Abstract
Olives are very rich in phenolic compounds with important health-promoting properties. The profile and content of phenols in olive pulp and virgin olive oil are strongly influenced by the fruit ripening degree, but little is known concerning the evolution of phenolic compounds in the seed. In this work, the phenolic composition of seed from Tuscan cultivars (Frantoio, Moraiolo, Leccino) was studied over maturation. Starting from each seed sample, a phenolic extract was prepared and analyzed by HPLC-DAD-MS. Nüzhenide and nüzhenide 11-methyl oleoside were by far the most abundant phenolic compounds; their content reached up to 46 g/kg in dry seeds, although this diminished in the final stage of fruit maturation. At the same time, the phenolic composition of the pulp was also characterized over the course of maturation, showing that oleuropein was by far the most abundant compound, with concentrations comparable to those of nüzhenide and nüzhenide 11-methyl oleoside in the seeds. Overall, the total amount of phenols in seed dry extracts was significant, reaching approx. 100 g/kg. The chemically characterized dry phenolic extracts from seeds could be used for future biological assays aimed at evaluating the potential bioactivities of these phytocomplexes.
1. Introduction
In recent years, much evidence has supported the important health beneficial effects of the phenolic compounds present in extra virgin olive oil (EVOO) and in related by-products [,,]. These compounds are mainly constituted by the secoiridoid derivatives, a class of phenolic compounds which is typical of Olea europaea L., but also by lignans and lower amounts of flavonoids, phenolic acids and phenolic alcohols [,,,,]. Due to the well-documented effects against inflammation, diabetes, cardiovascular and neurodegenerative diseases exerted by the different components extracted from different parts of the olive tree [,], the production and characterization of such extracts is required []. Among the different types of secondary metabolites from the olive tree (e.g., sterols, phenolic compounds, tocopherols, pigments, triterpenoids, hydrocarbons), phenolic compounds are the most widely studied, as they have shown the most promising health-promoting effects. This was clearly confirmed by observations of the in vivo protection from oxidative damage of low-density lipoproteins (LDL) exerted by olive oil phenolic compounds [,], leading the European Food Safety Authority (EFSA) to approve a health claim for olive oil polyphenols []. Other well-recognized health benefits attributed to phenols from Olea europaea L. have been described in the literature as antioxidant and anti-inflammatory properties, as well as the abilities to help in maintaining low levels of cholesterol, normal blood pressure and normal gastrointestinal tract function and to strengthen the immune system [].
In the context of the circular economy, based on the concept of re-using and valorizing by-products which are therefore no longer considered as waste materials, recovering value from the various olive oil production by-products is receiving more and more attention [,,]. According to the “zero waste” model, each residue of the olive oil production chain should be treated for use in traditional or innovative applications, e.g., as an energy source, compost, cosmetic, animal feed or nutraceutical ingredient for human consumption [,].
The olive fruit is constituted by an exocarp (i.e., the skin), a mesocarp (i.e., the pulp) and an endocarp (i.e., the pit) which is, in turn, constituted by the woody shell which encloses the seed []. The woody shell and the seed can be considered as a by-product of the production of VOO from de-stoned olives, but also of the pitted table olives [,]. It has been reported that specific machines are now available which are able to recover the entire seed from the whole endocarp after de-stoning olives []. The woody part is quite poor in phenolic compounds, and it is mainly used as a source of energy, while it has been reported that the seed is rich in phenolic compounds [].
Concerning seed components, some pioneer studies have highlighted beneficial healthy effects exerted by proteins extracted from olive seeds []. Other studies in the literature have shown some preliminary health-promoting effects exerted by nüzhenide, one of the main phenolic components in the seed [], or by phenolic extracts from olive seeds []. However, in order to highlight the possible advantages in recovering phenols from olive seeds, further detailed studies on their composition and biological activities are required.
In this sense, the first step is the acquisition of deeper knowledge of the phenolic profiles of olive seeds and the influence of the variety and ripening time. To date, only a few manuscripts in the literature have described the phenolic composition of Olea europaea L. seeds. Overall, it has been reported that the main phenolic compounds present in the seeds are bitter glucosides such as nüzhenide and nüzhenide-11-methyl oleoside. These molecules bear in their chemical structure at least two glucose moieties, which make them poorly liposoluble, and consequently, they are not extracted into the olive oil []. However, some authors have reported the presence of nüzhenide but not of nüzhenide-11-methyl oleoside [] while detected observed neither nüzhenide nor nüzhenide-11-methyl oleoside in olive seeds []. Furthermore, some studies have reported a clear prevalence of phenolic compounds in the glycosylated form [,,], while other authors have reported the presence of phenolic compounds other than secoiridoids and in non-glycosylated forms []. From a quantitative point of view, to the authors’ knowledge, there is a lack of data in the literature. In one manuscript, the authors only reported a total phenolic compounds content of 2.79 mg/g seeds, with no specification of the method used for quantitation and with no details on the amount of each phenol []. In another work [], only six molecules were detected, and these seemed to be the molecules that are typically found in the olive pulp rather than those associated with olive seed (the only exception was nüzhenide). In the work of Elbir et al. [], the total phenolic content of the whole stone was reported, albeit showing only the percentage chromatographic areas of the single detected phenols. In a study of the seeds from the olives of six Portuguese cultivars, considered at the optimum ripening degree according to the skin color, the secoiridoids extracted from seeds were identified as an oleuropein equivalent, i.e., nüzhenide 11-methyl oleoside was the most abundant compound in the Lentisca cultivar (reaching 16.1 gole/kg), followed by nüzhenide (12.2 gole/kg). The same work reported that olive seed phenols were almost all secoiridoids, with the exception of tyrosol []. These literature data make it necessary to clarify the composition of this part of the olive fruit. The phenolic composition of the olive seed is certainly of interest in efforts to define possible uses of this by-product. In the literature, only the phenolic compositions of olive seeds from a handful of different samples have been described, and no study has reported the evolution of the phenolic composition of olive seeds over the course of maturation of the fruits of different cultivars. To the best of the authors’ knowledge, only one study has examined the qualitative phenolic composition of olive seeds at different ripening stages, but no quantitative data were provided [].
