Regioselective Reaction of 2-Indolylmethanols with Enamides
Abstract
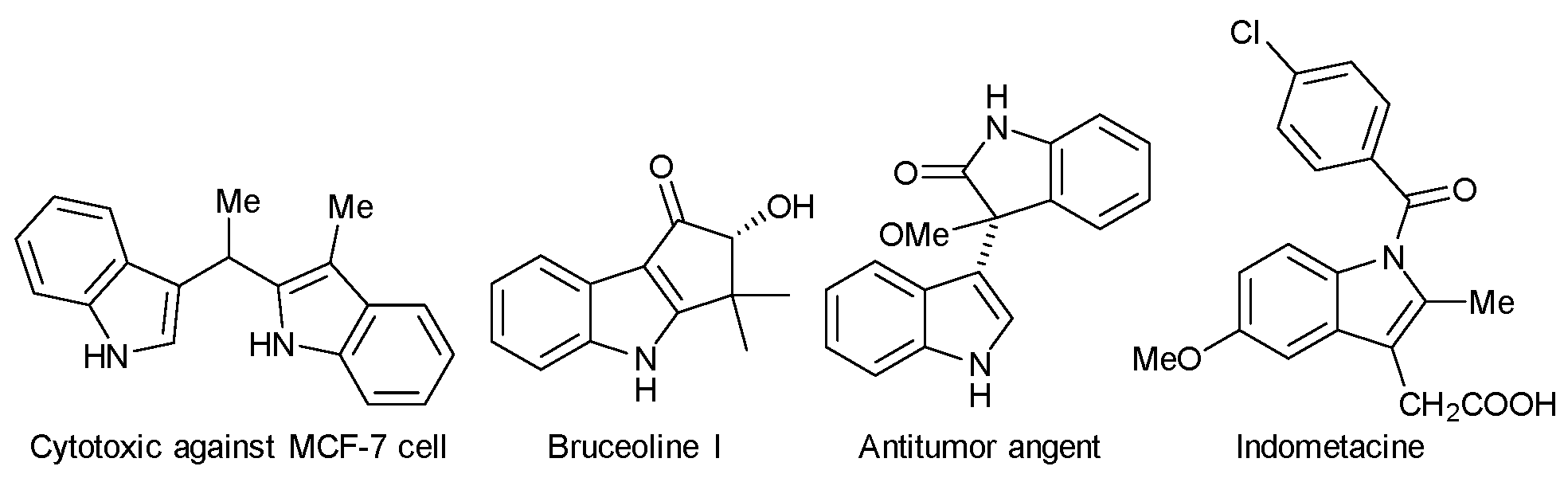
1. Introduction
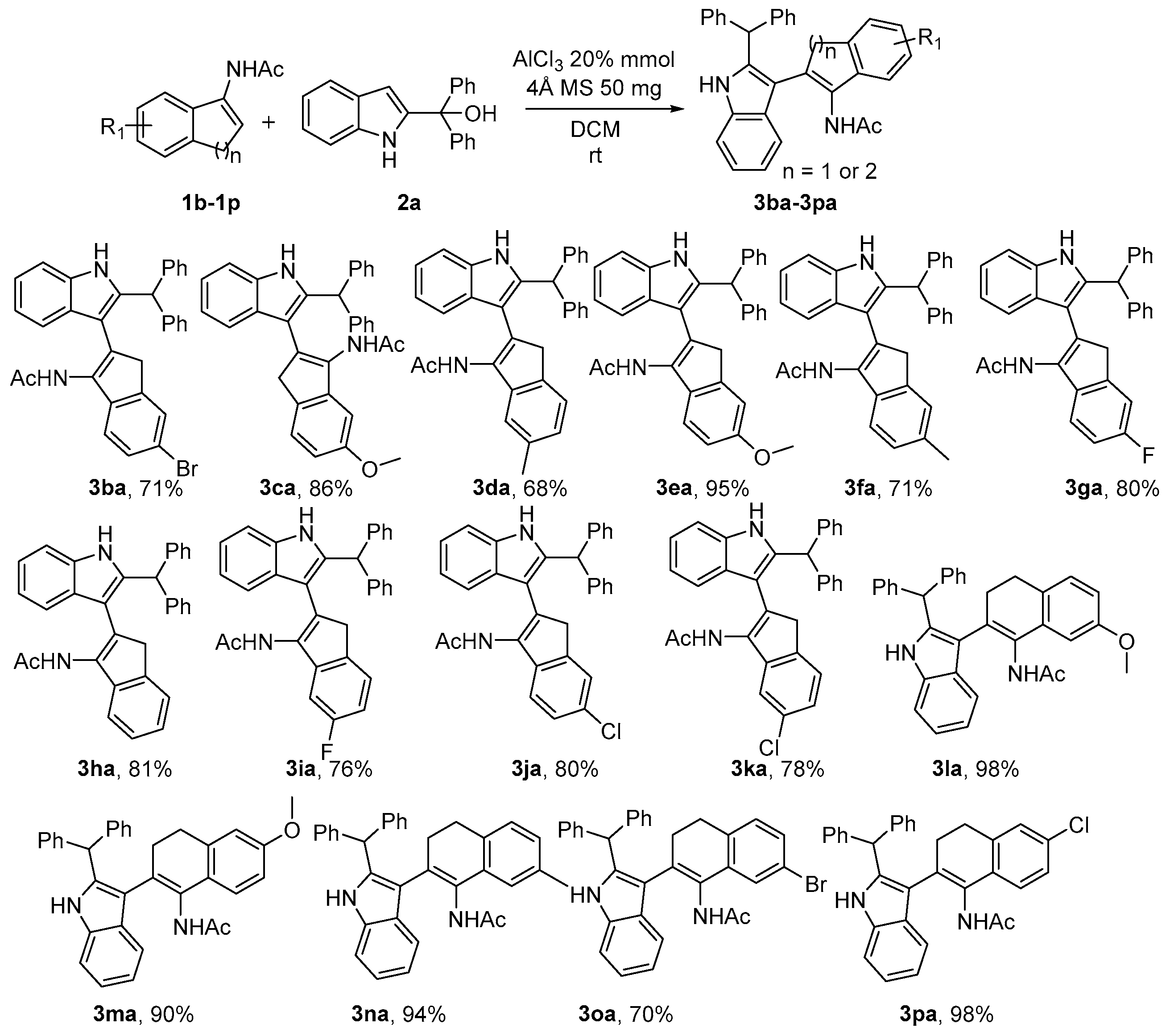
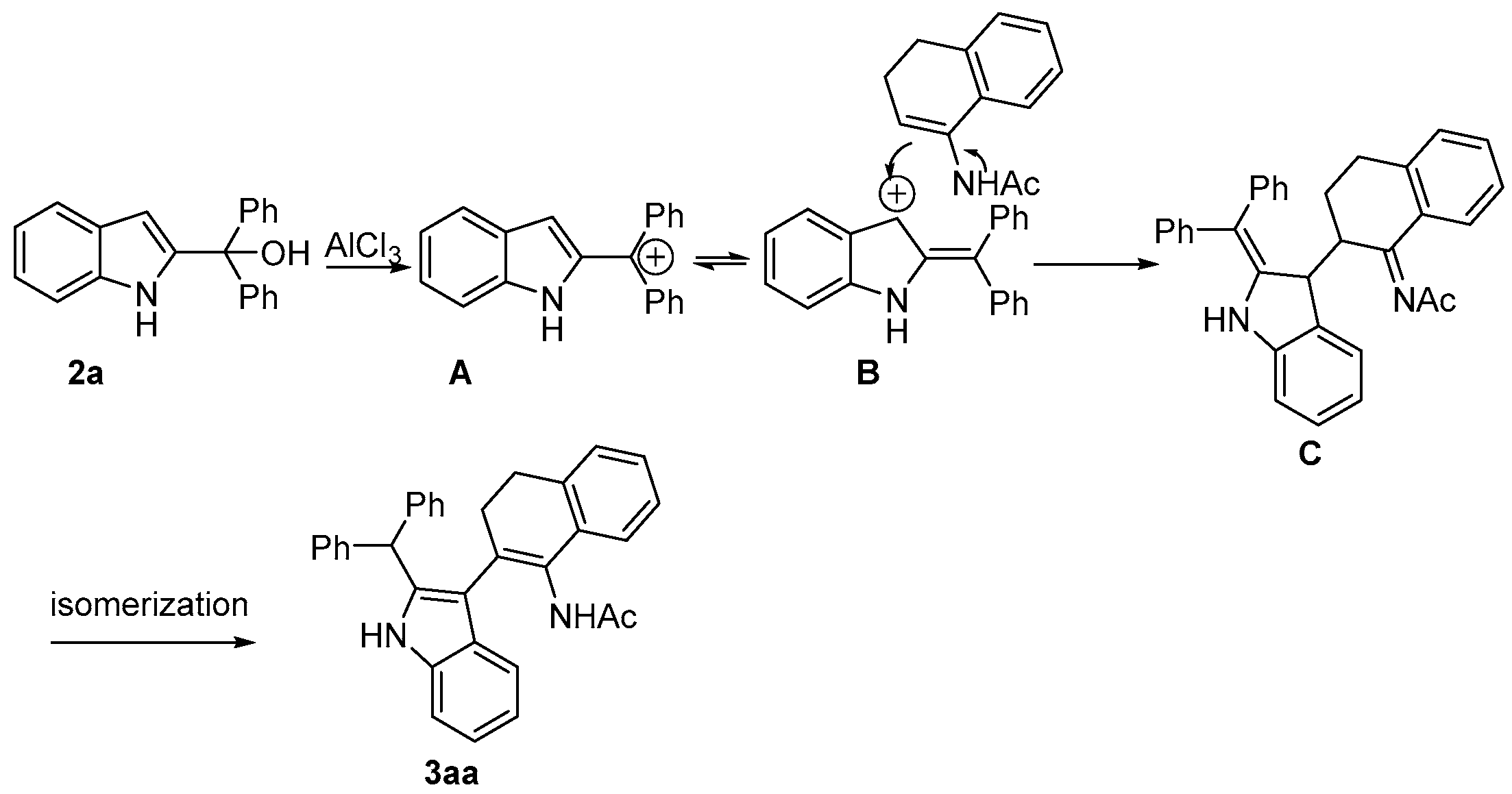
2. Results
3. Experimental Section
3.1. General Procedures
3.2. Typical Procedure for Synthesis of 3aa
- 2-(2-benzhydryl-1H-indol-3-yl)-3,4-dihydronaphthalen-1-amine (3aa). The product 3aa was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 4:1) as a green solid (82.4 mg, yield: 88%); mp 289–290 °C; 1H NMR (500 MHz, CDCl3) δ 7.92 (s, 1H), 7.41 (d, J = 7.8 Hz, 1H), 7.35 (t, J = 7.4 Hz, 2H), 7.31–7.21 (m, 8H), 7.19–7.14 (m, 3H), 7.13–7.03 (m, 4H), 6.37 (s, 1H), 5.71 (s, 1H), 2.93–2.82 (m, 1H), 2.77–2.67 (m, 1H), 2.66–2.56 (m, 1H), 2.42–2.32 (m, 1H), 1.61 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.9, 142.4, 141.6, 136.5, 136.0, 135.8, 131.8, 131.6, 129.2, 129.1, 128.8, 127.5, 127.3, 127.2, 127.2, 127.0, 126.9, 126.5, 126.3, 123.5, 122.2, 120.4, 119.6, 113.0, 111.2, 48.7, 29.7, 28.2, 23.3; HRMS (ESI) m/z: [M + H]+ calculated for C33H29ON2, 469.2274; found, 469.2275.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-1H-inden-3-yl)acetamide (3ba). The product 3ba was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 2:1) as a white solid (73.6 mg, yield: 81%); mp 306–307 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.04 (s, 1H), 9.34 (s, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.43 (d, J = 7.3 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.31 (t, J = 7.5 Hz, 4H), 7.25–7.20 (m, 3H), 7.20–7.14 (m, 6H), 7.10–7.04 (m, 1H), 7.00–6.95 (m, 1H), 5.72 (s, 1H), 3.72 (s, 2H), 1.48 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 169.0, 142.8, 142.6, 141.6, 137.8, 136.7, 134.3, 130.0, 128.9, 128.3, 127.2, 126.4, 125.7, 124.1, 123.4, 121.1, 120.3, 119.2, 119.0, 111.4, 108.3, 47.8, 40.1, 22.0; HRMS (ESI) m/z: [M + H]+ calculated for C32H26BrON2, 533.1223; found, 533.1224.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-5-methoxy-1H-inden-3-yl)acetamide (3ca). The product 3ca was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a gray solid (83.4 mg, yield: 86%); mp 314–315 °C; 1H NMR (500 MHz, CDCl3) δ 8.05 (s, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.35–7.26 (m, 8H), 7.20 (t, J = 7.5 Hz, 1H), 7.18–7.10 (m, 5H), 7.02 (d, J = 2.4 Hz, 1H), 6.82 (dd, J = 8.2, 2.3 Hz, 1H), 6.66 (s, 1H), 5.74 (s, 1H), 3.84 (s, 3H), 3.64 (s, 2H), 1.57 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 169.1, 158.9, 142.8, 142.2, 137.4, 136.1, 135.6, 134.6, 129.3, 129.0, 128.9, 128.0, 127.2, 124.0, 122.4, 120.5, 119.8, 112.0, 111.3, 108.6, 106.8, 55.8, 48.5, 40.0, 23.0; HRMS (ESI) m/z: [M + H]+ calculated for C33H29O2N2, 485.2224; found, 485.2223.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-5-methyl-1H-inden-3-yl)acetamide (3da). The product 3da was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a white solid (63.7 mg, yield: 68%); mp 306–307 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.04 (s, 1H), 9.32 (s, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.34–7.28 (m, 5H), 7.23 (t, J = 7.3 Hz, 2H), 7.19 (d, J = 7.5 Hz, 4H), 7.10–7.05 (m, 1H), 7.01–6.96 (m, 3H), 5.73 (s, 1H), 3.66 (s, 2H), 2.35 (s, 3H), 1.51 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 168.9, 143.0, 142.6, 138.7, 137.7, 136.6, 134.6, 134.2, 130.4, 128.9, 128.3, 127.3, 126.4, 124.9, 123.1, 121.1, 120.7, 119.2, 119.0, 111.4, 108.4, 47.8, 22.1, 21.3; HRMS (ESI) m/z: [M + H]+ calculated for C33H29ON2, 469.2274; found, 469.2275.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-6-methoxy-1H-inden-3-yl)acetamide (3ea). The product 3ea was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a brown solid (92.1 mg, yield: 95%); mp 306–307 °C; 1H NMR (500 MHz, CDCl3) δ 8.01 (s, 1H), 7.48 (d, J = 7.9 Hz, 1H), 7.36 (d, J = 8.5 Hz, 1H), 7.32 (t, J = 7.4 Hz, 5H), 7.27 (d, J = 7.3 Hz, 2H), 7.19 (t, J = 7.4 Hz, 1H), 7.17–7.09 (m, 5H), 7.01 (d, J = 1.8 Hz, 1H), 6.88 (dd, J = 8.4, 2.1 Hz, 1H), 6.66 (s, 1H), 5.74 (s, 1H), 3.84 (s, 3H), 3.65 (s, 2H), 1.57 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 169.0, 158.2, 144.3, 142.3, 137.3, 136.1, 135.4, 134.5, 129.0, 128.9, 128.1, 127.2, 125.0, 122.4, 122.3, 120.5, 119.8, 112.2, 111.2, 110.0, 108.6, 55.8, 48.5, 40.6, 23.0; HRMS (ESI) m/z: [M + H]+ calculated for C33H29O2N2, 485.2224; found, 485.2223.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-6-methyl-1H-inden-3-yl)acetamide (3fa). The product 3fa was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a brown solid (66.2 mg, yield: 71%); mp 296–297 °C; 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H), 7.48 (d, J = 7.9 Hz, 1H), 7.35–7.27 (m, 8H), 7.24 (s, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.16–7.10 (m, 6H), 6.64 (s, 1H), 5.73 (s, 1H), 3.65 (s, 2H), 2.41 (s, 3H), 1.58 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.0, 142.7, 142.3, 138.8, 137.4, 136.1, 135.7, 134.9, 129.1, 128.9, 128.1, 127.2, 127.2, 126.4, 124.5, 122.4, 121.4, 120.5, 119.9, 111.2, 108.7, 48.5, 40.5, 23.1, 21.6; HRMS (ESI) m/z: [M + H]+ calculated for C33H29ON2, 469.2274; found, 469.2273.