Abstract
Chalcones are interesting anticancer drug candidates which have attracted much interest due to their unique structure and their extensive biological activity. Various functional modifications in chalcones have been reported, along with their pharmacological properties. In the current study, novel chalcone derivatives with the chemical base of tetrahydro-[1,2,4]triazolo[3,4-a]isoquinolin-3-yl)-3-arylprop-2-en-1-one were synthesized, and the structure of their molecules was confirmed through NMR spectroscopy. The antitumor activity of these newly synthesized chalcone derivatives was tested on mouse (Luc-4T1) and human (MDA-MB-231) breast cancer cell lines. The antiproliferative effect was evaluated through SRB screening and the MTT assay after 48 h of treatment at different concentrations. Interestingly, among the tested chalcone derivatives, chalcone analogues with a methoxy group were found to have significant anticancer activity and displayed gradient-dependent inhibition against breast cancer cell proliferation. The anticancer properties of these unique analogues were examined further by cytometric analysis of the cell cycle, quantitative PCR, and the caspases-Glo 3/7 assay. Chalcone methoxy derivatives showed the capability of cell cycle arrest and increased Bax/Bcl2 mRNA ratios as well as caspases 3/7 activity. The molecular docking analysis suggests that these chalcone methoxy derivatives may inhibit anti-apoptotic proteins, particularly cIAP1, BCL2, and EGFRK proteins. In conclusion, our findings confirm that chalcone methoxy derivatives could be considered to be potent drug candidates against breast cancer.
Keywords:
breast cancer; chalcones; methoxy group effect; MDA; Luc4T1; cell cycle; cytotoxicity; docking; cIAP1 1. Introduction
Breast cancer is the most prevalent type of cancer in women worldwide, with a total of 2.26 million cases each year, 11.7% of all cancer cases, and 24.5% of the cancer cases in women [1]. Additionally, it accounts for 15.5% of annual cancer deaths among women [1]. The use of cytotoxic chemotherapeutic drugs is the currently established treatment option for breast cancer, either alone or in combination with other medical procedures such as surgery and radiotherapy. A number of FDA-approved chemotherapeutic drugs such as 5-fluorouracil (5-FU), capecitabine, docetaxel, doxorubicin, epirubicin, gemcitabine, methotrexate, paclitaxel, tamoxifen citrate, and nucleosides [2] are available as therapeutic options for cancer. However, they are still considered toxic agents and are associated with long-term adverse effects [2,3].
A continuous research effort is ongoing to develop potent new therapeutics or to improve chemotherapeutics. Modern oncology aims to develop novel natural or synthetic compounds with antitumor properties. Such potential compounds are chalcones, which belong to a class group of flavonoids [4]. Chalcones typical structure is a central 1,3-diphenyl-prop-2-en-1-one structure known as a chalcone core, which consists of two aromatic rings jointed by the three-carbon α, β-unsaturated carbonyl system [5]. Chalcones (1-(2′-hydroxyphenyl)-3-phenylprop-2-en-1-ones) and their derivatives are often obtained from natural sources such as citrus fruits, vegetables, and spices [6,7] or as a result of chemical synthesis [8,9]. Both natural and synthetic analogs are reported to exhibit various biological properties including anticancer, anti-inflammatory, and antioxidant activities [10,11,12,13].
Recently, chalcones have been shown to induce apoptosis and cell cycle arrest in various cancer cells, including breast cancer [14,15]. Inhibitory effects of some chalcones have been linked to the tumor suppressor p53 [16]. The functional groups of synthetic chalcone derivatives might influence such bioactivity depending on their position on the aryl rings (A and B). The chalcones exist in two isomers: trans (E) and cis (Z). The E isomer is the most stable and hence the most prevalent structure of the chalcones [17]. Trimethoxy chalcone exhibits anticancer activity in a variety of human cancer cell lines [18], making it an attractive scaffold for studying its anticancer activity.
Motivated by the above-mentioned facts and in continuation of our interest in the synthesis of bioactive heterocycles [19,20,21,22,23,24,25,26,27,28], in the present study, we synthesized a new series of chalcone derivatives. The cytotoxic, antiproliferative, and proapoptotic effects of newly synthesized compounds were analyzed. The anticancer activity of new chalcone analogues with a methoxy group tested on mouse and human breast cancer cell lines showed a greater significant anticancer potency than the parent compound. Compounds 3a and 5a exhibited a strong suppression of cell growth in association with cell cycle arrests and apoptosis.
2. Results
2.1. Synthesis of the Chalcones
A high yield and purity of starting 3-acetyl-8,9-dimethoxy-1-(4-methoxyphenyl)-1,5,6,10b-tetrahydro-[1,2,4]triazolo[3,4-a]isoquinoline 1a, 3-acetyl-8,9-dimethoxy-1-(4-chlorophenyl)-1,5,6,10b-tetrahydro-[1,2,4]triazolo[3,4-a]isoquinoline 1b, and 3-acetyl-8,9-dimethoxy-1-(4-bromophenyl)-1,5,6,10b-tetrahydro-[1,2,4]triazolo[3,4-a]isoquinoline 1c, were synthesized using the described procedure in the scheme at (Figure 1A) through the reactions of nitrilimine ii with 3,4-dihydro-6,7-dimethoxyisoquinoline iii. Then, Claisen–Schmidt condensation of compound (1a–c) with equimolar amounts of substituted aldehydes 2 and 4 in the presence of potassium hydroxide solution leads to the formation of the corresponding chalcone derivatives (3a–c) shown in scheme Figure 1B and (5a–c) shown in scheme Figure 1C. The structures of the formed products were elucidated by inspection of their spectral data.
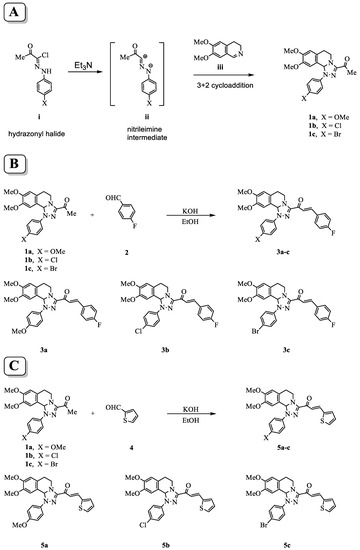
Figure 1.
Scheme for the synthesis of tetrahydro-[1,2,4]triazolo[3,4-a]isoquinolin-3-yl)-3-arylprop-2-en-1-one chalcone derivatives. (A) Procedures of the reaction to form the parent compounds (1a–c) through the reactions of nitrilimine ii with 3,4-dihydro-6,7-dimethoxyisoquinoline iii. (B) Formation of the chalcone derivatives (3a–c) by Claisen–Schmidt condensation of compounds (1a–c) with 4-fuorobenzaldehyde 2. (C) Formation of the (5a–c) by Claisen–Schmidt condensation of compound (1a–c) with thiophene-2-carbaldehyde 4.
