Evaluation of Novel Rapid Analytical Methods to Categorize Extra Virgin Olive Oil Based on the Coulometrically Determined Antioxidant Capacity and on the Spectrophotometric Assessment of Phenolic Compounds
Abstract
1. Introduction
2. Results and Discussion
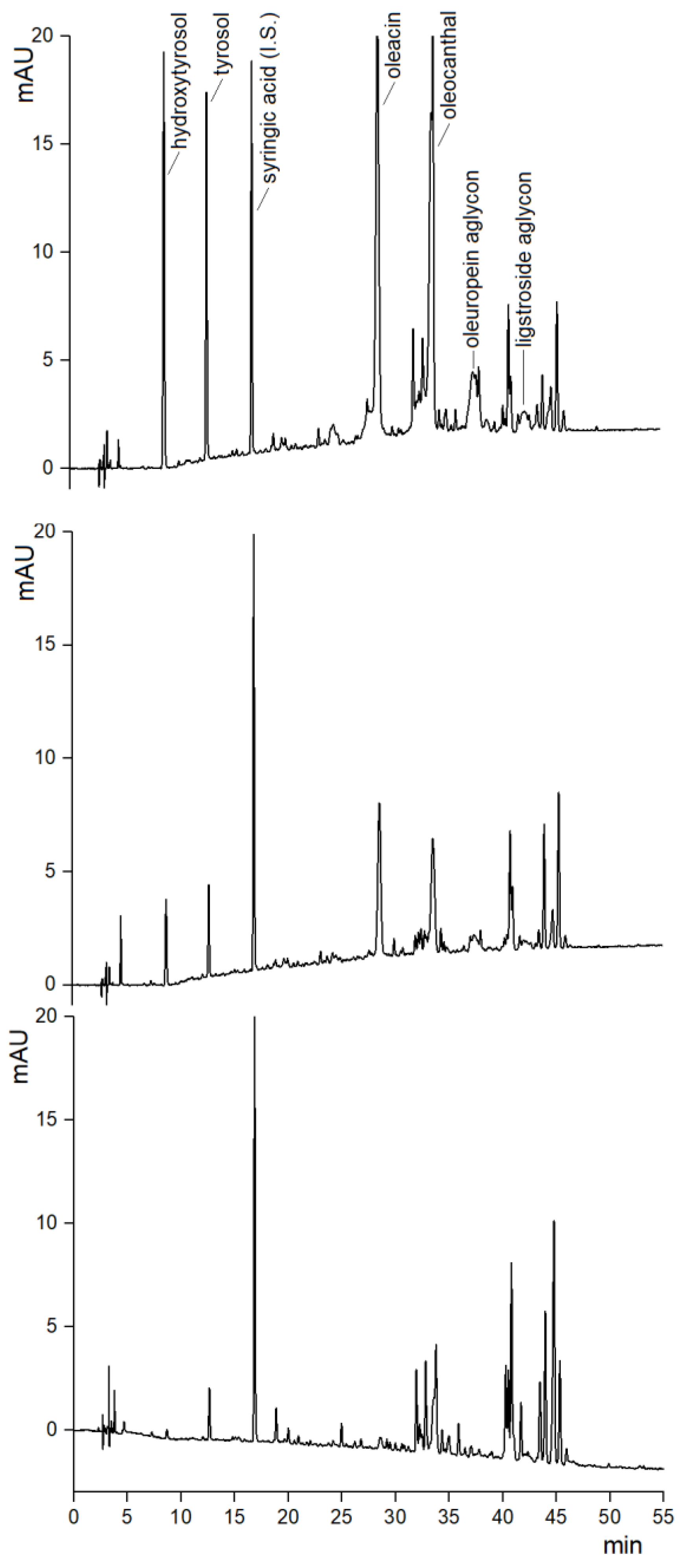
2.1. HPLC-UV Analysis
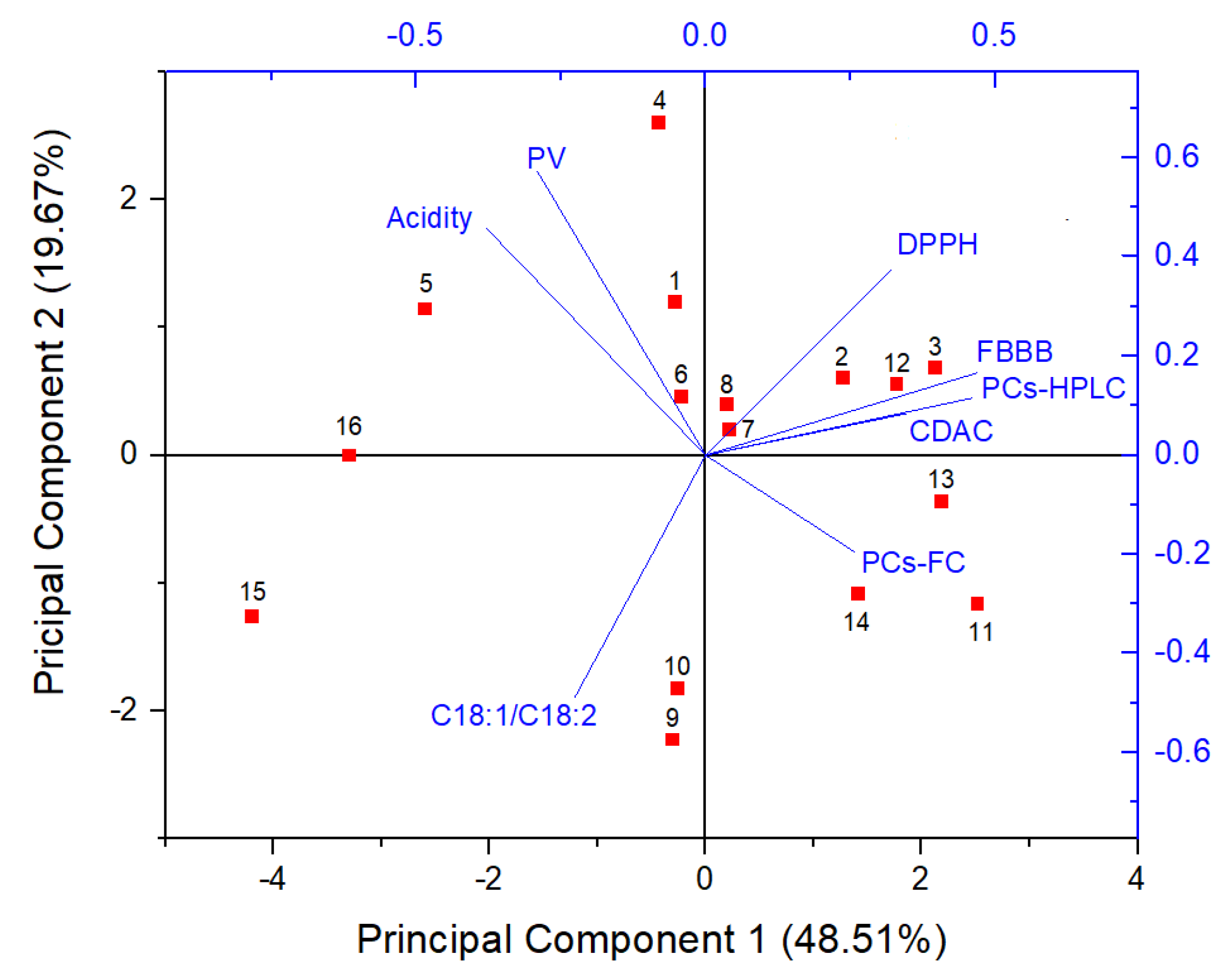
2.2. Principal Component Analysis (PCA)
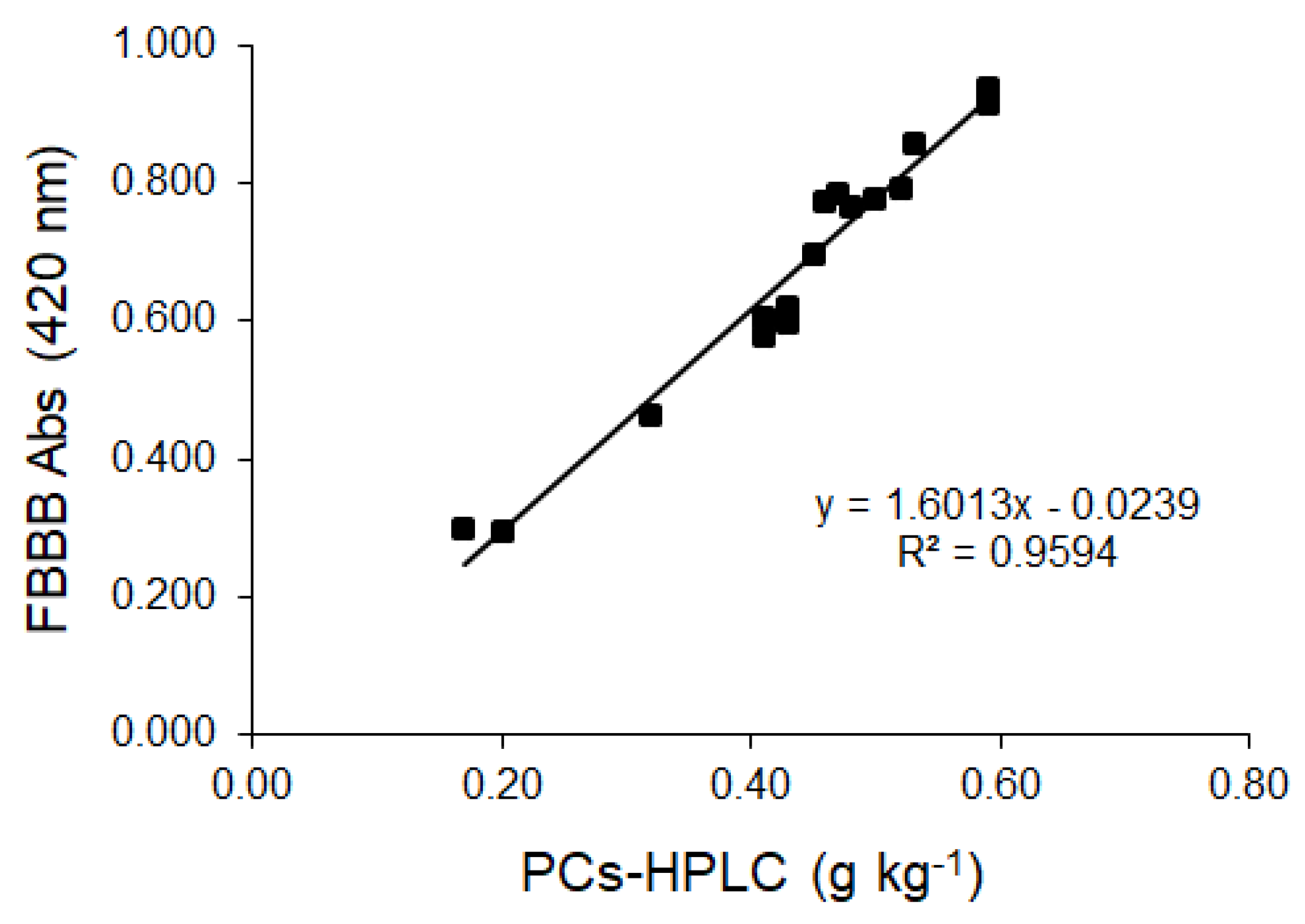
2.3. FBBB—PCs-HPLC Correlation and FBBB—CDAC Association
3. Materials and Methods
3.1. Acidity and Peroxide Value Analysis
3.2. Extraction of Polar Compounds from EVOO Samples
3.3. DPPH Radical Scavenging Capacity
3.4. Determination of Total Phenolic Compounds with the Folin–Ciocalteu Method (PCs-FC)
3.5. CDAC
3.6. Fast Blue BB
3.7. GC Analysis of Fatty Acids
3.8. HPLC-UV Determination of Total Phenolic Compounds (PCs-HPLC)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gomez-Caravaca, A.; Segura-Cerretero, A.; Fernandez-Gutierrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Muraglia, M.; Crupi, P.; Hbaieb, R.H.; De Santis, S.; Desantis, A.; Corbo, F. The Tower of Babel of Pharma-Food Study on Extra Virgin Olive Oil Polyphenols. Foods 2022, 11, 1915. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. From Olive Fruits to Olive Oil: Phenolic Compound Transfer in Six Different Olive Cultivars Grown under the Same Agronomical Conditions. Int. J. Mol. Sci. 2016, 17, 337. [Google Scholar] [CrossRef]
- López-Huertas, E.; Lozano-Sánchez, J.; Segura-Carretero, A. Olive oil varieties and ripening stages containing the antioxidants hydroxytyrosol and derivatives in compliance with EFSA health claim. Food Chem. 2021, 342, 128291. [Google Scholar] [CrossRef]
- Sánchez de Medina, V.; Priego-Capote, F.; Jiménez-Ot, C.; Luque de Castro, M.D. Quality and stability of edible oils enriched with hydrophilic antioxidants from the olive tree: The role of enrichment extracts and lipid composition. J. Agric. Food Chem. 2011, 59, 11432–11441. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of Phenolic Compounds and Their Contribution to Sensory Properties of Olive Oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef]
- European Union Commission. European Commission Regulation (ECC) No. 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Communities L 1991, 248, 1–83. [Google Scholar]
- European Union Commission. Commission Implementing Regulation (EU) 2019/1604 of 27 September 2019 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Communities L 2019, 250, 14–48. [Google Scholar]
- EFSA. Guidance on the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health. EFSA J. 2012, 9, 2474. [Google Scholar]
- European Union Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Communities L 2012, 136, 1–40. [Google Scholar]
- Fiorini, D.; Boarelli, M.C.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; Gabbianelli, R.; Pacetti, D. Chemical and sensory differences between high price and low price extra virgin olive oils. Food Res. Int. 2018, 105, 65–75. [Google Scholar] [CrossRef]
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; De Gennaro, B. Are health claims a useful tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Technol. 2017, 68, 176–181. [Google Scholar] [CrossRef]
- Mastralexi, A.; Nenadis, N.; Tsimidou, M.Z. Addressing analytical requirements to support health claims on “olive oil polyphenols” (EC Regulation 432/2012). J. Agric. Food Chem. 2014, 62, 2459–2461. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Boskou, D. The health claim on “olive oil polyphenols” and the need for meaningful terminology and effective analytical protocols. Eur. J. Lipid Sci. Technol. 2015, 117, 1091–1094. [Google Scholar] [CrossRef]
- International Olive Council. COI/T.20/Doc. No. 29. Official Method of Analysis. Determination of Biophenols in Olive Oil by HPLC. 2009. Available online: http://www.internationaloliveoil.org (accessed on 8 March 2023).
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef]
- International Olive Council. COI/T.20/Doc. No 29/Rev.1. Official Method of Analysis. Determination of Biophenols in Olive Oil by HPLC. 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-29-Rev-1-2017.pdf (accessed on 8 March 2023).