With this in mind, the aim of this research was to determine the phenolic composition of the olive seeds of three Tuscan cultivars (i.e., Frantoio, Moraiolo, Leccino) over the course of maturation. To this end, fruit samples of the three cultivars were collected at different levels of ripeness in the period of 15 September–17 November 2020. Dry phenolic extracts of the seeds were produced, allowing us to determine the final yields and the phenolic profiles by HPLC-DAD-MS. Bearing in mind the possible uses of the dried extracts from seeds for future biological assays, the best mode of quantitation is also discussed.
2. Results and Discussion
The study mainly aimed to provide a detailed characterization of the phenolic profile of olive seeds, also evaluating its evolution during maturation in three typical Tuscan cultivars: Frantoio, Moraiolo and Leccino. The importance of this type of characterization is underlined by the well-documented health-promoting properties of the phenolic compounds in Olea europaea L.; these properties have already been widely investigated for the phenols from olive fruit pulp, virgin olive oil, olive leaves and olive oil production by-products [], but to date, they have not been thoroughly investigated for the phenols from the olive seed. As a further objective, we wanted to compare the phenolic concentrations in seeds with those in the whole fruit in order to provide useful information about the advantages of preparing phenolic extracts from the seed with respect to the whole fruit and to provide useful information for the use of the pit as a source of specific phenolic components. The steps toward these objectives have been:
- collecting samples of olive fruits of the three cultivars to evaluate the biodiversity and evolution during ripening of the phenolic contents of seeds;
- characterizing these samples in terms of yield with respect to the weight of the different parts of the fruit (Table 1);
 Table 1. For each of the collected olive fruit samples, the table reports the weights of 100 whole fruits and of the corresponding seeds, the percentage of the seed weight with respect to whole fruit, the pulp/stone ratio, the % yield of the phenolic extracts from seeds relative to the whole seed weight, and the fruit moisture content.
Table 1. For each of the collected olive fruit samples, the table reports the weights of 100 whole fruits and of the corresponding seeds, the percentage of the seed weight with respect to whole fruit, the pulp/stone ratio, the % yield of the phenolic extracts from seeds relative to the whole seed weight, and the fruit moisture content. - defining a protocol for the extraction of phenols from olive seeds that enables both the analysis of phenols by HPLC-DAD-MS and the preparation of a dried extract suitable for future biological assays;
- characterizing the phenolic extracts of the seed from both qualitative and quantitative standpoints;
- characterizing the phenolic profile of the pulp and comparing the results with those previously reported in the literature, and with those for seeds.
2.1. Characteristics of the Collected Olive Samples
The olive fruit samples of the three Tuscan cultivars were collected at seven sampling dates from September to November. Table 1 reports the composition of the fruits in terms of whole fruit weight, seed weight (also as % with respect to the whole fruit), pulp/stone ratio and moisture of the whole fruit. The table also reports the yield % of the phenolic extracts obtained from the seed samples.
Overall, the weight of 100 whole fruits increased over the course of ripening for all three cultivars, passing from 101.5 to 166.6 g (+64%) for the Frantoio cultivar, from 108.6 to 159.2 g (+46%) for the Moraiolo cultivar and from 106.0 to 136.2 g (+28%) for the Leccino cultivar. Concerning the data of the Leccino cultivar, the datum related to the sixth sampling point appears to be an outlier. The weight of the 100 seeds (which were those from the same 100 whole olives) showed different behavior for those of the three cultivars: it increased from 2.195 to 4.323 g (+97%) for the Frantoio cultivar, and from 2.515 to 4.418 g (+76%) for the Leccino cultivar. On the other hand, for the Moraiolo cultivar, it did not increase, passing from 3.150 g at the first sampling to 3.082 g at the last sampling, and fluctuating from a minimum of 2.790 g to a maximum of 3.395 g. Of course, this different behavior of the seed weigh resulted in a different behavior of the seed % weight, which showed an increasing trend for the Frantoio and Leccino cultivars and a decreasing trend for the Moraiolo cultivar. As for the pulp/stone ratio, an increasing trend was observed for the three cultivars, with comparable values for Frantoio (from 1.62 to 2.98) and Leccino (from 1.75 to 2.84) and higher values for Moraiolo (from 2.38 to 3.95). Finally, the moisture content increased for the three cultivars, albeit with slightly different trends: for Frantoio, it increased from 44.7% to 57.6%, with the highest increases observed at the second, sixth and seventh sampling points; for Moraiolo, it increased from 48.1% to 54.8%, with the increase having already largely occurred by the second sampling point (i.e., 53.3%); similarly, for Leccino, it increased from 48.0% to 56.8%, with the increase having similarly almost completely occurred by the second sampling point (i.e., 56.0%).