2.2. In Vitro Cytotoxicity Screening of Chalcone-Based Compounds
The in vitro cytotoxic screening was performed against the six newly synthesized chalcone compounds 3a, 3b, 3c, 5a, 5b, and 5c, using the SRB assay. The mouse breast cancer cells Luc-4T1 and the human breast cancer cells MDA-MB-231 were treated with or without each compound independently for 48 h at a final concentration of 20 µg/mL. In order to evaluate the drug’s activity, we used 5-FU, an approved chemotherapeutic drug, as a positive control. Results indicated that out of six tested compounds, five showed a lower viability percentage than the 5-FU in both mouse and human cell lines (Table 1). The cell viability percentages of Luc-4T1 were 52.5 ± 11.9, 64.3 ± 15.6, 57.6 ± 13.2, 79.6 ± 9.3, 92.1 ± 2.1, 81.6 ± 4.3, and 88.5 ± 0.6 for 3a, 3b, 3c, 5a, 5b, 5c, and 5-FU, respectively. The cell viability percentages of MDA-MB-231 were 49.7 ± 12.7, 30.9 ± 8.3, 40.6 ± 6.8, 39.4 ± 12.5, 31.7 ± 11.5, 25.9 ± 3.3, and 64 ± 11.6 for 3a, 3b, 3c, 5a, 5b, 5c, and 5-FU, respectively. These data indicated that screening of the chalcone compounds 3a, 3b, 3c, 5a, and 5c except 5b showed a better cytotoxic effect compared to 5-FU.

Table 1.
SRB screening for six chalcone compounds on mouse and human breast cancer cell lines.
Subsequently, we tested the five compounds with different doses to see the dose-dependent effect with the increasing of concentration (Figure 2). Cytotoxic evaluations of the chalcone derivatives and 5-FU as a positive control were performed against the Luc-4T1 cell line at different concentrations (20, 40, 50, and 100 μg/mL). After a 48 h treatment with different concentrations under the same experimental conditions, compounds 3a and 5a showed a gradual cytotoxic effect with increasing concentrations, but not the other tested compounds 3b, 3c, 5b, and 5c. In contrast, the positive control 5-FU showed a slight gradual cytotoxic effect, but its level of cytotoxicity was much lower than that of compounds 3a and 5a. Therefore, to ascertain these data and calculate the IC50, we repeated the experiment with a wider range of concentrations between (10-200 μg/mL) for the two promising compounds (3a and 5a) as well as the positive control 5-FU on the cell lines from the two origins of mouse and human (Figure 3). As the same as before, both compounds showed better cytotoxic effects with lower IC50s against the two cell lines when compared to 5-FU. The IC50 on Luc4T1 was 4.4, 27.1, and 188.8 μg/mL for compounds 3a, 5a, and 5-FU, respectively (Figure 3A,C). In addition, the IC50 on MDA-MB-231 was 24.5, 29.5, and 122.2 μg/mL for compounds 3a, 5a, and 5-FU, respectively (Figure 3B,C). These data indicated that the introduction of a methyl group at position 4 of the phenyl group (compounds 3a and 5a) significantly improved the growth-inhibitory activity of breast carcinoma.
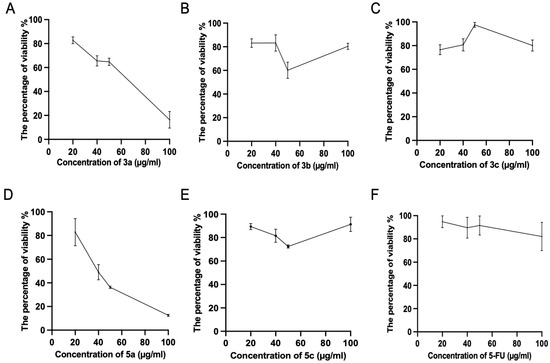
Figure 2.
Cytotoxic evaluations of the tested chalcone compounds against the Luc-4T1 cell line. The cell line was treated for 48 h with different concentrations (20, 40, 50, and 100 μg/mL) of the individual chalcones: (A) compound 3a, (B) compound 3b, (C) compound 3c, (D) compound 5a, (E) compound 5c, and (F) 5-FU, and the percentage of viability was determined through MTT assay. The values are means ± SD (n = 4), and two independent experiments were examined.
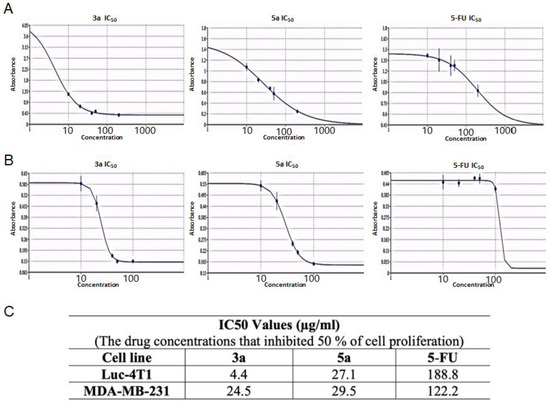
Figure 3.
The IC50 values of the chalcone methoxy derivatives 3a and 5a. IC50 was determined through the MTT assay by non-linear regression using (A) Mouse Luc-4t1 and (B) Human MDA-MB-231 cell lines after 48 h of treatment, with 5-FU as a positive control against. (C) Summary of the IC50 values. The Quest Graph™ IC50 Calculator [29] was used to calculate the IC50.
2.3. Chalcone Analogues with the Methoxy Group Induced Cell Cycle Arrests and Apoptosis in Luc4T1 Cells
The compounds with the strongest activity in the MTT test (compounds 3a and 5a) were selected for further research on the nature of cell death. Anti-proliferation is an important mechanism for therapeutic agents to suppress tumor growth. The effects of the compounds 3a and 5a on the Luc-4T1 cell cycle progression were examined after 24 h of treatment with a concentration around the IC50 (20 μg/mL). FACS profiles indicated that compound 3a induced cell cycle arrest at the G2/M phase (50.0 ± 7.3%) as well as compound 5a (52.5 ± 4.5%) in comparison to the non-treated cells (28.1 ± 4.1%), while the positive control 5-FU induced cell cycle arrest at G2/M phase lower than the tested chalcone derivatives (40.9 ± 9.8%) (Figure 4). The cell cycle distribution of the non-treated cells (G0G1 = 67.3 ± 4.7%, S = 4.06 ± 2.2%, G2/M = 28.1 ± 4.1%), 5-FU (G0G1 = 55.4 ± 6.8%, S = 2.68 ± 3.4%, G2/M = 40.9 ± 9.8%), compound 3a (G0G1 = 51.4 ± 9.6%, S = 1.03 ± 1.6%, G2/M = 50.0 ± 7.3%), and compound 5a (G0G1 = 47.1 ± 6.6%, S = 1.51 ± 2.9%, G2M = 52.5 ± 4.5%). The transition from one phase to another phase in the cell cycle is a very important point for controlling cell proliferation. These data suggest that compounds 3a and 5a cause mitotic arrest, as shown by the accumulation of cells in the G2/M phase (Figure 4).
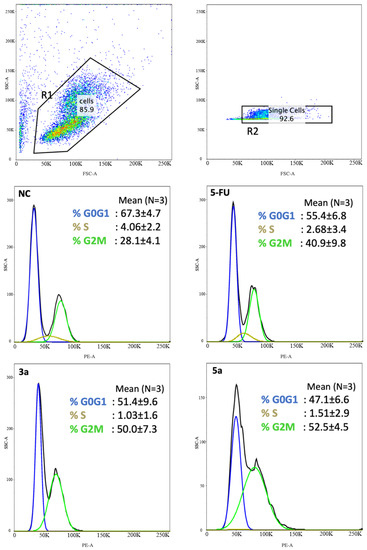
Figure 4.
Chalcone methoxy derivatives induce cell cycle arrest in Luc-4T1 cells. Cells were treated for 24 h with 20 μg/mL of compound 3a, compound 3c, 5-FU as a positive control, or untreated as controls (NC). The cells were then harvested, fixed in 70% ethanol, and stained with PI for FACS analysis. The percentage of cells in each phase of the cell cycle was shown as mean ± SD over the histograms. Two independent experiments were performed. NC; non-treated cells.