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-6-fluoro-1H-inden-3-yl)acetamide (3ga). The product 3ga was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a white solid (75.4 mg, yield: 80%); mp 356–357 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.06 (s, 1H), 9.38 (s, 1H), 7.50 (dd, J = 7.7, 3.0 Hz, 1H), 7.38 (dd, J = 7.7, 4.7 Hz, 1H), 7.34–7.28 (m, 5H), 7.26–7.18 (m, 6H), 7.17–7.13 (m, 1H), 7.12–7.06 (m, 2H), 7.02–6.96 (m, 1H), 5.72 (s, 1H), 3.75 (s, 2H), 1.50 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 169.0, 161.2, 159.6, 143.9 (d, J = 8.8 Hz), 142.6, 139.0, 137.8, 136.7, 133.6, 129.7 (d, J = 3.5 Hz), 128.9, 128.3, 127.2, 126.4, 121.2, 121.2, 119.1 (d, J = 17.0 Hz), 112.5 (d, J = 22.6 Hz), 111.4, 110.9 (d, J = 23.0 Hz), 108.1, 47.8, 40.1, 22.0; 19F NMR (471 MHz, DMSO-d6) δ −119.15–−119.20 (m, 1F); HRMS (ESI) m/z: [M + H]+ calculated for C32H26ON2F, 473.2024; found, 473.2024.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-6-chloro-1H-inden-3-yl)acetamide (3ha). The product 3ha was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a white solid (78.2 mg, yield: 80%); mp 334–335 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.09 (s, 1H), 9.41 (s, 1H), 7.51–7.46 (m, 2H), 7.37 (d, J = 8.1 Hz, 1H), 7.34–7.28 (m, 5H), 7.25–7.21 (m, 2H), 7.18 (d, J = 7.4 Hz, 4H), 7.14 (d, J = 8.2 Hz, 1H), 7.11–7.05 (m, 1H), 7.02–6.96 (m, 1H), 5.70 (s, 1H), 3.77 (s, 2H), 1.48 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 169.1, 143.8, 142.5, 141.7, 138.0, 136.7, 133.6, 130.8, 129.0, 128.9, 128.4, 127.1, 126.4, 125.8, 123.6, 121.6, 121.2, 119.2, 119.2, 111.5, 108.0, 47.9, 40.0, 22.0; HRMS (ESI) m/z: [M + H]+ calculated for C32H27ON2, 455.2118; found, 455.2116.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-6-bromo-1H-inden-3-yl)acetamide (3ia). The product 3ia was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a yellow solid (75.2 mg, yield: 71%); mp 296–297 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.09 (s, 1H), 9.41 (s, 1H), 7.63 (s, 1H), 7.49 (dd, J = 7.9, 2.3 Hz, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.38 (dd, J = 7.8, 4.0 Hz, 1H), 7.31 (t, J = 7.4 Hz, 4H), 7.23 (t, J = 7.3 Hz, 2H), 7.20–7.17 (m, 4H), 7.12–7.06 (m, 2H), 7.03–6.92 (m, 1H), 5.71 (s, 1H), 3.76 (s, 2H), 1.50 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 169.1, 144.1, 142.5, 142.0, 138.0, 136.7, 133.6, 130.8, 128.9, 128.5, 128.3, 127.1, 126.4, 126.4, 122.0, 121.2, 119.2, 117.4, 111.5, 107.9, 47.8, 40.0, 22.0; HRMS (ESI) m/z: [M + H]+ calculated for C32H26ON2F, 473.2024; found, 473.2023.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-5-fluoro-1H-inden-3-yl)acetamide (3ja). The product 3ja was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a brown solid (71.2 mg, yield: 76%); mp 331–332 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.08 (s, 1H), 9.40 (s, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.42 (dd, J = 8.1, 5.2 Hz, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.31 (t, J = 7.6 Hz, 4H), 7.23 (t, J = 7.3 Hz, 2H), 7.19 (d, J = 7.5 Hz, 4H), 7.10–7.05 (m, 1H), 7.01–6.95 (m, 2H), 6.91 (dd, J = 9.5, 2.4 Hz, 1H), 5.71 (s, 1H), 3.72 (s, 2H), 1.50 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 169.1, 162.2, 160.6, 144.9 (d, J = 9.4 Hz), 142.5, 137.9, 137.3 (d, J = 1.5 Hz), 136.6, 133.7 (d, J = 2.9 Hz), 132.6, 128.9, 128.3, 127.1, 126.4, 124.4 (d, J = 9.0 Hz), 121.2, 119.2 (d, J = 8.5 Hz), 111.4, 110.6 (d, J = 22.8 Hz), 108.0, 107.1 (d, J = 23.9 Hz), 47.8, 40.1, 22.0; 19F NMR (471 MHz, DMSO-d6) δ −117.67–−117.72 (m, 1F); HRMS (ESI) m/z: [M + H]+ calculated for C32H26ON2Cl, 489.1728; found, 489.1727.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-5-chloro-1H-inden-3-yl)acetamide (3ka). The product 3ka was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a white solid (75.9 mg, yield: 78%); mp 327–328 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.11 (s, 1H), 9.45 (s, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 8.1 Hz, 1H), 7.33–7.29 (m, 4H), 7.25–7.18 (m, 7H), 7.17 (d, J = 2.0 Hz, 1H), 7.12–7.07 (m, 1H), 7.03–6.97 (m, 1H), 5.72 (s, 1H), 3.76 (s, 2H), 1.52 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 169.3, 144.8, 142.5, 140.4, 138.1, 136.7, 133.3, 132.3, 130.8, 128.9, 128.4, 127.1, 126.5, 124.8, 123.8, 121.3, 120.1, 119.2, 111.5, 108.0, 47.9, 39.8, 22.0; HRMS (ESI) m/z: [M + H]+ calculated for C32H26ON2Cl, 489.1728; found, 489.1727.