Liquid-Phase Dehydration of Glycerol to Acrolein with ZSM-5-Based Catalysts in the Presence of a Dispersing Agent
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of Reaction Parameters
2.2. Comparison of Various Solid Acid Catalysts
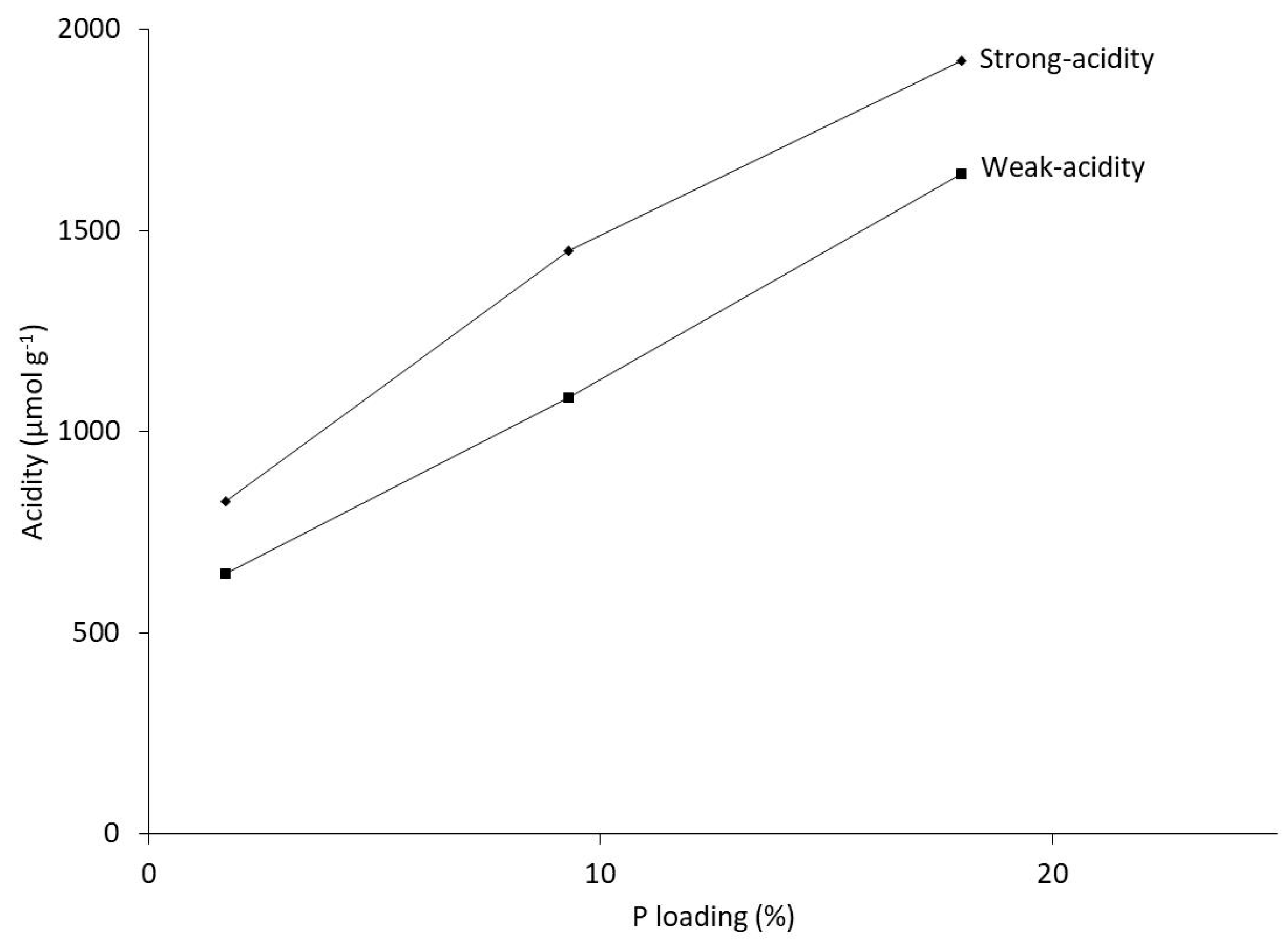
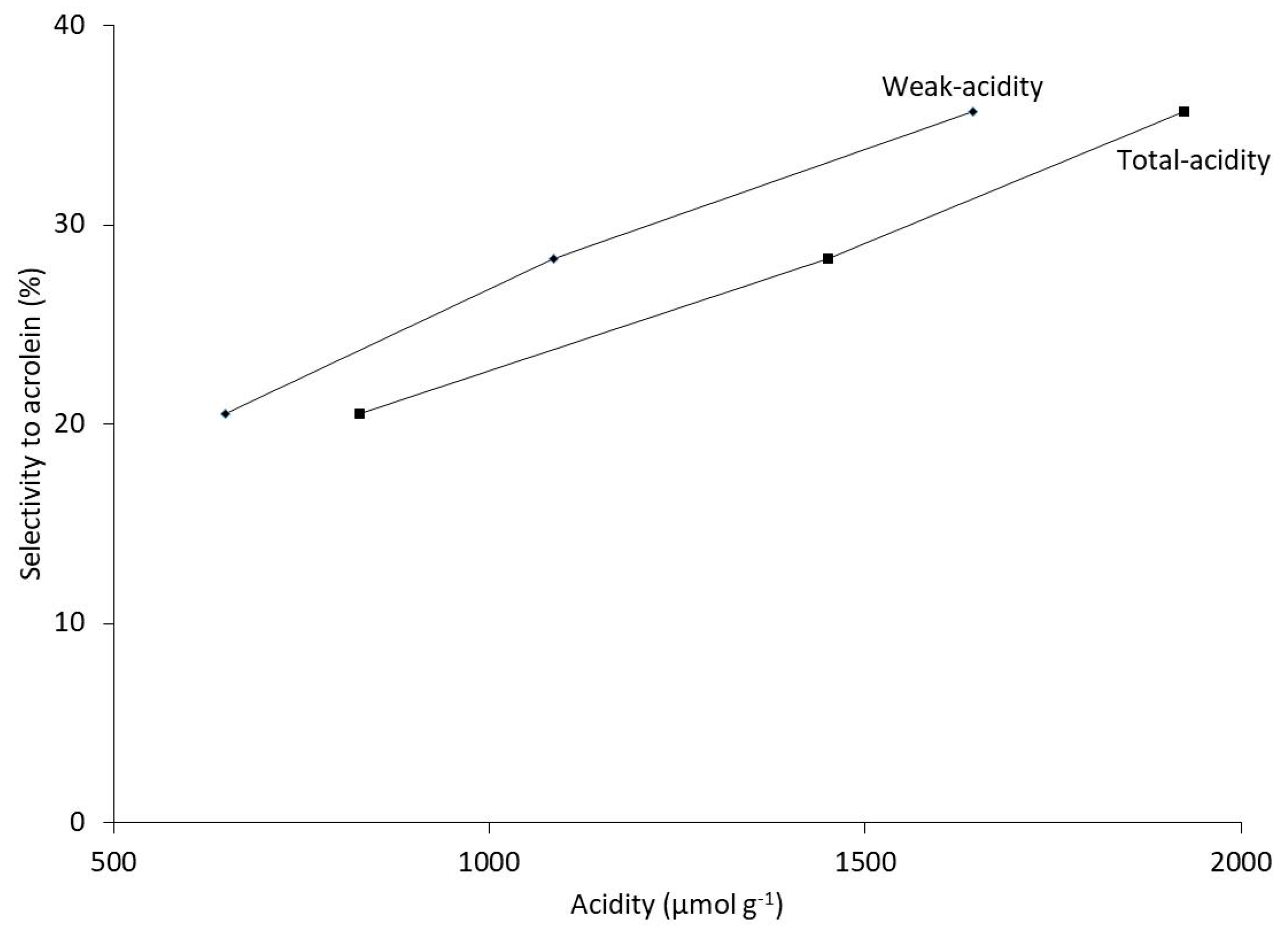
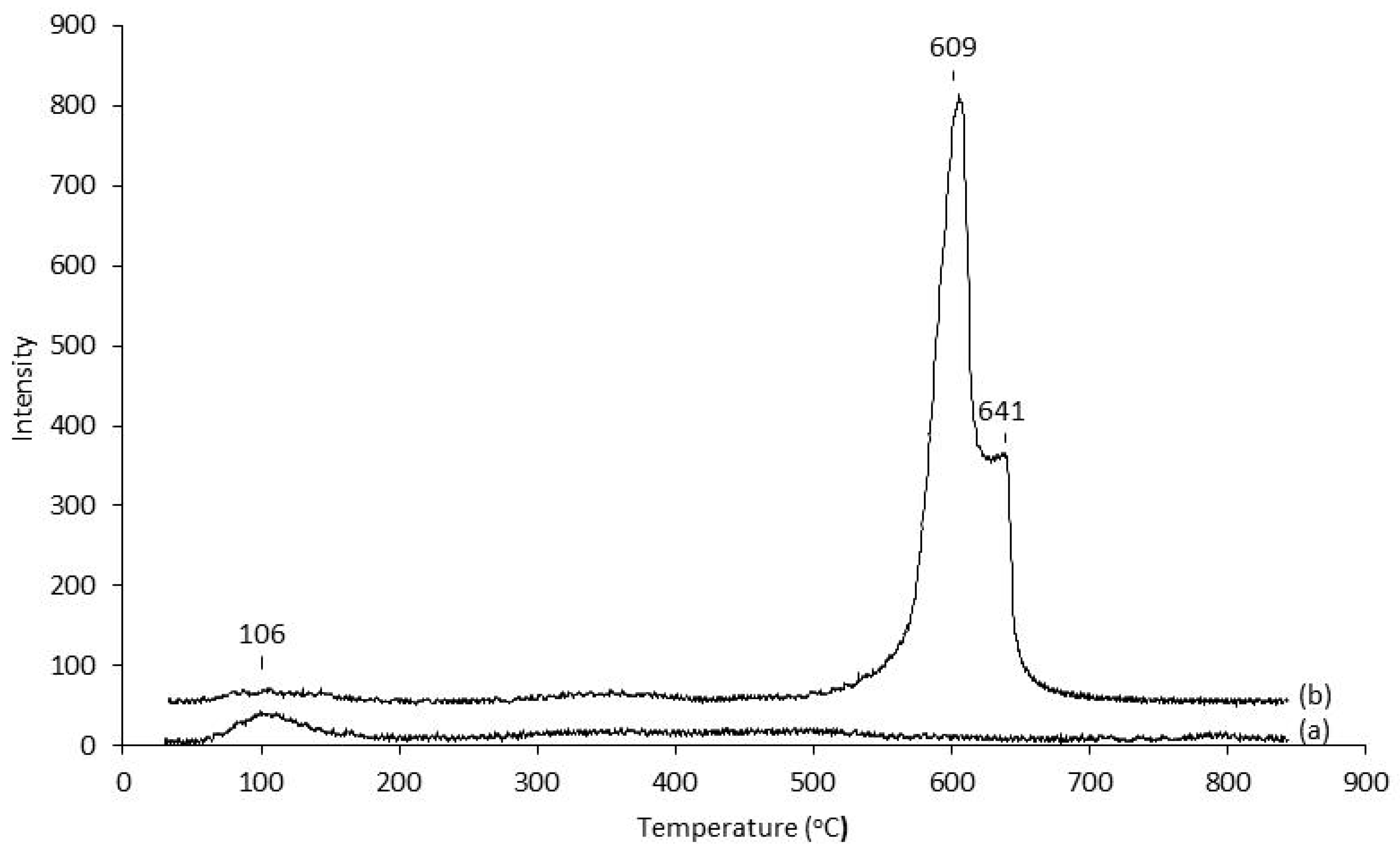
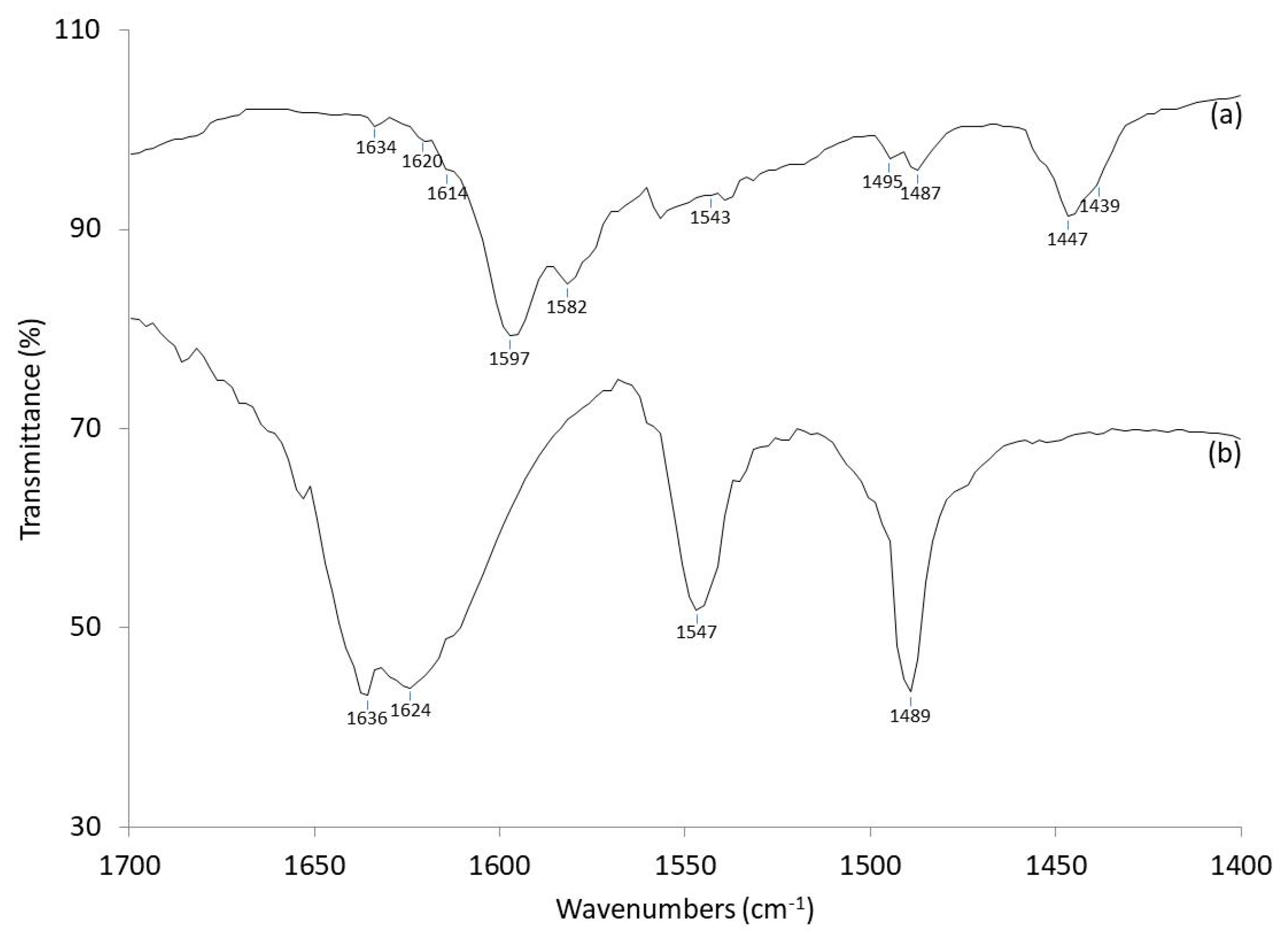
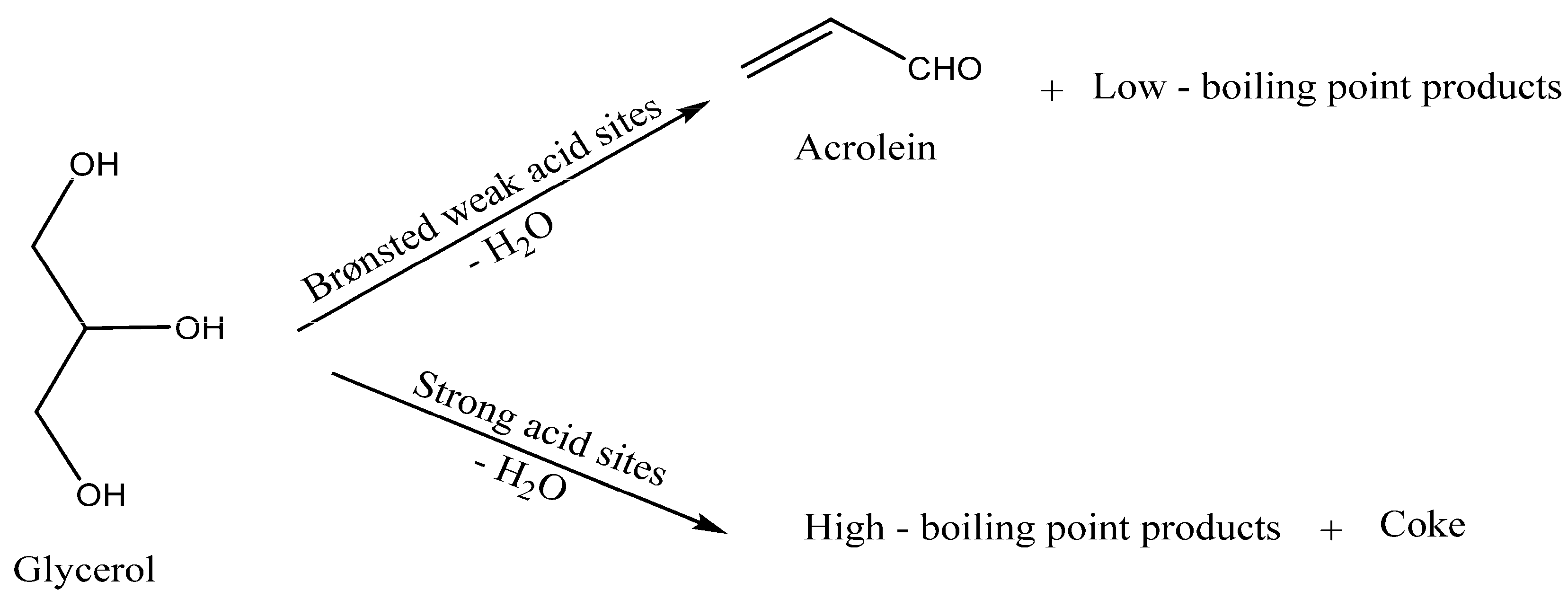
2.3. Correlation of Catalytic Performance with Catalyst Acid Properties
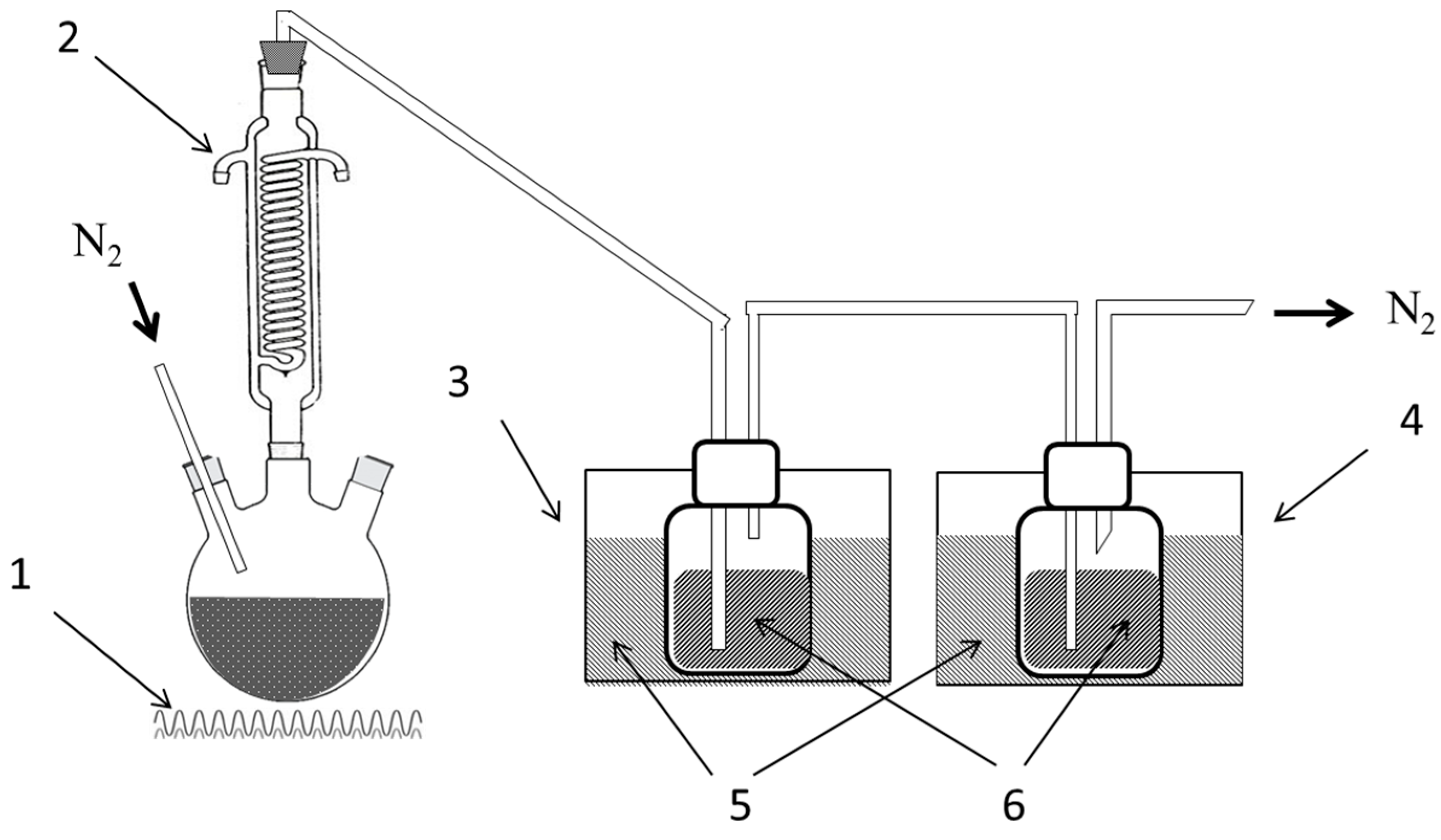
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huber, C.W.; Iborra, S.A. Corma, Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, X.; Shen, Y. Commodity chemicals derived from glycerol, an important biorefinery feedstock. Chem. Rev. 2008, 108, 5253–5277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Beltramini, J.N.; Fana, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Hezaveh, H. Glycerol for renewable acrolein production by catalytic dehydration. Renew. Sustain. Energy Rev. 2014, 40, 28–59. [Google Scholar] [CrossRef]