Pro-apoptosis is another important mechanism for therapeutic agents to eliminate malignant cells. The analysis for apoptotic key elements was performed using quantitative PCR. Bax and Bcl2 are two important regulator genes in the mitochondrial apoptotic pathway. The Bcl2 gene product is thought to contribute to oncogenesis by suppressing signals that induce apoptotic cell death. We checked the effects of chalcone methoxy derivatives (3a and 5a) on Bax and Bcl2 expression. Luc-4T1 cells were treated with a slightly lower concentration (10 μg/mL) of 3a, 5a, or the vehicle, for 24 h to monitor molecular changes before the cells entered cell cycle arrest or died due to cytotoxicity. The mRNA expression level of the proapoptotic gene Bax was found to be significantly up-regulated when treated with compound 3a (p < 0.001) and compound 5a (p < 0.05) relative to the control (Figure 5A), while the expression of the anti-apoptotic gene Bcl2 was significantly down-regulated when treated with compound 3a (p < 0.001) and compound 5a (p < 0.01) relative to the control (Figure 5B). Because the experimental evidence suggests that the balance between anti-apoptotic and pro-apoptotic members of the Bcl2 family is a much better determinant of the sensitivity to apoptosis, we evaluated the Bax/Bcl2 expression ratio. The ratio of Bax/Bcl2 was significantly increased (Figure 5C). Thus, up-regulation of the Bax/Bcl2 expression ratio by the two compounds 3a and 5a relative to the control promoted the apoptotic death of the Luc-4T1 cell line. Concentrations of active caspase-3/7, the main enzymes involved in the process of apoptosis, were measured to confirm the apoptotic cascade (Figure 5D). Luc-4T1 cells were treated with the same concentration (10 μg/mL) of 3a, 5a, or the vehicle for 24 h. By using the Caspase-Glo 3/7 assay kit, we measured Caspase 3/7 activity. Our results showed a significant increase in relative luminescence (p < 0.05) in the case of both compounds 3a and 5a compared with the control vehicle (Figure 5D). These data suggested that both compounds 3a and 5a strongly stimulated apoptotic induction of Luc-4T1. Taken together, the data above indicated that compounds 3a and 5a could induce apoptosis and cell cycle arrest at the G2/M phase in Luc-4T1 breast cancer cells.
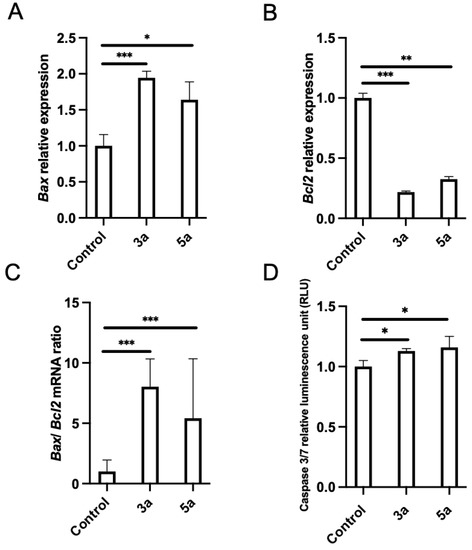
Figure 5.
Effect of chalcone methoxy derivatives on the levels of apoptotic markers in Luc-4T1 cells. The cells were cultured with 10 µg/mL of compound 3a, compound 5a, or a control vehicle for 24h, and then either RNA extraction or the Caspase-Glo 3/7 assay was performed. The relative transcription levels of (A) Bax, (B) Bcl2, and (C) Bax/Bcl2 mRNA ratio were determined. Gene expression levels were normalized to Hprt. (D) Caspace 3/7 relative luminescence. Bars represent the mean ± SD of four replicates from two independent experiments. * p < 0.05, ** p < 0.01, and *** p < 0.001 represent a statistical difference.
2.4. Modeling Simulation Analysis of Chalcone Analogues with the Methoxy Group
Compounds 3a and 5a were found to inhibit the expression of the anti-apoptotic gene Bcl2. To gain insight into their potential mode of action, we performed molecular docking studies to investigate the binding modes of these promising compounds against targeted anti-apoptotic proteins. This was important because inhibiting these proteins is a crucial strategy for reducing cancer growth. The targeted proteins were epidermal growth factor receptor tyrosine kinase (EGFRTK), cyclin-dependent kinase 2 (CDK2), cellular inhibitor of apoptosis protein 1 (cIAP1), mouse double minute 2 (MDM2), and B-cell lymphoma 2 (BCL2). The PDB IDs of the targeted proteins were 1m17, 2c6o, 4kmn, 4wt2, and 2w3l, respectively. Table 2 and Table 3 showed different binding energy readings of the co-crystallized standard ligand in comparison to the energy readings of the tested compounds 3a and 5a. The two tested compounds 3a and 5a proved a strong binding affinity toward the targeted anti-apoptotic protein c-IAP1 with binding energies (S) −22.12 and −24.08 Kcal/mol, respectively, in comparison to their co-crystallized ligand −14.40 Kcal/mol. Moreover, compound 5a ensured affinity (−20.07 Kcal/mol) much more than standard ligand (−18.25 Kcal/mol) regarding the anti-apoptotic BCL2 protein, while compound 3a exhibited almost the same binding affinity against that protein with binding energy (−18.09 Kcal/mol). On the other hand, compounds 3a and 5a proposed comparable binding activities toward the EGFRTK protein with binding energies of −23.60 and −22.56 Kcal/mol, respectively, with respect to the standard ligand of −23.91 Kcal/mol. Regarding the CDK2 active domain, the two compounds showed moderate effects relative to the standard ligand. In contrast, the two compounds 3a and 5a illustrated similar and weak binding affinities of −24.72 and −24.60 Kcal/mol toward MDM2 protein considering the standard ligand −41.29 Kcal/mol.

Table 2.
Standard ligand energy readings against (1m17, 2c6o, 4kmn, 4wt2, and 2w31) domains.

Table 3.
Tested compounds 3a and 5a energy readings against (1m17, 2c6o, 4kmn, 4wt2, and 2w31) domains.
Compound 3a exposed the best affinity and selectivity toward the active site 4kmn of the anti-apoptotic protein (cIAP1). As illustrated in Figure 6C, compound 3a fitted well and blocked active site 4kmn through seven different types of interactions (conventional H bonds, halogen (fluorine), Pi-cation, Amid-Pi stacked, Pi-alkyl, Van der Waals, and carbon-hydrogen bond). Compound 3a blocked the active site of EGFRTK with a binding energy of −23.60 Kcal/mol comparable to that of a standard ligand −23.91 Kcal/mol. As exposed in Figure 6A, compound 3a exerted its action through five different interactions as follows: conventional H bonds, Amid-Pi stacked, Pi-alkyl, Van der Waals, and carbon-hydrogen bond. Moreover, compound 3a confirmed compatible activity against the BCL2 domain −18.09 Kcal/mol relative to the standard ligand −18.25 Kcal/mol with six interactions as illustrated in Figure 6E. Interactions achieved by 3a with the active site of 2w3l were conventional H bonds, Alkyl, Pi-alkyl, Van der Waals, Pi-anion, and Pi-donor H bonds. On the other hand, compound 3a showed a moderate effect toward cdk2 protein with a binding energy −21.54 Kcal/mol regarding the standard ligand −26.73 Kcal/mol. However, it showed good binding affinity toward the 2c6o domain, as illustrated in Figure 6B. The compound demonstrated different types of interactions toward that domain such as conventional H bonds, carbon-hydrogen bonds, Pi-alkyl, Van der Waals, and Amide-Pi stacked. Regarding the 4wt2 domain, compound 3a was weakly fitted into that active site with a binding affinity of −24.72 Kcal/mol compared to the standard ligand −41.29 Kcal/mol. As seen in (Figure 6D), interactions considered in that active canter by compound 3a were only C-H bond, Pi-Alkyl, and Van der Waals.