- International Olive Council. COI/T.20/Doc. No 29/Rev.2. Official Method of Analysis. Determination of Biophenols in Olive Oil by HPLC. 2022. Available online: https://www.interna.tionaloliveoil.org/wp-content/uploads/2022/06/Doc.-No-29-REV-2_ENK.pdf (accessed on 8 March 2023).
- Tsimidou, M.; Sotiroglou, M.; Mastralexi, A.; Nenadis, N.; García-Gonzalez, D.; Gallina Toschi, T. In house validated UHPLC protocol for the determination of the total hydroxytyrosol and tyrosol content in virgin olive oil fit for the purpose of the health claim introduced by the EC regulation 432/2012 for “olive oil polyphenols”. Molecules 2019, 24, 1044. [Google Scholar] [CrossRef]
- Siano, F.; Picariello, G.; Vasca, E. Coulometrically determined antioxidant capacity (CDAC) as a possible parameter to categorize extra virgin olive oil. Food Chem. 2021, 354, 129564. [Google Scholar] [CrossRef]
- Siano, F.; Vasca, E.; Picariello, G. Accurate determination of total biophenols in unfractionated extra-virgin olive oil with the fast blue BB assay. Food Chem. 2022, 370, 130990. [Google Scholar] [CrossRef]
- Aparicio, R.; Roda, L.; Albi, M.A.; Gutiérrez, F. Effect of various compounds on virgin olive oil stability measured by Rancimat. J. Agric. Food Chem. 1999, 47, 4150–4155. [Google Scholar] [CrossRef]
- Hernández, M.L.; Sicardo, M.D.; Belaj, A.; Martínez-Rivas, J.M. The Oleic/Linoleic Acid Ratio in Olive (Olea europaea L.) Fruit Mesocarp Is Mainly Controlled by OeFAD2-2 and OeFAD2-5 Genes Together With the Different Specificity of Extraplastidial Acyltransferase Enzymes. Front. Plant Sci. 2021, 12, 653997. [Google Scholar] [CrossRef]
- Beltrán, G.; del Río, C.; Sánchez, S.; Martínez, L. Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I. Authorised EU health claims forpolyphenols in olive oil. In Foods, Nutrients and Food Ingredients with Authorised EU Health Claims; Sadler, M.J., Ed.; Woodhead Publishing: Cambridge, UK, 2014; Chapter 10; pp. 212–228. [Google Scholar]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive Analysis of Polyphenols in 55 Extra Virgin Olive Oils by HPLC-ECD and Their Correlation with Antioxidant Activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive oil polyphenols: A quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef]
- Olmo-García, L.; Fernández-Fernández, C.; Hidalgo, A.; Vílchez, P.; Fernández-Gutiérrez, A.; Marchal, R.; Carrasco-Pancorbo, A. Evaluating the reliability of specific and global methods to assess the phenolic content of virgin olive oil: Do they drive to equivalent results? J. Chromatogr. A 2019, 1585, 56–69. [Google Scholar] [CrossRef]
- Alessandri, S.; Ieri, F.; Romani, A. Minor polar compounds in extra virgin olive oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu spectrophotometric method. J. Agric. Food Chem. 2014, 62, 826–835. [Google Scholar] [CrossRef]
- European Economic Community (EEC). Commission Regulation (EEC) No. 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur Commnities 1991, L248, 1–82. [Google Scholar]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Siano, F.; Addeo, F.; Volpe, M.G.; Paolucci, M.; Picariello, G. Oxidative Stability of Pomegranate (Punica granatum L.) Seed Oil to Simulated Gastric Conditions and Thermal Stress. J. Agric. Food Chem. 2016, 64, 8369–8378. [Google Scholar] [CrossRef]
