Fermented Aronia melanocarpa Inhibits Melanogenesis through Dual Mechanisms of the PI3K/AKT/GSK-3β and PKA/CREB Pathways
Abstract
1. Introduction
2. Results
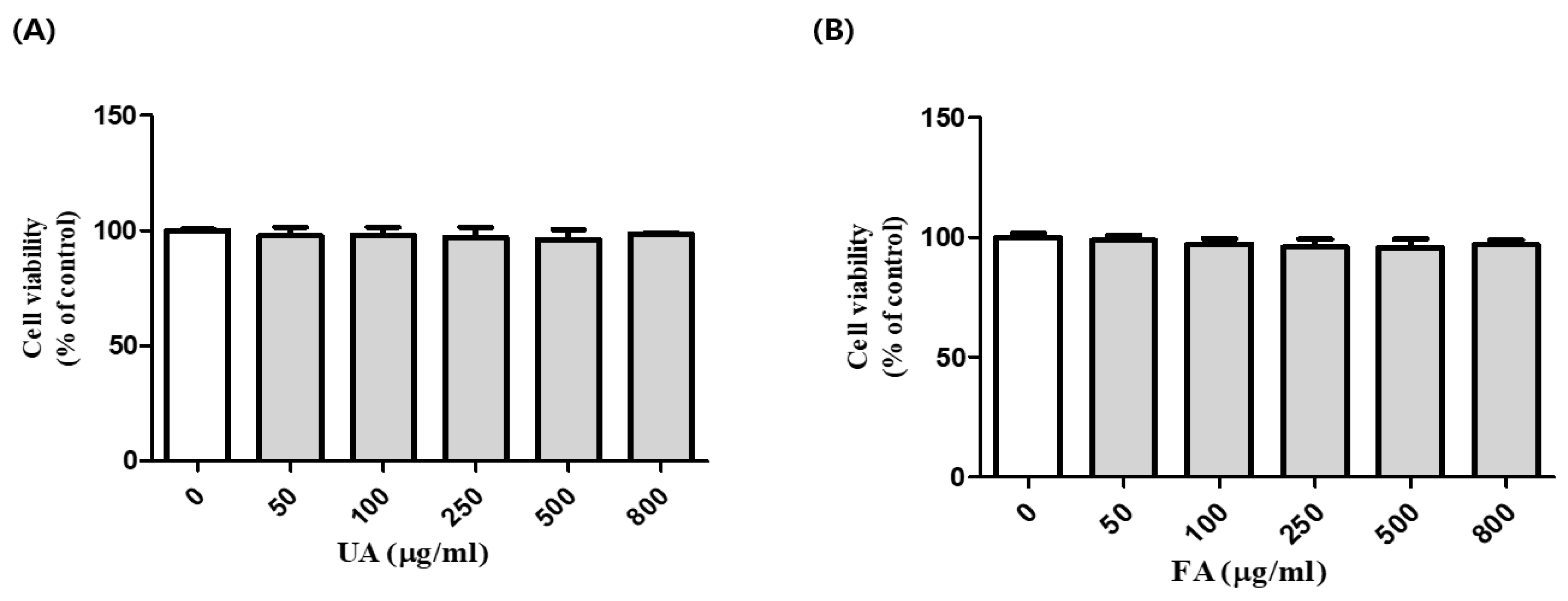
2.1. Cell Viability of B16F10 Cells with Unfermented Aronia Melanocarpa (UA) and Fermented Aronia melanocarpa (FA)
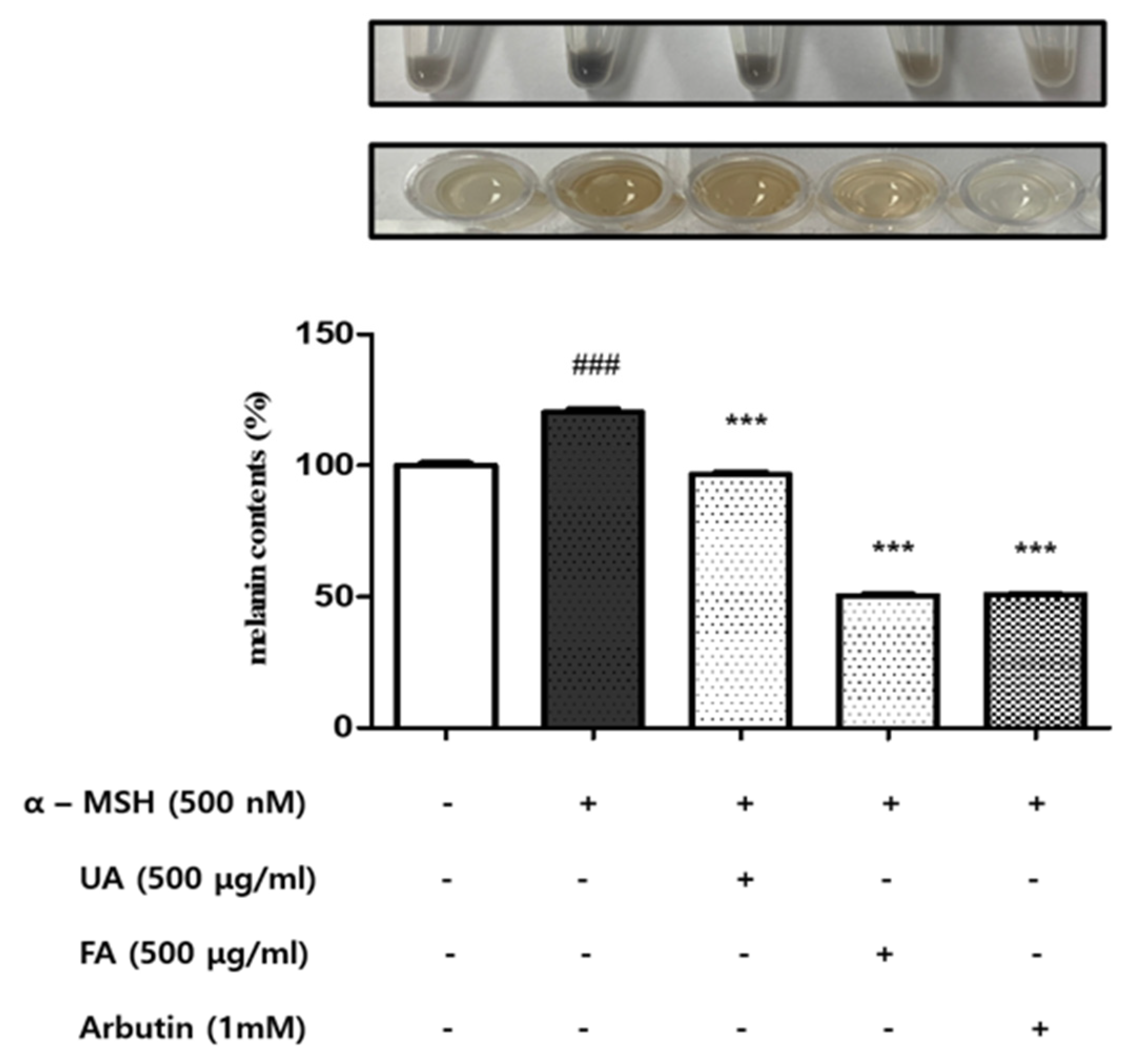
2.2. UA and FA Inhibited Melanin Biosynthesis in B16F10 Cells
2.3. UA and FA Inhibited Protein Expression Levels of Tyrosinase, TRP-1, TRP-2, and MITF in B16F10 Cells
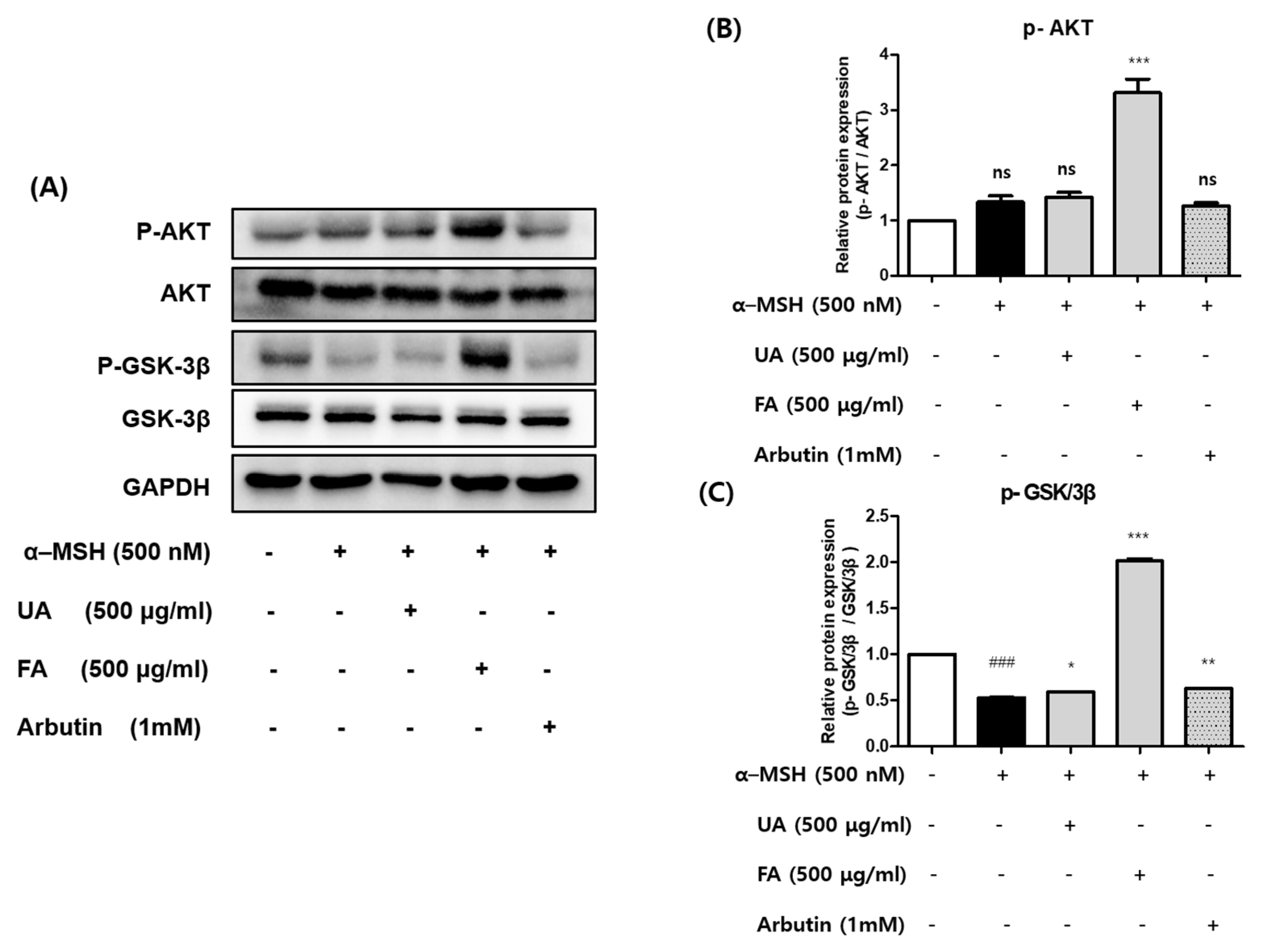
2.4. UA and FA Inhibited Melanogenesis by Upregulating p-AKT and p-GSK-3β
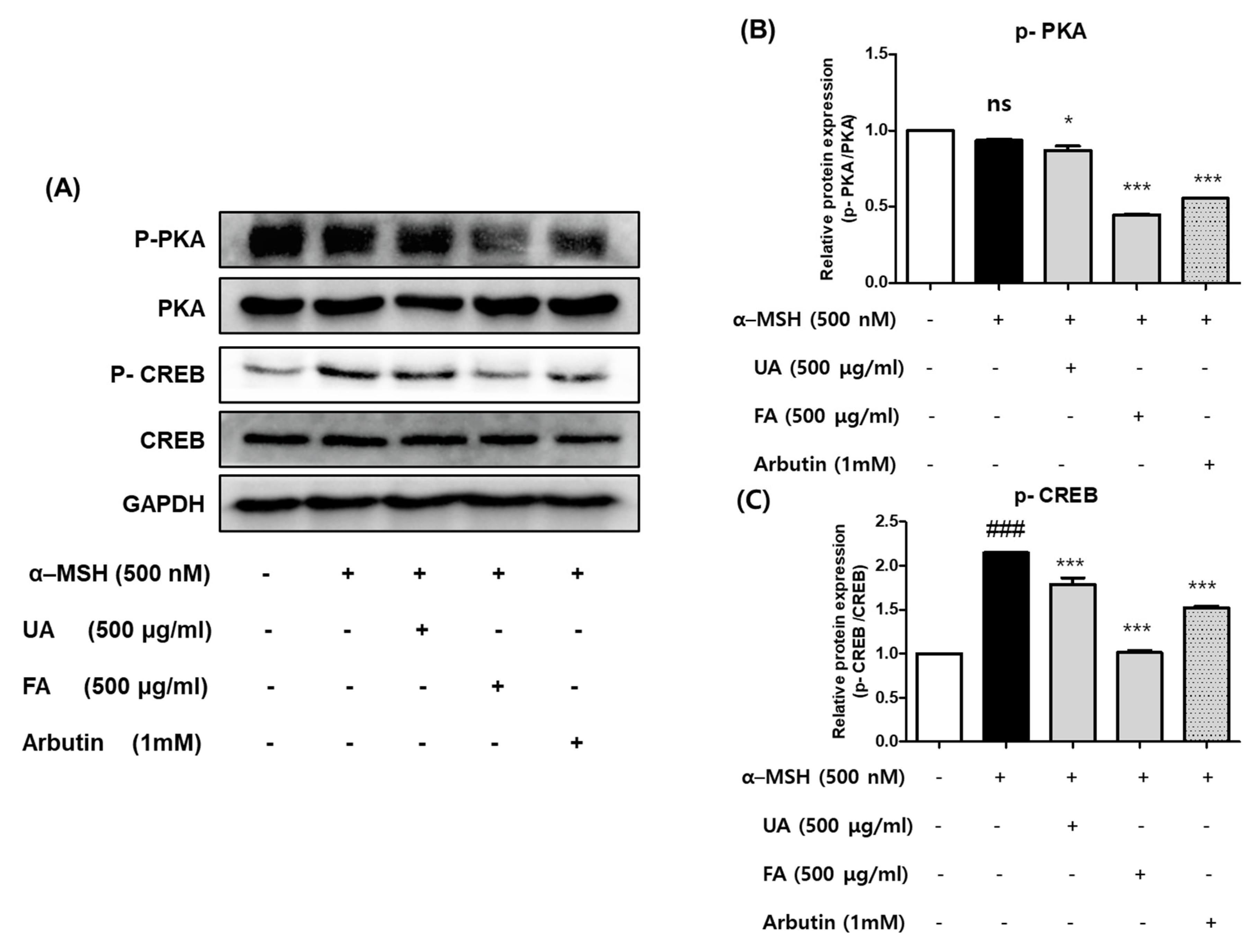
2.5. UA and FA Inhibited Melanogenesis by Downregulating p-PKA and p-CREB
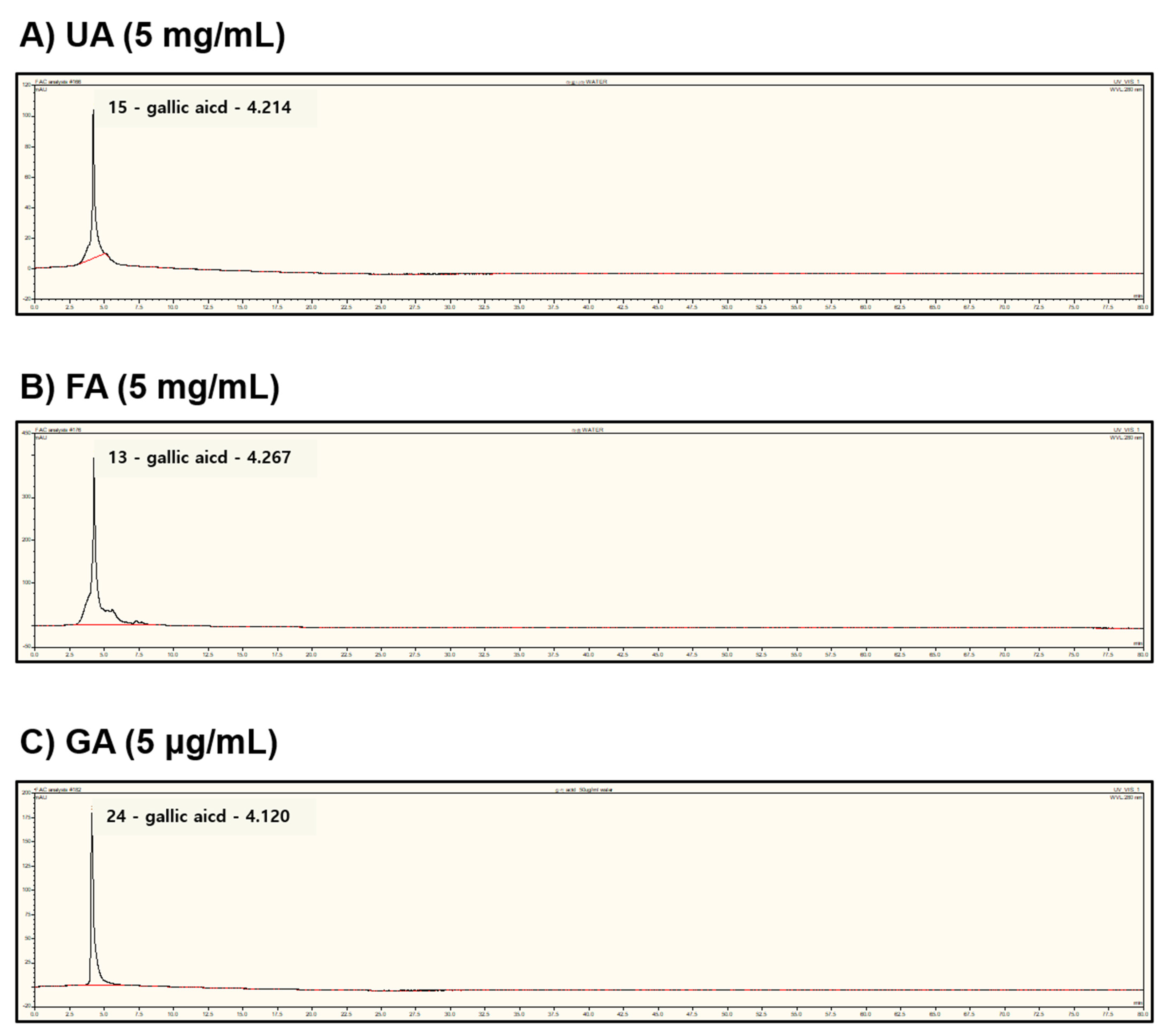
2.6. Fermentation of Monascus purpureus Increased Gallic Acid Content in Aronia melanocarpa
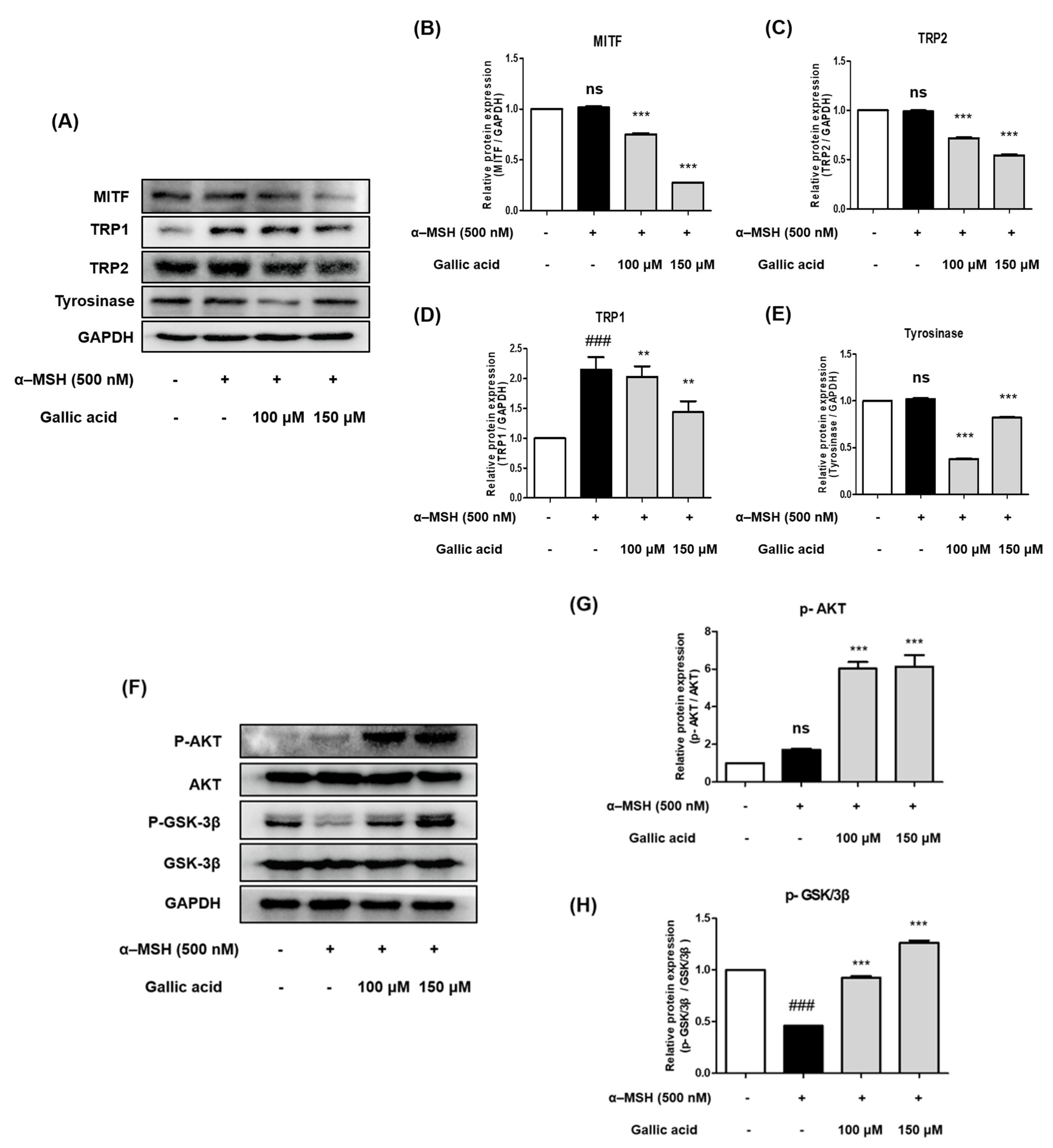
2.7. Gallic Acid Inhibited Protein Expression Levels of Tyrosinase, TRP-1, TRP-2, and MITF in B16F10 Cells
2.8. Gallic Acid Inhibited Melanogenesis by Upregulating p-AKT and p-GSK-3β
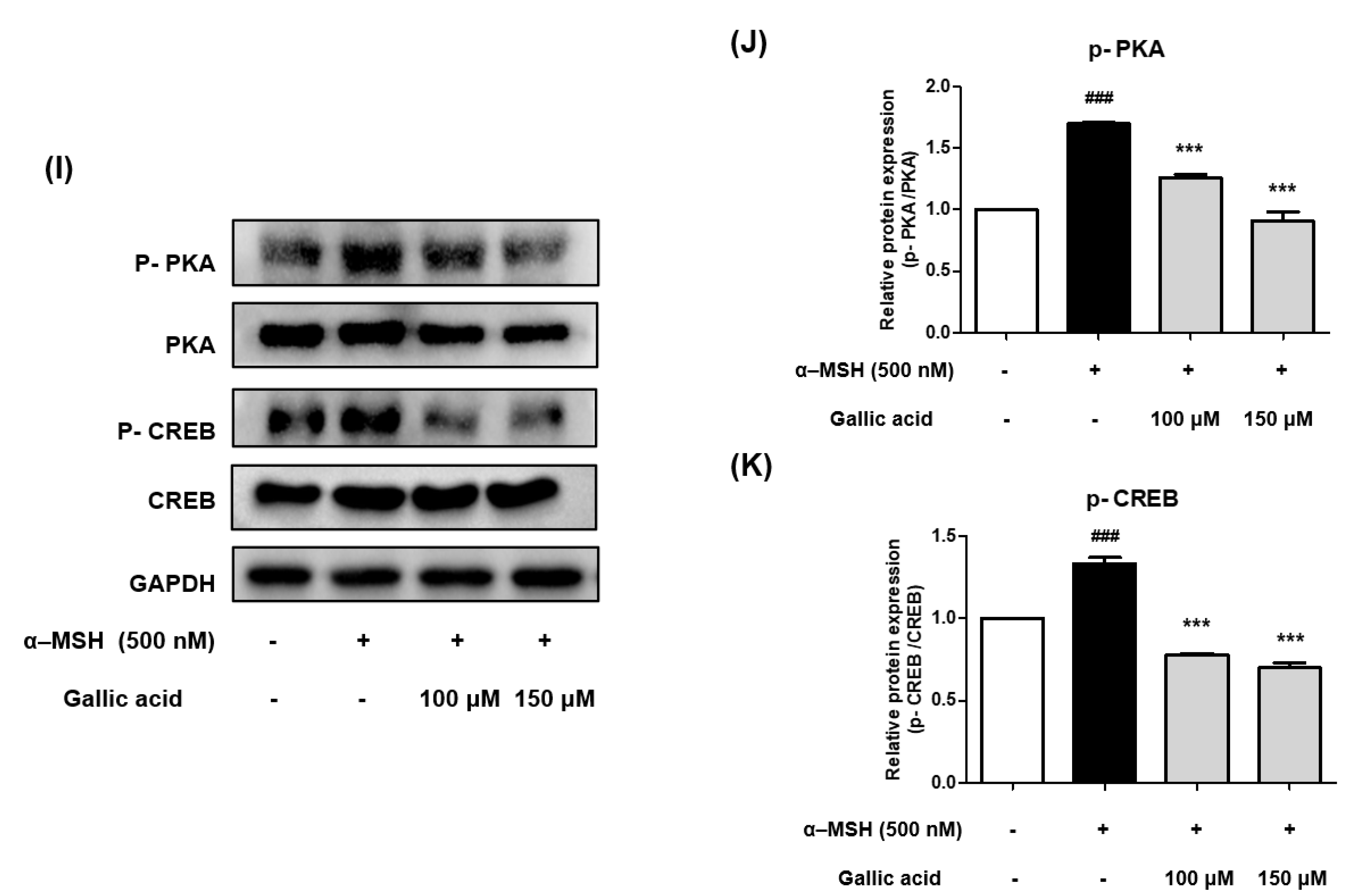
2.9. Gallic Acid Inhibited Melanogenesis by Downregulating p-PKA and p-CREB
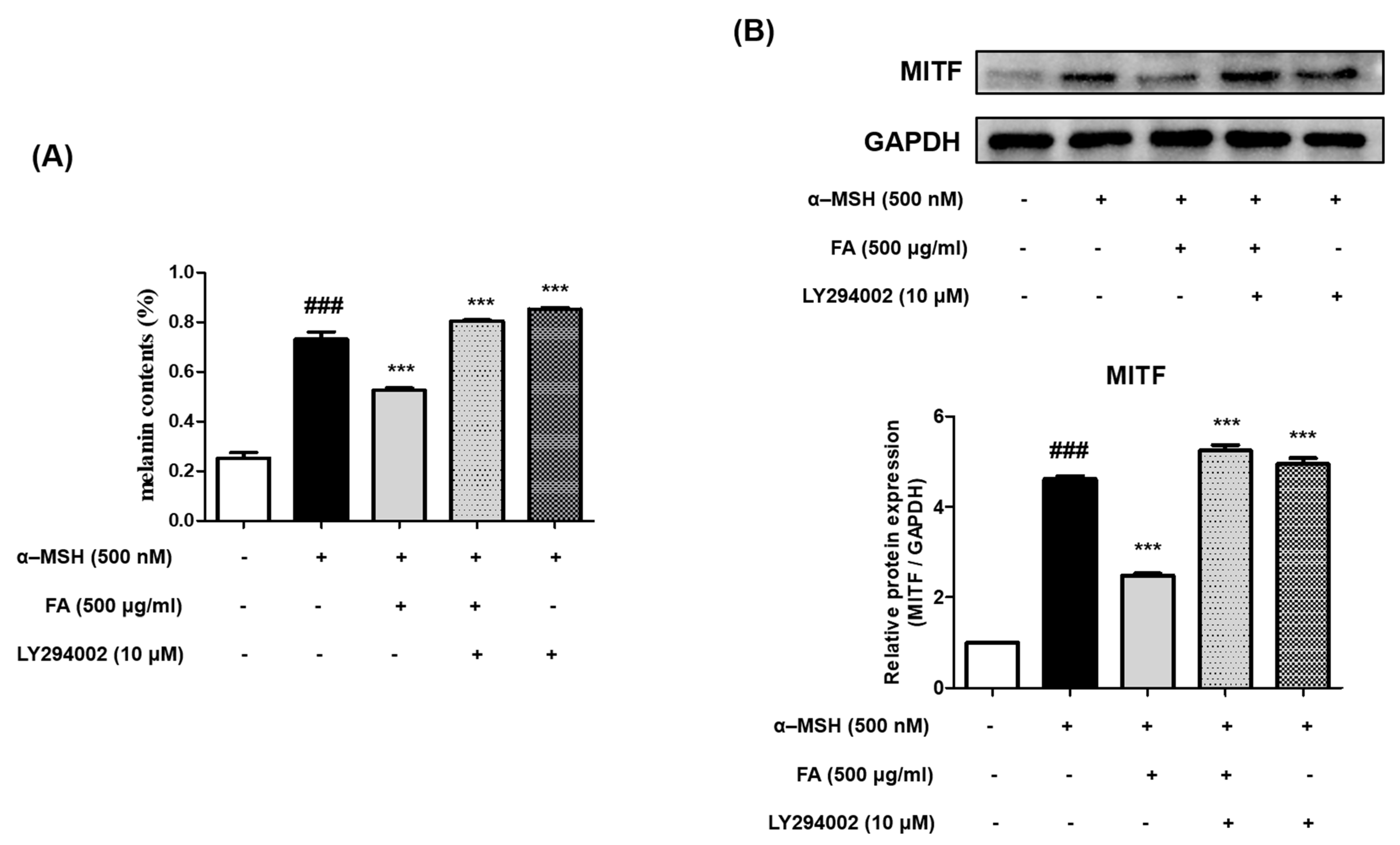
2.10. Inhibition of Melanogenesis by FA Was Associated with PI3K/AKT/GSK-3β Regulation
