Peroxymonosulfate Activation by Facile Fabrication of α-MnO2 for Rhodamine B Degradation: Reaction Kinetics and Mechanism
Abstract
:1. Introduction
2. Results and Discussion
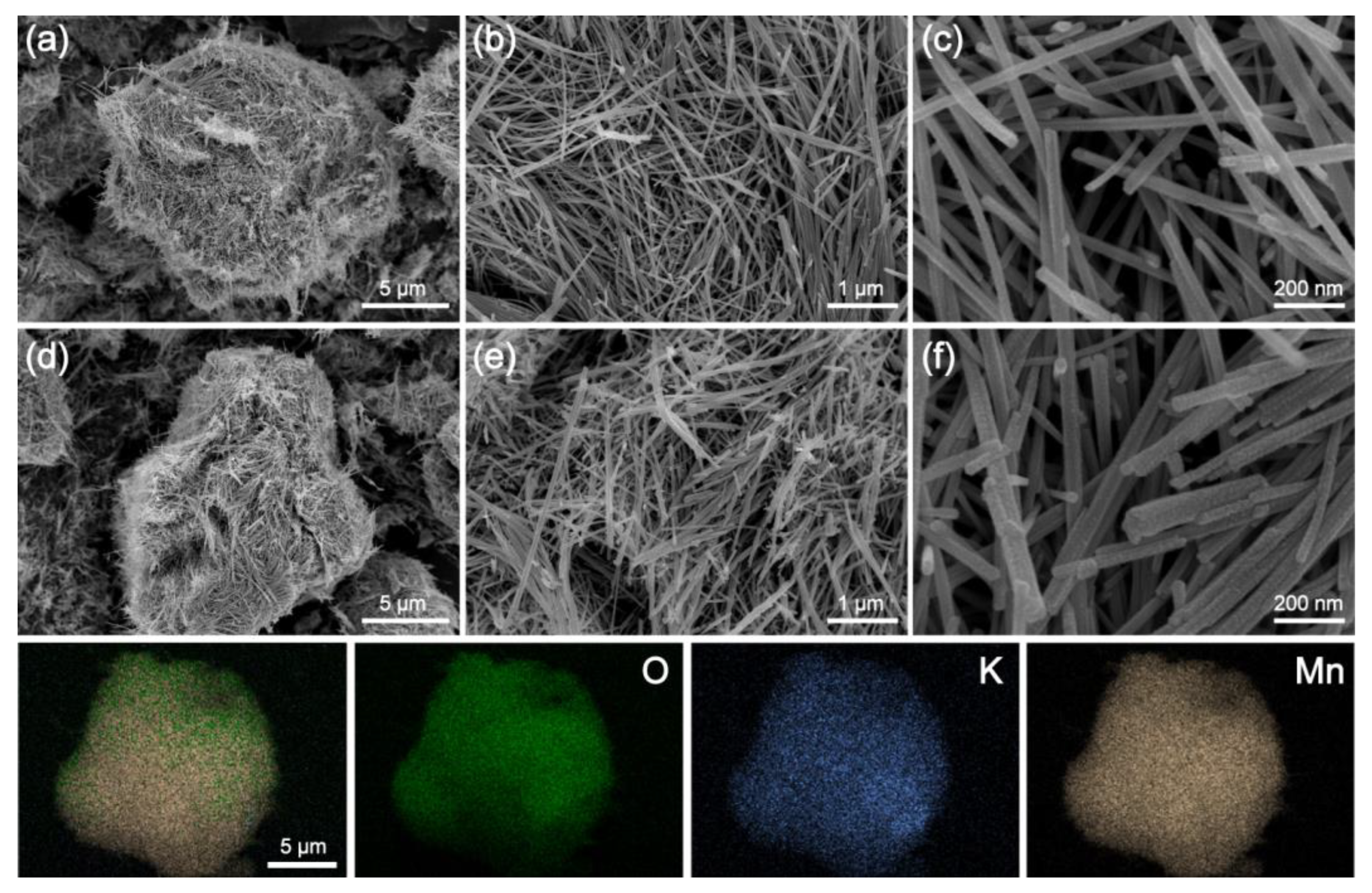
2.1. Catalysts’ Characterization Result
2.2. α-MnO2 Catalytic PMS Activation Performance
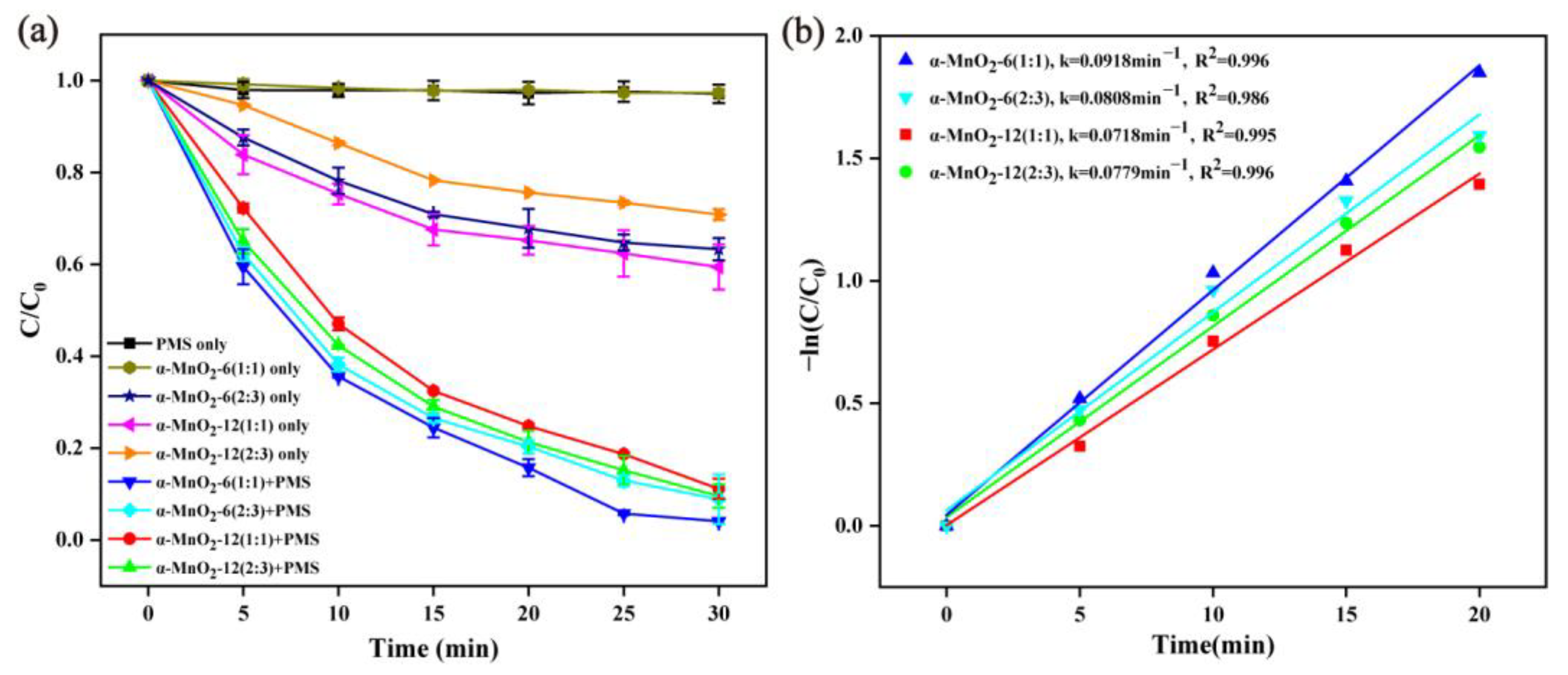
2.2.1. Effect of Hydrothermal Preparation Parameters
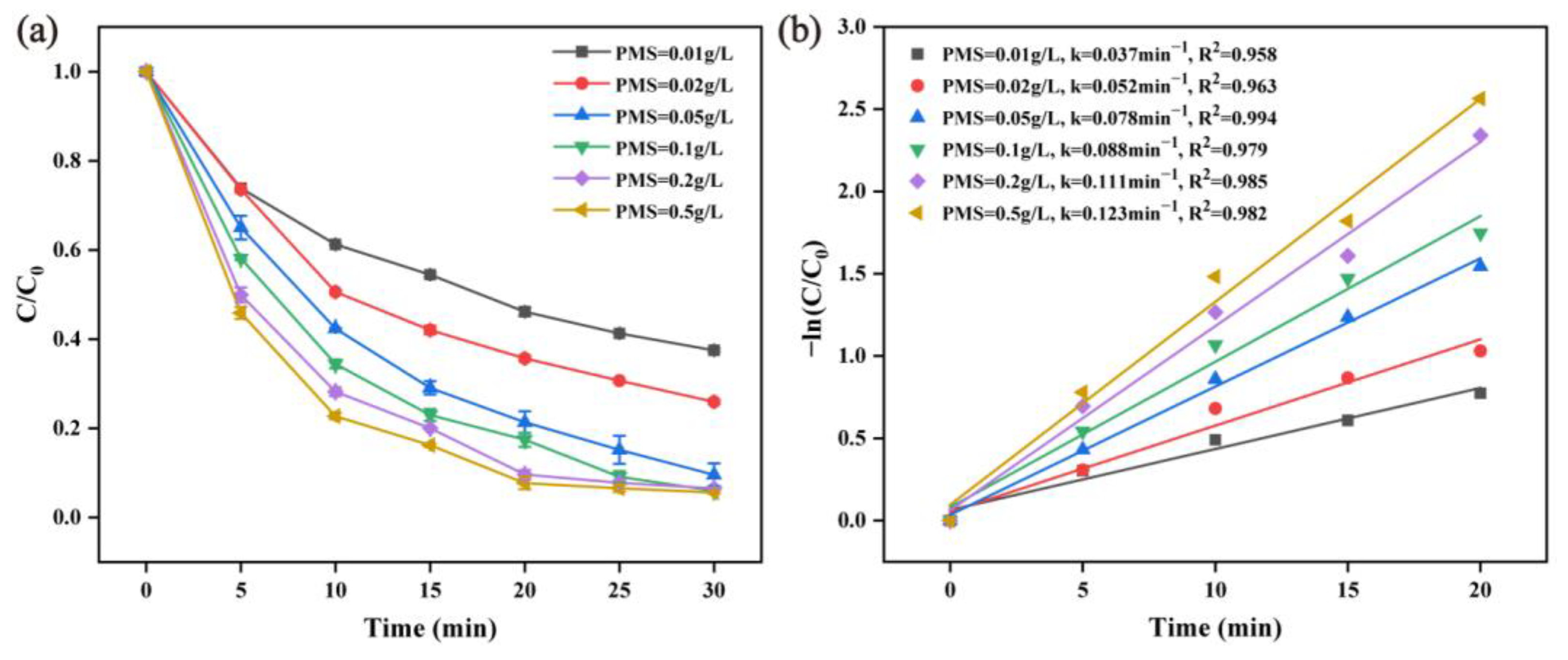
2.2.2. Effect of PMS Dosage
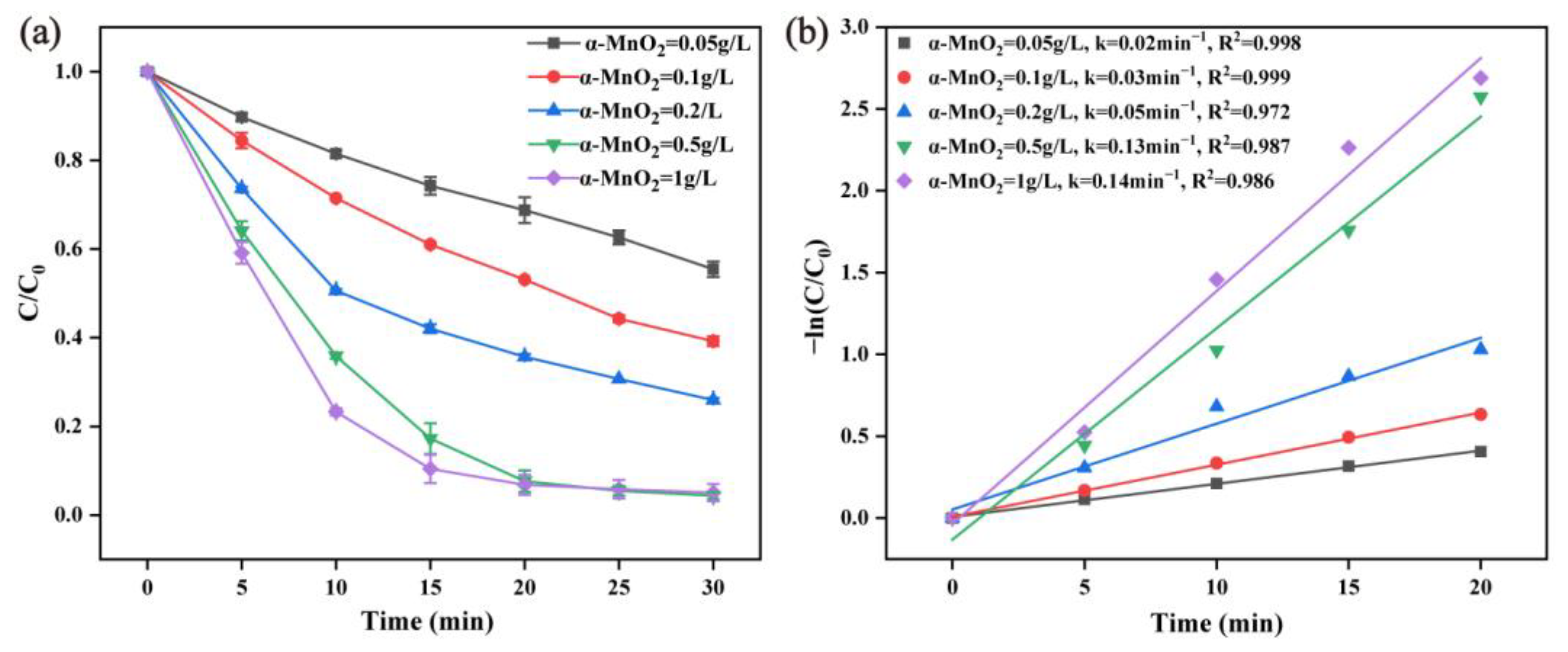
2.2.3. Effect of α-MnO2 Dosage
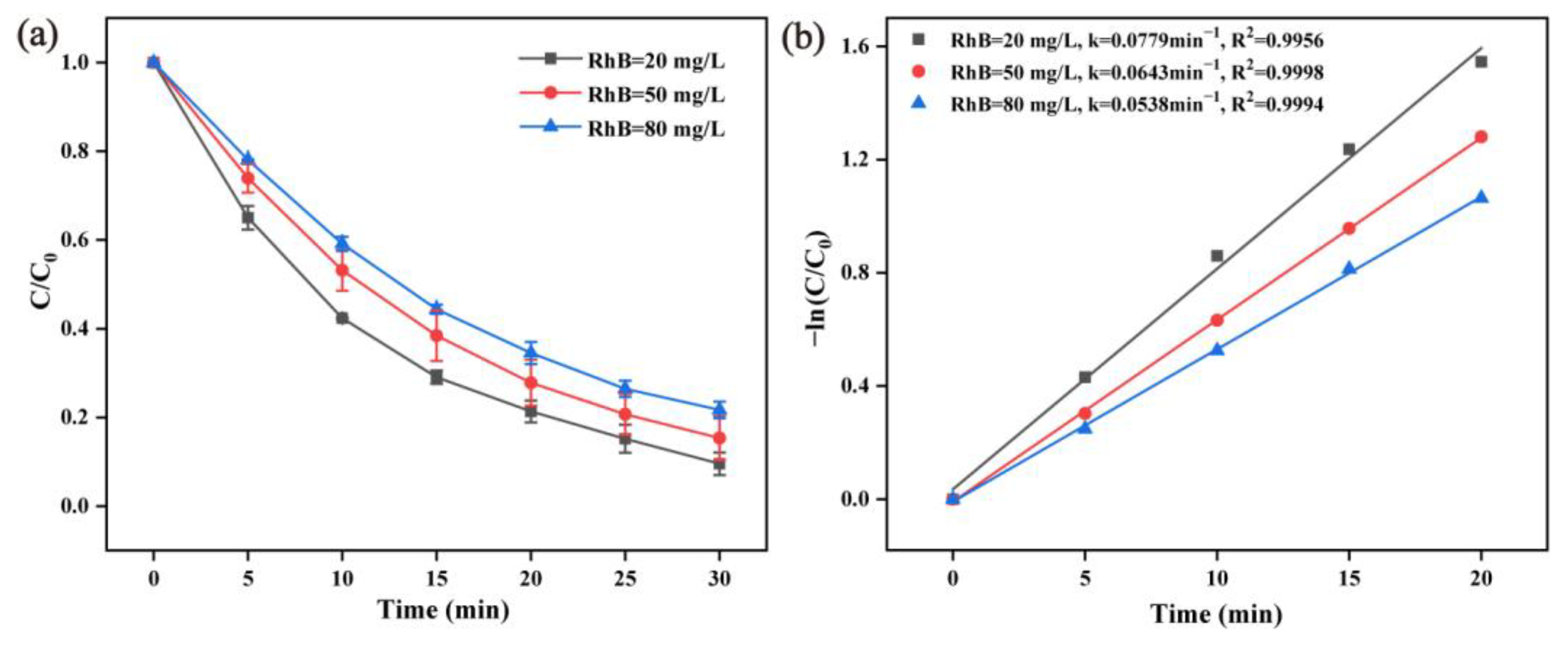
2.2.4. Effect of RhB Initial Concentration
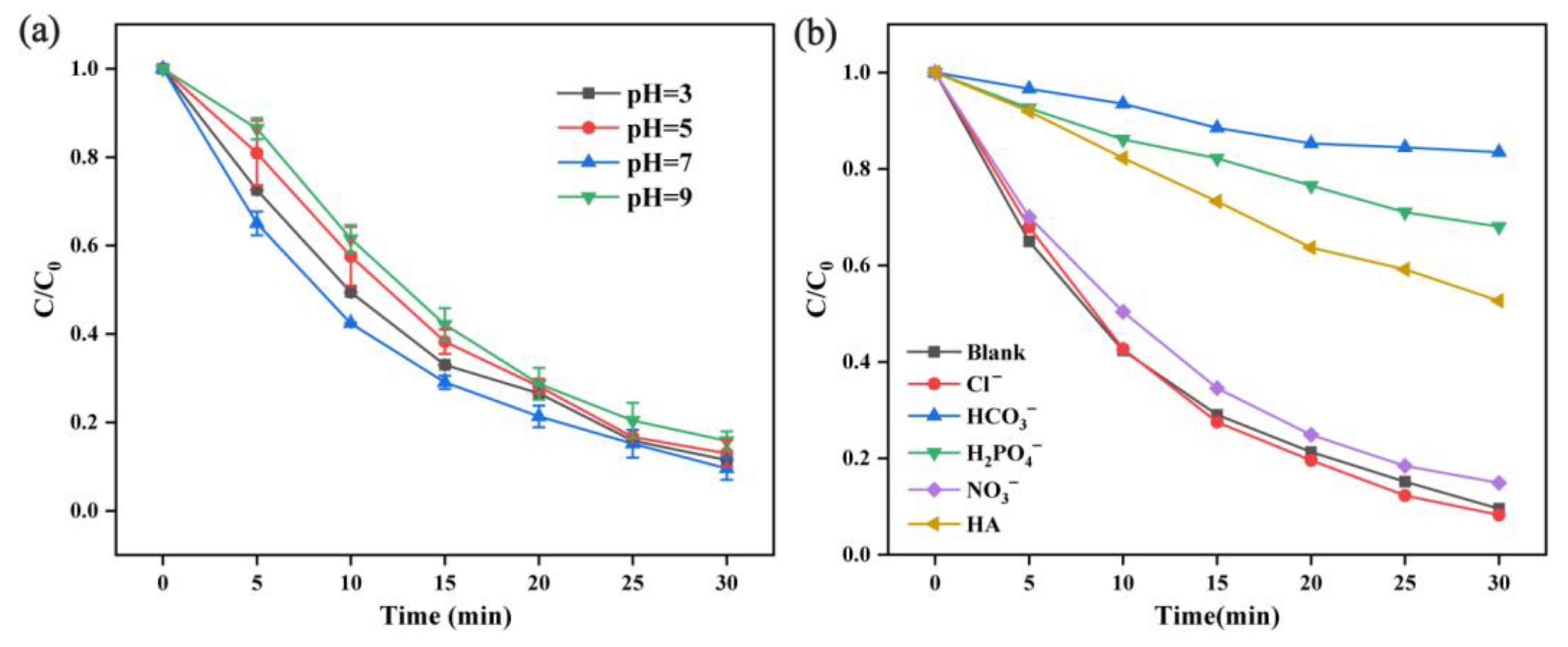
2.2.5. Effect of pH Value and Cations
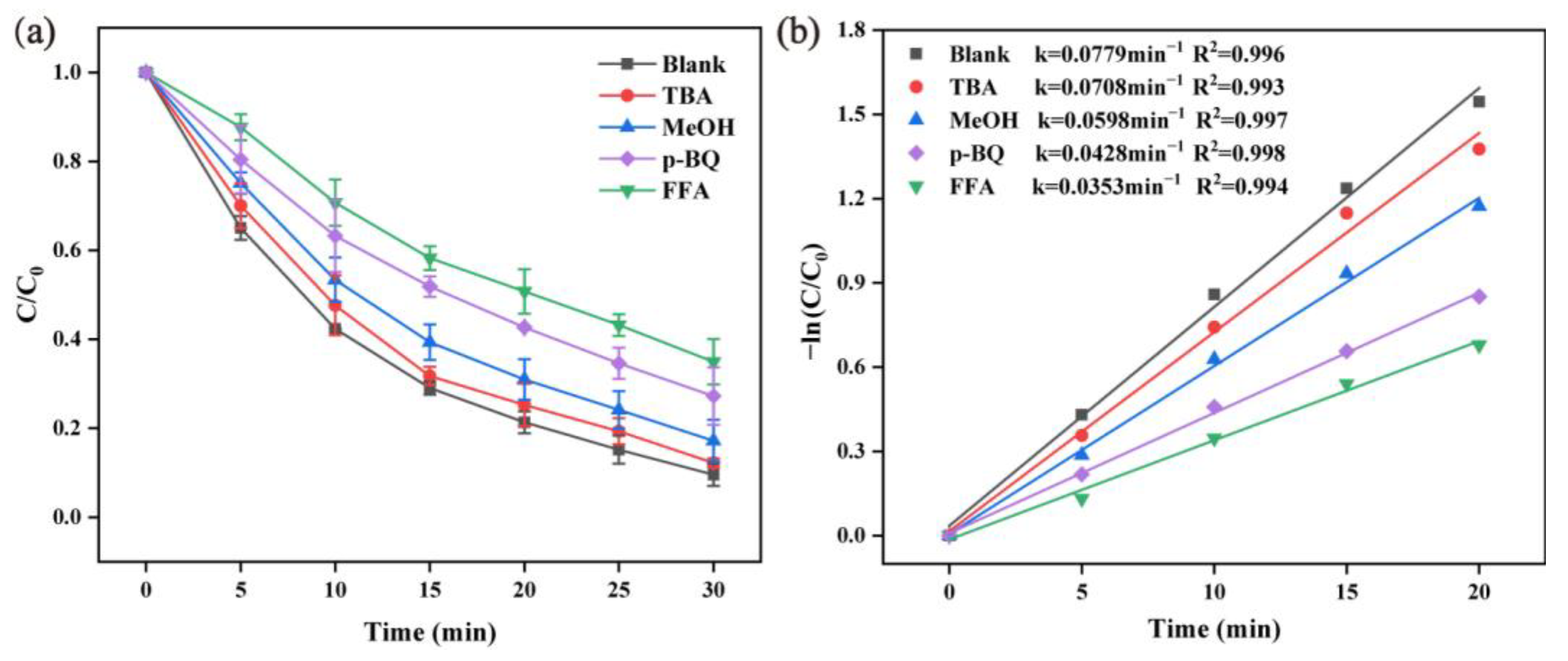
2.2.6. Radical Scavenger Tests
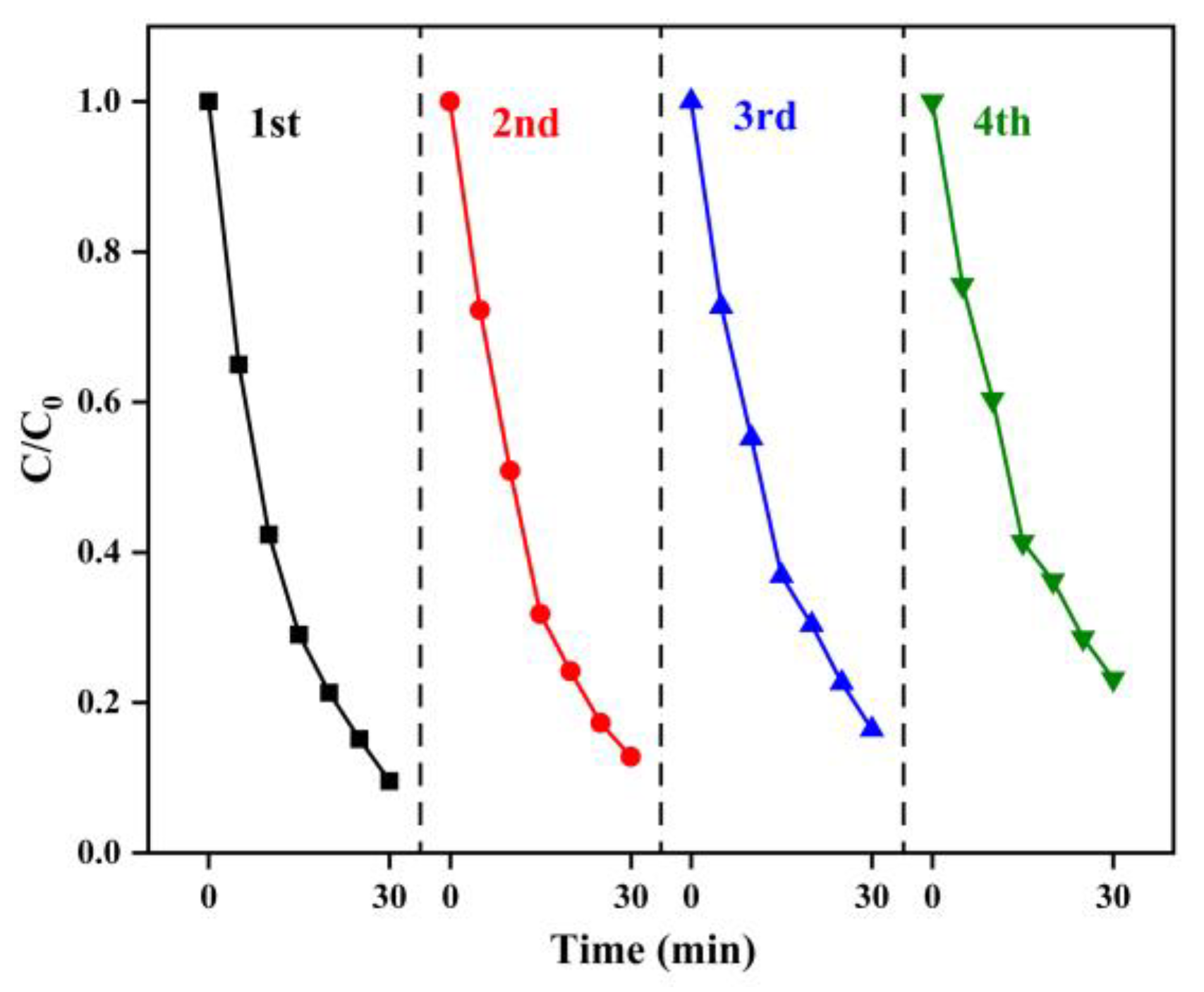
2.2.7. Repeatability
2.3. RhB Degradation Mechnism
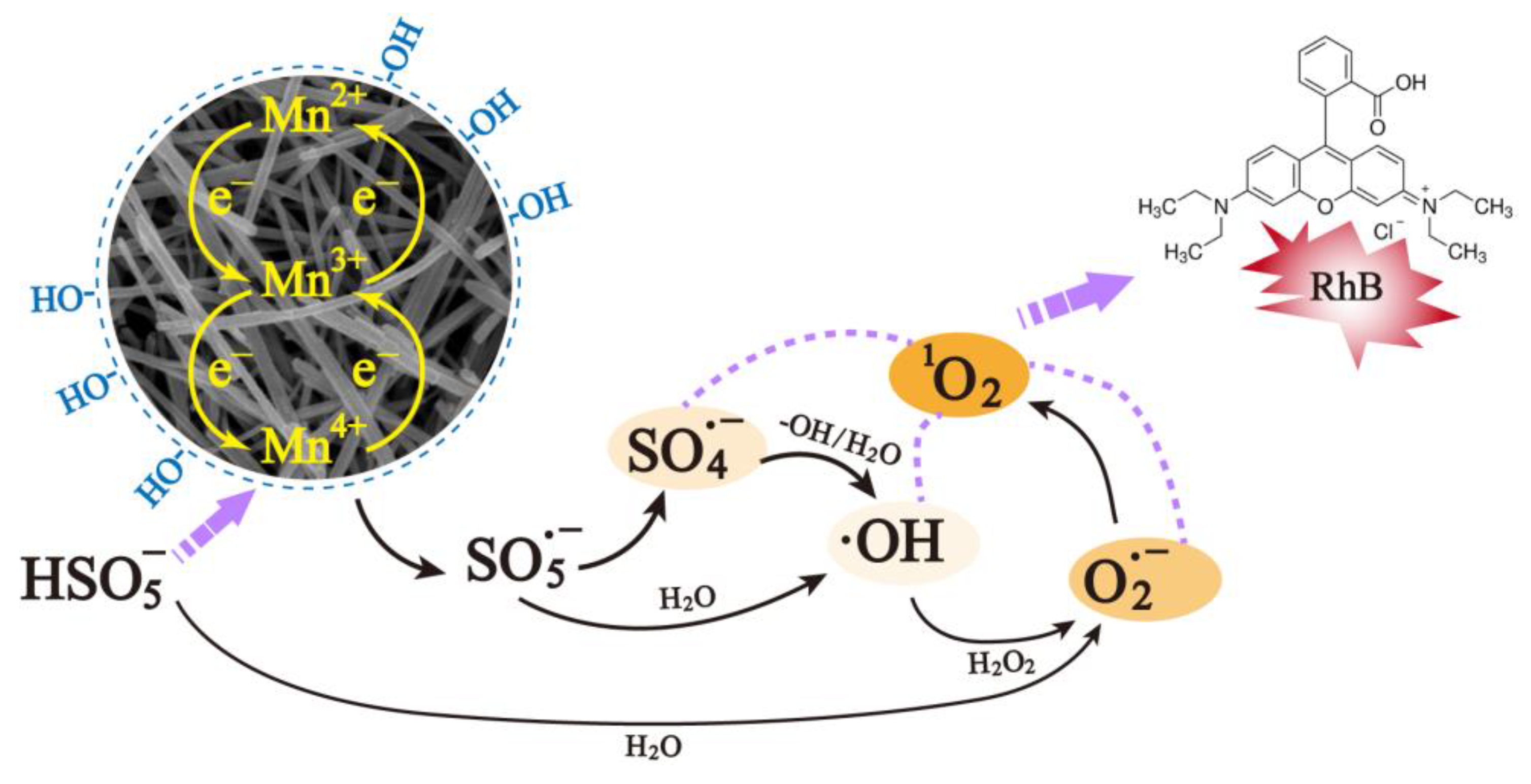
2.4. Possible Activation Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Catalysts’ Preparation
3.3. Analytical Instruments
3.4. Experimental Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bal, G.; Thakur, A. Distinct approaches of removal of dyes from wastewater: A review. Mater. Today Proc. 2021, 50, 1575–1579. [Google Scholar] [CrossRef]
- Akar, S.T.; Aslan, S.; Akar, T. Conversion of natural mineral to effective geosorbent by coating MnO2 and its application potential for dye contaminated wastewaters. J. Clean. Prod. 2018, 189, 887–897. [Google Scholar] [CrossRef]
