Selenized Chickpea Sprouts Hydrolysates as a Potential Anti-Aging Ingredient
Abstract
1. Introduction
2. Results
2.1. Protein and Selenium Content in Chickpea Sprouts Flour
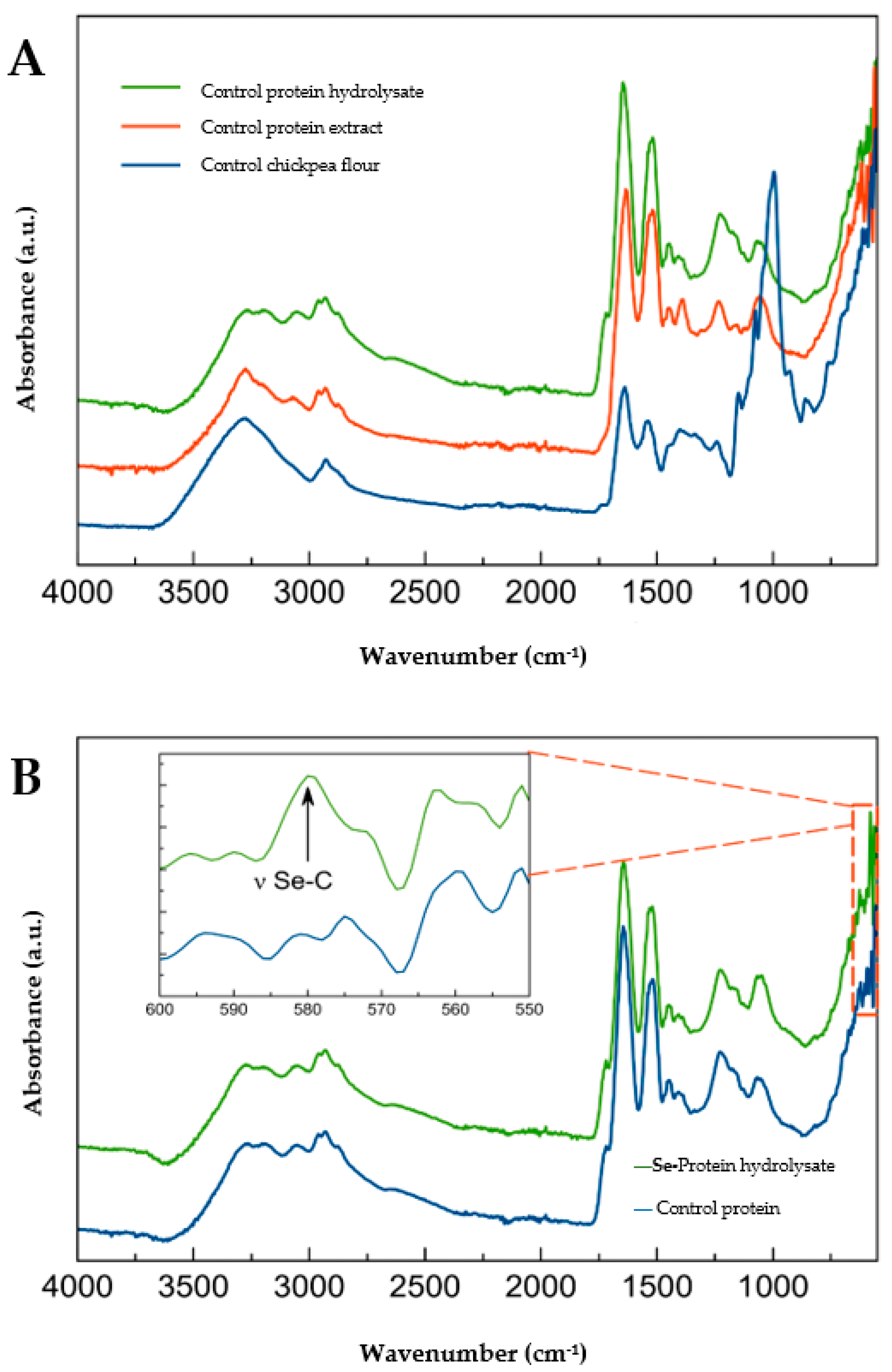
2.2. Protein Structure by FTIR Spectroscopy
2.3. Enzymatic Hydrolysis and Screening of Antioxidant Activity of Selenized Protein Hydrolysates
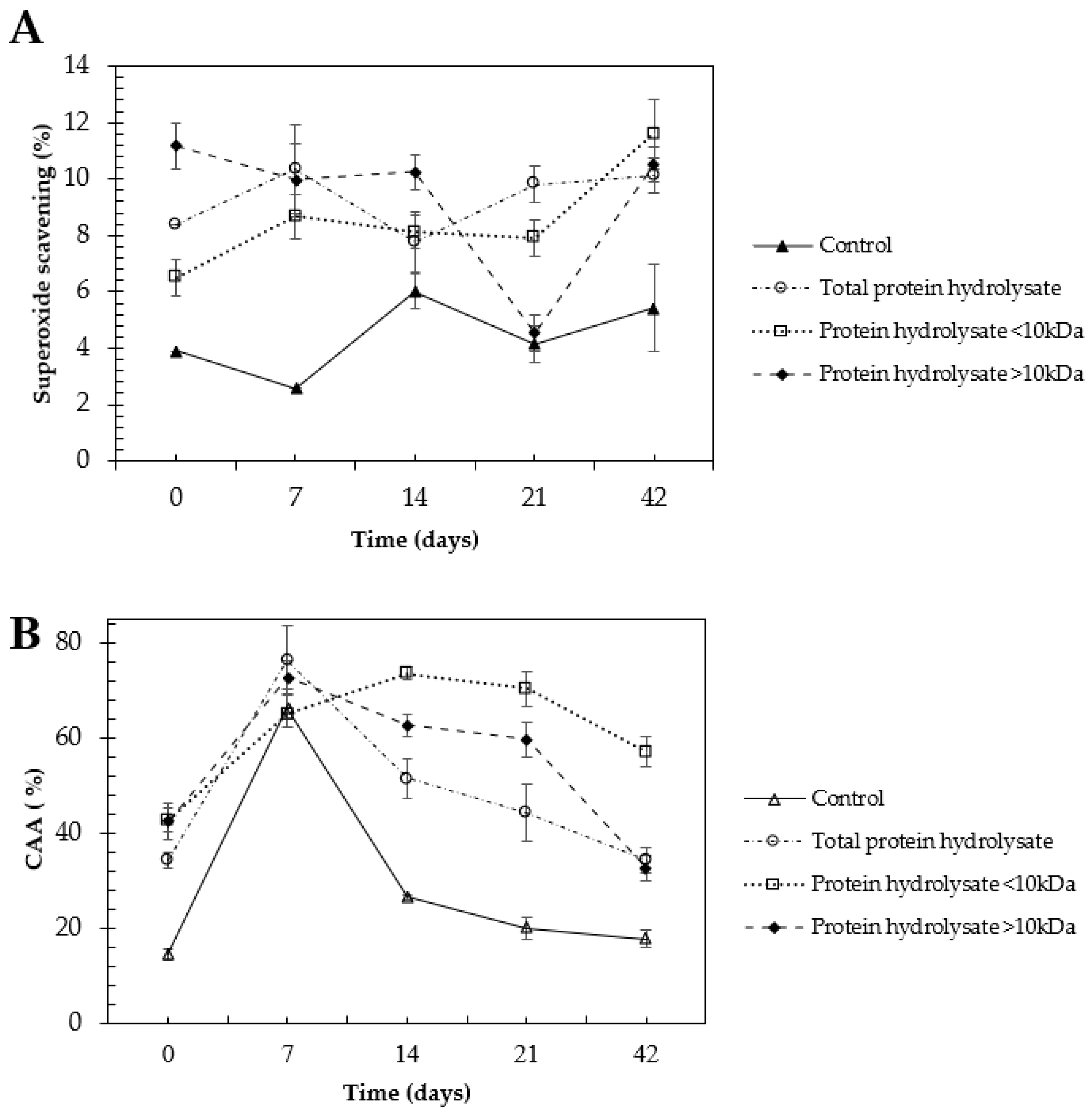
2.4. Selenized Hydrolysates Stability through Time
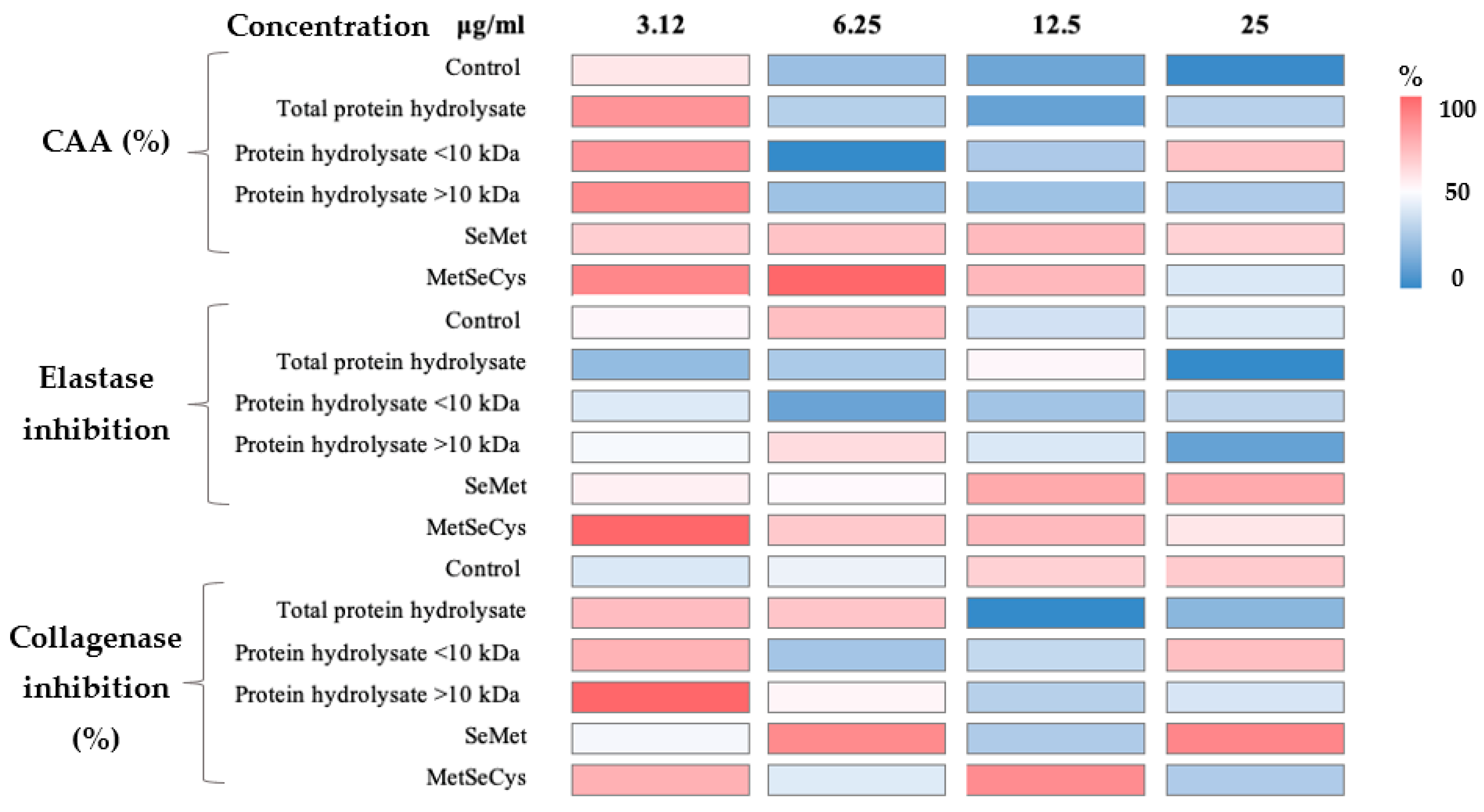
2.5. Cosmeceutical Effect of Selenized Hydrolysates
3. Discussion
3.1. Protein and Selenium Content in Chickpea Sprouts Flour
3.2. Protein Structure by FTIR Spectroscopy
3.3. Enzymatic Hydrolysis and Screening of Antioxidant Activity of Selenized Protein Hydrolysates
3.4. Selenized Hydrolysates Stability through Time
3.5. Cosmeceutical Effect of Selenized Hydrolysates
4. Materials and Methods
4.1. Biological Material
4.2. Chemical Reagents
4.3. Germination of Chickpea in Presence of Selenium (Se)
4.4. Sprouted Chickpea Flour Production
4.5. Protein Extraction
4.6. Total Selenium Quantification
4.7. Enzymatic Hydrolysis of Protein Fraction
4.8. Fourier Transform-Infrared Spectroscopy
4.9. DPPH Radical Scavenging Assay
4.10. Oxygen Radical Absorbance Capacity Assay (ORAC)
4.11. Hydroxyl Radical (·OH) Scavenging Assay
4.12. Superoxide Radical Assay
4.13. Cell Viability Assay
4.14. Cellular Antioxidant Activity Assay
4.15. Cosmeceutical Effect of Selenized Hydrolysates
4.15.1. Protein Fractioning
4.15.2. Elastase Inhibitory Activity
4.15.3. Collagenase Inhibitory Activity
4.15.4. Anti-Photoaging Activity
4.15.5. Collagen Quantification
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Choi, D.; Min, S.-G.; Jo, Y.-J. Functionality of Porcine Skin Hydrolysates Produced by Hydrothermal Processing for Liposomal Delivery System. J. Food Biochem. 2018, 42, e12464. [Google Scholar] [CrossRef]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Bai, F.; Wang, J.; Li, D.; Li, Y.; Jin, W.; Yuan, L. Sturgeon Protein-Derived Peptides Exert Anti-Inflammatory Effects in LPS-Stimulated RAW264.7 Macrophages via the MAPK Pathway. J. Funct. Foods 2020, 72, 104044. [Google Scholar] [CrossRef]