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-7-methoxy-3,4-dihydronaphthalen-1-yl)acetamide (3la). The product 3la was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a white solid (97.5 mg, yield: 98%); mp 324–325 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.91 (s, 1H), 8.93 (s, 1H), 7.38–7.27 (m, 6H), 7.29–7.22 (m, 3H), 7.22–7.18 (m, 3H), 7.11 (d, J = 8.2 Hz, 1H), 7.08–7.02 (m, 1H), 6.97–6.92 (m, 1H), 6.74 (dd, J = 8.2, 2.6 Hz, 1H), 6.58 (d, J = 2.6 Hz, 1H), 5.72 (s, 1H), 3.71 (s, 3H), 2.78–2.57 (m, 3H), 2.23–2.15 (m, 1H), 1.46 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 157.9, 143.5, 142.1, 136.8, 136.6, 134.2, 129.9, 129.1, 128.8, 128.7, 128.5, 128.1, 127.9, 127.7, 126.4, 126.3, 126.3, 120.8, 119.5, 118.7, 112.9, 111.4, 111.0, 109.6, 55.0, 47.8, 30.2, 26.9, 22.2; HRMS (ESI) m/z: [M + H]+ calculated for C34H31O2N2F, 499.2380; found, 499.2376.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-6-methoxy-3,4-dihydronaphthalen-1-yl)acetamide (3ma). The product 3ma was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a brown solid (89.7 mg, yield: 90%); mp 321–322 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.86 (s, 1H), 8.87 (s, 1H), 7.37–7.23 (m, 9H), 7.20 (t, J = 8.7 Hz, 3H), 7.04 (t, J = 7.6 Hz, 1H), 6.97–6.91 (m, 2H), 6.81 (d, J = 2.3 Hz, 1H), 6.73 (dd, J = 8.5, 2.5 Hz, 1H), 5.72 (s, 1H), 3.76 (s, 3H), 2.76–2.67 (m, 3H), 2.23–2.11 (m, 1H), 1.44 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 158.0, 143.6, 142.2, 137.4, 136.6, 136.5, 129.7, 129.1, 128.8, 128.5, 128.1, 126.5, 126.4, 126.3, 126.0, 125.1, 124.5, 120.8, 119.5, 118.6, 113.0, 112.9, 111.4, 111.1, 55.1, 47.8, 29.7, 28.2, 22.2; HRMS (ESI) m/z: [M + H]+ calculated for C34H31O2N2, 499.2380; found, 499.2377.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-7-methyl-3,4-dihydronaphthalen-1-yl)acetamide (3na). The product 3na was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a black solid (90.5 mg, yield: 94%); mp 316–317 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.88 (s, 1H), 8.91 (s, 1H), 7.36–7.31 (m, 4H), 7.32–7.26 (m, 2H), 7.27–7.22 (m, 3H), 7.22–7.17 (m, 3H), 7.10–7.01 (m, 2H), 6.98–6.91 (m, 2H), 6.84 (s, 1H), 5.72 (s, 1H), 2.72–2.64 (m, 3H), 2.25 (s, 3H), 2.21–2.12 (m, 1H), 1.47 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 143.5, 142.1, 136.7, 136.6, 134.7, 133.0, 132.7, 130.0, 129.1, 128.8, 128.5, 128.1, 128.1, 127.0, 126.9, 126.4, 126.4, 126.3, 123.6, 120.8, 119.5, 118.7, 112.9, 111.4, 47.8, 30.0, 27.4, 22.2, 21.1; HRMS (ESI) m/z: [M + H]+ calculated for C34H31ON2, 483.2431; found, 483.2425.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-7-bromo-3,4-dihydronaphthalen-1-yl)acetamide (3oa). The product 3oa was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a yellow solid (76.5 mg, yield: 70%); mp 301–302 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.95 (s, 1H), 9.03 (s, 1H), 7.38–7.31 (m, 5H), 7.29 (d, J = 7.4 Hz, 2H), 7.25 (t, J = 8.0 Hz, 3H), 7.20 (t, J = 7.3 Hz, 3H), 7.16 (d, J = 8.0 Hz, 1H), 7.10 (d, J = 2.0 Hz, 1H), 7.09–7.03 (m, 1H), 6.98–6.93 (m, 1H), 5.68 (s, 1H), 2.78–2.60 (m, 3H), 2.27–2.17 (m, 1H), 1.46 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 169.0, 143.4, 142.0, 136.9, 136.6, 135.4, 134.9, 130.0, 129.2, 129.1, 128.8, 128.8, 128.5, 128.2, 126.5, 126.4, 126.2, 125.4, 121.0, 119.5, 119.2, 118.8, 112.4, 111.5, 47.9, 29.5, 27.1, 22.1; HRMS (ESI) m/z: [M + H]+ calculated for C33H28ON2Br, 547.1380; found, 547.1379.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-6-chloro-3,4-dihydronaphthalen-1-yl)acetamide (3pa). The product 3pa was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a yellow solid (98.6 mg, yield: 98%); mp 301–302 °C. 1H NMR (500 MHz, DMSO-d6) δ 10.92 (s, 1H), 9.00 (s, 1H), 7.36–7.31 (m, 4H), 7.31–7.25 (m, 3H), 7.24 (d, J = 10.5 Hz, 2H), 7.24–7.15 (m, 5H), 7.08–7.01 (m, 1H), 6.98 (d, J = 8.3 Hz, 1H), 6.97–6.91 (m, 1H), 5.67 (s, 1H), 2.79–2.68 (m, 3H), 2.24–2.15 (m, 1H), 1.43 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 169.0, 143.5, 142.0, 138.0, 136.9, 136.6, 131.9, 130.5, 129.2, 129.1, 128.8, 128.6, 128.5, 128.2, 126.8, 126.5, 126.3, 126.3, 125.8, 124.9, 120.9, 119.5, 118.8, 112.5, 111.5, 47.9, 29.4, 27.4, 22.1; HRMS (ESI) m/z: [M + H]+ calculated for C33H28ON2Cl, 503.1885; found, 503.1883.
- N-(2-(2-benzhydryl-5-methyl-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ab). The product 3ab was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 3:1) as a yellow solid (81.9 mg, yield: 85%); mp 303–304 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.73 (s, 1H), 8.88 (s, 1H), 7.34–7.27 (m, 4H), 7.24–7.18 (m, 5H), 7.18–7.10 (m, 6H), 7.00 (dd, J = 7.2, 1.6 Hz, 1H), 6.86 (dd, J = 8.3, 1.2 Hz, 1H), 5.68 (s, 1H), 2.76–2.68 (m, 3H), 2.31 (s, 3H), 2.23–2.12 (m, 1H), 1.45 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 143.6, 142.2, 136.7, 135.6, 134.9, 133.0, 129.9, 129.1, 128.8, 128.4, 128.1, 128.1, 127.1, 127.0, 126.5, 126.4, 126.3, 126.2, 126.0, 123.0, 122.2, 119.2, 112.3, 111.1, 47.8, 29.7, 27.7, 22.1, 21.2; HRMS (ESI) m/z: [M + H]+ calculated for C34H31ON2, 483.2431; found, 483.2430.
