron Microscopic (TEM)
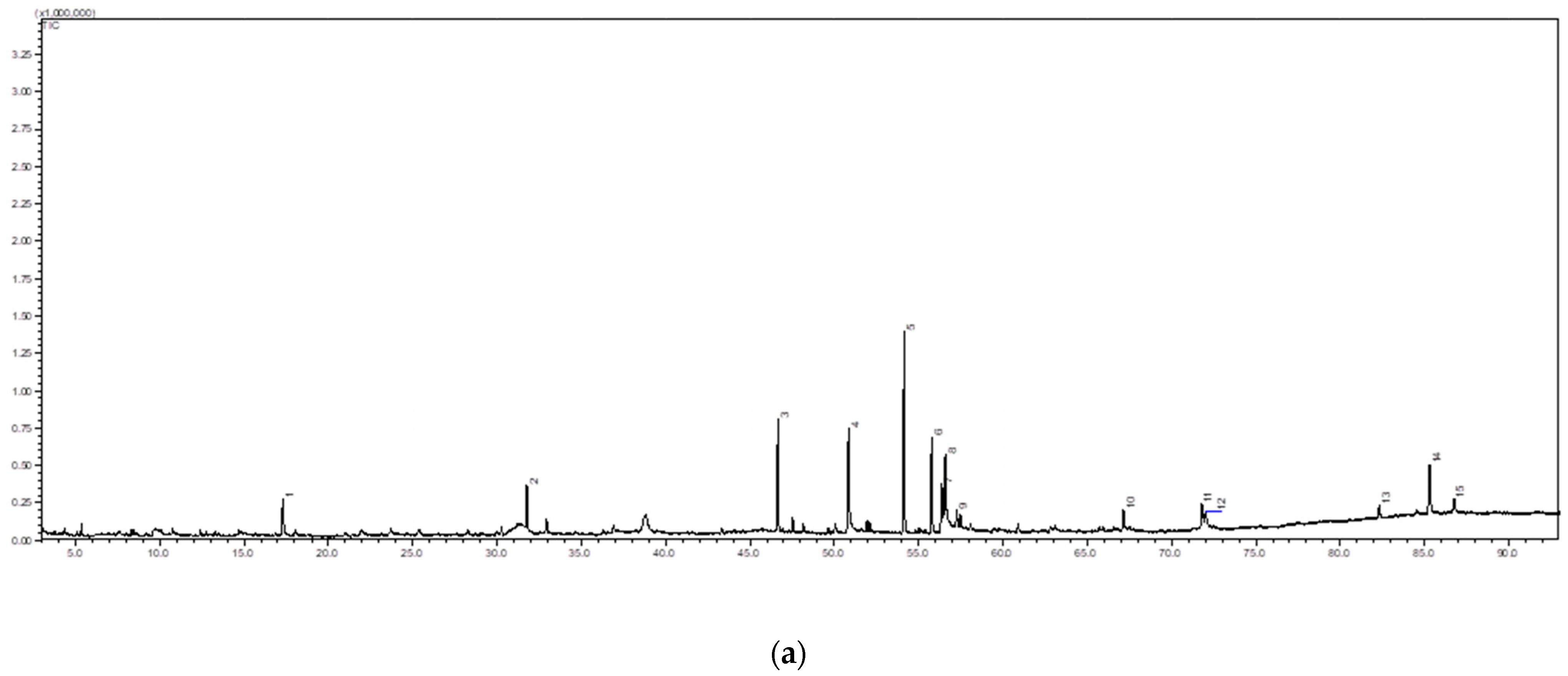
2.4. Phytochemical Screening of Centella asiatica
2.5. Quantification of the Triterpenes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Sample
3.3. Washing (Pre-Treatment)
3.4. Apparatus
3.5. Pulsed Light Treatment
3.6. Sensory Evaluation
3.6.1. Acceptability Test
3.6.2. Survey Question
3.7. Scanning Electron Microscopy (SEM)
3.8. Transmission Electron Microscopy (TEM)
3.9. Crude Extracts Preparation
3.9.1. Sonication-Assisted Solvent Extraction
3.9.2. Preparation of Samples and Standards
3.10. Gas Chromatography-Mass Spectrometry (GC-MS)
3.11. Ultra-High Pressure Liquid Chromatography (UHPLC)
3.12. Experimental Design
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- De São José, J.F.B.; de Andrade, N.J.; Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Santos, J.; Herrero, M.; Mendiola, J.A. Fresh-cut aromatic herbs: Nutritional quality stability during shelf-life. LWT Food Sci. Technol. 2014, 59, 101–107. [Google Scholar] [CrossRef]
- Baker, S.L.; McCabe, S.D.; Swithers, S.E.; Payne, C.R.; Kranz, S. Do healthy, child-friendly fruit and vegetable snacks appeal to consumers? A field study exploring adults’ perceptions and purchase intentions. Food Qual. Prefer. 2015, 39, 202–208. [Google Scholar] [CrossRef]
- You, Y.; Shahar, S.; Haron, H.; Yahya, H. More ulam for your brain: A review on the potential role of ulam in protecting against cognitive decline, (Lebihkan ulam untuk otak anda: Suatu kajian tentang potensi ulam dalam lindungi penyusutan fungsi kognitif). Sains Malays. 2018, 47, 2713–2729. [Google Scholar] [CrossRef]