2.2. Preparation of the Dry Phenolic Extracts from the Olive Seeds
In order to prepare a phenolic extract from the seeds which would be useful both for HPLC analysis and for future biological tests, we defined a protocol, as illustrated in Figure 1. After some preliminary extraction trials, performed in a previous study, the EtOH:H2O 90:10 extractive solution was selected (this gave the same extraction yields as using lower percentages of EtOH (e.g., 70%), but the samples were more easily and quickly concentrated during vacuum evaporation), with an extractive ratio of 50 g of extractive solution for 1 g of the seed powder prepared as described in Section 3.3. In order to reduce the extraction time and to maximize yield, after some preliminary trials, the combined use of the Ultraturrax (2 min) and of the ultrasounds bath (20 min) was selected as the best extraction approach. After the separation of the liquid extract from the solid residue by centrifugation, the supernatant was defatted twice with n-hexane to remove any fatty residue, as this would hinder the solubility of the sample in the aqueous media commonly used for biological assays. In the successive step, the defatted extract was vacuum dried and recovered with water to a total of 10 mL. The aqueous phenolic extract so obtained was split into two aliquots: a first 1-mL aliquot to be used for the HPLC-DAD-MS analysis after centrifugation at 14,000 rpm, and a second 9-mL aliquot which was lyophilized, thus yielding a light-yellow dried extract (Figure 2). The dried extracts were easily pulverizable and maintained the same characteristics for at least 12 months after lyophilization, confirming their storability.
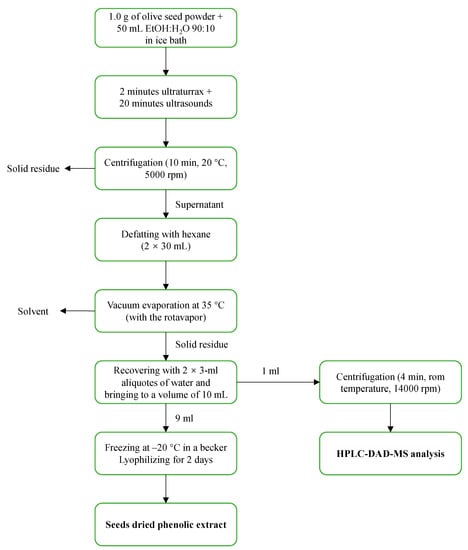
Figure 1.
Procedure for the preparation of dry phenolic extracts from seeds.

Figure 2.
Dried phenolic extract from the seeds of the Moraiolo cultivar at the fourth sampling point.
2.3. Characterization of the Phenolic Profile of Olive Seeds over the Course of Ripening
Table 2 reports the evolution over the course of ripening of the content of the molecules identified in olive seeds of the three cultivars, Frantoio (Table 2A), Moraiolo (Table 2B) and Leccino (Table 2C), based on UV and mass spectra analyses and literature data. An example of a chromatogram of the phenolic profile of seeds of the Moraiolo cultivar is given in Figure 3. Overall, 19 molecules were tentatively identified, 16 of which bore at least one phenolic moiety in their chemical structure. The remaining three molecules (i.e., oleoside 11-methyl ester, oleoside 11-methyl ester isomer and bis(oleoside 11-methyl ester) glucoside) are constituted by residues of glucose and elenolic acid, typical non-phenolic residues of the secoiridoids phenols from Olea europaea L. Interestingly, the phenolic moiety of 15 out of the 16 phenolic molecules identified (i.e., all the tyrosol derivatives, including salidroside oleoside, nüzhenide and its derivatives, and ligstroside oleoside) was tyrosol, while only in the case of verbascoside was it hydroxytyrosol. This situation is very different for the other products from Olea europaea L. (e.g., olive fruit pulp, virgin olive oil, olive leaves and virgin olive oil production by-products), in which hydroxytyrosol derivatives usually prevail, or are at least present in comparable amounts with tyrosol derivatives [].

Table 2.
Evolution of the content of phenolic compounds in seeds of olive fruits of the (A) Frantoio, (B) Moraiolo and (C) Leccino cultivars. Results are expressed in mg/kg of seed. The RSD was <5% (values determined as a mean of five replicates of a mixture of different samples).
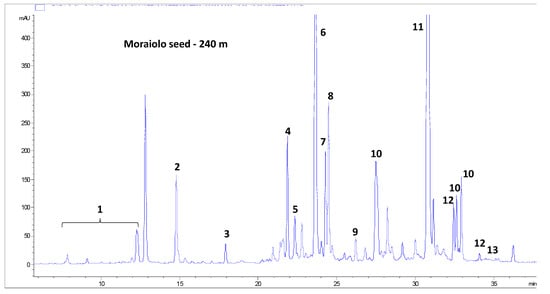
Figure 3.
Chromatographic profile at 240 nm of the phenolic compounds in seeds of the Moraiolo cultivar at the fourth sampling date. 1, tyrosol derivatives; 2, oleoside 11-methyl ester; 3, oleoside 11-methyl ester isomer; 4, nüzhenide derivative; 5, verbascoside; 6, nüzhenide; 7, bis(oleoside 11-methyl ester) glucoside; 8, salidroside oleoside; 9, nüzhenide isomer; 10, nüzhenide di-(11-methyl oleoside) isomers; 11, nüzhenide 11-methyl oleoside; 12, nüzhenide di-(11-methyl oleoside) isomer 1; 13, nüzhenide di-(11-methyl oleoside) isomer 1; 14, ligstroside oleoside.