2.11. Inhibition of Melanogenesis by FA Was Associated with PKA/CREB Regulation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. Preparation of UA and FA
4.5. High-Performance Liquid Chromatography (HPLC) of Sample
4.6. Measurement of Melanin Content in B16F10 Cells
4.7. Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: Perspective of anti-pigmenting agents. Int. J. Mol. Sci. 2014, 15, 8293–8315. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Na, J.I.; Choi, J.Y.; Park, K.C. Melasma: Updates and perspectives. Exp. Dermatol. 2019, 28, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.P. Diagnosis and Management of Lentigo Maligna: Clinical Presentation and Comprehensive Review. J. Skin Cancer 2021, 2021, 7178305. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Hearing, V.J. Milestones in melanocytes/melanogenesis. J. Investig. Dermatol. 2011, 131, E1. [Google Scholar] [CrossRef]
- Yun, C.Y.; You, S.T.; Kim, J.H.; Chung, J.H.; Han, S.B.; Shin, E.Y.; Kim, E.G. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β-catenin/MITF pathways. J. Investig. Dermatol. 2015, 135, 1385–1394. [Google Scholar] [CrossRef]
- Feng, Z.C.; Riopel, M.; Popell, A.; Wang, R. A survival Kit for pancreatic beta cells: Stem cell factor and c-Kit receptor tyrosine kinase. Diabetologia 2015, 58, 654–665. [Google Scholar] [CrossRef]