- Purkait, S.; Abraham, T.J.; Karmakar, S.; Dey, B. Inhibition of fish pathogenic Aeromonas hydrophila and Edwardsiella tarda by Centella asiatica in-vitro Surveillance of diseases of aquacultured finfish and shellfish in West Bengal and development of disease management strategies. J. Aquac. Res. Dev. 2018, 9, 1000524. [Google Scholar] [CrossRef]
- Awang-Kanak, F.; Abu, M.F. Traditional vegetable salad (ulam) of Borneo as source of functional food. Food Res. 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Yasurin, P.; Sriariyanun, M.; Phusantisampan, T. The bioavailability activity of Centella asiatica. KMUTNB Int. J. Appl. Sci. Technol. 2015, 9, 1–9. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front. Pharmacol. 2020, 11, 1–24. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2014, 72, 546. [Google Scholar] [CrossRef]
- Ogunka-Nnoka, C.U.; Igwe, F.U.; Agwu, J.; Peter, O.J.; Wolugbom, P.H. Nutrient and phytochemical composition of Centella asiatica leaves. Med. Aromat. Plants 2020, 9. [Google Scholar] [CrossRef]
- Harun, N.H.; Septama, A.W.; Wan Ahmad, W.A.N.; Suppian, R. The potential of Centella asiatica (Linn.) Urban as an anti-microbial and immunomodulator agent: A review. Nat. Prod. Sci. 2019, 25, 92–102. [Google Scholar] [CrossRef]
- De Silva, M.L.E.; David, J.P.; Silva, L.C.R.C.; Santos, R.A.F.; David, J.M.; Lima, L.S.; Reis, P.S.; Fontana, R. Bioactive oleanane, lupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef] [PubMed]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef]
- Jeyaletchumi, P.; Tunung, R.; Selina, P.M.; Chai, L.C.; Radu, S.; Farinazleen, M.G.; Cheah, Y.K.; Mitsuaki, N.; Yoshitsugu, N.; Kumar, M.P. Evaluation of Listeria spp. and Listeria monocytogenes in selected vegetable farms. J. Trop. Agric. Food Sci. 2011, 39, 255–266. Available online: http://rac1.mardi.gov.my/jtafs/39-2/Evaluation%20of%20Listeria.pdf (accessed on 14 March 2020).