For all the three cultivars, two phenolic molecules were largely prevalent in the phenolic profile of the olive seeds: nüzhenide and nüzhenide 11-methyl oleoside. This result is in agreement with the literature [,,,]. The UV spectrum of these molecules presents a high absorption at 240 nm but a low absorption (or no absorption, as in the case of nüzhenide 11-methyl oleoside) at 280 nm (Figure 4).
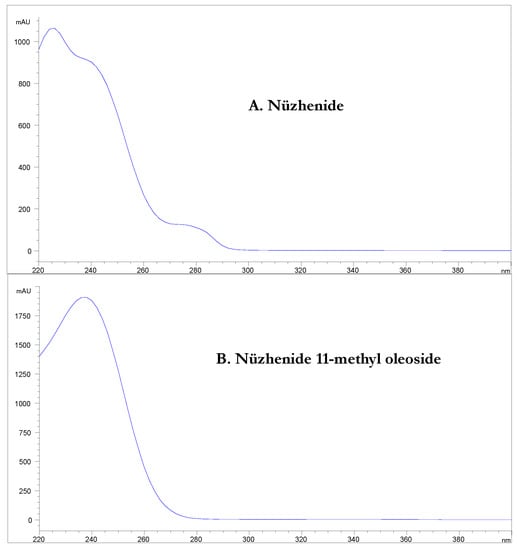
Figure 4.
UV-Vis spectrum of (A) nüzhenide and (B) nüzhenide 11-methyl oleoside.
For this reason, in order to be as reliable as possible, the quantitation of nüzhenide and its derivatives was performed using the calibration line built with the nüzhenide commercial standard at 240 nm. To the best of the authors’ knowledge, this manuscript is the first report in which the typical nüzhenide derivatives present in the olive seeds of specific cultivars over the course of maturation are quantitated with this approach. Nüzhenide 11-methyl oleoside was the most abundant molecule in all three cultivars, with values of up to 42,976 mg/kg for Frantoio, 46,531 mg/kg for Moraiolo and 42,597 mg/kg for Leccino, followed by nüzhenide, with values of up to 32,112 mg/kg for Frantoio, 20,919 mg/kg for Moraiolo and 30,491 mg/kg for Leccino. These data indicated that the highest concentration of nüzhenide 11-methyl oleoside was in the Moraiolo samples, the same samples in which the concentration of nüzhenide was the lowest, indicating a different relative concentration of these two molecules in the olive seeds of the cultivars analyzed in this study.
Concerning changes over the course of ripening, the concentration of nüzhenide 11-methyl oleoside increased in all three cultivars from the first to the second/third sampling date and then decreased, particularly at the last sampling date. Nüzhenide showed a trend similar to that of nüzhenide 11-methyl oleoside for Frantoio and Leccino, while in Moraiolo, it slightly decreased from the first to the fifth sampling date, with a final sharp decrease at the two last sampling points (Table 2A–C). Overall, a rather trend for Moraiolo with respect to Frantoio and Leccino was observed; this was also confirmed by the nüzhenide 11-methyl oleoside/nüzhenide ratio, which ranged from 1.20 to 1.49 for Frantoio, 1.39 to 1.93 for Leccino and 1.75 to 2.34 for Moraiolo.
Based on our data, to obtain olive seed extracts with the highest concentration of nüzhenide 11-methyl oleoside, olives of the Moraiolo cultivar should be harvested. For extracts with the highest concentration in nüzhenide, olives of the Frantoio or Leccino cultivars should be chosen. In both the cases, the highest amounts were from fruits harvested in the first part of October.
As for total phenolic compounds, the highest content during the first five sampling dates was observed in Leccino cv, followed by Frantoio and Moraiolo. This higher content was mainly due to the concentrations of nüzhenide 11-methyl oleoside isomers, which were higher in Leccino than in Moraiolo or Frantoio. At the last two sampling dates, the decrease in the main phenols was sharper in Leccino than in Moraiolo or Frantoio; therefore, on these two dates, the cultivar with the highest phenolic concentration was Frantoio.
Concerning molecules other than nüzhenide and nüzhenide 11-methyl oleoside, isomers of these two molecules showed the highest concentrations, with values ranging from a minimum range of 434–699 mg/kg for nüzhenide isomer in Moraiolo (in agreement with the lowest concentration of nüzhenide in this cultivar) to a maximum of 5229–10,138 mg/kg for nüzhenide 11-methyl oleoside isomer 1 in Leccino. Concerning tyrosol derivatives, their concentration was higher in Frantoio and Leccino than in Moraiolo, while the opposite behavior was observed for salidroside oleoside, which is still a tyrosol derivative. It could be hypothesized that some cultivars accumulate tyrosol in certain forms while others do so in other forms. Ligstroside oleoside was only present in low amounts in the samples from the Leccino variety at the first four sampling dates; isomers of nüzhenide di-(11-methyl oleoside) were present in low concentrations, with slightly increasing trends, mainly for Frantoio and Leccino. Verbascoside showed the highest concentrations in the Frantoio cultivar, with values up to 2080 mg/kg, followed by Leccino (up to 1745 mg/kg), while in Moraiolo, the values ranged from 321 to 787 mg/kg.
The data reported in Figure 5 highlight very high percentages of phenols in the dried seeds extracts, i.e., ranging from 45.5% for Moraiolo (M5) to 66.3% for Leccino (L5). These high phenolic percentages are driven by the abundance of two complex secoiridoids, i.e., as nüzhenide 11-methyl oleoside and nüzhenide, and by minor amounts of a number of other secoiridoids.