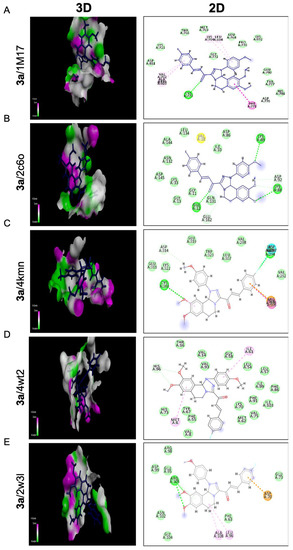
Figure 6.
Molecular docking analysis of chalcone compound 3a. Representation of 3D and 2D modeling into the active site of (A) epidermal growth factor receptor tyrosine kinase domain (1m17), (B) cyclin-dependent kinase 2 domain (2c6o), (C) cellular inhibitor of apoptosis protein 1 domain (4kmn), (D) mouse double minute 2 domain (4wt2), and (E) B-cell lymphoma 2 domain (2w3l).
Referring to compound 5a, it represents the best binding activities toward two proteins; cIAP1 and BCL2 with binding energies of −24.08 and −20.07 Kcal/mol relative to the co- crystallized ligands of −14.40 and −18.25 Kcal/mol, respectively. Seven different bindings toward the 4kmn domain were shown (Figure 7C) as follows: conventional H bonds, carbon-hydrogen bond, Pi-alkyl, Van der Waals, Pi-sulfur, Pi-Cation, and Pi-Anion. Moreover, compound 5a was strongly fitted into the 2w3l domain through four interactions (Pi-Pi T shaped, Pi-Alkyl, Alkyl, and Van der Waals) as outlined in Figure 7E. With respect to the 1m17 domain, compound 5a offered comparable binding affinity against that domain with the energy binding of −22.56 Kcal/mol as the same as the standard ligand −23.91 Kcal/mol. Figure 7A revealed that compound 5a was interacting with the active site of the EGFRTK domain within different interactions such as conventional H bonds, carbon-hydrogen bonds, Pi-alkyl, and Van der Waals. On the other hand, compound 5a indicated moderate binding affinity toward CDK2 protein with binding energy of −20.47 Kcal/mol compared to the standard ligand −26.73 Kcal/mol and by blotting 5a versus the 2C6O domain through several interactions: conventional H bonds, carbon-hydrogen bond, Pi-alkyl, and Van der Waals (Figure 7B). In addition, compound 5a showed a weak binding affinity toward the MDM2 protein with binding energy of −24.60 Kcal/mol in comparison to the standard ligand −41.29 Kcal/mol. Compound 5a was bonded through only Pi-Alkyl and Van der Waals bonds as shown (Figure 7D). This proposed that fluorobenzene and thiophene moieties were responsible for all the above-mentioned interactions, especially those involving the anti-apoptotic proteins cIAP1, BCL2, and EGFRK, which regulate metastasis and the cell cycle.
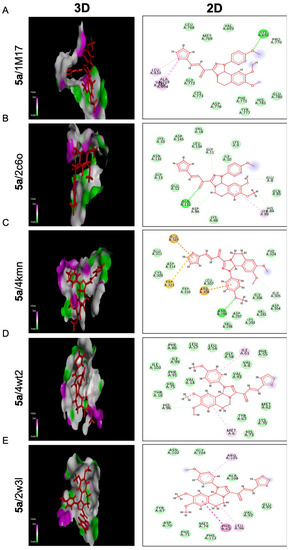
Figure 7.
Molecular docking analysis of chalcone compound 5a. Representation of 3D and 2D modeling into the active site of (A) epidermal growth factor receptor tyrosine kinase domain (1m17), (B) cyclin-dependent kinase 2 domain (2c6o), (C) cellular inhibitor of apoptosis protein 1 domain (4kmn), (D) mouse double minute 2 domain (4wt2), and (E) B-cell lymphoma 2 domain (2w3l).
3. Discussion
An investigation has been conducted on the potential therapeutic effects of novel chalcone derivatives on breast carcinoma. Breast cancer is a major health problem throughout the world. Current therapeutic options are not fully effective as a result of the complexity of the disease and the long-term adverse effects [3,30]. Chalcones are a class of flavonoids that have shown promise in various bioactivities, including anticancer properties [10,11,12,13]. Therefore, the modification was made to synthesize chalcone analogues containing a methoxy, chloro, or bromine group at position 4 of the phenyl group and to see how such a modification affects the antitumor activity in vitro. In the current study, we first screened the antiproliferative activity of the newly synthesized compounds (3a–c and 5a–c) in breast cancer cell lines originating from mouse (Luc-4T1) and human (MDA-MB-231). As shown in Table 1 and Figure 2 and Figure 3, using SRB and MTT assays, the authors found that two out of the six tested compounds, 3a and 5a, displayed dose-dependent inhibition effects with a lower IC50 than the positive control 5-FU against both cell lines. Interestingly, the two most active derivatives were chalcones with methoxy analogues, indicating that the introduction of a methyl group at position 4 of the phenyl group significantly improved their growth-inhibitory activity against breast carcinoma. In accordance with our finding, a recent study by Pawlak et al. demonstrated that the introduction of a methoxy functional group further improved chalcone’s cytotoxic potency against canine lymphoma or leukemia [18], although their strongest antitumor activity is shown by compounds containing a methoxy group in position 2 or 3 more than position 4 of the B ring. It has been demonstrated that methoxylated flavonoids easily penetrate the cells [31], resulting in better bioavailability and absorption. Moreover, the prolonged metabolism of methoxylated flavonoids might contribute to their promising cytotoxic activity. In this connection, further analysis of the chalcone with methoxy analogs (compounds 3a and 5a) was found to induce cell cycle arrest at the G2/M phase and induce apoptosis (Figure 4 and Figure 5). Cell cycle progression is a crucial process for controlling cancer cell proliferation. Current results showed that compounds 3a and 5a induced G2/M phase cell cycle arrest in breast cancer cells, which may have contributed to their anti-proliferative activity. This finding matches with previous studies showing that chalcone derivatives can induce cell cycle arrest at different phases in various cancer cell lines [13,15,32]. The specific mechanism by which compounds 3a and 5a induce cell cycle arrest in the G2/M phase remains to be explained, but it may involve the disruption of microtubule dynamics or the activation of checkpoint pathways. Apoptosis is another critical process for eliminating malignant cells, and the induction of apoptosis is an important mechanism of action for anticancer therapeutic agents. Our study reported that compounds 3a and 5a induced apoptosis in breast cancer cells, as evidenced by an increased Bax/Bcl2 expression ratio and the increase in active caspase-3/7, confirming the apoptotic cascade. These results suggest that chalcone with methoxy analogues may promote apoptosis in breast cancer cells through the mitochondrial apoptotic pathway.
The precise mechanisms of chalcone activating proapoptotic and anti-proliferative pathways need more investigation, however; we investigated the molecular binding modes of two compounds (3a and 5a) against five targeted anti-apoptotic proteins that play a central role in fighting cancer. Molecular docking investigations were conducted to evaluate the compounds’ binding activities against the epidermal growth factor receptor tyrosine kinase (EGFRTK), cyclin-dependent kinase 2 (CDK2), cellular inhibitor of apoptosis protein 1 (cIAP1), mouse double minute 2 (MDM2), and B-cell lymphoma 2 (BCL2), as illustrated in Table 2 and Table 3, Figure 6 and Figure 7. The binding energies of the compounds were compared to the binding energies of co-crystallized standard ligands. The results showed that compound 3a had the best affinity and selectivity toward the active site 4kmn of the anti-apoptotic protein (cIAP1). It also showed compatible activity against the EGFRTK and BCL2 domains. Moreover, compound 5a displayed the best binding activities toward two proteins: cIAP1 and BCL2. All these binding modes confirmed our suggestion that the methoxy group played an important role in the enhanced binding activity of compounds 3a and 5a into the active site of the target proteins and hence inhibit cancer progression.