- Klubthawee, N.; Adisakwattana, P.; Hanpithakpong, W.; Somsri, S.; Aunpad, R. A Novel, Rationally Designed, Hybrid Antimicrobial Peptide, Inspired by Cathelicidin and Aurein, Exhibits Membrane-Active Mechanisms against Pseudomonas Aeruginosa. Sci. Rep. 2020, 10, 9117. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-Y.; Je, J.-G.; Lee, H.-G.; Kim, E.-A.; Kang, S.I.; Lee, J.-S.; Jeon, Y.-J. Anti-Hypertensive Activity of Novel Peptides Identified from Olive Flounder (Paralichthys olivaceus) Surimi. Foods 2020, 9, 647. [Google Scholar] [CrossRef]
- Pan, L.-L.; Liang, W.; Ren, Z.; Li, C.; Chen, Y.; Niu, W.; Fang, X.; Liu, Y.; Zhang, M.; Diana, J.; et al. Cathelicidin-Related Antimicrobial Peptide Protects against Ischaemia Reperfusion-Induced Acute Kidney Injury in Mice. Br. J. Pharmacol. 2020, 177, 2726–2742. [Google Scholar] [CrossRef]
- Yaghoubzadeh, Z.; Peyravii Ghadikolaii, F.; Kaboosi, H.; Safari, R.; Fattahi, E. Antioxidant Activity and Anticancer Effect of Bioactive Peptides from Rainbow Trout (Oncorhynchus mykiss) Skin Hydrolysate. Int. J. Pept. Res. Ther. 2020, 26, 625–632. [Google Scholar] [CrossRef]
- Mada, S.B.; Ugwu, C.P.; Abarshi, M.M. Health Promoting Effects of Food-Derived Bioactive Peptides: A Review. Int. J. Pept. Res. Ther. 2020, 26, 831–848. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- López-Barrios, L.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A. Changes in Antioxidant and Antiinflammatory Activity of Black Bean (Phaseolus vulgaris L.) Protein Isolates Due to Germination and Enzymatic Digestion. Food Chem. 2016, 203, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive Peptides in Cereals and Legumes: Agronomical, Biochemical and Clinical Aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef]
- Selamassakul, O.; Laohakunjit, N.; Kerdchoechuen, O.; Yang, L.; Maier, C.S. Bioactive Peptides from Brown Rice Protein Hydrolyzed by Bromelain: Relationship between Biofunctional Activities and Flavor Characteristics. J. Food Sci. 2020, 85, 707–717. [Google Scholar] [CrossRef]