- Moravkova, M.; Kralik, P.; Verbikova, V.; Huvarova, V.; Babak, V.; Cahlikova, H.; Karpiskova, R. Occurrence of Cronobacter Spp. in ready-to-eat vegetable products, frozen vegetables, and sprouts examined using cultivation and real-time PCR methods. J. Food Sci. 2018, 83, 3054–3058. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Garbowska, M.; Stefańska, I.; Pluta, A.; Stefa, I.; Stefańska, I.; Pluta, A. Microbiological quality of selected ready-to-eat leaf vegetables, sprouts and non-pasteurized fresh fruit-vegetable juices including the presence of Cronobacter spp. Food Microbiol. 2017, 65, 221–230. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, S.; Wu, W.; Yang, K.; Zhang, Y.; Tumukunde, E.; Wang, S.; Wang, Y. Functional analysis of peptidyl-prolyl cis-trans isomerase from Aspergillus flavus. Int. J. Mol. Sci. 2019, 20, 2206. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; James Feng, L.X.; Bang, W.S.; Yuk, H.G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolysed water, ultraviolet light or/and ultrasounds. Food Control 2017, 74, 54–60. [Google Scholar] [CrossRef]
- Tawema, P.; Han, J.; Vu, K.D.; Salmieri, S.; Lacroix, M. Antimicrobial effects of combined UV-C or gamma radiation with natural antimicrobial formulations against Listeria monocytogenes, Escherichia coli O157:H7, and total yeasts/molds in fresh cut cauliflower. LWT Food Sci. Technol. 2016, 65, 451–456. [Google Scholar] [CrossRef]
- Režek Jambrak, A.; Nutrizio, M.; Djekić, I.; Pleslić, S.; Chemat, F. Internet of nonthermal food processing technologies (IoNTP): Food industry 4.0 and sustainability. Appl. Sci. 2021, 11, 686. [Google Scholar] [CrossRef]
- Siti Zaharah, R.; Noranizan, M.A.; Son, R.; Karim, R.; Yusof, N.; Koh, P.C.; Nor Hasni, H. Microbiological and physical properties of pennywort (Centella asiatica) leaves using pulsed light technology. Int. Food Res. J. 2020, 27, 16–27. [Google Scholar]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A.; Rosli, S.Z.; Nor Hasni, H. Enzymatic activity of alginate coated and pulsed light treated fresh-cut cantaloupes (Cucumis Melo L. Var. Reticulatus Cv. Glamour) during chilled storage. Int. Food Res. J. 2019, 26, 547–556. [Google Scholar] [CrossRef]
- Salehi, F. Application of pulsed light technology for fruits and vegetables disinfection: A review. J. Appl. Microbiol. 2021, 132, 2521–2530. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Pateiro, M.; Gavahian, M.; Franco, D.; Zhang, W.; Mousavi Khaneghah, A.; Guerrero-Sánchez, Y.; Lorenzo, J.M. Impact of pulsed light processing technology on phenolic compounds of fruits and vegetables. Trends Food Sci. Technol. 2021, 115, 1–11. [Google Scholar] [CrossRef]
- Agüero, M.V.; Jagus, R.J.; Martín-Belloso, O.; Soliva-Fortuny, R. Surface decontamination of spinach by intense pulsed light treatments: Impact on quality attributes. Postharvest Biol. Technol. 2016, 121, 118–125. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Cao, J.; Liu, Y.; Li, J.; Zhang, J.; Luo, S.; Yu, H. Revealing the structures of cellulose nanofiber bundles obtained by mechanical nanofibrillation via TEM observation. Carbohydr. Polym. 2015, 117, 950–956. [Google Scholar] [CrossRef]