- N-(2-(2-benzhydryl-5-methoxy-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ac). The product 3ac was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (71.2 mg, yield: 72%); mp 313–314 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.70 (s, 1H), 8.90 (s, 1H), 7.31 (q, J = 7.8 Hz, 4H), 7.25–7.20 (m, 4H), 7.18 (d, J = 7.9 Hz, 4H), 7.16–7.12 (m, 2H), 7.03–7.00 (m, 1H), 6.80 (d, J = 2.4 Hz, 1H), 6.68 (dd, J = 8.7, 2.4 Hz, 1H), 5.69 (s, 1H), 3.69 (s, 3H), 2.92–2.80 (m, 1H), 2.77–2.63 (m, 3H), 1.52 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 169.0, 153.3, 143.4, 142.3, 137.4, 135.7, 133.1, 131.5, 129.9, 129.1, 128.8, 128.5, 128.2, 127.9, 127.1, 126.6, 126.5, 126.4, 126.3, 126.0, 123.1, 112.8, 112.1, 111.0, 101.3, 55.4, 48.0, 29.6, 27.8, 22.4; HRMS (ESI) m/z: [M + H]+ calculated for C34H31O2N2, 499.2380; found, 499.2378.
- N-(2-(2-benzhydryl-5-fluoro-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ad). The product 3ad was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (76.7 mg, yield: 79%); mp 313–314 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.96 (s, 1H), 8.96 (s, 1H), 7.37–7.29 (m, 5H), 7.28–7.13 (m, 9H), 7.09–7.01 (m, 2H), 6.93–6.84 (m, 1H), 5.72 (s, 1H), 2.78–2.57 (m, 3H), 2.25–2.06 (m, 1H), 1.52 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 157.8, 156.0, 143.1, 142.0, 138.9, 135.7, 133.0 (d, J = 13.3 Hz), 130.2, 129.1, 128.8, 128.5, 128.2, 127.5, 127.1, 126.6, 126.5, 126.5, 126.4, 126.0, 123.1, 113.2 (d, J = 4.4 Hz), 112.2 (d, J = 9.6 Hz), 108.7 (d, J = 25.8 Hz), 104.2 (d, J = 23.4 Hz), 47.9, 29.4, 27.7, 22.2; 19F NMR (471 MHz, CDCl3) δ −124.89–−124.94 (m, 1F); HRMS (ESI) m/z: [M + H]+ calculated for C33H28ON2F, 487.2180; found, 487.2177.
- N-(2-(2-benzhydryl-5-chloro-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ae). The product 3ae was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (79.8 mg, yield: 80%); mp 314–315 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.07 (s, 1H), 8.97 (s, 1H), 7.40–7.29 (m, 6H), 7.28–7.21 (m, 4H), 7.20–7.12 (m, 5H), 5.74 (s, 1H), 2.81–2.58 (m, 3H), 2.28–2.12 (m, 1H), 1.55 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 143.0, 141.9, 138.6, 135.7, 134.9, 132.9, 130.5, 129.1, 128.8, 128.5, 128.2, 127.4, 127.2, 127.1, 126.6, 126.4, 126.0, 123.4, 123.1, 120.7, 118.6, 112.9, 112.8, 47.9, 29.5, 27.7, 22.3; HRMS (ESI) m/z: [M + H]+ calculated for C33H28ON2Cl, 503.1885; found, 503.1886.
- N-(2-(2-benzhydryl-5-bromo-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3af). The product 3af was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (90.5 mg, yield: 83%); mp 326–327 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.06 (s, 1H), 8.95 (s, 1H), 7.46 (d, J = 1.4 Hz, 1H), 7.37–7.28 (m, 5H), 7.27 (d, J = 7.2 Hz, 1H), 7.25–7.19 (m, 3H), 7.19–7.13 (m, 6H), 7.05 (d, J = 7.5 Hz, 1H), 5.72 (s, 1H), 2.78–2.56 (m, 3H), 2.25–2.15 (m, 1H), 1.54 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 142.9, 141.9, 138.4, 135.7, 135.1, 132.9, 130.5, 129.0, 128.7, 128.6, 128.2, 128.1, 127.1, 126.6, 126.4, 126.0, 123.2, 123.1, 121.5, 113.4, 112.7, 111.4, 47.9, 29.5, 27.6, 22.3; HRMS (ESI) m/z: [M + H]+ calculated for C33H28ON2Br, 547.1380; found, 547.1381.
- N-(2-(2-benzhydryl-6-chloro-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ag). The product 3ag was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 4:1) as a yellow solid (75.4 mg, yield: 75%); mp 316–317 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.01 (s, 1H), 8.96 (s, 1H), 7.36–7.29 (m, 6H), 7.26 (t, J = 7.3 Hz, 1H), 7.24–7.20 (m, 3H), 7.19–7.13 (m, 5H), 7.03 (dd, J = 7.2, 1.5 Hz, 1H), 6.95 (dd, J = 8.4, 2.0 Hz, 1H), 5.71 (s, 1H), 2.76–2.62 (m, 3H), 2.21–2.12 (m, 1H), 1.49 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 143.1, 141.8, 137.9, 136.9, 135.7, 132.9, 130.3, 129.0, 128.7, 128.6, 128.2, 127.3, 127.1, 126.6, 126.4, 126.1, 125.5, 125.1, 123.1, 120.8, 118.9, 113.1, 110.9, 47.8, 29.5, 27.7, 22.2; HRMS (ESI) m/z: [M + H]+ calculated for C33H28ON2Cl, 503.1885; found, 503.1886.
- N-(2-(2-(di-p-tolylmethyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ah). The product 3ah was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (64.5 mg, yield: 65%); mp 327–328 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.87 (s, 1H), 8.91 (s, 1H), 7.34 (dd, J = 8.0, 3.6 Hz, 2H), 7.24–7.15 (m, 3H), 7.16–7.12 (m, 2H), 7.09–6.98 (m, 7H), 6.98–6.90 (m, 2H), 5.62 (s, 1H), 2.79–2.72 (m, 3H), 2.26 (s, 3H), 2.23 (s, 3H), 2.18–2.13 (m, 1H), 1.44 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 140.6, 139.3, 137.3, 136.5, 135.6, 135.3, 135.2, 133.0, 129.9, 129.0, 128.6, 128.6, 128.0, 127.0, 126.3, 126.3, 126.0, 123.1, 120.7, 119.4, 118.6, 112.5, 111.3, 47.1, 29.7, 27.7, 22.2, 20.6; HRMS (ESI) m/z: [M + H]+ calculated for C35H33ON2, 497.2587; found, 497.2586.
- N-(2-(2-(bis(4-methoxyphenyl)methyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ai). The product 3ai was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (63.2 mg, yield: 60%); mp 311–312 °C;1H NMR (500 MHz, DMSO-d6) δ 10.81 (s, 1H), 8.91 (s, 1H), 7.34 (dd, J = 7.9, 3.7 Hz, 2H), 7.21–7.12 (m, 5H), 7.09 (d, J = 8.6 Hz, 2H), 7.06–7.01 (m, 2H), 6.93 (t, J = 7.5 Hz, 1H), 6.88 (dd, J = 17.5, 8.7 Hz, 4H), 5.60 (s, 1H), 3.74 (s, 3H), 3.70 (s, 3H), 2.76–2.66 (m, 3H), 2.25–2.16 (m, 1H), 1.51 (s, 3H);13C NMR (125 MHz, DMSO-d6) δ 168.8, 157.8, 137.7, 136.5, 135.8, 135.6, 134.4, 133.1, 130.1, 129.8, 129.7, 128.0, 127.0, 126.4, 126.3, 126.0, 123.1, 120.7, 119.4, 118.6, 113.8, 113.5, 112.3, 111.3, 55.1, 55.0, 46.2, 29.7, 27.8, 22.3; HRMS (ESI) m/z: [M + H]+ calculated for C35H33O3N2, 529.2486; found, 529.2483.
- N-(2-(2-(bis(4-fluorophenyl)methyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3aj). The product 3aj was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 7:1) as a yellow solid (91.2 mg, yield: 91%); mp 333–334 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.04 (s, 1H), 9.04 (s, 1H), 7.38 (q, J = 7.0 Hz, 4H), 7.28–6.84 (m, 12H), 5.77 (s, 1H), 2.76 (s, 3H), 2.24 (s, 1H), 1.49 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.9, 163.2 (d, J = 6.8 Hz), 161.3 (d, J = 5.8 Hz), 145.6 (d, J = 6.8 Hz), 144.4 (d, J = 6.9 Hz), 136.6, 135.7, 135.3, 132.9, 130.6 (d, J = 8.3 Hz), 130.2, 130.1, 130.1, 127.6, 127.1, 126.5, 126.1 (d, J = 13.7 Hz), 125.2 (d, J = 2.6 Hz), 125.0 (d, J = 2.4 Hz), 123.1, 121.2, 119.7, 118.9, 115.9 (d, J = 22.1 Hz), 115.3 (d, J = 21.6 Hz), 113.7 (d, J = 20.8 Hz), 113.4, 113.3, 111.4, 47.2, 29.7, 27.8, 22.1; 19F NMR (471 MHz, DMSO-d6) δ −112.64–−112.70 (m, 1F), −113.50–−113.56 (m, 1F); HRMS (ESI) m/z: [M + H]+ calculated for C33H27ON2F2, 505.2086; found, 505.2084.