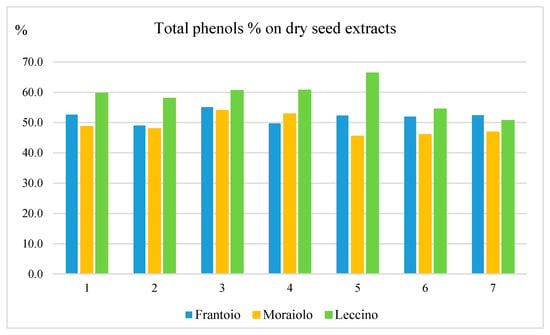
Figure 5.
Percentage of total phenols in dry seed extracts for the three cultivars over the course of ripening. In the abscissa, the ripening period is indicated.
2.4. Phenolic Profile of the Olive Pulp
As a final step of this work, we characterized the profile of the typical phenols present in olive pulp in the same samples used for seeds analysis. This step was aimed to complete the phenolic characterization of the collected samples, to compare the total content of phenols in the seeds with that of the typical phenols of the pulp and to compare the phenolic content of these samples with those analyzed over the course of ripening for the same cultivars in a previous study [].
Table 3 reports the evolution over the course of ripening of the content of the molecules identified in olive pulps of the three cultivars, i.e., Frantoio (Table 3A), Moraiolo (Table 3B) and Leccino (Table 3C). Overall, 11 phenolic molecules were quantitated; oleuropein was by far the most abundant compound for the samples of the three cultivars. For Frantoio, the values decreased from 44,565 to 18,520 mg/kg over the course of ripening; for Moraiolo, the values increased from 23,056 mg/kg at the first sampling date to 40,597 mg/kg at the third, and then decreased to 33,262 mg/kg; for Leccino, this value ranged from 38,273 to 43,352 mg/kg in the first three sampling dates and then constantly decreased to 13,258 mg/kg. Overall, these values were slightly greater than those of the olive fruit samples harvested from the same field in a previous study []. Concerning the other molecules, the amounts were at least one order of magnitude lower than those of oleuropein over the entire ripening period, with only a few exceptions, as follows. Demethyloleuropein, which was initially absent, increased over time in fruit samples of the three cultivars, but with different trends: for the Frantoio cultivar, this compound was detected starting from the second sampling date; its abundance constantly increased thereafter, reaching values of 13,286 mg/kg, i.e., not much lower than those of oleuropein (18,520 mg/kg). For Moraiolo, this compound was detected only from the fifth sampling date, reaching values of up to 3610 mg/kg at the last sampling point, i.e., an order of magnitude lower than the value for oleuropein (33,262 mg/kg). In contrast, for Leccino, demethyloleuropein was detected starting from the third sampling date, reaching values of 21,053 mg/kg at the last sampling date, i.e., even higher than oleuropein (13,258 mg/kg) (Table 3A–C). This behavior is similar to what we observed in a previous study using samples from the same cultivars [], confirming that demethyloleuropein is a degradation product of oleuropein, due to the action of endogenous esterase, and that it is cultivar dependent. Indeed, in both studies, the final values of demethyloleuropein were the lowest for Moraiolo and the greatest for Leccino. Another molecule that reached significant amounts was comselogoside. In the Frantoio samples, it decreased from 5112 mg/kg to 1223 mg/kg; in those of Leccino, it increased from 770 to 3721 mg/kg at the second sampling point and then constantly decreased to approx. 2000 mg/kg, while in the samples of Moraiolo, it increased from 1009 to 7677 mg/kg at the fourth sampling point and then decreased to 5376 (Table 3A–C). We do not want to speculate about the way this molecule is formed but, based on these data, we hypothesize that in the Moraiolo cultivar, it is likely synthesized via a diverse enzymatic activity.

Table 3.
Evolution of the content of phenolic compounds in the pulp of whole lyophilized olive fruits of (A) Frantoio cultivar, (B) Moraiolo cultivar, (C) Leccino cultivar. Results are expressed in mg/kg of whole dried olives. The RSD was <5% (values determined as a mean of five replicates of a mixture of different samples).
Concerning the comparison among pulp and seed, the total concentration of phenols was comparable (Table 2 and Table 3), with values decreasing from 120,675 to 61,326 mg/kg in fruits of Frantoio and from 104,067 to 66,567 mg/kg in fruits of Leccino but increasing from 74,749 to 92,979 mg/kg at the fourth sampling date and then decreasing to 78,861 mg/kg at the end of sampling date in fruits of the Moraiolo cultivar. These findings disagree with previous works, which stated that phenolic compounds were present in the seeds in concentrations well below those in other tissues []. Our data also indicated that the content of the main phenolic compounds in the olive pulp (i.e., oleuropein) was comparable to those of nüzhenide and nüzhenide 11-methyl oleoside in the seeds of the same samples (Table 2).
3. Materials and Methods
3.1. Chemicals
A Milli-Q-system (Millipore SA, Molsheim, France) was used to produce deionized water. Acetonitrile (HPLC-MS grade) was purchased from Panreac (Barcellona, Spain). Formic acid, hexane, methanol and ethanol were purchased from Merck (Darmstadt, Germany). The commercial standard of tyrosol was from Merck (Darmstadt, Germany), whereas those of oleuropein, nüzhenide, luteolin-7-O-glucoside, rutin, and verbascoside were from Extrasynthese Corporation (Genay, France).
3.2. Olive Fruit Samples Collection
Olive fruit samples of three typical Tuscan cultivars were harvested during ripening in the 2020 olive oil campaign (first sampling date: 9 September; last sampling date: 17 November).