- Tsimoidou, M.L. A Critical Appraisal of the Separation Protocols Proposed for the Implementation of the Health Claim on “Olive Oil Polyphenols” (EC Regulation 432/2012). Separations 2022, 9, 351. [Google Scholar] [CrossRef]

| N. | Acidity (%) | PV (mEqO2 kg−1) | C18:1/C18:2 | PCs-HPLC (g kg−1) | PCs-FC (g kg−1) | FB BB (Abs) | CDAC (mmol e− kg−1) | DPPH (% I) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.49 | 11 | 7.64 | 0.45 | 0.23 | 0.698 | 12 | 17 |
| 2 | 0.40 | 10 | 9.26 | 0.53 | 0.43 | 0.859 | 13 | 18 |
| 3 | 0.38 | 11 | 8.33 | 0.59 | 0.70 | 0.915 | 13 | 18 |
| 4 | 0.61 | 16 | 9.07 | 0.50 | 0.26 | 0.779 | 13 | 18 |
| 5 | 0.64 | 13 | 8.61 | 0.32 | 0.13 | 0.464 | 12 | 14 |
| 6 | 0.39 | 11 | 7.08 | 0.48 | 0.31 | 0.765 | 11 | 14 |
| 7 | 0.29 | 10 | 7.93 | 0.41 | 0.32 | 0.605 | 13 | 17 |
| 8 | 0.47 | 8 | 7.36 | 0.43 | 0.33 | 0.619 | 13 | 17 |
| 9 | 0.23 | 7 | 13.49 | 0.43 | 0.36 | 0.596 | 12 | 14 |
| 10 | 0.22 | 8 | 13.96 | 0.41 | 0.36 | 0.577 | 12 | 16 |
| 11 | 0.20 | 6 | 8.52 | 0.59 | 0.48 | 0.937 | 15 | 14 |
| 12 | 0.18 | 8 | 4.27 | 0.47 | 0.46 | 0.785 | 10 | 20 |
| 13 | 0.20 | 8 | 8.40 | 0.46 | 0.61 | 0.774 | 14 | 19 |
| 14 | 0.22 | 6 | 8.20 | 0.52 | 0.48 | 0.794 | 11 | 16 |
| 15 | 0.56 | 12 | 13.99 | 0.20 | 0.31 | 0.295 | 9 | 12 |
| 16 | 0.62 | 12 | 8.68 | 0.17 | 0.56 | 0.301 | 8 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siano, F.; Picariello, G.; Sammarco, A.S.; Celano, G.; Caruso, T.; Vasca, E. Evaluation of Novel Rapid Analytical Methods to Categorize Extra Virgin Olive Oil Based on the Coulometrically Determined Antioxidant Capacity and on the Spectrophotometric Assessment of Phenolic Compounds. Molecules 2023, 28, 3108. https://doi.org/10.3390/molecules28073108
Siano F, Picariello G, Sammarco AS, Celano G, Caruso T, Vasca E. Evaluation of Novel Rapid Analytical Methods to Categorize Extra Virgin Olive Oil Based on the Coulometrically Determined Antioxidant Capacity and on the Spectrophotometric Assessment of Phenolic Compounds. Molecules. 2023; 28(7):3108. https://doi.org/10.3390/molecules28073108
Chicago/Turabian StyleSiano, Francesco, Gianluca Picariello, Anna Sofia Sammarco, Giuseppe Celano, Tonino Caruso, and Ermanno Vasca. 2023. "Evaluation of Novel Rapid Analytical Methods to Categorize Extra Virgin Olive Oil Based on the Coulometrically Determined Antioxidant Capacity and on the Spectrophotometric Assessment of Phenolic Compounds" Molecules 28, no. 7: 3108. https://doi.org/10.3390/molecules28073108
APA StyleSiano, F., Picariello, G., Sammarco, A. S., Celano, G., Caruso, T., & Vasca, E. (2023). Evaluation of Novel Rapid Analytical Methods to Categorize Extra Virgin Olive Oil Based on the Coulometrically Determined Antioxidant Capacity and on the Spectrophotometric Assessment of Phenolic Compounds. Molecules, 28(7), 3108. https://doi.org/10.3390/molecules28073108