- Cao, M.; Zhuang, Z.; Liu, Y.; Zhang, Z.; Xuan, J.; Zhang, Q.; Wang, W. Peptide-mediated green synthesis of the MnO2@ZIF-8 core–shell nanoparticles for efficient removal of pollutant dyes from wastewater via a synergistic process. J. Colloid Interface Sci. 2022, 608, 2779–2790. [Google Scholar] [CrossRef] [PubMed]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.A.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Wang, J. A critical review of various adsorbents for selective removal of nitrate from water: Structure, performance and mechanism. Chemosphere 2022, 291, 132728. [Google Scholar] [CrossRef]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and Improving Microplastic Removal during Water Treatment: Impact of Coagulation and Flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef]
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.-H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230, 115877. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Yu, M.; Xu, H.; Lv, J.; Yang, S.; Zhu, X.; Li, L. One-pot pyrolysis method for synthesis of Fe/N co-doped biochar as an effective peroxymonosulfate activator for RhB degradation. J. Taiwan Inst. Chem. Eng. 2021, 128, 209–219. [Google Scholar] [CrossRef]
- Luo, H.; Guo, J.; Shen, T.; Zhou, H.; Liang, J.; Yuan, S. Study on the catalytic performance of LaMnO3 for the RhB degradation. J. Taiwan Inst. Chem. Eng. 2020, 109, 15–25. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. The role of hydroxyl radical reactions in ozonation processes in aqueous solutions. Water Res. 1976, 10, 377–386. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Long, L.; Bai, C.; Zhou, X.; Zhang, S.; Zhang, Y.; Chen, C.; He, J.; Song, C.; Yang, G. A novel strategy for promoting PMS activation: Enhanced utilization of side reactions. Sep. Purif. Technol. 