- N-(2-(2-(bis(4-chlorophenyl)methyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ak). The product 3ak was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 7:1) as a yellow solid (79.5 mg, yield: 74%); mp 315–316 °C; 1H NMR (600 MHz, DMSO-d6) δ 10.91 (s, 1H), 8.99 (s, 1H), 7.41 (dd, J = 16.9, 8.5 Hz, 4H), 7.38–7.32 (m, 2H), 7.24 (d, J = 8.5 Hz, 2H), 7.21–7.13 (m, 5H), 7.08–7.01 (m, 2H), 6.96 (t, J = 7.4 Hz, 1H), 5.74 (s, 1H), 2.77–2.66 (m, 3H), 2.24–2.16 (m, 1H), 1.53 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 168.9, 141.9, 140.7, 136.6, 135.7, 135.6, 133.0, 131.3, 131.3, 130.9, 130.5, 130.1, 128.6, 128.2, 127.8, 127.1, 126.5, 126.3, 126.0, 123.0, 121.1, 119.6, 118.8, 113.2, 111.4, 46.6, 29.6, 27.7, 22.2; HRMS (ESI) m/z: [M + H]+ calculated for C33H27ON2Cl2, 537.1495; found, 537.1496.
- N-(2-(2-(bis(4-(trifluoromethyl)phenyl)methyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3al). The product 3al was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (77.3 mg, yield: 64%); mp 313–314 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.05 (s, 1H), 9.04 (s, 1H), 7.74 (t, J = 8.4 Hz, 4H), 7.47 (d, J = 8.2 Hz, 2H), 7.43–7.28 (m, 4H), 7.22–7.12 (m, 3H), 7.11–7.05 (m, 1H), 7.04–7.01 (m, 1H), 6.99–6.94 (m, 1H), 5.93 (s, 1H), 2.79–2.70 (m, 3H), 2.31–2.13 (m, 1H), 1.46 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 169.0, 147.3, 146.1, 136.7, 135.7, 134.8, 132.9, 130.2, 129.9, 129.6, 127.8, 127.6, 127.5, 127.4, 127.3, 127.1, 126.6, 126.2, 126.1, 125.7 (q, J = 3.7 Hz), 125.5, 125.4, 125.2 (q, J = 3.8 Hz), 123.3, 123.2, 123.1, 121.3, 119.7, 119.0, 113.8, 111.5, 47.5, 29.6, 27.7, 22.1; 19F NMR (471 MHz, DMSO-d6) δ −60.82 (s, 3F), −60.86 (s, 3F); HRMS (ESI) m/z: [M + H]+ calculated for C35H27ON2F6, 605.2022; found, 605.2015.
- N-(2-(2-(bis(3-fluorophenyl)methyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3am). The product 3am was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 7:1) as a yellow solid (78.6 mg, yield: 78%); mp 325–326 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.03 (s, 1H), 9.03 (s, 1H), 7.43–7.34 (m, 4H), 7.21–7.18 (m, 1H), 7.17–7.14 (m, 2H), 7.12 (s, 1H), 7.11–7.02 (m, 5H), 7.01–6.94 (m, 3H), 5.76 (s, 1H), 2.81–2.65 (m, 3H), 2.34–2.12 (m, 1H), 1.48 (s, 3H);13C NMR (125 MHz, DMSO-d6) δ 168.9, 163.2 (d, J = 6.7 Hz), 161.3 (d, J = 5.8 Hz), 145.6 (d, J = 6.8 Hz), 144.4 (d, J = 7.0 Hz), 136.6, 135.7, 135.3, 132.9, 130.7 (d, J = 8.3 Hz), 130.2, 130.1, 130.1, 127.7, 127.1, 126.5, 126.1 (d, J = 12.1 Hz), 125.2 (d, J = 2.2 Hz), 125.0 (d, J = 2.2 Hz), 123.1, 121.2, 119.7, 118.9, 115.9 (d, J = 22.1 Hz), 115.3 (d, J = 21.6 Hz), 113.7 (d, J = 20.9 Hz), 113.5, 113.3, 111.4, 47.2, 29.7, 27.8, 22.1; 19F NMR (471 MHz, DMSO-d6) δ −112.65–−112.71 (m, 1F), −113.51–−113.56 (m, 1F); HRMS (ESI) m/z: [M + H]+ calculated for C33H27ON2F2, 505.2086; found, 505.2084.
- N-(2-(2-(bis(3-chlorophenyl)methyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3an). The product 3an was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 6:1) as a yellow solid (104.2 mg, yield: 97%); mp 313–314 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.09 (s, 1H), 9.10 (s, 1H), 7.41–7.34 (m, 5H), 7.34–7.28 (m, 2H), 7.27–7.21 (m, 1H), 7.22–7.19 (m, 2H), 7.19–7.13 (m, 2H), 7.12–7.04 (m, 2H), 7.06–7.00 (m, 1H), 7.01–6.94 (m, 1H), 5.76 (s, 1H), 2.82–2.73 (m, 3H), 2.24–2.14 (m, 1H), 1.49 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.9, 145.1, 143.9, 136.6, 135.6, 135.1, 133.2, 133.1, 132.9, 130.6, 130.1, 128.7, 128.3, 127.8, 127.7, 127.5, 127.1, 126.8, 126.6, 126.5, 126.2, 126.1, 123.1, 121.3, 119.7, 118.9, 113.5, 111.5, 47.1, 29.6, 27.7, 22.1; HRMS (ESI) m/z: [M + H]+ calculated for C33H27ON2Cl2, 537.1495; found, 537.1494.
- N-(2-(2-(bis(3-methoxyphenyl)methyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ao). The product 3ao was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (64.4 mg, yield: 61%); mp 313–314 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.91 (s, 1H), 8.94 (s, 1H), 7.37–7.32 (m, 2H), 7.28–7.22 (m, 1H), 7.24–7.17 (m, 2H), 7.18–7.11 (m, 2H), 7.08–7.01 (m, 1H), 7.04–6.98 (m, 1H), 6.97–6.91 (m, 1H), 6.87–6.79 (m, 2H), 6.81–6.73 (m, 3H), 6.75 (d, J = 8.2 Hz, 1H), 5.63 (s, 1H), 3.70 (s, 3H), 3.67 (s, 3H), 2.80–2.67 (m, 3H), 2.26–2.15 (m, 1H), 1.44 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 159.3, 159.1, 145.0, 143.4, 136.6, 136.5, 135.6, 133.0, 130.0, 129.5, 129.1, 127.9, 127.1, 126.4, 126.3, 126.0, 123.1, 121.6, 121.1, 120.9, 119.5, 118.7, 115.7, 114.8, 112.8, 111.4, 111.0, 55.0, 47.8, 29.7, 27.8, 22.1; HRMS (ESI) m/z: [M + H]+ calculated for C35H33O3N2, 529.2486; found, 529.2481.
- N-(2-(2-(di-m-tolylmethyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3ap). The product 3ap was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (70.5 mg, yield: 71%); mp 321–322 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.81 (s, 1H), 8.90 (s, 1H), 7.33 (dd, J = 8.0, 3.9 Hz, 2H), 7.20–7.16 (m, 1H), 7.17–7.08 (m, 8H), 7.06 (d, J = 8.5 Hz, 2H), 7.04–7.00 (m, 2H), 6.95–6.90 (m, 1H), 5.62 (s, 1H), 2.76–2.66 (m, 3H), 2.29 (s, 3H), 2.25 (s, 3H), 2.23–2.16 (m, 1H), 1.48 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 140.6, 139.3, 137.3, 136.5, 135.6, 135.3, 135.2, 133.0, 129.9, 129.0, 128.6, 128.0, 127.0, 126.3, 126.3, 126.0, 123.1, 120.7, 119.4, 118.6, 112.5, 111.4, 47.1, 29.7, 27.8, 22.2, 20.6; HRMS (ESI) m/z: [M + H]+ calculated for C35H33ON2, 497.2587; found, 497.2587.
- N-(2-(2-(di-o-tolylmethyl)-1H-indol-3-yl)-3,4-dihydronaphthalen-1-yl)acetamide (3aq). The product 3aq was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a yellow solid (86.3 mg, yield: 87%); mp 321–322 °C; 1H NMR (600 MHz, DMSO-d6) δ 10.52 (s, 1H), 8.54 (s, 1H), 7.39–7.33 (m, 2H), 7.22–7.13 (m, 5H), 7.12–7.07 (m, 5H), 7.04 (t, J = 7.5 Hz, 1H), 6.93 (t, J = 7.4 Hz, 1H), 6.91–6.87 (m, 1H), 6.86–6.83 (m, 1H), 5.67 (s, 1H), 2.86–2.77 (m, 1H), 2.72–2.65 (m, 1H), 2.64–2.55 (m, 1H), 2.27–2.23 (m, 1H), 2.22 (s, 3H), 1.95 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 168.7, 141.9, 140.3, 137.3, 136.5, 135.7, 135.6, 134.5, 132.7, 130.5, 130.4, 130.0, 128.4, 128.0, 127.3, 127.0, 126.6, 126.5, 126.3, 126.2, 126.0, 125.8, 125.7, 123.2, 120.7, 119.7, 118.6, 112.6, 111.4, 42.7, 29.7, 27.6, 21.4, 19.0; HRMS (ESI) m/z: [M + Na]+ calculated for C35H32N2ONa, 519.2407; found, 519.2415.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-1-phenylvinyl)acetamide (5a). The product 5a was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a white solid (58.6 mg, yield: 66%); mp 321–322 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.01 (s, 1H), 9.22 (s, 1H), 7.37–7.26 (m, 10H), 7.25–7.16 (m, 7H), 7.06–7.00 (m, 1H), 6.98–6.92 (m, 1H), 6.55 (s, 1H), 5.82 (s, 1H), 1.67 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 142.1, 139.5, 139.0, 136.4, 133.7, 129.0, 128.4, 128.0, 127.0, 126.5, 125.9, 125.5, 121.0, 120.0, 118.9, 114.2, 111.4, 109.5, 48.0, 22.8; HRMS (ESI) m/z: [M + Na]+ calculated for C31H26N2ONa, 465.1938; found, 465.1950.