- Abdullah, A.; Abdullah, A.Z.; Ahmed, M.; Khan, J.; Shahadat, M.; Umar, K.; Alim, M.A. A review on recent developments and progress in sustainable acrolein production through catalytic dehydration of bio-renewable glycerol. J. Clean. Prod. 2022, 341, 130876. [Google Scholar] [CrossRef]
- Groll, H.; Hearne, G. Process of converting a Polyhydric Alcohol to a Carbonyl Compound. U.S. Patent 2,042,224, 26 May 1936. [Google Scholar]
- Hoyt, H.; Manninen, T. Production of Acrolein from Glycerol. U.S. Patent 2,558,520, 26 June 1951. [Google Scholar]
- Neher, A.; Haas, T.; Dietrich, A.; Klenk, H.; Girke, W. Process for the Production of Acrolein and Its Use. DE Patent 4,238,493, 21 April 1994. [Google Scholar]
- Neher, A.; Haas, T.; Dietrich, A.; Klenk, H.; Girke, W. Process for the Production of Acrolein. U.S. Patent 5,387,720, 7 February 1995. [Google Scholar]
- Suzuki, N.; Takahashi, M. Method for Producing Acrolein. JP Patnet 2,006,290,815, 26 October 2006. [Google Scholar]
- Takanori, A.; Masayuki, Y. Method for Producing Acrolein and Method for Producing Acrylic Acid. JP Patent 2,009,292,773, 17 December 2009. [Google Scholar]