2022, 297, 121432. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, H.; Li, X.; Ma, J. Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem. Eng. J. 2017, 315, 201–209. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, X.; Ma, J.; Tan, H.; Ye, W. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity. Chem. Eng. J. 2014, 249, 6–14. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Ioannidi, A.A.; Mantzavinos, D.; Frontistis, Z. Heat-activated persulfate for the degradation of micropollutants in water: A comprehensive review and future perspectives. J. Environ. Manag. 2022, 318, 115568. [Google Scholar] [CrossRef]
- Manz, K.E.; Adams, T.J.; Carter, K.E. Furfural degradation through heat-activated persulfate: Impacts of simulated brine and elevated pressures. Chem. Eng. J. 2018, 353, 727–735. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Novel magnetic MnO2/MnFe2O4 nanocomposite as a heterogeneous catalyst for activation of peroxymonosulfate (PMS) toward oxidation of organic pollutants. Sep. Purif. Technol. 2019, 213, 456–464. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Li, W.; Lan, Y.; Chen, C. Performance comparison and mechanism investigation of Co3O4-modified different crystallographic MnO2 (α, β, γ, and δ) as an activator of peroxymonosulfate (PMS) for sulfisoxazole degradation. Chem. Eng. J. 2022, 427, 130888. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, S.; Duan, X.; Zheng, W.; Shao, C.; Wu, W.; Jiang, Z.; Lai, W. MnO2/UIO-66 improves the catalysed degradation of oxytetracycline under UV/H2O2/PMS system. J. Solid State Chem. 2021, 300, 122231. [Google Scholar] [CrossRef]