- N-(2-(2-benzhydryl-5-chloro-1H-indol-3-yl)-1-phenylvinyl)acetamide (5b). The product 5b was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a white solid (49.6 mg, yield: 52%); mp 321–322 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.19 (s, 1H), 9.30 (s, 1H), 7.37–7.28 (m, 10H), 7.26–7.21 (m, 7H), 7.06 (dd, J = 8.6, 2.1 Hz, 1H), 6.55 (s, 1H), 5.86 (s, 1H), 1.75 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 141.7, 141.4, 138.8, 134.8, 133.8, 129.0, 128.4, 128.0, 127.1, 126.8, 126.6, 125.5, 123.6, 120.7, 119.5, 113.5, 112.9, 109.4, 47.9, 22.7; HRMS (ESI) m/z: [M + Na]+ calculated for C31H25N2ONaCl, 499.1548; found, 499.1560.
- N-(2-(2-benzhydryl-6-methyl-1H-indol-3-yl)-1-phenylvinyl)acetamide (5c). The product 5c was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a white solid (79.1 mg, yield: 87%); mp 321–322 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.88 (s, 1H), 9.20 (s, 1H), 7.37–7.27 (m, 8H), 7.25–7.19 (m, 8H), 7.13 (s, 1H), 6.90–6.86 (m, 1H), 6.55 (s, 1H), 5.81 (s, 1H), 2.31 (s, 3H), 1.70 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 142.1, 139.6, 139.1, 134.7, 133.3, 129.0, 128.3, 128.0, 127.2, 126.9, 126.5, 126.0, 125.4, 122.3, 120.0, 114.4, 111.1, 109.1, 48.0, 22.8, 21.2; HRMS (ESI) m/z: [M + Na]+ calculated for C32H28N2ONa, 479.2094; found, 479.2101.
- N-(2-(2-(di-p-tolylmethyl)-1H-indol-3-yl)-1-phenylvinyl)acetamide (5d). The product 5d was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a white solid (63.6 mg, yield: 68%); mp 321–322 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.91 (s, 1H), 9.20 (s, 1H), 7.36–7.26 (m, 6H), 7.23 (t, J = 7.0 Hz, 1H), 7.09 (s, 8H), 7.05–7.01 (m, 1H), 6.97–6.92 (m, 1H), 6.51 (s, 1H), 5.72 (s, 1H), 2.26 (s, 6H), 1.71 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.8, 140.0, 139.2, 139.0, 136.3, 135.5, 133.4, 128.9, 128.0, 126.9, 125.9, 125.4, 120.8, 119.9, 118.8, 114.3, 111.4, 109.2, 47.3, 22.9, 20.6; HRMS (ESI) m/z: [M + Na]+ calculated for C33H30N2ONa, 493.2251; found, 493.2257.
- N-(2-(2-(bis(4-fluorophenyl)methyl)-1H-indol-3-yl)-1-phenylvinyl)acetamide (5e). The product 5e was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a white solid (66.7 mg, yield: 70%); mp 321–322 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.08 (s, 1H), 9.26 (s, 1H), 7.41–7.28 (m, 8H), 7.25–7.21 (m, 1H), 7.09–7.01 (m, 7H), 7.00–6.93 (m, 1H), 6.59 (s, 1H), 5.91 (s, 1H), 1.67 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.7, 163.2, 161.3, 144.4 (d, J = 7.0 Hz), 138.9, 137.9, 136.5, 134.1, 130.4 (d, J = 8.4 Hz), 128.0, 127.1, 125.8, 125.6, 125.2 (d, J = 2.3 Hz), 121.3, 120.1, 119.1, 115.7 (d, J = 21.9 Hz), 113.8, 113.7, 113.5, 111.5, 110.1, 47.2, 22.8; HRMS (ESI) m/z: [M + Na]+ calculated for C31H24N2ONaF2, 501.1749; found, 501.1758.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-1-(4-fluorophenyl)vinyl)acetamide (5f). The product 5f was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a white solid (72.0 mg, yield: 78%); mp 321–322 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.02 (s, 1H), 9.25 (s, 1H), 7.39–7.36 (m, 2H), 7.35–7.28 (m, 6H), 7.26–7.21 (m, 6H), 7.15–7.10 (m, 2H), 7.07–7.03 (m, 1H), 6.98–6.94 (m, 1H), 6.52 (s, 1H), 5.83 (s, 1H), 1.69 (s, 3H);13C NMR (150 MHz, DMSO-d6) δ 168.9, 160.6, 142.1, 139.5, 136.4, 135.5, 132.7, 129.0, 128.3, 127.3 (d, J = 8.0 Hz), 126.5, 125.8, 121.0, 119.9, 118.9, 114.7 (d, J = 21.4 Hz), 114.0, 111.4, 109.4, 48.0, 22.8; HRMS (ESI) m/z: [M + Na]+ calculated for C31H25N2ONaF, 483.1844; found, 483.1843.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-1-(2-chlorophenyl)vinyl)acetamide (5g). The product 5g was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a white solid (61.8 mg, yield: 65%); mp 344–345 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.05 (s, 1H), 9.30 (s, 1H), 7.40–7.27 (m, 11H), 7.27–7.20 (m, 7H), 7.10–7.03 (m, 1H), 7.00–6.96 (m, 1H), 6.18 (s, 1H), 5.78 (s, 1H), 1.53 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.2, 142.1, 138.8, 138.4, 136.4, 132.6, 131.1, 130.9, 129.5, 129.0, 128.4, 126.7, 126.5, 126.3, 121.1, 119.6, 118.9, 116.1, 111.4, 108.6, 48.1, 22.3; HRMS (ESI) m/z: [M + H]+ calculated for C31H26ClN2O, 477.1728; found, 477.1729.
- N-(2-(2-benzhydryl-1H-indol-3-yl)-1-(o-tolyl)vinyl)acetamide (5h). The product 5h was obtained by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1) as a white solid (68.3 mg, yield: 75%); mp 301–302 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.97 (s, 1H), 9.23 (s, 1H), 7.38–7.28 (m, 6H), 7.27–7.20 (m, 7H), 7.17–7.12 (m, 3H), 7.09–7.03 (m, 1H), 7.00–6.94 (m, 1H), 6.02 (s, 1H), 5.78 (s, 1H), 2.28 (s, 3H), 1.53 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 168.0, 142.2, 139.9, 138.4, 136.4, 135.1, 134.7, 130.0, 129.0, 128.8, 128.3, 126.8, 126.5, 126.4, 125.3, 120.9, 119.6, 118.8, 114.9, 111.4, 108.9, 48.1, 22.4, 20.3; HRMS (ESI) m/z: [M + H]+ calculated for C32H29N2O, 457.2274; found, 457.2271.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humphrey, G.R.; Kuethe, J.T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Kochanowska, A.J.; Hamann, M.T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A Privileged Scaffold for the Design of Anti-cancer Agents. Eur. J. Med. Chem. 2019, 183, 111691–111692. [Google Scholar] [CrossRef] [PubMed]
- Bandini, M.; Eichholzer, A. Catalytic Functionalization of Indoles in a New Dimension. Angew. Chem. Int. Ed. 2009, 48, 9608–9644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, L.; Wang, L.; Xiao, J. Recent advances in asymmetric synthesis of optically active indole derivatives from 3-indolylmethanols. Chin. J. Org. Chem. 2016, 36, 1229–1240. [Google Scholar] [CrossRef]
- Palmieri, A.; Petrini, M.; Shaikh, R.R. Synthesis of 3-Substituted Indoles via Reactive Alkylideneindolenine Intermediates. Org. Biomol. Chem. 2010, 8, 1259–1270. [Google Scholar] [CrossRef]
- Chen, L.; Yin, X.P.; Wang, C.H.; Zhou, J. Catalytic FunctionAlization of Tertiary Alcohols to Fully Substituted Carbon Centres. Org. Biomol. Chem. 2014, 12, 6033–6048. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Xiao, J. Alkylideneindoleninium Ions and Alkylideneindolenines: Key Intermediates for the Asymmetric Synthesis of 3-Indolyl Derivatives. Asian J. Org. Chem. 2014, 3, 1036–1052. [Google Scholar]
- Cozzi, P.G.; Benfatti, F.; Zoli, L. Organocatalytic Asymmetric Alkylation of Aldehydes by SN1-Type Reaction of Alcohols. Angew. Chem. Int. Ed. 2009, 48, 1313–1316. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Z.J.; Antilla, J.C. Chiral Brønsted Acid Catalyzed Pinacol Rearrangement. Angew. Chem. Int. Ed. 2010, 49, 9734–9736. [Google Scholar] [CrossRef]
- Wang, D.S.; Tang, J.; Zhou, Y.G.; Chen, M.W.; Yu, C.B.; Duan, Y.; Jiang, G.F. Dehydration Triggered Asymmetric Hydrogenation of 3-(α-Hydroxyalkyl)Indoles. Chem. Sci. 2011, 2, 803–806. [Google Scholar] [CrossRef]