The proapoptotic activity of chalcones is still a frequently discussed subject of scientific research. There are numerous scientific reports that describe the different mechanisms of proapoptotic actions of compounds from this group [33,34,35]. In our study, we primarily focused on the structure-function of chalcones containing an alteration at position 4 of the phenyl group with methoxy, chloro, or bromine on breast cancer cell death and, secondarily, investigated their possible anticancer mechanisms of action. To the best of our knowledge, the current study is the first to demonstrate that newly synthesized chalcone analogues containing a methoxy group over a chloro or bromine group could potentially be an effective treatment option for breast cancer. Further studies are needed to elucidate the molecular mechanisms and to evaluate their efficacy and safety in preclinical and clinical trials.
4. Materials and Methods
4.1. Chemistry and Structure Elucidation
A Stuart melting point device was used to measure each compound’s melting points and they were uncorrected. DMSO-d6 was used as a solvent to record the 1H and 13C (the attached proton test, APT) NMR spectra at 300 MHz and 75 MHz, respectively, on a Varian Gemini NMR spectrometer using TMS as an internal standard. Chemical shifts are reported in δ units (ppm). The IR spectra were recorded as KBr using a Bruker-vector 22 spectrophotometer FTIR. A Shimadzu GMSS -QP-1000 EX mass spectrometer was used to measure the mass spectra at 70 eV. All the elemental analyses were performed at the Microanalytical Center, Cairo University (Giza, Egypt).
4.2. Synthesis of Chalcones (3a–c) and (5a–c) and NMR Analysis
A mixture of [1,2,4]triazolo[3,4-a]isoquinolin-3-yl)ethan-1-one 1 (0.351 g, 1 mmol) and appropriate aldehydes 2 or 4 (1 mmol) was dissolved in 20 mL ethanol. KOH (20%, 5 mL) was added to the mixture at 0–5 °C. The reaction mixture was stirred at room temperature for 5 h, then poured over ice containing HCl. The yellow solid obtained was then filtered and washed with water. After precipitation, the yellow solid was separated using a vacuum pump, then dried at a constant temperature (80–90 °C) in an oven. The crude product was crystallized in a proper solvent to yield chalcones.
- (E)-1-(8,9-Dimethoxy-1-(4-methoxyphenyl)-1,5,6,10b-tetrahydro-[1,2,4]triazolo[3,4-a]isoquinolin-3-yl)-3-(4-fluorophenyl)prop-2-en-1-one (3a). Yield: (78%) as a pale-yellow solid (from acetonitrile); m.p 138–140 °C. IR (KBr, cm−1): 1665 (CO); 1H NMR (400 MHz, DMSO-d6): δ, ppm: 2.6–2.8 (m, 2H, H6), 3.4 (s, 3H, OMe), 3.5–3.6 (m, 1H, H5), 3.7 (s, 3H, OMe), 3.8 (s, 3H, OMe), 4.3–4.4 (m, 1H, H5), 6.6 (s, 1H, H10b), 6.7 (s, 1H, H7), 6.9 (s, 1H, H10), 7.0–7.9 (m, 10H, 2 vinyl-H + Ar-H); 13C NMR (100 MHz, DMSO-d6): δ, ppm: 27.3, 41.9, 55.7, 55.8, 55.9, 79.5, 109.8, 112.5, 115.1, 116.6, 118.4, 123.0, 126.7, 129.1, 131.4, 131.7, 137.0, 140.0, 147.3, 148.9, 149.5, 155.2, 162.5, 164.9, 178.8; MS (EI): m/z = 487 (M+). Anal. Calcd. for C28H26FN3O4 (487.53): C, 68.98; H, 5.38; N, 8.62. Found: C, 69.12; H, 5.51; N, 8.83.
- (E)-1-(1-(4-Chlorophenyl)-8,9-dimethoxy-1,5,6,10b-tetrahydro-[1,2,4]triazolo[3,4-a]isoquinolin-3-yl)-3-(4-fluorophenyl)prop-2-en-1-one (3b). Yield: (85%) as a pale-yellow solid (from dioxane); m.p 178–180 °C. IR (KBr, cm−1): 1668 (CO); 1H NMR (400 MHz, DMSO-d6): δ, ppm: 2.7–2.9 (m, 2H, H6), 3.5 (s, 3H, OMe), 3.6–3.7 (m, 1H, H5), 3.7 (s, 3H, OMe), 4.1–4.2 (m, 1H, H5), 6.6 (s, 1H, H10b), 6.8 (s, 1H, H7), 6.9 (s, 1H, H10), 7.3–7.9 (m, 10H, 2 vinyl-H + Ar-H); 13C NMR (100 MHz, DMSO-d6): δ, ppm: 27.4, 41.9, 55.8, 56.0, 77.8, 109.1, 112.5, 116.5, 116.6, 122.7, 124.9, 127.4, 128.8, 129.6, 131.6, 141.1, 142.7, 147.6, 149.1, 150.2, 156.5, 162.7, 165.1, 179.5; MS (EI): m/z = 491 (M+). Anal. Calcd. for C27H23ClFN3O3 (491.95): C, 65.92; H, 4.71; N, 8.54. Found: C, 66.15; H, 4.56; N, 8.76.
- (E)-1-(1-(4-Bromophenyl)-8,9-dimethoxy-1,5,6,10b-tetrahydro-[1,2,4]triazolo[3,4-a]isoquinolin-3-yl)-3-(4-fluorophenyl)prop-2-en-1-one (3c). Yield: (88%) as a pale-yellow solid (from dioxane); m.p 174–176 °C. IR (KBr, cm−1): 1666 (CO); 1H NMR (400 MHz, DMSO-d6): δ, ppm: 2.7–2.8 (m, 2H, H6), 3.5 (s, 3H, OMe), 3.7 (s, 3H, OMe), 3.9–3.9 (m, 1H, H5), 4.1–4.2 (m, 1H, H5), 6.6 (s, 1H, H10b), 6.8 (s, 1H, H7), 6.9 (s, 1H, H10), 7.3–7.9 (m, 10H, 2 vinyl-H + Ar-H); 13C NMR (100 MHz, DMSO-d6): δ, ppm: 27.4, 41.9, 55.8, 56.0, 66.8, 77.7, 109.1, 112.5, 112.6, 116.4, 116.7, 116.8, 127.4, 128.8, 131.6, 132.4, 141.1, 143.1, 147.6, 149.1, 150.2, 162.7, 165.2, 179.5; MS (EI): m/z = 536 (M+). Anal. Calcd. for C27H23BrFN3O3 (536.40): C, 60.46; H, 4.32; N, 7.83. Found: C, 60.61; H, 4.57; N, 7.97.