- Koh, P.C.; Noranizan, M.A.; Abedin, Z.; Hanani, N.; Karim, R. Application of edible coatings and repetitive pulsed light for shelf life extension of fresh-cut cantaloupe (Cucumis melo L. reticulatus cv. Glamour). Postharvest Biol. Technol. 2017, 129, 64–78. [Google Scholar] [CrossRef]
- Valdivia-Nájar, C.G.; Martín-Belloso, O.; Giner-Seguí, J.; Soliva-Fortuny, R. Modeling the inactivation of Listeria innocua and Escherichia coli in fresh-cut tomato treated with pulsed light. Food Bioprocess Technol. 2017, 10, 266–274. [Google Scholar] [CrossRef]
- Uesugi, A.R.; Moraru, C.I. Reduction of Listeria on ready-to-eat sausages after exposure to a combination of pulsed light and nisin. J. Food Prot. 2009, 72, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Cullen, P.J.; Muhammad, A.I.; Jiang, Z.; Ye, X.; Liu, D. Cold Plasma-based hurdle interventions: New strategies for improving food safety. Food Eng. Rev. 2020, 12, 321–332. [Google Scholar] [CrossRef]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Khan, I.; Nku, C.; Miskeen, S.; Lee, B.H.; Oh, D. Hurdle technology: A novel approach for enhanced food quality and safety: A review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Imtiyaz, H.; Soni, P.; Yukongdi, V. Role of sensory appeal, nutritional quality, safety, and health determinants on convenience food choice in an academic environment. Foods 2021, 10, 3455. [Google Scholar] [CrossRef]
- Gil, M.I.; Gómez-López, V.M.; Hung, Y.C.; Allende, A. Potential of electrolysed water as an alternative disinfectant agent in the fresh-cut industry. Food Bioprocess Technol. 2015, 8, 1336–1348. [Google Scholar] [CrossRef]
- Issa-Zacharia, A.; Kamitani, Y.; Muhimbula, H.S.; Ndabikunze, B.K. A review of microbiological safety of fruits and vegetables and the introduction of electrolysed water as an alternative to sodium hypochlorite solution. African J. Food Sci. 2010, 4, 778–789. [Google Scholar]
- Siti Zaharah, R.; Noranizan, M.A.; Radu, S.; Karim, R.; Mohd Adzahan, N.; Aadil, R.M.; Koh, P.C. Impact of sanitizer solutions on microbial reduction and quality of fresh-cut pennywort (Centella Asiatica) leaves. J. Food Sci. Technol. 2021, 59, 1211–1220. [Google Scholar] [CrossRef]
- Apolot, M.G.; Ssozi, J.; Namutebi, A.; Masanza, M.; Elizabeth, K.; Rees, D.; Hedwig, A. Changes in sensory and quality characteristics of S. Aethiopicum (Shum) and A. Lividus (Linn) leafy vegetables along the supply chain. Am. J. Food Sci. Technol. 2018, 6, 161–166. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Mahomud, A.S. High carbon-di-oxide modified atmospheric packaging on quality of ready-to-eat minimally processed fresh-cut iceberg lettuce. Food Sci. Biotechnol. 2021, 30, 413–421. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Xu, Y.; Zhou, J.; Zhang, H.; Karimi-Maleh, H.; Lai, G.; Zhao, S.; et al. Development of an electrochemical biosensor for phylogenetic analysis of amaryllidaceae based on the enhanced electrochemical fingerprint recorded from plant tissue. Biosens. Bioelectron. 2020, 159, 112212. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Zenhausern, F. The emerging role of decellularized plant-based scaffolds as a new biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, M.; Hou, R.; Shen, R.; Qiu, S.; Ouyang, Z. Effects of experimental warming on stomatal traits in leaves of maize (Zea may L.). Ecol. Evol. 2013, 3, 3095–3111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, D.; Guan, H.; Sun, Y.; Ye, M.; Zhang, L.; Gu, T.; Chen, J.; Wang, S.; Zhang, C.; et al. Transmission electron microscopy analysis on microbial ultrathin sections prepared by the ultra-low lead staining technique. Microsc. Microanal. 2021, 27, 1265–1272. [Google Scholar] [CrossRef]
- Valdés García, A.; Mart, D.; Landete, M.P.; Moya, P.; Beltr, A. Potential of industrial pineapple (Ananas comosus (L.) Merrill) by-products as aromatic and antioxidant sources. Antioxidants 2021, 10, 1767. [Google Scholar] [CrossRef]
- Chen, G.W.; Chen, Y.A.; Chang, H.Y.; Huang, T.C.; Chen, T.Y. Combined impact of high-pressure processing and slightly acidic electrolysed water on Listeria monocytogenes proteomes. Food Res. Int. 2021, 147, 110494. [Google Scholar] [CrossRef]
- Teoh, Y.P.; Don, M.M. Mycelia growth and production of total flavonoids and 4H-Pyran-4-One, 2, 3-Dihydro-3, 5-Dihydroxy-6-Methyl- by Schizophyllum commune using a bubble column bioreactor considering aeration effect and mass transfer study. Chem. Biochem. Eng. Q. 2014, 28, 553–559. [Google Scholar] [CrossRef]
- Chong, N.J.; Aziz, Z. A systematic review on the chemical constituents of Centella asiatica. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 445–459. [Google Scholar]
- James, J.T.; Dubery, I.A. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef] [PubMed]
- Muffler, K.; Leipold, D.; Scheller, M.; Haas, C.; Steingroewer, J.; Bley, T.; Neuhaus, H.E.; Antonio, M.; Schrader, J.; Ulber, R. Biotransformation of triterpenes. Process Biochem. 2011, 46, 1–15. [Google Scholar] [CrossRef]
- Furtado, N.A.J.C.; Pirson, L.; Miranda, L.M.; Loira-pastoriza, C.; Preat, V.; Larondelle, Y.; Andr, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2009; p. 546. [Google Scholar]
- Bylka, W.; Znajdek-Awizeń, P.; Studzińska-Sroka, E.; Brzezińska, M. Centella asiatica in cosmetology. Postep. Dermatol. Alergol. 2013, 30, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.; Handayani, F.; Darusman, L.K.; Rohaeti, E.; Wahyu, Y.; Sulistiyani; Honda, K.; Putri, S.P. A combination of simultaneous quantification of four triterpenes and fingerprint analysis using HPLC for rapid identification of Centella asiatica from its related plants and classification based on cultivation ages. Ind. Crops Prod. 2018, 122, 93–97. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Artiaga, L.N.; Morales, F.F.; Girón, F. Effect of pulsed light on quality of shelled walnuts effect of pulsed light on quality of shelled walnuts. Foods 2022, 11, 1186. [Google Scholar] [CrossRef]