- Li, P.H.; Liu, L.H.; Chang, C.C.; Gao, R.; Leung, C.H.; Ma, D.L.; DavidWang, H.M. Silencing stem cell factor gene in fibroblasts to regulate paracrine factor productions and enhance c-kit expression in melanocytes on melanogenesis. Int. J. Mol. Sci. 2018, 19, 1475. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Lee, T.H.; Lee, W.J.; Shim, W.S.; Yeo, E.J.; Kim, S.; Kim, S.Y. A novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI 3K and ERK pathways and by regulating Ca2+ influx via TRPM 1 channels. Pigment Cell Melanoma Res. 2016, 29, 81–91. [Google Scholar] [CrossRef]
- Feng, D.; Fang, Z.; Zhang, P. The melanin inhibitory effect of plants and phytochemicals: A systematic review. Phytomedicine 2022, 107, 154449. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.N.; Al Ramahi, R. Depigmentation and Anti-aging Treatment by Natural Molecules. Curr. Pharm. Des. 2019, 25, 2292–2312. [Google Scholar]
- Kanlayavattanakul, M.; Lourith, N. Plants and Natural Products for the Treatment of Skin Hyperpigmentation—A Review. Planta Med. 2018, 84, 988–1006. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic disease. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Rahmani, J.; Clark, C.; Kord Varkaneh, H.; Lakiang, T.; Vasanthan, L.T.; Onyeche, V.; Mousavi, S.M.; Zhang, Y. The effect of Aronia consumption on lipid profile, blood pressure, and biomarkers of inflammation: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1981–1990. [Google Scholar] [CrossRef]