- (E)-1-(8,9-Dimethoxy-1-(4-methoxyphenyl)-1,5,6,10b-tetrahydro-[1,2,4]triazolo [3,4-a]isoquinolin-3-yl)-3-(thiophen-2-yl)prop-2-en-1-one (5a). Yield: (80%) as a red solid (from acetonitrile); m.p 168–170 °C. IR (KBr, cm−1): 1670 (CO); 1H NMR (300 MHz, DMSO-d6): δ, ppm: 2.6–2.8 (m, 2H, H6), 3.4 (s, 3H, OMe), 3.7 (s, 3H, OMe), 3.7–3.7 (m, 1H, H5), 4.2–4.3 (m, 1H, H5), 6.6 (s, 1H, H10b), 6.7 (s, 1H, H7), 6.9 (s, 1H, H10), 7.0–7.00 (m, 2H, Ar-H and vinyl-H), 7.2–7.2 (dd, 1H, thiophene-H, J = 3.6, 5.0 Hz), 7.3–7.4 (m, 3H, Ar-H), 7.6 (d, 1H, thiophene-H, J = 3.6 Hz), 7.7 (d, 1H, thiophene-H, J = 5.0 Hz), 7.80 (d, 1H, vinyl-H, J = 15.6 Hz);13C NMR (75 MHz, DMSO-d6): δ, ppm: 27.3, 41.9, 55.7, 55.8, 55.9, 79.6, 109.7, 112.4, 115.2, 118.3, 121.5, 126.6, 129.1, 129.3, 130.4, 133.3, 134.1, 137.1, 140.2, 147.3, 148.9, 149.6, 155.3, 178.4; MS (EI): m/z = 483 (M+). Anal. Calcd. for C26H25N3O4S (475.56): C, 65.67; H, 5.30; N, 8.84. Found: C, 65.79; H, 5.47; N, 8.98.
- (E)-1-(1-(4-Chlorophenyl)-8,9-dimethoxy-1,5,6,10b-tetrahydro-[1,2,4]triazolo[3,4-a]isoquinolin-3-yl)-3-(thiophen-2-yl)prop-2-en-1-one (5b). Yield: (84%) as a red solid (from acetonitrile); m.p 178–180 °C. IR (KBr, cm−1): 1671 (CO); 1H NMR (300 MHz, DMSO-d6): δ, ppm: 2.7–2.9 (m, 2H, H6), 3.5 (s, 3H, OMe), 3.6–3.7 (m, 1H, H5), 3.7 (s, 3H, OMe), 4.1–4.2 (m, 1H, H5), 6.6 (s, 1H, H10b), 6.8 (s, 1H, H7), 6.9 (s, 1H, H10), 7.2–7.2 (m, 1H, thiophene-H), 7.3–7.4 (m, 5H, vinyl-H), 7.6 (d, 1H, thiophene-H, J = 3.6 Hz), 7.8 (d, 1H, thiophene-H, J = 5.0 Hz), 7.9 (d, 1H, vinyl-H, J = 15.7 Hz);13C NMR (75 MHz, DMSO-d6): δ, ppm: 27.3, 41.9, 55.8, 56.1, 77.8, 109.1, 112.5, 116.4, 121.2, 124.8, 127.4, 128.8, 129.4, 129.7, 130.9, 133.8, 135.2, 140.0, 142.7 147.6, 149.1, 150.3, 178.9; MS (EI): m/z = 479 (M+). Anal. Calcd. for C25H22ClN3O3S (479.98): C, 62.56; H, 4.62; N, 8.75. Found: C, 62.41; H, 4.81; N, 8.95.
- (E)-1-(1-(4-Bromophenyl)-8,9-dimethoxy-1,5,6,10b-tetrahydro-[1,2,4]triazolo[3,4-a]isoquinolin-3-yl)-3-(thiophen-2-yl)prop-2-en-1-one (5c). Yield: (78%) as a red solid (from acetonitrile); m.p 182–184 °C. IR (KBr, cm−1): 1668 (CO); 1H NMR (300 MHz, DMSO-d6): δ, ppm: 2.7–2.8 (m, 2H, H6), 3.5 (s, 3H, OMe), 3.9 (s, 3H, OMe), 4.1–4.2 (m, 2H, H5), 6.6 (s, 1H, H10b), 6.8 (s, 1H, H7), 6.9 (s, 1H, H10), 7.2–7.9 (m, 8H, Ar + thiophene-H + vinyl-H), 7.9 (d, 1H, vinyl-H, J = 15.7 Hz);13C NMR (75 MHz, DMSO-d6): δ, ppm: 27.4, 41.9, 55.8, 55.8, 56.0, 77.7, 109.0, 112.5, 112.6, 116.7, 121.2, 128.8, 129.4, 131.0, 132.5, 133.8, 135.2, 140.0, 143.1 147.6, 149.1, 150.3, 179.0; MS (EI): m/z = 524 (M+). Anal. Calcd. for C25H22BrN3O3S (524.43): C, 57.26; H, 4.23; N, 8.01. Found: C, 57.42; H, 4.41; N, 8.13.
4.3. Cell Culture
Experimental work and the handling of cell lines were performed according to Nagoya City University guidelines. Mouse Luc-4T1 was obtained from the JCRB Cell Bank (Accession number #JCRB1447) and human MDA-MB-231 was obtained from the ATCC (Accession number #HTB-26). Both are breast cancer cells that were maintained as a monolayer culture in RPMI-1640 (Sigma, St. Louis, MO, USA) supplemented with L-glutamine, sodium pyruvate, non-essential amino acids, 1% penicillin-streptomycin, and 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA). Cells were grown at 37 °C in a humidified atmosphere containing 5% CO2.
4.4. SRB Assay
The SRB assay is used for the toxicity screening of compounds on adherent cells in a 96-well plate. The cells were cultured with the tested compounds, or 5-FU (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), for 48 h at a final concentration of 20 µg/mL. After the incubation period, cells were fixed with ice-cold absolute ethanol for 1 h at −20 °C, followed by staining with 0.4% (w/vol) sulforhodamine b (code) for 30 min in the dark at RT. Cells were repeatedly washed to remove the excess dye with 1% (v/v) acetic acid. The protein-bound dye was resolved with a 10 mM unbuffered Tris solution for optical density determination at 565 nm using a microplate reader. Cell viability was expressed as a percentage of the control values.
4.5. MTT Assay
Cell viability was estimated by a colorimetric 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, cells were seeded for 24 h at 1 × 104 cells/well in 96-well plates, followed by treatment with different doses of the tested compounds or 5-FU for another 24 h. The culture medium was then replaced with fresh serum-free medium containing MTT solution (MTT cell proliferation assay kit, Nacalai tesque, No. 23506-80) and incubated for 3 h at 37 °C. The formed formazan crystals were solubilized by MTT solvent on a shaker and the absorbance was measured at 590 nm on a microplate reader (Spectra MAX 340, Molecular Devices Co., Sunnyvale, CA, USA). Cells were treated with a medium containing 0.1% DMSO as a vehicle and survival was expressed as a percentage of the absorbance relative to that of control cells.
4.6. Cell Cycle Analysis
The cell cycle was analyzed using FACS Canto II (BD Biosciences, San Jose, CA, USA). Briefly, cells were seeded for 24 h at 1 × 105 cells/well in a 12-well plate, followed by treatment with 20 µg/mL of the tested compounds, 5-FU, or vehicle for 24 h. Thereafter, the medium was removed, and the cells were harvested by trypsinization, washed with PBS, and fixed with 70% ethanol for 30 min at 4 °C. The cells were then washed twice with PBS and stained with propidium iodide (50 μg/mL) and RNase A (100 μg/mL) in PBS at room temperature for 1 h. The stained cells were immediately analyzed on the flow cytometer for relative DNA content. The resulting data were analyzed using FlowJo software v10.8.2 to determine cell cycle distribution.