For each of the three cultivars, 10 olive plants were selected at the Società Agricola Buonamici (Fiesole, Firenze) before the first sampling date. For each cultivar and for each of the sampling dates reported in Table 4, approx. 600 g of olive fruits were manually harvested from 10 selected plants. Olives were randomly harvested along the whole circumference of all the plants at a height close to 150–190 cm. Immediately after arriving in the laboratory, each olive sample was split into two aliquots, which were treated as described in the following paragraphs.

Table 4.
The olive fruit samples of three Tuscan cultivars collected over the course of ripening in the 2020 crop season.
3.3. Pulp/Stone Ratio, Seed Yield and Lyophilization of the Whole Fruit
For each sample, a first aliquot of 100 olive fruits, randomly selected from the whole sample, was weighed. The olives were treated using a laboratory destoner (Toscana Enologica Mori, Tavarnelle Val di Pesa, Firenze, Italia), thus separating the stone from the pulp. The stones, which remained unbroken after pulp separation, were weighed, and the pulp/stone ratio was calculated as follows:
where P/S is the pulp/stone ratio, m100wf is the mass of 100 whole fruits before destoning and m100s is the mass of 100 stones.
In the following step, the 100 stones were lyophilized after freezing using liquid nitrogen. The lyophilized stones were then manually broken using a hammer, thus obtaining the olive seeds. The 100 seeds thus obtained were weighed and the percentage mass of the seeds with respect to the whole olive fruit was calculated. The seeds were then minced using a M20 Universal Mill (IKA-Werke Corporation, Staufen, Germany), and the obtained powder was used to prepare the phenolic extracts to be used for the HPLC-DAD-MS analysis as described in the following paragraphs.
A further aliquot of each olive fruit sample was lyophilized according to the method described in a previous study []. The lyophilized olives were crushed in a laboratory miller (Toscana Enologica Mori, Tavarnelle Val di Pesa, Firenze, Italia), and the obtained olive paste was used to characterize the phenolic composition of the olive samples, as described in the following paragraphs.
3.4. Preparation of Phenolic Extracts from Olive Seeds
Phenolic compounds were extracted from olive seed powder using an extraction procedure suitable for both HPLC-DAD-MS analysis of phenolic compounds and for the preparation of dried extracts to be used for biological tests (which beyond the score of this manuscript) in the future steps of this study.
Approx. 1 g of seed powder was added to 50 mL EtOH:H2O 90:10. The obtained mixture was first cold extracted with the aid of an Ultraturrax (2 min) and then with the aid of an ultrasound bath (20 min). Next, the mixture was centrifuged for 10 min at 20 °C and 5000 rpm. The supernatant was defatted twice with 30 mL of hexane and transferred into a 250-mL flask. The solvent was vacuum evaporated at 35 °C, and then the residue was recovered with three 2-mL aliquots of water with the aid of ultrasounds and transferred in a 10-mL volumetric flask, which was subsequently brought to volume. An aliquot of 1 mL of the solution was withdrawn, centrifuged for 4 min at room temperature and 14,000 rpm and immediately used for the HPLC-DAD-MS analysis. The remaining part (9 mL) was transferred into a previously weighed beaker, frozen at −20 °C and lyophilized for two days. The lyophilized extract was weighed to calculate the yield of seed phenolic extract. The dried extract was stored under vacuum for future biological assays (schematic in Figure 1).
3.5. Extraction of Phenolic Compounds from Lyophilized Olive Fruits
The olive pastes, obtained as described in Section 3.3, were used to characterize the phenolic composition of the whole olive fruit in order to make comparisons with the phenolic composition of olive fruits of the same cultivars from different crop seasons [] but also to compare the abundances of phenolic compounds in the seed and the whole fruit.
Phenolic compounds were extracted from the olive fruit pastes using the following extraction procedure. Approx. 1 g of olive paste was cold extracted twice with 30 mL EtOH:H2O 80:20 by mixing for 4 min with an Ultraturrax. After each extraction cycle, the mixture was centrifuged for 10 min at 0 °C and 5000 rpm, and the supernatant was recovered. The obtained phenolic extract was defatted twice with 30 mL of hexane, then the hydroalcoholic solvent was evaporated under vacuum at 35 °C. The residue was recovered with 8 mL of MeOH:H2O 80:20 and the obtained suspension was centrifuged for 4 min at room temperature and 14,000 rpm in order to remove the insoluble residue. The supernatant was immediately used for the HPLC-DAD-MS analysis.
3.6. HPLC-DAD-MS Analysis of Phenolic Extracts
A chromatographic analysis of phenolic compounds extracted from both seeds and whole olives was performed using a previously described method [] with slight modifications. The HPLC was a 1260 Infinity II LC System provided with two types of detectors: a Diode Array Detector (DAD) and a Mass Spectrometry Detector (MSD) equipped with an API-electrospray interface (InfinityLab LC/MSD) (both from Agilent, Santa Clara, CA, USA). The column was a Poroshell 120, EC-C18 (150 mm × 3.0 mm, 2.7 µm, from Agilent technology) which worked at a temperature of 26 °C and was safeguarded by a precolumn with the same stationary phase. The mobile phase was acetonitrile (A) and acidic H2O (formic acid, pH 3.2) (B). A multistep linear gradient was applied as follows: solvent A varied from 5% to 40% in the first 40 min, stayed at 40% for 5 min, then varied from 40% to 100% in 5 min; next, it stayed at 100% for three minutes before returning to 5% over 2 min, for a total analysis time of 55 min, followed by a post run reconditioning of 10 min. The flow rate was 0.4 mL min−1 and the injection volume was 2 µL. Chromatograms were recorded at 240, 280 and 350 nm. Regarding the MSD conditions, the ESI parameters were set as follows: nitrogen with a 10.5 L/min flow rate was used as a drying gas at a temperature of 350 °C; the pressure of the nebulizer was 1811 Torr; the capillary voltage was 3500 V. The acquisition was performed in an m/z range 150–2000 Th in full spectrum scan mode/negative ionization mode, applying the fragmentor at 200 V.