- Takanori, A.; Masayuki, Y. Method for Producing Acrolein and Acrylic Acid. JP Patnet 2,009,292,774, 17 December 2009. [Google Scholar]
- De Oliveira, A.S.; Vasconcelos, S.J.S.; de Sousa, J.R.; de Sousa, F.F.; Filho, J.M.; Oliveira, A.C. Catalytic conversion of glycerol to acrolein over modified molecular sieves: Activity and deactivation studies. Chem. Eng. J. 2011, 168, 765–774. [Google Scholar] [CrossRef]
- Estevez, R.; Lopez-Pedrajas, S.; Blanco-Bonilla, F.; Luna, D.; Bautista, F.M. Production of acrolein from glycerol in liquid phase on heterogeneous catalysts. Chem. Eng. J. 2015, 282, 179–186. [Google Scholar] [CrossRef]
- Shen, L.; Yin, H.; Wang, A.; Feng, Y.; Shen, Y.; Wu, Z.; Jiang, T. Liquid phase dehydration of glycerol to acrolein catalyzed by silicotungstic, phosphotungstic, and phosphomolybdic acids. Chem. Eng. J. 2012, 180, 277–283. [Google Scholar] [CrossRef]
- Shen, L.; Yin, H.; Wang, A.; Lu, X.; Zhang, C.; Chen, F.; Wang, Y.; Chen, H. Liquid phase catalytic dehydration of glycerol to acrolein over Brønsted acidic ionic liquid catalysts. J. Ind. Eng. Chem. 2014, 20, 759–766. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jung, K.D.; Park, E.D. Gas-phase dehydration of glycerol over ZSM-5 catalysts. Micropor. Mesopor. Mater. 2010, 131, 28–36. [Google Scholar] [CrossRef]
- Jia, C.-J.; Liu, Y.; Schmidt, W.; Lu, A.-H.; Schüth, F. Small-sized HZSM-5 zeolite as highly active catalyst for gas phase dehydration of glycerol to acrolein. J. Catal. 2010, 269, 71–79. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Z.; Huang, L.; Zhang, H.; Song, K.; Wang, L.; Shi, Z.; Ma, J.; Zhuang, Y.; Shen, W.; et al. Dehydration of Glycerol to Acrolein over Hierarchical ZSM-5 Zeolites: Effects of Mesoporosity and Acidity. ACS Catal. 2015, 5, 2548–2558. [Google Scholar] [CrossRef]
- Fernandes, J.O.; Neves, T.M.; da Silva, E.D.; da Rosa, C.A.; Mortola, V.B. Influence of reaction parameters on glycerol dehydration over HZSM-5 catalyst. React. Kinet. Mech. Catal. 2021, 132, 485–498. [Google Scholar] [CrossRef]