- Koo, P.-L.; Jaafar, N.F.; Yap, P.-S.; Oh, W.-D. A review on the application of perovskite as peroxymonosulfate activator for organic pollutants removal. J. Environ. Chem. Eng. 2022, 10, 107093. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, B.; An, H.; Dong, G.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. Enhanced activation of peroxymonosulfate by Sr-doped LaFeO3 perovskite for Orange I degradation in the water. Sep. Purif. Technol. 2021, 256, 117838. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Z.; Deng, L.; Duan, Y. Efficient degradation of sulfadiazine using magnetically recoverable MnFe2O4/δ-MnO2 hybrid as a heterogeneous catalyst of peroxymonosulfate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125637. [Google Scholar] [CrossRef]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Fu, L.; Peng, X.; Pan, C.; Mao, Q.; Wang, C.; Yan, J. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective. Sci. Total Environ. 2021, 765, 142794. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, C.; Waite, T.D. Hydroxyl radicals in anodic oxidation systems: Generation, identification and quantification. Water Res. 2022, 217, 118425. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, P.-S.; Jiang, Y.-X.; Sun, L.; Sun, X.-H. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, J.-L.; Li, X.; Yang, S.; Luo, W.; Zhang, Y.; Kim, S.-H.; Kim, D.-H.; Shinde, S.S.; Li, Y.-F.; et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 2021, 4, 1012–1023. [Google Scholar] [CrossRef]