- Merga, B.; Haji, J. Economic Importance of Chickpea: Production, Value, and World Trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Faridy, J.-C.M.; Stephanie, C.-G.M.; Gabriela, M.-M.O.; Cristian, J.-M. Biological Activities of Chickpea in Human Health (Cicer arietinum L.). A Review. Plant Foods Hum. Nutr. 2020, 75, 142–153. [Google Scholar] [CrossRef]
- Monk, J.M.; Lepp, D.; Wu, W.; Graf, D.; McGillis, L.H.; Hussain, A.; Carey, C.; Robinson, L.E.; Liu, R.; Tsao, R.; et al. Chickpea-Supplemented Diet Alters the Gut Microbiome and Enhances Gut Barrier Integrity in C57Bl/6 Male Mice. J. Funct. Foods 2017, 38, 663–674. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Antunes-Ricardo, M.; Rocha-Pizaña, M.R.; Martínez-Torres, A.-C.; Gutiérrez-Uribe, J.A.; Serna Saldivar, S.O. Chickpea (Cicer arietinum L.) Sprouts Containing Supranutritional Levels of Selenium Decrease Tumor Growth of Colon Cancer Cells Xenografted in Immune-Suppressed Mice. J. Funct. Foods 2019, 53, 76–84. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Guardado-Félix, D.; Serna-Saldivar, S.O.; Cuevas-Rodríguez, E.O.; Jacobo-Velázquez, D.A.; Gutiérrez-Uribe, J.A. Effect of Sodium Selenite on Isoflavonoid Contents and Antioxidant Capacity of Chickpea (Cicer arietinum L.) Sprouts. Food Chem. 2017, 226, 69–74. [Google Scholar] [CrossRef]
- Serrano-Sandoval, S.N.; Guardado-Félix, D.; Gutiérrez-Uribe, J.A. Changes in Digestibility of Proteins from Chickpeas (Cicer arietinum L.) Germinated in Presence of Selenium and Antioxidant Capacity of Hydrolysates. Food Chem. 2019, 285, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Catron, B.; Zhang, Y.; Zhao, L.; Caruso, J.A.; Hu, Q. Distribution and in Vitro Availability of Selenium in Selenium-Containing Storage Protein from Selenium-Enriched Rice Utilizing Optimized Extraction. J. Agric. Food Chem. 2010, 58, 9731–9738. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Yin, H.; Hou, T. Selenium-Containing Proteins/Peptides from Plants: A Review on the Structures and Functions. J. Agric. Food Chem. 2020, 68, 15061–15073. [Google Scholar] [CrossRef]