- Rosalizan, M.S.; Rohani, M.Y.; Khatijah, I.; Shukri, M.A. Physical characteristics, nutrient contents and triterpene compounds of ratoon crops of Centella asiatica at three different stages of maturity. J. Trop. Agric. Food Sci. 2008, 36, 43–51. [Google Scholar]
- Faour-Klingbeil, D.; Kuri, V.; Todd, E.C.D.D. The influence of pre-wash chopping and storage conditions of parsley on the efficacy of disinfection against S. Typhimurium. Food Control 2016, 65, 121–131. [Google Scholar] [CrossRef]
- Xu, W.; Wu, C. The impact of pulsed light on decontamination, quality, and bacterial attachment of fresh raspberries. Food Microbiol. 2016, 57, 135–143. [Google Scholar] [CrossRef]
- Ondeko, D.A.; Juma, B.F.; Baraza, L.D.; Nyongesa, P.K. LC-ESI/MS and GC-MS methanol extract analysis, phytochemical and antimicrobial activity studies of Centella asiatica. Asian J. Chem. Sci. 2020, 8, 32–51. [Google Scholar] [CrossRef]



| Day/ Treatment | 0 | 12 |
|---|---|---|
| Acidic EW | 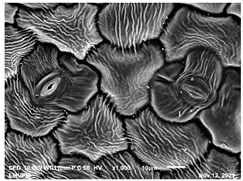 |  |
| Acidic EW 1.5 J/cm2 |  | 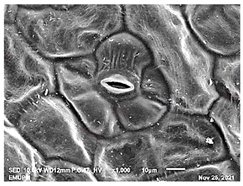 |
| Acidic EW 4.2 J/cm2 | 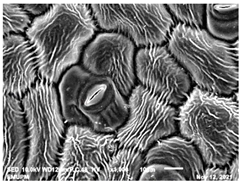 | 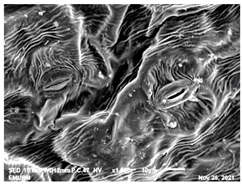 |
| Acidic EW 6.9 J/cm2 |  |  |
| (a) Peak No. | Name of Compound | Functional Compound | Molecular Formula | MW | High % |
| 1 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4h-pyran-4-one | Flavonoid | C6H8O4 | 144 | 3.93 |
| 2 | Bergamotene <beta-, trans-> | Sesquiterpenoids | C15H24 | 204 | 4.98 |
| 3 | Neophytadiene | Diterpenes | C20H38 | 278 | 13.1 |
| 4 | Hexadecanoic acid <n-> | Fatty acids | C16H32O2 | 256 | 11.9 |
| 5 | Panaxynone | Fatty acids | C17H22O | 242 | 23.8 |
| 6 | Phytol | Fatty acids | C20H40O | 296 | 11.3 |
| 7 | 9,12-Octadecadienoic acid (Z,Z)- | Fatty acids | C18H33O2 | 280 | 5.31 |
| 8 | Linolenate <methyl-> | Fatty acids | C19H32O2 | 292 | 8.31 |
| 9 | Ethyl-9,12-octadecadienoate | Fatty acids | C20H36O2 | 308 | 2.06 |
| 10 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | Fatty acids | C19H38O4 | 330 | 2.32 |
| 11 | 9,12-Octadecadienoic acid (Z,Z)-, 2,3-dihydroxypropyl ester | Fatty acids | C21H38O4 | 354 | 2.65 |
| 12 | Methyl (Z)-5,11,14,17-eicosatetraenoate | Fatty acids | C21H34O2 | 318 | 1.72 |
| 13 | (+/−)-.alpha.-Tocopherol | Sterol | C29H50O2 | 430 | 1.37 |
| 14 | Stigmasterol | Sterol | C29H48O | 412 | 5.71 |
| 15 | Rhamnol | Sterol | C29H50O | 414 | 1.54 |
| (b) Peak No. | Name of Compound | Functional Compound | Molecular Formula | MW | High % |
| 1 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4h-pyran-4-one | Flavonoid | C6H8O4 | 144 | 4.67 |
| 2 | Neophytadiene | Diterpenes | C20H38 | 278 | 1.84 |
| 3 | 7,11,15-Trimethyl-3-methylenehexadec-1-ene | Diterpenes | C20H39 | 278 | 1.84 |
| 4 | Hexadecanoic acid <n-> | Fatty acids | C16H32O2 | 256 | 12.5 |
| 5 | Isopropyl palmitate | Fatty acids | C19H38O2 | 298 | 3.77 |