- Jiang, Z.-R.; Wang, P.; Zhou, Y.-X.; Wang, C.; Jiang, J.; Lan, Y.; Chen, C. Fabrication of a 3D-blocky catalyst (CoMnOx@sponge) via mooring Co-Mn bimetallic oxide on sponge to activate peroxymonosulfate for convenient and efficient degradation of sulfonamide antibiotics. Chem. Eng. J. 2022, 446, 137306. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J.; et al. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Qi, C.; Wen, Y.; Zhao, Y.; Dai, Y.; Li, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of organic contaminants by Fe(III)/peroxymonosulfate process with l-cysteine. Chin. Chem. Lett. 2022, 33, 2125–2128. [Google Scholar] [CrossRef]
- Pan, M.; Wang, N.; Weng, Z.; Zou, X.; Huang, X. The synergistic activation of peroxymonosulfate for the degradation of Acid Scarlet GR by palygorskite/MnO2/Fe3O4 nanocomposites. Dalton Trans. 2023, 52, 1009–1020. [Google Scholar] [CrossRef]
- Wang, T.; Qian, X.; Yue, D.; Yan, X.; Yamashita, H.; Zhao, Y. CaMnO3 perovskite nanocrystals for efficient peroxydisulfate activation. Chem. Eng. J. 2020, 398, 125638. [Google Scholar] [CrossRef]
- Manos, D.; Miserli, K.; Konstantinou, I. Perovskite and Spinel Catalysts for Sulfate Radical-Based Advanced Oxidation of Organic Pollutants in Water and Wastewater Systems. Catalysts 2020, 10, 1299. [Google Scholar] [CrossRef]
- Guo, M.; Fang, R.; Liu, X.; Yang, Z. Experimental study of volatile organic compounds catalytic combustion on Cu-Mn catalysts with different carriers. Int. J. Energy Res. 2021, 45, 8749–8762. [Google Scholar] [CrossRef]