- Kim, B.; Ku, C.S.; Pham, T.X.; Park, Y.; Martin, D.A.; Xie, L.; Taheri, R.; Lee, J.; Bolling, B.W. Aronia melanocarpa (chokeberry) polyphenol–rich extract improves antioxidant function and reduces total plasma cholesterol in apolipoprotein E knockout mice. Nutr. Res. 2013, 33, 406–413. [Google Scholar] [CrossRef]
- Rodriguez-Werner, M.; Winterhalter, P.; Esatbeyoglu, T. Phenolic composition, radical scavenging activity and an approach for authentication of Aronia melanocarpa berries, juice, and pomace. J. Food Sci. 2019, 84, 1791–1798. [Google Scholar] [CrossRef]
- Carletti, G.; Nervo, G.; Cattivelli, L. Flavonoids and Melanins: A common strategy across two kingdoms. Int. J. Biol. Sci. 2014, 10, 1159–1170. [Google Scholar] [CrossRef]
- Maack, A.; Pegard, A. Populus nigra (Salicaceae) absolute rich in phenolic acids, phenylpropanoïds and flavonoids as a new potent tyrosinase inhibitor. Fitoterapia 2016, 111, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Choi, M.; Sim, J.; Viji, M.; Li, S.; Lee, Y.H.; Kim, Y.; Seo, S.Y.; Zhou, Y.; Lee, K.; et al. Synthesis and biological evaluation of caffeic acid derivatives as potent inhibitors of α-MSH-stimulated melanogenesis. Bioorganic Med. Chem. Lett. 2017, 27, 3374–3377. [Google Scholar] [CrossRef] [PubMed]