- Díaz, O.; Ferreiro, T.; Rodríguez-Otero, J.L.; Cobos, Á. Characterization of Chickpea (Cicer arietinum L.) Flour Films: Effects of PH and Plasticizer Concentration. Int. J. Mol. Sci. 2019, 20, 1246. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of Germination on Lignan Biosynthesis, and Antioxidant and Antiproliferative Activities in Flaxseed (Linum usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef]
- Marcoux, J.; Thierry, E.; Vivès, C.; Signor, L.; Fieschi, F.; Forest, E. Investigating Alternative Acidic Proteases for H/D Exchange Coupled to Mass Spectrometry: Plasmepsin 2 but Not Plasmepsin 4 Is Active Under Quenching Conditions. J. Am. Soc. Mass Spectrom. 2010, 21, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Manea, M.; Mezo, G.; Hudecz, F.; Przybylski, M. Mass Spectrometric Identification of the Trypsin Cleavage Pathway in Lysyl-Proline Containing Oligotuftsin Peptides. J. Pept. Sci. 2007, 13, 227–236. [Google Scholar] [CrossRef]
- Chand, A.; Sahoo, D.K.; Rana, A.; Jena, S.; Biswal, H.S. The Prodigious Hydrogen Bonds with Sulfur and Selenium in Molecular Assemblies, Structural Biology, and Functional Materials. Acc. Chem. Res. 2020, 53, 1580–1592. [Google Scholar] [CrossRef]
- Zhang, L.; Chou, C.P.; Moo-Young, M. Disulfide Bond Formation and Its Impact on the Biological Activity and Stability of Recombinant Therapeutic Proteins Produced by Escherichia Coli Expression System. Biotechnol. Adv. 2011, 29, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Giles, G.I.; Giles, N.M.; Sies, H. Sulfur and Selenium: The Role of Oxidation State in Protein Structure and Function. Angew. Chem. Int. Ed. Engl. 2003, 42, 4742–4758. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.K.; Singh, S.K.; Kumar, S.; Singh, G.; Sarkar, B.; Madhusudhan, M.S.; Das, A. Water-Mediated Selenium Hydrogen-Bonding in Proteins: PDB Analysis and Gas-Phase Spectroscopy of Model Complexes. J. Phys. Chem. A 2019, 123, 5995–6002. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Zhang, X.; Guo, M.; Sun, M.; Yu, H.; Chen, Q.; Yang, W.; Wang, M.; Zuo, S.; et al. Probing the Impact of Sulfur/Selenium/Carbon Linkages on Prodrug Nanoassemblies for Cancer Therapy. Nat. Commun. 2019, 10, 3211. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, Identification and Characterization of Two Novel Antioxidant Peptides from Finger Millet (Eleusine coracana) Protein Hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar] [CrossRef]