- Qin, C.; Guo, M.; Jiang, C.; Yu, R.; Huang, J.; Yan, D.; Li, S.; Dang, X. Simultaneous oxidation of toluene and ethyl acetate by dielectric barrier discharge combined with Fe, Mn and Mo catalysts. Sci. Total Environ. 2021, 782, 146931. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Zong, Y.; Wu, R.; Zhang, M.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. Activation of peroxymonosulfate by α-MnO2 for Orange Ⅰ removal in water. Environ. Res. 2022, 210, 112919. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Nie, F.; Zhang, R.; Fang, X.; Wang, Y. The role of MnO2 crystal morphological scale and crystal structure in selective catalytic degradation of azo dye. Environ. Sci. Pollut. Res. Int. 2023, 30, 15377–15391. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; He, J.; Fang, X.; Hong, P.; Nie, M.; Yang, W.; Xie, C.; Wu, Z.; Zhang, K.; et al. Nano-hybrids of needle-like MnO2 on graphene oxide coupled with peroxymonosulfate for enhanced degradation of norfloxacin: A comparative study and probable degradation pathway. J. Colloid Interface Sci. 2020, 562, 1–11. [Google Scholar] [CrossRef]
- Yi, H.; Wang, Y.; Diao, L.; Xin, Y.; Chai, C.; Cui, D.; Ma, D. Ultrasonic treatment enhances the formation of oxygen vacancies and trivalent manganese on α-MnO2 surfaces: Mechanism and application. J. Colloid Interface Sci. 2022, 626, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Hu, C.; Xin, Y.; Ma, D.; Gao, M.; Xie, H. Enhanced MnO2/peroxymonosulfate activation for phthalic acid esters degradation: Regulation of oxygen vacancy. Chem. Eng. J. 2022, 433, 134048. [Google Scholar] [CrossRef]