- Alhanash, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Gas-Phase Dehydration of Glycerol to Acrolein Catalysed by Caesium Heteropoly Salt. Appl. Catal. A Gen. 2010, 378, 11–18. [Google Scholar] [CrossRef]
- Qureshi, B.A.; Lan, X.; Arslan, M.T.; Wang, T. Highly Active and Selective Nano H-ZSM-5 Catalyst with Short Channels along b-Axis for Glycerol Dehydration to Acrolein. Ind. Eng. Chem. Res. 2019, 58, 12611–12622. [Google Scholar] [CrossRef]
- Chen, X.; Sun, W.; Xiao, N.; Yan, Y.; Liu, S. Experimental study for liquid phase selective hydrogenation of furfuryl alcohol to tetrahydrofurfuryl alcohol on supported Ni catalysts. Chem. Eng. J. 2007, 126, 5–11. [Google Scholar] [CrossRef]
- Sievers, C.; Noda, Y.; Qi, L.; Albuquerque, E.M.; Rioux, R.M.; Scott, S.L. Phenomena Affecting Catalytic Reactions at Solid-Liquid Interfaces. ACS Catal. 2016, 6, 8286–8307. [Google Scholar] [CrossRef]
- Sulfolane. The Wikipedia Online. Available online: https://en.wikipedia.org/wiki/Sulfolane (accessed on 7 April 2023).
- Glycerine. The Microkat Online. Available online: https://www.microkat.gr/msdspd90-99/Glycerine.html (accessed on 7 April 2023).