- Sandoval-Sicairos, E.S.; Milán-Noris, A.K.; Luna-Vital, D.A.; Milán-Carrillo, J.; Montoya-Rodríguez, A. Anti-Inflammatory and Antioxidant Effects of Peptides Released from Germinated Amaranth during in vitro Simulated Gastrointestinal Digestion. Food Chem. 2021, 343, 128394. [Google Scholar] [CrossRef]
- Cotabarren, J.; Rosso, A.M.; Tellechea, M.; García-Pardo, J.; Rivera, J.L.; Obregón, W.D.; Parisi, M.G. Adding Value to the Chia (Salvia hispanica L.) Expeller: Production of Bioactive Peptides with Antioxidant Properties by Enzymatic Hydrolysis with Papain. Food Chem. 2019, 274, 848–856. [Google Scholar] [CrossRef]
- Guo, H.; Guo, S.; Liu, H. Antioxidant Activity and Inhibition of Ultraviolet Radiation-Induced Skin Damage of Selenium-Rich Peptide Fraction from Selenium-Rich Yeast Protein Hydrolysate. Bioorg. Chem. 2020, 105, 104431. [Google Scholar] [CrossRef]
- Hernández-Grijalva, M.I.; Serrano-Sandoval, S.N.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O.; Milán-Carrillo, J.; Antunes-Ricardo, M.; Villela-Castrejón, J.; Guardado-Félix, D. Application of Protein Fractions from Selenized Sprouted Chickpeas as Emulsifying Agents and Evaluation of Their Antioxidant Properties. Food Bioprod. Process. 2022, 136, 59–66. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, D.; Sun, J.; Luo, X.; Li, H.; Sun, X.; Zheng, F. Analysis of Antioxidant Effect of Two Tripeptides Isolated from Fermented Grains (Jiupei) and the Antioxidative Interaction with 4-Methylguaiacol, 4-Ethylguaiacol, and Vanillin. Food Sci. Nutr. 2019, 7, 2391–2403. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential Role of Natural Bioactive Peptides for Development of Cosmeceutical Skin Products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef] [PubMed]