- Bei, Q.; Chen, G.; Lu, F.; Wu, S.; Wu, Z. Improving free, conjugated, and bound phenolic fractions in fermented oats (Avena sativa L.) with Monascus anka. Food Chem. 2018, 245, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bei, Q.; Wu, Z.; Chen, G. Dynamic changes in the phenolic composition and antioxidant activity of oats during simultaneous hydrolysis and fermentation. Food Chem. 2020, 305, 125269. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wang, O.; Wang, M.; He, J.; Wang, Y.; Zhang, D.; Zhou, F.; Ji, B. In vitro inhibitory effect on pancreatic lipase activity of subfractions from ethanol extracts of fermented Oats (Avena sativa L.) and synergistic effect of three phenolic acids. J. Agric. Food Chem. 2012, 60, 7245–7251. [Google Scholar] [CrossRef]
- Liu, A.; Juan Chen, A.; Liu, B.; Wei, Q.; Bai, J.; Hu, Y. Investigation of citrinin and monacolin K gene clusters variation among pigment producer Monascus species. Fungal Genet. Biol. 2022, 160, 103687. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Lee, J.Y.; Park, J.; Park, S.N. An inhibitory mechanism of action of a novel syringic-acid derivative on α-melanocyte–stimulating hormone (α-MSH)-induced melanogenesis. Life Sci. 2017, 191, 52–58. [Google Scholar] [CrossRef]
- Zhou, S.; Sakamoto, K. Pyruvic acid/ethyl pyruvate inhibits melanogenesis in B16F10 melanoma cells through PI3K/AKT, GSK3beta, and ROS-ERK signaling pathways. Genes Cells 2019, 24, 60–69. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Bei, Q.; Shi, K.; Wu, Z. Fingerprint profiles of flavonoid compounds from different Psidium guajava leaves and their antioxidant activities. J. Sep. Sci. 2017, 40, 3817–3829. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.; Vani, M.G.; Wang, S.Y.; Liao, J.W.; Hsu, L.S.; Yang, H.L.; Hseu, Y.C. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013, 39, 259–270. [Google Scholar] [CrossRef]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Zheng, Y.Q.; Su, C.C.; Wu, Y.J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Seelam, S.D.; Agsar, D.; Shetty, P.R.; Vemireddy, S.; Reddy, K.M.; Umesh, M.K.; Rajitha, C.H. Characterization and photoprotective potentiality of lime dwelling Pseudomonas mediated melanin as sunscreen agent against UV-B radiations. J. Photochem. Photobiol. B 2021, 216, 112126. [Google Scholar] [CrossRef]
- Maddodi, N.; Jayanthy, A.; Setaluri, V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef]
- Fajuyigbe, D.; Young, A.R. The impact of skin colour on human photobiological responses. Pigment Cell Melanoma Res. 2016, 29, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Rzepka, Z.; Kowalska, J.; Banach, K.; Beberok, A.; Wrześniok, D. Molecular and Biochemical Basis of Minocycline-Induced Hyperpigmentation-The Study on Normal Human Melanocytes Exposed to UVA and UVB Radiation. Int. J. Mol. Sci. 2021, 22, 3755. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Miyamura, Y.; Coelho, S.G.; Schlenz, K.; Batzer, J.; Smuda, C.; Choi, W.; Brenner, M.; Passeron, T.; Zhang, G.; Kolbe, L.; et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res. 2011, 24, 136–147. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.J.; Kim, E.Y.; Park, J.E.; Jo, S.Y.; Bang, S.H.; Chang, E.J.; Chang, S.E. Microtubule-associated protein light chain 3 is involved in melanogenesis via regulation of MITF expression in melanocytes. Sci. Rep. 2016, 6, 19914. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Erickson, C.A. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development 2009, 136, 1849–1858. [Google Scholar] [CrossRef]
- Li, C.Y.; Wang, Q.; Shen, S.; Wei, X.L.; Li, G.X. Oridonin inhibits migration, invasion, adhesion and TGF-β1-induced epithelial-mesenchymal transition of melanoma cells by inhibiting the activity of PI3K/Akt/GSK-3β signaling pathway. Oncol. Lett. 2018, 15, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, J.H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, Y.R.; Park, J.H.; Ahn, E.K.; Jeong, W.; Shin, H.S.; Kim, M.S.; Yang, S.H.; Oh, J.S. Anti-melanogenic and anti-oxidant activities of ethanol extract of Kummerowia striata: Kummerowia striata regulate anti-melanogenic activity through down-regulation of TRP-1, TRP-2 and MITF expression. Toxicol. Rep. 2018, 6, 10–17. [Google Scholar] [CrossRef]