| 6 | Panaxynone | Fatty acids | C17H22O | 242 | 22.2 |
| 7 | Phytol | Fatty acids | C20H40O | 296 | 5.34 |
| 8 | Linoelaidic acid | Fatty acids | C18H32O2 | 292 | 5.99 |
| 9 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z) | Fatty acids | C20H36O2 | 308 | 11.1 |
| 10 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | Fatty acids | C19H38O4 | 330 | 2.65 |
| 11 | 9,12-Octadecadienoic acid (Z,Z)-, 2,3-dihydroxypropyl ester | Fatty acids | C21H38O4 | 354 | 2.84 |
| 12 | Linolenic acid, methyl ester | Fatty acids | C19H32O2 | 292 | 1.99 |
| 13 | (+/−)-.alpha.-Tocopherol | Sterol | C29H50O2 | 430 | 1.78 |
| 14 | Stigmasterol | Sterol | C29H48O | 412 | 6.67 |
| 15 | Rhamnol | Sterol | C29H50O | 414 | 2.47 |
| Storage | Day 0 | |||
|---|---|---|---|---|
| Bioactive Compound/Treatment | MS | MA | AS | AA |
| Untreated (tap water) | 11.65 ± 0.05 Aa | 1.16 ± 0.01 Db | 8.22 ± 0.27 Aa | 1.16 ± 0.01 Db |
| Acidic EW | 10.05 ± 0.05 ABa | 1.95 ± 0.01 Cb | 5.95 ± 0.07 Ba | 1.95 ± 0.01 Cb |
| Acidic EW 1.5 J/cm2 | 7.63 ± 0.12 ABa | 2.69 ± 0.03 Bb | 5.75 ± 0.07 BCb | 2.69 ± 0.03 Bb |
| Acidic EW 4.2 J/cm2 | 10.05 ± 0.05 ABa | 2.73 ± 0.09 Bb | 5.73 ± 0.38 BCa | 2.73 ± 0.09 Bb |
| Acidic EW 6.9 J/cm2 | 6.41 ± 0.09 Ba | 2.94 ± 0.01 Ab | 5.05 ± 0.05 Ca | 2.94 ± 0.01 Ab |
| Storage | Day 12 | |||
| Untreated (tap water) | 3.52 ± 0.21 Cb | 2.63 ± 0.06 Ca | 1.79 ± 0.03 Cb | 2.63 ± 0.06 Ca |
| Acidic EW | 2.63 ± 0.02 Cb | 2.21 ± 0.01 Da | 2.38 ± 0.06 Cb | 2.21 ± 0.01 Da |
| Acidic EW 1.5 J/cm2 | 7.22 ± 0.18 Aa | 3.46 ± 0.06 Ba | 6.27 ± 0.02 Aa | 3.46 ± 0.06 Ba |
| Acidic EW 4.2 J/cm2 | 5.86 ± 0.13 ABa | 3.72 ± 0.07 ABa | 3.41 ± 0.08 Bb | 3.72 ± 0.07 ABa |
| Acidic EW 6.9 J/cm2 | 4.13 ± 1.14 BCa | 3.87 ± 0.12 Aa | 2.36 ± 0.48 Cb | 3.87 ± 0.11 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosli, S.-Z.; Mohd Adzahan, N.; Karim, R.; Mahmud Ab Rashid, N.-K. Effect of Acidic Electrolysed Water and Pulsed Light Technology on the Sensory, Morphology and Bioactive Compounds of Pennywort (Centella asiatica L.) Leaves. Molecules 2023, 28, 311. https://doi.org/10.3390/molecules28010311
Rosli S-Z, Mohd Adzahan N, Karim R, Mahmud Ab Rashid N-K. Effect of Acidic Electrolysed Water and Pulsed Light Technology on the Sensory, Morphology and Bioactive Compounds of Pennywort (Centella asiatica L.) Leaves. Molecules. 2023; 28(1):311. https://doi.org/10.3390/molecules28010311
Chicago/Turabian StyleRosli, Siti-Zaharah, Noranizan Mohd Adzahan, Roselina Karim, and Nor-Khaizura Mahmud Ab Rashid. 2023. "Effect of Acidic Electrolysed Water and Pulsed Light Technology on the Sensory, Morphology and Bioactive Compounds of Pennywort (Centella asiatica L.) Leaves" Molecules 28, no. 1: 311. https://doi.org/10.3390/molecules28010311
APA StyleRosli, S.-Z., Mohd Adzahan, N., Karim, R., & Mahmud Ab Rashid, N.-K. (2023). Effect of Acidic Electrolysed Water and Pulsed Light Technology on the Sensory, Morphology and Bioactive Compounds of Pennywort (Centella asiatica L.) Leaves. Molecules, 28(1), 311. https://doi.org/10.3390/molecules28010311