- King, C.J.; Hsueh, L.; Mao, K.W. Liquid Phase Diffusion of Non-electrolytes at High Dilution. J. Chem. Eng. Data 1965, 10, 348–350. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable Production of Acrolein: Investigation of Solid Acid-Base Catalysts for Gas-Phase Dehydration of Glycerol. Green Chem. 2007, 9, 1130–1136. [Google Scholar] [CrossRef]
- Sawa, M.; Niwa, M.; Murakami, Y. Relationship between acid amount and framework aluminum content in mordenite. Zeolites 1990, 10, 532–538. [Google Scholar] [CrossRef]
- Sato, H. Acidity Control and Catalysis of Pentasil Zeolites. Catal. Rev. Sci.-Eng. 1997, 39, 395–424. [Google Scholar] [CrossRef]
- Lónyi, F.; Valyon, J. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite. Micropor. Mesopor. Mater. 2001, 47, 293–301. [Google Scholar] [CrossRef]
- Parry, E.P. An Infrared Study of Pyridine Adsorbed on Acidic Solids. Characterization of Surface Acidity. J. Catal. 1963, 2, 371–391. [Google Scholar] [CrossRef]
- Basila, M.R.; Kantner, T.R.; Rhee, K.H. The Nature of the Acidic Sites on a Silica-Alumina. Characterization by Infrared Spectroscopic Studies of Trimethylamine and Pyridine Chemisorption. J. Phys. Chem. 1964, 68, 3197–3207. [Google Scholar] [CrossRef]
- Connerton, J.; Joyner, R.W.; Padley, M.B. Characterisation of the Acidity of Well Defined Cu-ZSM-5 Catalysts using Pyridine as a Probe Molecule. J. Chem. Soc. Faraday Trans. 1995, 91, 1841–1844. [Google Scholar] [CrossRef]
- Buzzoni, R.; Bordiga, S.; Ricchiardi, G.; Lamberti, C.; Zecchina, A. Interaction of Pyridine with Acidic (H-ZSM5, H-β, H-MORD Zeolites) and Superacidic (H-Nafion Membrane) Systems: An IR Investigation. Langmuir 1996, 12, 930–940. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Yamazaki, H.; Yokoi, T.; Tatsumi, T.; Kondo, J.N. IR Characterization of Homogeneously Mixed Silica−Alumina Samples and Dealuminated Y Zeolites by Using Pyridine, CO, and Propene Probe Molecules. J. Phys. Chem. C 2013, 117, 14043–14050. [Google Scholar] [CrossRef]
- Meng, T.; Mao, D.; Guo1, Q.; Ma, Z. Effect of the Si/Al Ratios of Nanocrystalline HZSM-5 Zeolite on the Performance in Catalytic Conversion of Ethanol to Propylene. J. Nanosci. Nanotechnol. 2017, 17, 3779–3785. [Google Scholar] [CrossRef]
- Murphy, B.M.; Letterio, M.P.; Xu, B. Catalytic dehydration of methyl lactate: Reaction mechanism and selectivity control. J. Catal. 2016, 339, 21–30. [Google Scholar] [CrossRef]
- Gould, N.S.; Xu, B. Effect of liquid water on acid sites of NaY: An in situ liquid phase spectroscopic study. J. Catal. 2016, 342, 193–202. [Google Scholar] [CrossRef]
- Gould, N.S.; Xu, B. Catalyst characterization in the presence of solvent: Development of liquid phase structure–activity relationships. Chem. Sci. 2018, 9, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Katryniok, B.; Paul, S.; Dumeignil, F. Recent developments in the field of catalytic dehydration of glycerol to acrolein. ACS Catal. 2013, 3, 1819–1834. [Google Scholar] [CrossRef]
- Izumi, Y.; Ono, M.; Kitagawa, M.; Yoshiaki, M.; Urabe, K. Silica-included heteropoly compounds as solid acid catalysts. Micropor. Mater. 1995, 5, 255–262. [Google Scholar] [CrossRef]