- Borowska, S.; Brzoska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, G.S.; Park, S.; Kim, Y.H.; Kim, M.B.; Lee, W.S.; Jeong, S.W.; Lee, S.J.; Jin, J.S.; Shin, S.C. Determination of chokeberry (Aronia melanocarpa) polyphenol components using liquid chromatography-tandem mass spectrometry: Overall contribution to antioxidant activity. Food Chem. 2014, 146, 1–5. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Shin, D.W.; Lim, B.O. Fermented Aronia melanocarpa Inhibits Melanogenesis through Dual Mechanisms of the PI3K/AKT/GSK-3β and PKA/CREB Pathways. Molecules 2023, 28, 2981. https://doi.org/10.3390/molecules28072981
Kim DH, Shin DW, Lim BO. Fermented Aronia melanocarpa Inhibits Melanogenesis through Dual Mechanisms of the PI3K/AKT/GSK-3β and PKA/CREB Pathways. Molecules. 2023; 28(7):2981. https://doi.org/10.3390/molecules28072981
Chicago/Turabian StyleKim, Da Hee, Dong Wook Shin, and Beong Ou Lim. 2023. "Fermented Aronia melanocarpa Inhibits Melanogenesis through Dual Mechanisms of the PI3K/AKT/GSK-3β and PKA/CREB Pathways" Molecules 28, no. 7: 2981. https://doi.org/10.3390/molecules28072981
APA StyleKim, D. H., Shin, D. W., & Lim, B. O. (2023). Fermented Aronia melanocarpa Inhibits Melanogenesis through Dual Mechanisms of the PI3K/AKT/GSK-3β and PKA/CREB Pathways. Molecules, 28(7), 2981. https://doi.org/10.3390/molecules28072981