- Negari, I.P.; Keshari, S.; Huang, C.-M. Probiotic Activity of Staphylococcus Epidermidis Induces Collagen Type I Production through FFaR2/p-ERK Signaling. Int. J. Mol. Sci. 2021, 22, 1414. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Jung, H.-J.; Choi, J.-S.; Nam, T.-J. Anti-Wrinkle Effects of a Tuna Heart H2O Fraction on Hs27 Human Fibroblasts. Int. J. Mol. Med. 2016, 37, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C.; Beal, D.; Blouin, E.; Leccia, M.T.; Roussel, A.M.; Rachidi, W. Age-Dependent Protective Effect of Selenium against UVA Irradiation in Primary Human Keratinocytes and the Associated DNA Repair Signature. Oxid. Med. Cell. Longev. 2018, 2018, 5895439. [Google Scholar] [CrossRef]
- Lo, W.M.Y.; Li-Chan, E.C.Y. Angiotensin I Converting Enzyme Inhibitory Peptides from in Vitro Pepsin-Pancreatin Digestion of Soy Protein. J. Agric. Food Chem. 2005, 53, 3369–3376. [Google Scholar] [CrossRef]
- Rutherfurd, S.M. Food Composition and Additives: Methodology for Determining Degree of Hydrolysis of Proteins in Hydrolysates: A Review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S. In Vitro Stability of Bioactive Peptides Derived from Fermented Soy Milk against Heat Treatment, PH and Gastrointestinal Enzymes. LWT Food Sci. Technol. 2018, 91, 303–307. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Reig, M.; Toldrá, F. Stability of the Potent Antioxidant Peptide SNAAC Identified from Spanish Dry-Cured Ham. Food Res. Int. 2018, 105, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Sonklin, C.; Laohakunjit, N.; Kerdchoechuen, O. Assessment of Antioxidant Properties of Membrane Ultrafiltration Peptides from Mungbean Meal Protein Hydrolysates. PeerJ 2018, 6, e5337. [Google Scholar] [CrossRef]
- Lopez-Barrios, L.; Heredia-Olea, E.; Guajardo-Flores, D.; Perez-Carrillo, E.; Uribe, J.A.G. Bioactive Peptides by in Vitro Digestion of Germinated Bean Cotyledons Extrudates. J. Food Res. 2018, 7, 76. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, S.H.; Hong, Y.; Ahn, D.U.; Paik, H.D. Anti-Elastase and Anti-Hyaluronidase Activity of Phosvitin Isolated from Hen Egg Yolk. Br. Poult. Sci. 2020, 61, 17–21. [Google Scholar] [CrossRef]
- Hubert, J.; Angelis, A.; Aligiannis, N.; Rosalia, M.; Abedini, A.; Bakiri, A.; Reynaud, R.; Nuzillard, J.M.; Gangloff, S.C.; Skaltsounis, A.L.; et al. In Vitro Dermo-Cosmetic Evaluation of Bark Extracts from Common Temperate Trees. Planta Med. 2016, 82, 1351–1358. [Google Scholar] [CrossRef]