- Xiao, J.; Li, R.; Dong, H.; Li, Y.; Li, L.; Xiao, S.; Jin, Z. Activation of sulfite via zero-valent iron-manganese bimetallic nanomaterials for enhanced sulfamethazine removal in aqueous solution: Key roles of Fe/Mn molar ratio and solution pH. Sep. Purif. Technol. 2022, 297, 121479. [Google Scholar] [CrossRef]
- Feng, M.; Qu, R.; Zhang, X.; Sun, P.; Sui, Y.; Wang, L.; Wang, Z. Degradation of flumequine in aqueous solution by persulfate activated with common methods and polyhydroquinone-coated magnetite/multi-walled carbon nanotubes catalysts. Water Res. 2015, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Miao, J.; Ge, J.; Lang, J.; Yu, C.; Zhang, L.; Alvarez, P.J.J.; Long, M. Ultrahigh Peroxymonosulfate Utilization Efficiency over CuO Nanosheets via Heterogeneous Cu(III) Formation and Preferential Electron Transfer during Degradation of Phenols. Environ. Sci. Technol. 2022, 56, 8984–8992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, Y.; Fan, W.; Wang, Y.; Huang, L. Shell-core MnO2/Carbon@Carbon nanotubes synthesized by a facile one-pot method for peroxymonosulfate oxidation of tetracycline. Sep. Purif. Technol. 2021, 278, 119558. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Ji, J.; Li, X.; Yuan, X.; Duan, A.; Guan, X.; Jiang, L.; Li, Y. Recycling of waste power lithium-ion batteries to prepare nickel/cobalt/manganese-containing catalysts with inter-valence cobalt/manganese synergistic effect for peroxymonosulfate activation. J. Colloid Interface Sci. 2022, 626, 564–580. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Liang, G.; Zhang, X.; Xie, X. Catalyst bridging-mediated electron transfer for nonradical degradation of bisphenol A via natural manganese ore-cornstalk biochar composite activated peroxymonosulfate. Chem. Eng. J. 2021, 426, 131777. [Google Scholar] [CrossRef]