For the quantitative analysis, several calibration lines were built, using standards belonging to the chemical classes typical of the molecules identified in the analyzed phenolic extracts. In particular, they were: oleuropein (λ = 280 nm; linearity range 0–6.01 µg; R2 = 0.9985), nüzhenide (λ = 240 nm; 0–1.29 µg; R2 = 0.9999), luteolin-7-O-glucoside (λ = 280 nm; 0–2.79 µg; R2 = 0.9989), rutin (λ = 280 nm; 0–2.26 µg; R2 = 0.9997), tyrosol (λ = 280 nm; 0–1.22 µg; R2 = 1.0000) and verbascoside (λ = 280 nm; 0–1.98 µg; R2 = 0.9986). Tyrosol, hydroxytyrosol and their glycosylated derivatives were quantified using the calibration line of tyrosol and expressed as mgtyr/kg. Demethyloleuropein, ligstroside, oleuropein and their derivatives were quantified using the calibration line of oleuropein and expressed as mgole/kg. Nüzhenide, nüzhenide 11-methyl oleoside and their isomers and derivatives were quantified with the calibration line of nüzhenide and expressed as mgnuzh/kg. Rutin was quantified using the calibration line of rutin and expressed as mgrut/kg. Luteolin-7-O-glucoside was quantified using the calibration line of luteolin-7-O-glucoside and expressed as mglut/kg. Finally, verbascoside and comselogoside were quantified using the calibration line of verbascoside and expressed as mgverba/kg. All phenolic compounds were quantified at 280 nm with the exception of nüzhenide, nüzhenide 11-methyl oleoside and their isomers and derivatives, which do not absorb at 280 nm; consequently, these compounds were quantified at 240 nm.
3.7. Data Treatment
During the development phase of the method, one olive fruit sample and one olive seed sample (both constituted by a mixture of the available samples) were used to evaluate the precision, in terms of variability, of the quantitation of each phenolic compound in that matrix. To this end, the extraction and chromatographic analysis were repeated five times, and the obtained results were used to calculate the CV% of each phenol.
4. Conclusions
The work is a systematic study of olive seeds that sought to evaluate the biodiversity and the variability over time of the phenolic content in three cultivars. It provides, for the first time, the yields of the phenolic dry extracts of the seed and correlates the phenolic content in the pulp with that of the corresponding seed harvested at different ripening time. In all seed extracts, both nüzhenide and nüzhenide 11-methyl oleoside were consistently found to be the major phenolic compounds. Our results indicated that oleuropein, the main phenolic compound of the olive pulp, was present in amounts comparable to those of nüzhenide and nüzhenide 11-methyl oleoside in the seeds of the same samples. Knowledge of the correlation between the phenolic content in pulp and seed can help to evaluate the possibility of new uses of the whole fruit, and in particular, of the seed recovered from the pit, a by-product of the production of virgin olive oil, from de-stoned olives but also from pitted table olives. Finally, our study showed that the dried seed extracts are a rich source of total phenols, and in particular, of some complex secoiridoid compounds.
Author Contributions
Conceptualization, L.C. and N.M.; methodology, L.C., C.L. and B.Z.; formal analysis, L.C., G.G. and C.L.; investigation, G.G. and M.B.; resources, N.M.; data curation, L.C., G.G. and B.Z.; writing—original draft preparation, L.C., G.G. and N.M.; writing—review and editing, L.C., G.G. and N.M.; supervision, L.C., M.B. and N.M.; funding acquisition, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by AgriTech-PNRR MUR-M4C2, Investment 14 “National Research Centre for Agricultural Technologies”-AgriTech CUP HUB-B63D21015240004-CN2-Spoke 9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Scavone, F.; Bellumori, M.; Cecchi, L.; Nediani, C.; Maggini, N.; Sofi, F.; Giovannelli, L.; Mulinacci, N. Effects of an Olive By-Product Called Pâté on Cardiovascular Risk Factors. J. Am. Coll. Nutr. 2021, 40, 617–623. [Google Scholar] [CrossRef]
- De Bock, M.; Hodgkinson, S.; Curtfield, W.; Schlothauer, R.C. Methods and Uses of an Extract from Olive Leaf in Management of Type 2 Diabetes (WO/2014/038962); Patent Application Publication: Alexandria, VA, USA, 2014. [Google Scholar]
- Migliorini, M.; Cecchi, L.; Cherubini, C.; Trapani, S.; Cini, E.; Zanoni, B. Understanding degradation of phenolic compounds during olive oil processing by inhibitor addition. Eur. J. Lipid Sci. Technol. 2012, 114, 942–950. [Google Scholar] [CrossRef]
- Lopez-Biedma, A.; Sanchez-Quesada, C.; Delgado-Rodriguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Lavee, S. Changes in phenolic content of olive during maturation. Int. J. Food Sci. Technol. 1999, 34, 265–274. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1–2, 113–127. [Google Scholar] [CrossRef]
- Cecchi, L.; Innocenti, M.; Melani, F.; Migliorini, M.; Conte, L.; Mulinacci, N. New isobaric lignans from refined olive oils as quality markers for virgin olive oils. Food Chem. 2017, 219, 148–157. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Visioli, F.; Franco, M.; Toledo, E.; Luchsinger, J.; Willett, W.C.; Hu, F.B.; Martínez-González, M.A. Olive oil and prevention of chronic diseases: Summary of an International conference. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 649–656. [Google Scholar] [CrossRef]
- Olmo-Garcia, L.; Kessler, N.; Neuweger, H.; Wendt, K.; Olmo-Peinado, J.M.; Fernandez-Gutierrez, A.; Baessmann, C.; Carrasco-Pancorbo, A. Unravelling the distribution of secondary metabolites in Olea europaea L.: Exhaustive characterization of eight olive-tree derived matrices by complementary platforms (LC-ESI/APCI-MS and GC-APCI-MS). Molecules 2018, 23, 2419. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Marrugat, J.; et al. The effect of polyphenols in olive oil on heart disease risk factor: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- de la Torre-Carbot, K.; Chávez-Servìn, J.L.; Jauregui, O.; Castellote, A.I.; Lamuela-Raventòs, R.M.; Nurmi, T.; Poulsen, H.E.; Gaddi, A.V.; Kaikkonen, J.; Lopez-Sabater, M.C.; et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation, of plasma LDL. J. Nutr. 2010, 140, 501–508. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to polyphenols in olive oil and protection of LDL particles from oxidative damage. EFSA J. 2011, 9, 2033. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/2033.htm (accessed on 19 February 2023).