- Nakyai, W.; Saraphanchotiwitthaya, A.; Viennet, C.; Humbert, P.; Viyoch, J. An In Vitro Model for Fibroblast Photoaging Comparing Single and Repeated UVA Irradiations. Photochem. Photobiol. 2017, 93, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Atale, N.; Chakraborty, M.; Mohanty, S.; Bhattacharya, S.; Nigam, D.; Sharma, M.; Rani, V. Cardioprotective Role of Syzygium Cumini against Glucose-Induced Oxidative Stress in H9C2 Cardiac Myocytes. Cardiovasc. Toxicol. 2013, 13, 278–289. [Google Scholar] [CrossRef]
- Keira, S.M.; Ferreira, L.M.; Gragnani, A.; Duarte, I.d.S.; Barbosa, J. Experimental Model for Collagen Estimation in Cell Culture. Acta Cir. Bras. 2004, 19 (Suppl. S1), 17–22. [Google Scholar] [CrossRef]
| Source | mg Na2SeO3/100 g | Se Content (mg/kg Sample) |
|---|---|---|
| Germinated chickpea flour | 0 | 0.78 ± 0.12 c |
| 2 | 27.50 ± 1.98 a | |
| Protein extract | 0 | 0.50 ± 0.00 c |
| 2 | 8.84 ± 1.65 b |
| Degree of Hydrolysis (%) | ||||
|---|---|---|---|---|
| Enzyme | 30 min | 60 min | 120 min | 180 min |
| Alcalase | 14.29 ± 2.81 b | 17.18 ± 0.26 b | 18.63 ± 1.28 b | 18.63 ± 1.28 b |
| Pepsin | 31.30 ± 4.43 a | 33.49 ± 5.75 a | 36.12 ± 2.04 a | 37.12 ± 0.63 a |
| Trypsin | 11.50 ± 3.62 b | 13.10 ± 1.36 b | 13.52 ± 0.77 b | 14.48 ± 0.59 b |
| Antioxidant Activity (%) | ||||
|---|---|---|---|---|
| Hydrolisis Time (min) | Alcalase | Pepsin | Trypsin | |
| DPPH scavenging | 30 | 3.59 ± 0.69 bc | 13.62 ± 2.42 abc | 13.38 ± 1.38 abc |
| 60 | 12.40 ± 1.96 abc | 14.85 ± 6.23 ab | 7.01 ± 8.31 abc | |
| 120 | 7.99 ± 0.00 abc | 14.36 ± 2.13 a | 9.95 ± 0.00 abc | |
| 180 | 8.73 ± 3.81 abc | 12.40 ± 2.77 abc | 3.10 ± 1.38 c | |
| ORAC (%) | 30 | 78.27 ± 0.42 de | 84.42 ± 9.40 cd | 95.93 ± 0.00 ab |
| 60 | 69.19 ± 4.42 e | 81.89 ± 3.72 cd | 90.82 ± 0.00 abc | |
| 120 | 53.46 ± 7.16 f | 98.81 ± 2.67 a | 86.70 ± 0.00 bcd | |
| 180 | 57.92 ± 3.93 f | 88.19 ± 1.68 bc | 84.82 ± 0.00 cd | |
| OH scavenging (%) | 30 | 11.65 ± 1.50 d | 17.64 ± 2.29 abc | 22.63 ± 1.73 a |