- Xu, L.S.; Sun, X.B.; Hong, J.-M.; Zhang, Q. Peroxymonosulfate activation by α-MnO2/MnFe2O4 for norfloxacin degradation: Efficiency and mechanism. J. Phys. Chem. Solids 2021, 153, 110029. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Shi, Q.; Sun, M.; Liu, J.; Zhao, R.; Chen, J.; Wang, X.; Liu, Y.; Gong, W.; Liu, P.; et al. Peroxymonosulfate Activation by Facile Fabrication of α-MnO2 for Rhodamine B Degradation: Reaction Kinetics and Mechanism. Molecules 2023, 28, 4388. https://doi.org/10.3390/molecules28114388
Li J, Shi Q, Sun M, Liu J, Zhao R, Chen J, Wang X, Liu Y, Gong W, Liu P, et al. Peroxymonosulfate Activation by Facile Fabrication of α-MnO2 for Rhodamine B Degradation: Reaction Kinetics and Mechanism. Molecules. 2023; 28(11):4388. https://doi.org/10.3390/molecules28114388
Chicago/Turabian StyleLi, Juexiu, Qixu Shi, Maiqi Sun, Jinming Liu, Rui Zhao, Jianjing Chen, Xiangfei Wang, Yue Liu, Weijin Gong, Panpan Liu, and et al. 2023. "Peroxymonosulfate Activation by Facile Fabrication of α-MnO2 for Rhodamine B Degradation: Reaction Kinetics and Mechanism" Molecules 28, no. 11: 4388. https://doi.org/10.3390/molecules28114388
APA StyleLi, J., Shi, Q., Sun, M., Liu, J., Zhao, R., Chen, J., Wang, X., Liu, Y., Gong, W., Liu, P., & Chen, K. (2023). Peroxymonosulfate Activation by Facile Fabrication of α-MnO2 for Rhodamine B Degradation: Reaction Kinetics and Mechanism. Molecules, 28(11), 4388. https://doi.org/10.3390/molecules28114388