4.7. Quantitative PCR Analysis
Total RNA was extracted from Mouse Luc-4T1 cells after 24 h of treatment using ISOGEN II (311-07361, NIPPON GENE, Tokyo, Japan), and QuantiTect Reverse Transcription Kit (QIAGEN, 205313) was used to synthesize cDNA, each according to the manufacturer’s instructions. The quantitative PCR reaction was carried out on the QuantStudio 12K Flex Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) with PowerUp™ SYBR®-Green Master Mix (A25776, Thermo Fisher Scientific). The following primers were used: forward 5′-TTAGAGAGATGCGAGGAACCG-3′ and reverse 5′-GGGACAAGTAAACCTGGAAGAA-3′ for Bcl2; 5′-TGCAGAGGATGATTGCTGAC-3′ and reverse 5′-GATCAGCTCGGGCACTTTAG-3′ for Bax; and forward 5′-TTGTTGTTGGATATGCCCTTGACTA-3′ and reverse 5′-AGGCAGATGGCCACAGGACTA-3′ for Hprt. The results were expressed in Cycle threshold (Ct). Relative quantitation was assessed according to the calculation of delta-delta Ct. All qPCR analyses were performed in duplicate.
4.8. Caspase-Glo 3/7 Assay
Caspase-3/7 activity was measured using the Caspase-Glo® 3/7 assay kit (Promega Corporation, Madison, WI, USA, 8090) according to the manufacturer’s protocol. In brief, cells were seeded for 24 h at 1 × 104 cells/well in a white-walled 96-well plate, followed by treatment with 10 µg/mL of the tested compounds for another 24 h. The Caspase-Glo® 3/7 reagents were brought to room temperature for 30 min, added to the culture cells, and then incubated for 3 h at RT. The luminescence was measured using a SpectraMax Microplate Luminometer (Molecular Devices, CA, USA).
4.9. Molecular Docking Analysis
The molecular docking studies were performed using the Molecular Operating Environment (MOE) version 2009.10 program. The target compounds were drawn with the MOE builder interface and subjected to local energy minimization using the included MOPAC. Then, the resulting structure was subjected to global energy minimization via Systematic Conformational Search where the RMS gradient and RMS distance were set to be 0.01 kcal/mole and 0.1A°, respectively. After the energy calculation of our compound was achieved, the lowest value of energy was chosen for docking with epidermal growth factor receptor kinase (EGFRK), cyclin-dependent kinase 2, (CDK2), and Mouse double minute 2 homolog (MDM2) proteins. The X-ray crystallographic structure of the proteins was downloaded from the protein data bank (PDB ID: 1m17, 2C6O, and 4wt2, respectively). Before the molecular simulation step, the proteins were prepared as follows: Firstly, protonation of the target protein with its standard ligand. Then, unwanted co-ligands and water chains were removed from the protein. After that, the active site was selected by the MOE alpha site finder. Finally, the prepared proteins were docked with the target compound and the results were compared with the self-docking results where the proteins were docked with their co-crystallized ligands ([6,7-Bis(2-methoxy-ethoxy)quinazoline-4-yl]-(3-ethynylphenyl)amine), (O6-cyclohexylmethoxy-2-(4′-sulphamoylanilino)purine), and (4-({[(3R,5R,6S)-1-[(1S)-2-(tert-butylsulfonyl)-1-cyclopropylethyl]-6-(4-chloro-3fluorophenyl)-5-(3-chlorophenyl)-3-methyl-2-oxopiperidin-3-yl]acetyl}amino)-2-methoxybenzoic acid). The final results were saved as pdb format files, which were then visualized through BIOVIA Discovery Studio V6.1.0.15350 in 2D and 3D forms.
4.10. Statistical Analysis
All results are representative of at least two independent experiments. The data were presented as mean ± standard deviation (SD) and statistical comparisons between two groups were determined by a two-tailed Student’s t-test, while for comparing more than one group, a one-way ANOVA was used. A probability value of <0.05 was considered significant.
5. Conclusions
In the present study, novel chalcone derivatives were synthesized and evaluated for their antitumor activity against cancer cells of two origins (mouse and human) breast carcinoma. Results showed that chalcone with a methoxy group exhibited enhanced cytotoxicity, cell cycle arrest, and proapoptotic activity, leading to reduced cell growth. Their inhibitory effect on anti-apoptotic proteins, particularly cIAP1, BCL2, and EGFRK proteins was also confirmed by molecular docking.
Author Contributions
Methodology, M.I.M.D., A.M.M., A.M.Y., M.M., A.I.Y., H.H.S., M.F.M. and I.A.A.; Software, M.F.M.; Validation, M.M.; Formal analysis, I.A.A. and H.O.; Investigation, M.I.M.D., H.H.S. and I.A.A.; Resources, A.I.Y., A.E.O. and H.M.H.; Data curation, A.E.O.; Writing—original draft, M.M., H.H.S. and M.F.M.; Visualization, A.M.M., A.M.Y. and M.F.M.; Supervision, H.H.S., H.M.H., I.A.A. and H.O.; Project administration, H.O.; Funding acquisition, H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to support the findings of this study are included within the article.
Acknowledgments
We are grateful to the Egypt-Japan Education Partnership (EJEP) for the financial support of Mahmoud I.M. Darwish, Ahmed M. Moustafa, and Asmaa M. Youssef during their study at Nagoya City University, Japan.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds (3a–c) and (5a–c) are available from the authors.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2022, 10, 451. [Google Scholar]
- Hussain, S.; Singh, A.; Nazir, S.U.; Tulsyan, S.; Khan, A.; Kumar, R.; Bashir, N.; Tanwar, P.; Mehrotra, R. Cancer Drug Resistance: A Fleet to Conquer. J. Cell Biochem. 2019, 120, 14213–14225. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Microbial Transformations of 4′-Methylchalcones as an Efficient Method of Obtaining Novel Alcohol and Dihydrochalcone Derivatives with Antimicrobial Activity. RSC Adv. 2018, 8, 30379–30386. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, W.; Dharmaratne, H.; Dhammika Nanayakkara, N.P.; Khan, I.A. Kavalactones from Piper Methysticum, and Their 13C NMR Spectroscopic Analyses. Phytochemistry 2002, 59, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary Chalcones with Chemopreventive and Chemotherapeutic Potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and New Aspects of a Class of Natural Therapeutic Drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Huang, Y.C.; Thiyagarajan, V.; Mathew, D.C.; Lin, K.Y.; Chen, S.C.; Liu, J.Y.; Hsu, L.S.; Li, M.L.; Yang, H.L. Anticancer Activities of Chalcone Flavokawain B from Alpinia Pricei Hayata in Human Lung Adenocarcinoma (A549) Cells via Induction of Reactive Oxygen Species-Mediated Apoptotic and Autophagic Cell Death. J. Cell Physiol. 2019, 234, 17514–17526. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, X.; Ren, H.; Wang, K.; Chang, J. Isolation and Characterization of Three Chalcone Synthase Genes in Pecan (Carya Illinoinensis). Biomolecules 2019, 9, 236. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Gawande, N.M.; Khobragade, C.N. Synthesis and Biological Evaluation of a Novel Series of Pyrazole Chalcones as Anti-Inflammatory, Antioxidant and Antimicrobial Agents. Bioorg. Med. Chem. 2009, 17, 8168–8173. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Mohamed, M.S.; Shouman, S.A.; Fathi, M.M.; Abdelhamid, I.A. Synthesis and Biological Evaluation of a Novel Series of Chalcones Incorporated Pyrazole Moiety as Anticancer and Antimicrobial Agents. Appl. Biochem. Biotechnol. 2012, 168, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.R.; Foroumadi, A.; Amirabadi, A.; Samzadeh-Kermani, A.; Azimzadeh, B.S.; Eskandarizadeh, A. Evaluation of Anti-Inflammatory and Analgesic Activity of a Novel Rigid 3, 4-Dihydroxy Chalcone in Mice. Ann. N Y Acad. Sci. 2009, 1171, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Shenvi, S.; Kumar, K.; Hatti, K.S.; Rijesh, K.; Diwakar, L.; Reddy, G.C. Synthesis, Anticancer and Antioxidant Activities of 2,4,5-Trimethoxy Chalcones and Analogues from Asaronaldehyde: Structure–Activity Relationship. Eur. J. Med. Chem. 2013, 62, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, P.L.; Tzeng, W.S.; Lin, C.C. Chalcone Inhibits the Proliferation of Human Breast Cancer Cell by Blocking Cell Cycle Progression and Inducing Apoptosis. Food Chem. Toxicol. 2006, 44, 704–713. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Hassaneen, H.M.; Abdelhamid, I.A. Cytotoxicity, Molecular Modeling, Cell Cycle Arrest, and Apoptotic Induction Induced by Novel Tetrahydro-[1,2,4]Triazolo[3,4-a]Isoquinoline Chalcones. Eur. J. Med. Chem. 2018, 143, 532–541. [Google Scholar] [CrossRef]
- Moreira, J.; Almeida, J.; Saraiva, L.; Cidade, H.; Pinto, M. Chalcones as Promising Antitumor Agents by Targeting the P53 Pathway: An Overview and New Insights in Drug-Likeness. Molecules 2021, 26, 3737. [Google Scholar] [CrossRef]
- Evranos Aksöz, B.; Ertan, R. Chemical and Structural Properties of Chalcones I. Fabad J. Pharm. Sci. 2011, 36, 223–242. [Google Scholar]
- Pawlak, A.; Henklewska, M.; Suárez, B.H.; Łużny, M.; Kozłowska, E.; Obmińska-Mrukowicz, B.; Janeczko, T. Chalcone Methoxy Derivatives Exhibit Antiproliferative and Proapoptotic Activity on Canine Lymphoma and Leukemia Cells. Molecules 2020, 25, 4362. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Sroor, F.M.; Mohamed, M.F.; El-Naggar, M.E.; Saleh, F.M.; Hassaneen, H.M.; Abdelhamid, I.A. Molecular Docking Study, Cytotoxicity, Cell Cycle Arrest and Apoptotic Induction of Novel Chalcones Incorporating Thiadiazolyl Isoquinoline in Cervical Cancer. Anticancer Agents Med. Chem. 2019, 20, 70–83. [Google Scholar] [CrossRef]
- Sroor, F.M.; Aboelenin, M.M.; Mahrous, K.F.; Mahmoud, K.; Elwahy, A.H.M.; Abdelhamid, I.A. Novel 2-Cyanoacrylamido-4,5,6,7-Tetrahydrobenzo[b]Thiophene Derivatives as Potent Anticancer Agents. Arch. Pharm. 2020, 353, e2000069. [Google Scholar] [CrossRef]
- Sroor, F.M.; Abdelmoniem, A.M.; Abdelhamid, I.A. Facile Synthesis, Structural Activity Relationship, Molecular Modeling and In Vitro Biological Evaluation of New Urea Derivatives with Incorporated Isoxazole and Thiazole Moieties as Anticancer Agents. ChemistrySelect 2019, 4, 10113–10121. [Google Scholar] [CrossRef]
- Mansour, M.; Mohamed, M.F.; Elhalwagi, A.; El-Itriby, H.A.; Shawki, H.H.; Abdelhamid, I.A. Moringa Peregrina Leaves Extracts Induce Apoptosis and Cell Cycle Arrest of Hepatocellular Carcinoma. Biomed. Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.T.; Sroor, F.M.; Mahrous, K.F.; Mahmoud, K.; Hassaneen, H.M.; Saleh, F.M.; Abdelhamid, I.A.; Mohamed Teleb, M.A. Anticancer Activity of Novel 3-(Furan-2-Yl)Pyrazolyl and 3-(Thiophen-2-Yl)Pyrazolyl Hybrid Chalcones: Synthesis and in Vitro Studies. Arch. Pharm. 2022, 355, 2100381. [Google Scholar] [CrossRef] [PubMed]
- Fathi, E.M.; Sroor, F.M.; Mahrous, K.F.; Mohamed, M.F.; Mahmoud, K.; Emara, M.; Elwahy, A.H.M.; Abdelhamid, I.A. Design, Synthesis, In Silico and In Vitro Anticancer Activity of Novel Bis-Furanyl-Chalcone Derivatives Linked through Alkyl Spacers. ChemistrySelect 2021, 6, 6202–6211. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Sroor, F.M.; Ibrahim, N.S.; Salem, G.S.; El-Sayed, H.H.; Mahmoud, M.M.; Wagdy, M.A.M.; Ahmed, A.M.; Mahmoud, A.A.T.; Ibrahim, S.S.; et al. Novel [l,2,4]Triazolo[3,4-a]Isoquinoline Chalcones as New Chemotherapeutic Agents: Block IAP Tyrosine Kinase Domain and Induce Both Intrinsic and Extrinsic Pathways of Apoptosis. Invest. New Drugs 2021, 39, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.G.; Sroor, F.M.; Othman, A.M.; Mahrous, K.F.; Saleh, F.M.; Hassaneen, H.M.; Abdallah, T.A.; Abdelhamid, I.A.; Teleb, M.A.M. Structure-Based Design of Novel Pyrazolyl–Chalcones as Anti-Cancer and Antimicrobial Agents: Synthesis and in Vitro Studies. Monatsh. Chem. 2022, 153, 211–221. [Google Scholar] [CrossRef]
- Abdelaal, N.E.; Hassaneen, H.M.; Elzayat, E.M.; Abdelhamid, I.A. Design, synthesis, in silico studies and biological evaluation of EGFR inhibitors based on chalcones incorporating triazolo[3,4-a]isoquinoline and thiophene scaffolds targeting resistance in non-small cell lung cancer (NSCLC). 2023; Submitted for Publication. [Google Scholar]
- Mohamed, M.F.; Ibrahim, N.S.; Ibrahim, S.A.; El-Manawaty, M.A.; El-Hallouty, S.M.; Hassaneen, H.M.; Abdelhamid, I.A. Cytotoxic Activity, Apoptosis Induction and Cell Cycle Arrest in Human Breast Cancer (MCF7) Cells by a Novel Fluorinated Tetrahydro-[1,2,4]Triazolo[3,4-a]Isoquinolin Chalcones. Polycycl. Aromat. Compd. 2021, 43, 268–287. [Google Scholar] [CrossRef]
- AAT Bioquest Inc. Quest GraphTM IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 12 February 2023).
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria. Molecules 2018, 23, 2477. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.; Zhang, S.; Hu, J.; Huang, L.; Li, X. Synthesis, Evaluation, and Mechanism Study of Novel Indole-Chalcone Derivatives Exerting Effective Antitumor Activity Through Microtubule Destabilization in Vitro and in Vivo. J. Med. Chem. 2016, 59, 5264–5283. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-Specificity and Apoptosis-Inducing Activity of Stilbenes and Flavonoids. Anticancer Res. 2005, 25, 2055–2063. [Google Scholar] [PubMed]
- Quintin, J.; Desrivot, J.; Thoret, S.; Menez, P.L.; Cresteil, T.; Lewin, G. Synthesis and Biological Evaluation of a Series of Tangeretin-Derived Chalcones. Bioorg. Med. Chem. Lett. 2009, 19, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Tiamas, S.G.; Audet, F.; Samra, A.A.; Bignon, J.; Litaudon, M.; Fourneau, C.; Ariffin, A.; Awang, K.; Desrat, S.; Roussi, F. Asymmetric Total Synthesis and Biological Evaluation of Proapoptotic Natural Myrcene-Derived Cyclohexenyl Chalcones. Eur. J. Org. Chem. 2018, 2018, 5830–5835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).