- Suprun, W.; Lutecki, M.; Haber, T.; Papp, H. Acidic catalysts for the dehydration of glycerol: Activity and deactivation. J. Mol. Catal. A Gen. 2009, 309, 71–78. [Google Scholar] [CrossRef]
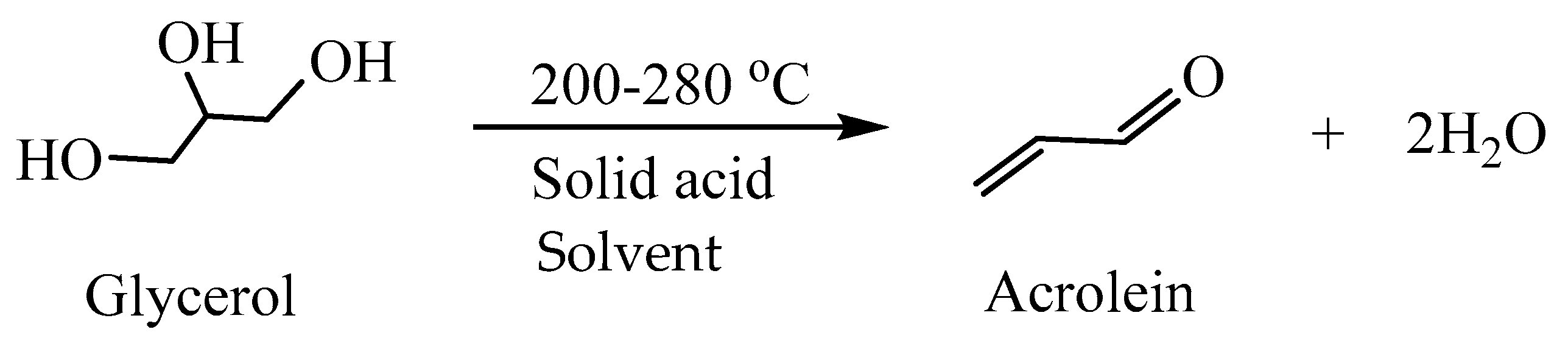

| Catalyst | Type of Reactor | T (°C) | P (bar) | Dispersing Agent | Acrolein Yield (%) | TON (gacr gcat−1) | Reference |
|---|---|---|---|---|---|---|---|
| H2SO4 | Semibatch | 190 | >1 | Nil | 49 | 0.57 | [7] |
| H3PO4/diatomaceous earth | Semibatch | 286 | 1 | Petroleum oil | 72 | 8.4 | [8] |
| H-ZSM-5 | Fixed-bed | 300 | 70 | Nil | 13 | 0.038 a | [10] |
| KHSO4 | Batch | 280 | 1 | Liquid paraffins | 80 | 1.6 | [11] |
| K2SO4 | Batch | 280 | 1 | Liquid paraffins | 75 | 1.5 | [11] |
| CuSO4 | Batch | 280 | 1 | Sulfolane | 85 | 2.1 | [12] |
| HY (SiO2/Al2O3 = 10 molar ratio) | Autoclave | 250 | 70 | Nil | 89 | 37 | [14] |
| SBA-15 (SiO2/Al2O3 = 60 molar ratio) | Autoclave | 250 | 70 | Nil | 34 | 14 | [14] |
| AlPO4 | Batch | 270 | 1 | Nil | 23 | 8.7 | [15] |
| HY (SiO2/Al2O3 = 5.2 molar ratio) | Batch | 270 | 1 | Nil | 4.3 | 1.6 | [15] |
| H-ZSM-5 (SiO2/Al2O3 = 30 molar ratio) | Batch | 270 | 1 | Nil | 4.6 | 1.7 | [15] |
| H-ZSM-5 (SiO2/Al2O3 = 50 molar ratio) | Batch | 270 | 1 | Nil | 3.5 | 1.4 | [15] |
| HSiW | Semibatch | 300 | 1 | Nil | 79 | 0.23 | [16] |
| HPW | Semibatch | 300 | 1 | Nil | 44 | 0.064 | [16] |
| [Bmim]H2PO4 | Semibatch | 300 | 1 | Nil | 57 | 22 | [17] |
| ZSM-5_80 | Batch | 280 | 1 | Sulfolane | 35 | 7.0 | This work |
| ZSM-5_280 | Batch | 280 | 1 | Sulfolane | 46 | 9.2 | This work |
| 2%P-ZSM-5_280 | Batch | 280 | 1 | Sulfolane | 19 | 3.8 | This work |
| 10%P-ZSM-5_280 | Batch | 280 | 1 | Sulfolane | 28 | 5.6 | This work |
| 15%P-ZSM-5_280 | Batch | 280 | 1 | Sulfolane | 30 | 6.0 | This work |
| 20%P-ZSM-5_280 | Batch | 280 | 1 | Sulfolane | 36 | 7.2 | This work |
| HPW | Batch | 280 | 1 | Sulfolane | 25 | 5.0 | This work |
| CsPW b | Batch | 280 | 1 | Sulfolane | 14 | 2.8 | This work |
| Acrolein | Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | Conv. (%) | TOF (ggly gcat−1 h−1) | Yield (%) | TON (gacr gcat−1) | Acrolein | Other Low-Boiling-Point Products | High-Boiling-Point Products | Coke |
| 200 † | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 200 | 16 | 0.64 | 0.93 | 0.19 | 5.8 | 0.44 | 84 | 10 |
| 280 † | 12 | 0.48 | 0.54 | 0.11 | 4.5 | 0 | 96 | 0 |
| 280 | 45 | 9.0 | 5.0 | 1.0 | 11 | 2.1 | 83 | 3.9 |
| Acrolein | Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Catalyst/Glycerol Mass Ratio | Conv. (%) | TOF (ggly gcat−1 h−1) | Yield (%) | TON (gacr gcat−1) | Acrolein | Other Low-Boiling-Point Products | High-Boiling-Point Products | Coke |
| 0 | 12 | – | 0.54 | – | 4.5 | 0 | 96 | 0 |
| 0.005 | 26 | 10 | 2.6 | 5.2 | 10 | 0 | 88 | 1.6 |
| 0.025 | 29 | 2.4 | 6.1 | 2.4 | 21 | 2.1 | 75 | 2.6 |
| 0.05 | 45 | 1.8 | 5.0 | 1.0 | 11 | 2.1 | 83 | 3.9 |
| Acrolein | Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sulfolane/Glycerol Mass Ratio | Conv. (%) | TOF (ggly gcat−1 h−1) | Yield (%) | TON (gacr gcat−1) | Acrolein | Other Low-Boiling-Point Products | High-Boiling-Point Products | Coke |