- Castillo-Luna, A.; Criado-Navarro, I.; Ledesma-Escobar, C.A.; Lopez-Bascon, M.A.; Priego-Capote, F. The decrease in the health benefits of extra virgin olive oil during storage is conditioned by the initial phenolic profile. Food Chem. 2021, 336, 127730. [Google Scholar] [CrossRef]
- Bartolomei, M.; Capriotti, A.L.; Li, Y.; Bollati, C.; Li, J.; Cerrato, A.; Cecchi, L.; Pugliese, R.; Bellumori, M.; Mulinacci, N.; et al. Exploitation of olive (Olea europaea L.) seed proteins as upgraded source of bioactive peptides with multifunctional properties: Focus on antioxidant and dipeptidyl-dipeptidase-IV inhibitory activities, and glucagon-like peptide 1 improved modulation. Antioxidants 2022, 11, 1730. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Influence of enzymes and technology on virgin olive oil composition. Crit. Rec. Food Sci. Nutr. 2017, 57, 3104–3126. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Garcia, J.; Granato, D.; Barros, A. Seed phytochemical profiling of three olive cultivars, antioxidant capacity, enzymatic inhibition, and effects on human neuroblastoma cells (SH-SY5Y). Molecules 2022, 27, 5057. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, A.; Marchegiani, D.; Pardi, D.; Contento, S.; Pardi, D.; Girardi, F.; Kotti, F. Evaluation of functional phytochemicals in destoned virgin olive oil. Food Bioprocess Technol. 2009, 2, 322–327. [Google Scholar] [CrossRef]
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jimenez-Lopez, J.; de Dios Alche, J. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019, 56, 4359–4370. [Google Scholar] [CrossRef]
- Silva, S.; Boross, P.; Soares, R.; Coelho, A.V.; Vilas Boas, L.; Bronze, M.R. Characterization of nuzhenide and related secoiridoids in Olea europaea L. seeds using MALDI-TOF mass spectrometry. In Proc. XXVIIIth IHC-IS on Emerging Health Topics in Fruits and Vegetables; Desjardins, Y., Ed.; Acta Hort.: Leuven, Belgium, 2012; Volume 939, ISHS 2012. [Google Scholar]
- Wang, Q.-Q.; Han, S.; Li, X.-X.; Yuan, R.; Zhuo, Y.; Chen, X.; Zhang, C.; Chen, Y.; Gao, H.; Zhao, L.-C.; et al. Nuezhenide exerts anti-inflammatory activity through the NF-kB pathway. Curr. Mol. Pharmacol. 2021, 14, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Alu’datt, M.H.; Alli, I.; Ereifj, K.; Alhamad, M.N.; Alsaad, A.; Rababeh, T. Optimisation and characterisation of various extraction conditions of phenolic compounds and antioxidant activity in olive seeds. Nat. Prod. Res. 2011, 25, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Elbir, M.; Es-Safi, N.E.; Amhoud, A.; Mbarki, M. Characterization of phenolic compounds in olive stones of three Moroccan varieties. Cienc. Y Tecnol. 2015, 17, 479–492. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitao, F.; Bronze, M.R.; Coelho, A.V.; Vilas Boas, L. Secoiridoids in olive seed: Characterization of nüzhenide and 11-methyl oleosides y liquid chromatography with diode array ad mass spectrometry. Grasas Y Aceites 2010, 61, 157–164. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Innocenti, M.; Mulinacci, N. Whole lyophilized olives as sources of unexpectedly high amounts of secoiridoids: The case of three Tuscan cultivars. J. Agric. Food Chem. 2015, 63, 1175–1185. [Google Scholar] [CrossRef]
- Cecchi, L.; Breschi, C.; Migliorini, M.; Canuti, V.; Fia, G.; Mulinacci, N.; Zanoni, B. Moisture in rehydrated olive paste affects oil extraction yield and phenolic compound content and profile of extracted olive oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1800449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).