- Song, L.; Guo, Q.X.; Li, X.C.; Tian, J.; Peng, Y.G. The Direct Asymmetric α-Alkylation of Ketones by Brønsted Acid Catalysis. Angew. Chem. Int. Ed. 2012, 51, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.Y.; Sarlah, D.; Carreira, E.M. Iridium-Catalyzed Enantioselective Allylic Vinylation. J. Am. Chem. Soc. 2013, 135, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.D.; Li, S.; Guo, R.; Nie, J.; Ma, J.A. Chiral Phosphoric Acid Catalyzed Enantioselective Decarboxylative Alkylation of β-Keto Acids with 3-Hydroxy-3-indolyloxindoles. Org. Lett. 2015, 17, 1389–1392. [Google Scholar] [CrossRef]
- Xu, B.; Shi, L.L.; Zhang, Y.Z.; Wu, Z.J.; Fu, L.N.; Luo, C.Q.; Zhang, L.X.; Peng, Y.G.; Guo, Q.X. Catalytic Asymmetric Direct α-Alkylation of Amino Esters by Aldehydes via Imine Activation. Chem. Sci. 2014, 5, 1988–1991. [Google Scholar] [CrossRef]
- Guo, Z.L.; Xue, J.H.; Fu, L.N.; Zhang, S.E.; Guo, Q.X. The Direct Asymmetric Alkylation of α-Amino Aldehydes with 3-Indolylmethanols by Enamine Catalysis. Org. Lett. 2014, 16, 6472–6475. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Xu, M.M.; Wan, Y.Y.; Mao, J.; Mei, G.J.; Shi, F. Application of 7-Indolylmethanols in Catalytic Asymmetric Arylations with Tryptamines: Enantioselective Synthesis of 7-indolylmethanes. Adv. Synth. Catal. 2018, 360, 1850–1860. [Google Scholar] [CrossRef]
- Silvia, V.; Aitor, L.; Antonia, M.; Iñaki, G.; Mikel, O.; Vadim, S. Catalytic Asymmetric α-Functionalization of α-Branched Aldehydes. Molecules 2023, 28, 2694. [Google Scholar]
- Antonio, D.V.; Arianna, S.; Valeria, N.; Giuliana, G.; Graziano, D.C.; Fabio, P. Synergistic Strategies in Aminocatalysis. Chem. Eur. J. 2022, 28, e202200818. [Google Scholar]
- Xu, M.M.; Wang, H.Q.; Wan, Y.; Wang, S.L.; Shi, F. Enantioselective Construction of Cyclopenta[b]indole Scaffolds via the Catalytic Asymmetric [3 + 2] Cycloaddition of 2-Indolylmethanols with p-Hydroxystyrenes. J. Org. Chem. 2017, 82, 10226–10233. [Google Scholar] [CrossRef]
- Tan, W.; Li, X.; Gong, Y.X.; Ge, M.D.; Shi, F. Highly Diastereo- and Enantioselective Construction of a Spiro[cyclopenta[b]Indole-1,3′-oxindole] Scaffold via Catalytic Asymmetric Formal [3 + 2] Cycloadditions. Chem. Commun. 2014, 50, 15901–15904. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Yu, L.; Sun, M.; Mei, G.J.; Shi, F. Regioselective [3 + 3] Cyclization of 2-Indolymethanols with Vinylcyclopropanes via Metal Catalysis. Adv. Synth. Catal. 2018, 360, 3109–3116. [Google Scholar] [CrossRef]
- Shi, Y.C.; Yan, X.Y.; Wu, P.; Jiang, S.; Xu, R.; Tan, W.; Shi, F. Design and Application of m-Hydroxybenzyl Alcohols in Regioselective (3 + 3) Cycloadditions of 2-Indolymethanols. Chin. J. Chem. 2023, 41, 27–36. [Google Scholar] [CrossRef]
- Qin, L.Z.; Cheng, Y.L.; Wen, X.A.; Xu, Q.L.; Zhen, L. Synthesis of Indole-fused Scaffolds via [3+3] Cyclization Reaction of 2-indolylmethanols with Quinone imines. Tetrahedron 2021, 77, 131742–131749. [Google Scholar] [CrossRef]
- Nawaz, S.; Huang, Y.; Bao, X.Z.; Wei, S.Q.; Wei, X.F.; Qu, J.P.; Wang, B.M. Construction of a Spiro[pyrazolone-4,2′-pyridoindole] Scaffold via a [3 + 3] Cycloaddition of 2-Indolylmethanol with a 4-Aminopyrazolone-derived Azomethine Ylide. Org. Biomol. Chem. 2021, 19, 8530–8538. [Google Scholar] [CrossRef]
- Qiu, Z.W.; Li, B.Q.; Liu, H.F.; Zhu, Z.Q.; Pan, H.P.; Feng, N.; Ma, A.J.; Peng, J.B.; Zhang, X.Z. Formal (3 + 4)-Annulation of Propargylic p-Quinone Methides with 2-Indolylmethanols: Synthesis of Polysubstituted Indole-Fused Oxepines. J. Org. Chem. 2021, 86, 7490–7499. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Li, T.Z.; Liu, S.J.; Zhang, Y.C.; Deng, S.; Jiao, Y.C.; Shi, F. Axially Chiral Aryl-Alkene-Indole Framework: A Nascent Member of the Atropisomeric Family and Its Catalytic Asymmetric Construction. Chin. J. Chem. 2020, 38, 543–552. [Google Scholar] [CrossRef]
- Sun, M.; Ma, C.; Zhou, S.J.; Lou, S.F.; Xiao, J.; Jiao, Y.C.; Shi, F. Catalytic Asymmetric (4 + 3) Cyclizations of In Situ Generated ortho-Quinone Methides with 2-Indolylmethanols. Angew. Chem. Int. Ed. 2019, 58, 8703–8708. [Google Scholar] [CrossRef]
- He, Y.Y.; Sun, X.X.; Li, G.H.; Mei, G.J.; Shi, F. Substrate-Controlled Regioselective Arylations of 2-Indolylmethanols with Indoles: Synthesis of Bis(indolyl)methane and 3,3′-Bisindole Derivatives. J. Org. Chem. 2017, 82, 2462–2471. [Google Scholar] [CrossRef]
- Tang, Z.C.; Hong, G.; Hu, C.; Wang, Q.; Zhong, Y.; Gong, Y.; Yang, P.; Wang, L.M. La(OTf)3 Facilitated Self-condensation of 2-Indolylmethanol: Construction of Highly Substituted Indeno[1,2-b]indoles. Org. Biomol. Chem. 2021, 19, 10337–10342. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, F.; Sheng, F.T.; Jiao, Y.; Mei, G.J.; Shi, F. Design and Catalytic Asymmetric Construction of Axially Chiral 3,3′-Bisindole Skeletons. Angew. Chem. Int. Ed. 2019, 58, 3014–3020. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, Y.; Han, F.S. Al-Catalyzed Facile Construction of Quaternary C—C Bonds by the Allylic Substitution of Tertiary Alcohols: A Concise and Formal Synthesis of (±)-Mersicarpine. Chem. Eur. J. 2012, 18, 9784–9788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Wang, C.S.; Li, C.; Mei, G.J.; Li, Y.X.; Shi, F. Design and Enantioselective Construction of Axially Chiral Naphthyl-Indole Skeletons. Angew. Chem. Int. Ed. 2017, 56, 116–121. [Google Scholar] [CrossRef]
- Chen, L.; Zou, Y.X.; Fang, X.Y.; Wu, J.; Sun, X.H. Brønsted Acid-catalysed Regiodivergent Phosphorylation of 2-Indolylmethanols to Synthesize Benzylic Site or C3-phosphorylated indole Derivatives. Org. Biomol. Chem. 2018, 16, 7417–7424. [Google Scholar] [CrossRef]
- Hu, C.; Hong, G.; He, Y.C.; Zhou, C.; Kozlowski, M.C.; Wang, L.M. Lewis Acid-Controlled Regioselective Phosphorylation of 2-Indolylmethanols with Diarylphosphine Oxides: Synthesis of Highly Substituted Indoles. J. Org. Chem. 2018, 83, 4739–4753. [Google Scholar] [CrossRef]
- Zhu, S.Z.; Zhang, Y.; Luo, J.Y.; Wang, F.; Cao, X.J.; Huang, S.L. Temperature-controlled Regioselective Thiolation of 2-Indolylmethanols under Aqueous Micellar Conditions. Green Chem. 2020, 22, 657–661. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.Q.; Xu, M.M.; Mei, G.J.; Shi, F. Direct C3-Arylations of 2-Indolylmethanols with Tryptamines and Tryptophols via an Umpolung Strategy. Org. Biomol. Chem. 2018, 16, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cao, W.B.; Zhang, L.L.; Xu, X.P.; Ji, S.J. Ag(I)-Promoted Dehydroxylation and Site-Selective 1,7-Disulfonylation of Diaryl(1H-indol-2-yl)methanols. J. Org. Chem. 2018, 83, 6056–6065. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Wang, H.Q.; Mao, Y.J.; Mei, G.J.; Wang, S.L.; Shi, F. Cooperative Catalysis-Enabled Asymmetric α-Arylation of Aldehydes Using 2-Indolylmethanols as Arylation Reagents. J. Org. Chem. 2018, 83, 5027–5034. [Google Scholar] [CrossRef]
- Fu, T.H.; Bonaparte, A.; Martin, S.F. Synthesis of β-Heteroaryl Propionates via Trapping of Carbocations with π-Nucleophiles. Tetrahedron Lett. 2009, 50, 3253–3257. [Google Scholar] [CrossRef]