| 0 | 45 | 1.8 | 5.0 | 1.0 | 11 | 2.1 | 83 | 3.9 |
| 1 | 92 | 3.6 | 18 | 3.6 | 19 | 1.7 | 78 | 1.2 |
| 3 | 100 | 4.0 | 46 | 9.2 | 46 | 2.3 | 47 | 4.7 |
| 5 | 95 | 3.8 | 44 | 8.8 | 46 | 2.5 | 50 | 1.5 |
| 7 | 80 | 3.2 | 25 | 5.0 | 31 | 1.2 | 67 | 0.92 |
| Acrolein | Selectivity (%) | ||||||
|---|---|---|---|---|---|---|---|
| Cycle | Conv. (%) | TOF (ggly gcat−1 h−1) | Yield (%) | TON (gacr gcat−1) | Acrolein | Other Low-Boiling-Point Products | High-Boiling-Point Products and Coke |
| 1 | 63 | 13 | 14 | 2.8 | 23 | 1.1 | 76 |
| 2 | 50 | 10 | 18 | 3.6 | 36 | 4.3 | 59 |
| 3 | 14 | 2.8 | 3.1 | 0.62 | 22 | 0 | 78 |
| 4 | 11 | 2.2 | 1.2 | 0.24 | 11 | 0 | 90 |
| Acrolein | Selectivity (%) | ||||||
|---|---|---|---|---|---|---|---|
| Catalyst | Conv. (%) | Yield (%) | TON (gacr gcat−1) | Acrolein | Other Low-Boiling-Point Products | High-Boiling-Point Products | Coke |
| ZSM-5_280 | 100 | 46 | 9.2 | 46 | 2.3 | 47 | 4.7 |
| ZSM-5_80 | 98 | 35 | 7.0 | 36 | 3.2 | 60 | 1.8 |
| 2% P-ZSM-5_280 | 91 | 19 | 3.8 | 21 | 0.63 | 54 | 1.8 |
| 10% P-ZSM-5_280 | 100 | 28 | 5.6 | 28 | 0.82 | 65 | 3.1 |
| 15% P-ZSM-5_280 | 100 | 30 | 6.0 | 30 | 0.84 | 65 | 4.5 |
| 20% P-ZSM-5_280 | 100 | 36 | 7.2 | 36 | 0.93 | 67 | 6.4 |
| HPW | 100 | 25 | 5.0 | 25 | 6.6 | 27 | 41 |
| CsPW | 100 | 14 | 2.8 | 14 | 0 | 74 | 12 |
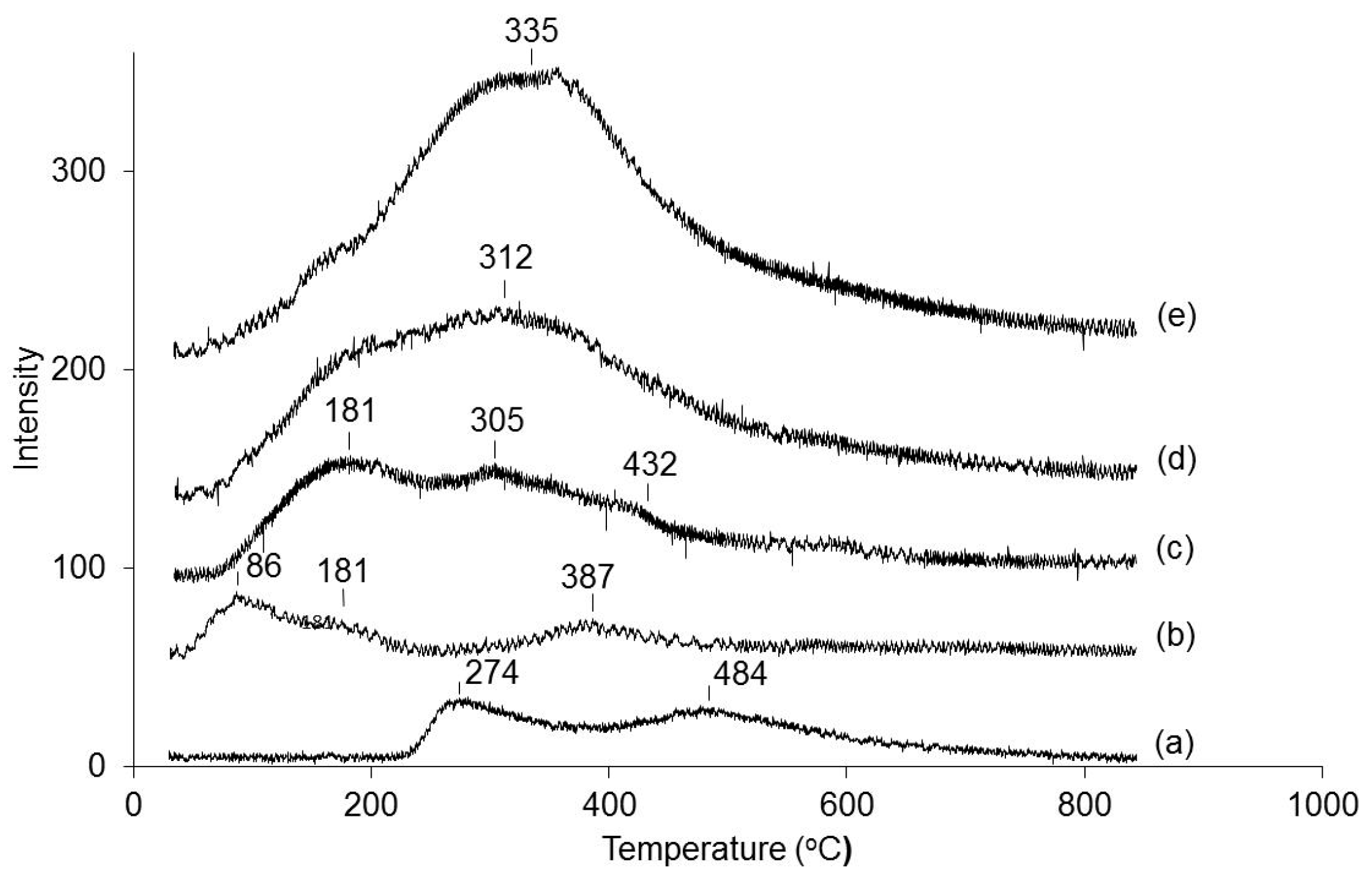
| Catalyst | Total-Acidity (µmol g−1) | Weak-Acidity (µmol g−1) a | Strong-Acidity (µmol g−1) a |
|---|---|---|---|
| ZSM-5_280 | 416 | 416 | 0 |
| ZSM-5_80 | 744 | 241 | 503 |
| 2%P-ZSM-5_280 | 828 | 649 | 179 |
| 10%P-ZSM-5_280 | 1450 | 1086 | 364 |
| 20%P-ZSM-5_280 | 1924 | 1643 | 281 |
| HPW | 1436 | 26 | 1382 |
| CsPW | 99 | 48 | 16 |
| Sample a | BET Surface Area (m2 g−1) b | Pore Volume (mL g−1) b | P Content (%) c |
|---|---|---|---|
| ZSM-5_80 | 345 | 0.24 | - |
| ZSM-5_280 | 363 | 0.21 | - |
| 2% P-ZSM-5_280 | 236 | 0.18 | 1.7 |
| 10% P-ZSM-5_280 | 90 | 0.11 | 9.3 |
| 15% P-ZSM-5_280 | 62 | 0.08 | 14 |
| 20% P-ZSM-5_280 | 43 | 0.06 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Wang, B.; Liu, L.; Borgna, A. Liquid-Phase Dehydration of Glycerol to Acrolein with ZSM-5-Based Catalysts in the Presence of a Dispersing Agent. Molecules 2023, 28, 3316. https://doi.org/10.3390/molecules28083316
Huang L, Wang B, Liu L, Borgna A. Liquid-Phase Dehydration of Glycerol to Acrolein with ZSM-5-Based Catalysts in the Presence of a Dispersing Agent. Molecules. 2023; 28(8):3316. https://doi.org/10.3390/molecules28083316
Chicago/Turabian StyleHuang, Lin, Bo Wang, Licheng Liu, and Armando Borgna. 2023. "Liquid-Phase Dehydration of Glycerol to Acrolein with ZSM-5-Based Catalysts in the Presence of a Dispersing Agent" Molecules 28, no. 8: 3316. https://doi.org/10.3390/molecules28083316
APA StyleHuang, L., Wang, B., Liu, L., & Borgna, A. (2023). Liquid-Phase Dehydration of Glycerol to Acrolein with ZSM-5-Based Catalysts in the Presence of a Dispersing Agent. Molecules, 28(8), 3316. https://doi.org/10.3390/molecules28083316