- Zhong, Y.; Hong, G.; Tang, Z.C.; Yang, P.; Wang, Q.; Gong, Y.; Wang, L.M. PFOA-Catalyzed Regioselective Alkylation of Indolylmethanols with 2-Alkylazaarenes. ChemistrySelect 2022, 7, e202200218. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, Z.Q.; Liu, J.X.; Yu, L.; Du, B.X.; Mei, G.J.; Shi, F. Brønsted Acid Catalyzed C3-Alkylation of 2-Indolylmethanols with Azlactones via an Umpolung Strategy. Synthesis 2017, 49, 4025–4034. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Shen, Y.; Liu, J.X.; Tao, J.Y.; Shi, F. Enantioselective Direct α-Arylation of Pyrazol-5-ones with 2-Indolylmethanols via Organo-Metal Cooperative Catalysis. Org. Lett. 2017, 19, 1542–1545. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.N.; Ma, C.; Lan, J.P.; Zhu, C.Q.; Mao, Y.J.; Mei, G.J.; Zhang, S.; Shi, F. Catalytic Enantioselective and Regioselective Substitution of 2,3-indolyldimethanols with Enaminones. Org. Chem. Front. 2018, 5, 2657–2667. [Google Scholar] [CrossRef]
- Tu, M.S.; Chen, K.W.; Wu, P.; Zhang, Y.C.; Liu, X.Q.; Shi, F. A Catalytic Asymmetric Interrupted Nazarov-type Cyclization of 2-Indolylmethanols with Cyclic Enaminones. Org. Biomol. Chem. 2018, 16, 5457–5464. [Google Scholar]
- Liu, Y.; Cao, M.; Zhang, S.X.; Wang, Z.X.; Dai, X.; Jiang, X.D.; Dong, Y.C.; Fu, J. Synthesis of C3-functionalized Indole Derivatives via Brønsted Acid-catalyzed Regioselective Arylation of 2-Indolylmethanols with Guaiazulene. Org. Biomol. Chem. 2022, 20, 1510–1517. [Google Scholar] [CrossRef]
- Wu, X.F.; Neumann, H.; Beller, M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef]
- Deiters, A.; Martin, S.F. Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis. Chem. Rev. 2004, 104, 2199–2238. [Google Scholar] [CrossRef]
- Fustero, S.; Sánchez, R.M.; Barrio, P.; Simón, F.A. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Liu, H.; Dagousset, G.; Masson, G.; Retailleau, P.; Zhu, J.P. Chiral Brønsted Acid-Catalyzed Enantioselective Three-Component Povarov Reaction. J. Am. Chem. Soc. 2009, 131, 4598–4599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Yu, S.W.; Hu, F.Z.; Liao, Y.J.; Liao, L.H.; Xu, X.Y.; Yuan, W.C.; Zhang, X.M. Highly Enantioselective [3 + 2] Coupling of Cyclic Enamides with Quinone Monoimines Promoted by a Chiral Phosphoric Acid. Chem. Commun. 2016, 52, 8757–8760. [Google Scholar] [CrossRef]
- Gelis, C.; Bekkaye, M.; Lebee, C.; Blanchard, F.; Masson, G.R. Chiral Phosphoric Acid Catalyzed [3 + 2] Cycloaddition and Tandem Oxidative [3 + 2] Cycloaddition: Asymmetric Synthesis of Substituted 3-Aminodihydrobenzofurans. Org. Lett. 2016, 18, 3422–3425. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhao, L.; Zhu, J.P.; Wang, M.X. Catalytic Asymmetric Tandem Reaction of Tertiary Enamides: Expeditious Synthesis of Pyrrolo[2,1-a]isoquinoline Alkaloid Derivatives. Angew. Chem. Int. Ed. 2016, 55, 3799–3803. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Hodík, T.; Schneider, C. Brønsted Acid Catalyzed Addition of Enamides to ortho-Quinone Methide Imines—An Efficient and Highly Enantioselective Synthesis of Chiral Tetrahydroacridines. Angew. Chem. Int. Ed. 2016, 55, 9788–9792. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Gao, L.M.; Wang, X.M.; Shi, R.J.; Ma, R.P.; Li, J.F.; Lan, X.S.; Zheng, Y.S.; Liu, J.K. [3 + 2] Cycloaddition of Nitrile Imines with Enamides: An Approach to Functionalized Pyrazolines and Pyrazoles. J. Org. Chem. 2021, 86, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, X.C.; Tao, J.Y.; Wu, X.D.; Wu, J.X.; Li, W.M.; Zhu, T.H.; Loh, T.P. Regio- and Stereoselective C(sp2)–H Acylation of Enamides with Aldehydes via Transition-metal-free Photoredox Catalysis. Green Chem. 2020, 22, 5497–5503. [Google Scholar] [CrossRef]
- Guo, J.Y.; Zhang, Z.Y.; Guan, T.; Mao, L.W.; Ban, Q.; Zhao, K.; Loh, T.P. Photoredox-catalyzed Stereoselective Alkylation of Enamides with N-hydroxyphthalimide Esters via Decarboxylative Cross-coupling Reactions. Chem. Sci. 2019, 10, 8792–8798. [Google Scholar] [CrossRef]
- Guo, J.Y.; Guan, T.; Tao, J.Y.; Zhao, K.; Loh, T.P. Stereoselective C(sp2)–H Alkylation of Enamides with Unactivated Aliphatic Carboxylic Acids via Decarboxylative Cross-Coupling Reactions. Org. Lett. 2019, 21, 8395–8399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.H.; Zhang, X.C.; Zhao, K.; Loh, T.P. Cu(OTf)2-mediated C(sp2)–H Arylsulfonylation of Enamides via the Insertion of Sulfur Dioxide. Org. Chem. Front. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Zhu, T.H.; Zhang, Z.Y.; Tao, J.Y.; Zhao, K.; Loh, T.P. Regioselective and Stereoselective Difluoromethylation of Enamides with Difluoromethyltriphenylphosphonium Bromide via Photoredox Catalysis. Org. Lett. 2019, 21, 6155–6159. [Google Scholar] [CrossRef]
- Zhu, T.H.; Zhang, X.C.; Cui, X.L.; Zhang, Z.Y.; Jiang, H.; Sun, S.S.; Zhao, L.L.; Zhao, K.; Loh, T.P. Direct C(sp2)-H Arylsulfonylation of Enamides via Iridium(III)-Catalyzed Insertion of Sulfur Dioxide with Aryldiazonium Tetrafluoroborates. Adv. Synth. Catal. 2019, 361, 3593–3598. [Google Scholar] [CrossRef]
- Zhu, W.J.; Zhao, L.; Wang, M.X. Synthesis of 2,3-Dihydro-1H-azepine and 1H-Azepin-2(3H)-one Derivatives From Intramolecular Condensation between Stable Tertiary Enamides and Aldehydes. J. Org. Chem. 2015, 80, 12047–12057. [Google Scholar] [CrossRef] [PubMed]
- Gigant, N.; Laetitia, C.B.; Belhomme, M.C.; Poisson, T.; Pannecoucke, X.; Gillaizeau, I. Copper-Catalyzed Direct Arylation of Cyclic Enamides Using Diaryliodonium Salts. Org. Lett. 2013, 15, 278–281. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.H.; Fan, T.; Shen, Y.; Wu, Q.; Shi, F. Brønsted Acid-catalyzed Regioselective Reactions of 2-indolylmethanols with Cyclic Enaminone and Anhydride Leading to C3-Functionalized Indole Derivatives. Org. Biomol. Chem. 2016, 14, 6932–6936. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Han, F.S. Organocatalytic Asymmetric Reaction of Indol-2-yl Carbinols with Enamides: Synthesis of Chiral 2-Indole-substituted 1,1-Diarylalkanes. Chem. Commun. 2015, 51, 11844–11847. [Google Scholar] [CrossRef] [PubMed]




| Entry | Catalyst | Solvent | Time | Yield (%) b |
|---|---|---|---|---|
| 1 | CPA | Toluene | 2 | 35 |
| 2 c | AcOH | Toluene | 72 | NR |
| 3 c | TFA | Toluene | 72 | NR |
| 4 | TsOH | Toluene | 8 | 38 |
| 5 | Sc(OTf)3 | Toluene | 3 | NR |
| 6 | Cu(OTf)2 | Toluene | 3 | NR |
| 7 | AlCl3 | Toluene | 2 | 40 |
| 8 | AlCl3 | THF | 36 | NR |
| 9 | AlCl3 | MeCN | 3 | 86 |
| 10 | AlCl3 | CHCl3 | 2 | 80 |
| 11 | AlCl3 | DCM | 8 | 70 |
| 12 | AlCl3 | DCM | 2 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; He, D.; Gao, L.; Zou, Y.; Liu, X.; Wang, Q.; Liang, E.; Zheng, Y. Regioselective Reaction of 2-Indolylmethanols with Enamides. Molecules 2023, 28, 3341. https://doi.org/10.3390/molecules28083341
Tian Y, He D, Gao L, Zou Y, Liu X, Wang Q, Liang E, Zheng Y. Regioselective Reaction of 2-Indolylmethanols with Enamides. Molecules. 2023; 28(8):3341. https://doi.org/10.3390/molecules28083341
Chicago/Turabian StyleTian, Yuting, Dongqing He, Limei Gao, Yu Zou, Xiaoshuang Liu, Qiang Wang, Enxiang Liang, and Yongsheng Zheng. 2023. "Regioselective Reaction of 2-Indolylmethanols with Enamides" Molecules 28, no. 8: 3341. https://doi.org/10.3390/molecules28083341
APA StyleTian, Y., He, D., Gao, L., Zou, Y., Liu, X., Wang, Q., Liang, E., & Zheng, Y. (2023). Regioselective Reaction of 2-Indolylmethanols with Enamides. Molecules, 28(8), 3341. https://doi.org/10.3390/molecules28083341