| 60 | 14.14 ± 1.32 cd | 17.80 ± 1.52 abc | 21.63 ± 1.41 ab | |
| 120 | 11.31 ± 0.76 d | 21.63 ± 3.53 ab | 18.30 ± 2.02 abc | |
| 180 | 13.14 ± 0.00 cd | 21.63 ± 2.12 ab | 17.39 ± 2.47 bcd | |
| Superoxide scavenging (%) | 30 | 5.41 ± 0.00 e | 14.97 ± 1.35 ab | 9.24 ± 0.00 cde |
| 60 | 7.80 ± 0.68 de | 18.31 ± 0.68 a | 14.01 ± 0.00 abc | |
| 120 | 7.32 ± 1.35 de | 6.37 ± 2.70 e | 11.62 ± 0.00 bcd | |
| 180 | 7.32 ± 0.00 de | 6.37 ± 2.70 e | 16.40 ± 0.00 ab | |
| CAA (%) | 30 | 18.53 ± 2.22 bc | 10.83 ± 1.16 def | 23.64 ± 2.62 ab |
| 60 | 5.63 ± 0.92 g | 13.19 ± 2.22 def | 6.30 ± 0.25 fg | |
| 120 | 8.97 ± 1.24 efg | 20.74 ± 1.19 abc | 4.13 ± 0.25 g | |
| 180 | 12.70 ± 1.40 de | 24.24 ± 3.76 a | 15.49 ± 1.78 cd | |
| Samples | Type I Collagen (µg/mL) | ROS Production (* RLU × 105) |
|---|---|---|
| Control | 0.78 ± 0.29 b | 20.2 ± 1.43 a |
| Total protein hydrolysate | 0.60 ± 0.22 b | 18.0 ± 2.64 a |
| Protein hydrolysate <10 kDa | 3.13 ± 0.57 a | 20.3 ± 1.00 a |
| Protein hydrolysate >10 kDa | 0.85 ± 0.32 b | 18.4 ± 1.45 a |
| SeMet | 4.88 ± 0.50 a | 9.77 ± 0.59 b |
| MeSeCys | 4.73 ± 0.64 a | 9.40 ± 0.34 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Sandoval, S.N.; Jiménez-Rodríguez, A.; Hernández-Pérez, J.; Chavez-Santoscoy, R.A.; Guardado-Félix, D.; Antunes-Ricardo, M. Selenized Chickpea Sprouts Hydrolysates as a Potential Anti-Aging Ingredient. Molecules 2023, 28, 3402. https://doi.org/10.3390/molecules28083402
Serrano-Sandoval SN, Jiménez-Rodríguez A, Hernández-Pérez J, Chavez-Santoscoy RA, Guardado-Félix D, Antunes-Ricardo M. Selenized Chickpea Sprouts Hydrolysates as a Potential Anti-Aging Ingredient. Molecules. 2023; 28(8):3402. https://doi.org/10.3390/molecules28083402
Chicago/Turabian StyleSerrano-Sandoval, Sayra N., Antonio Jiménez-Rodríguez, Jesús Hernández-Pérez, Rocio Alejandra Chavez-Santoscoy, Daniela Guardado-Félix, and Marilena Antunes-Ricardo. 2023. "Selenized Chickpea Sprouts Hydrolysates as a Potential Anti-Aging Ingredient" Molecules 28, no. 8: 3402. https://doi.org/10.3390/molecules28083402
APA StyleSerrano-Sandoval, S. N., Jiménez-Rodríguez, A., Hernández-Pérez, J., Chavez-Santoscoy, R. A., Guardado-Félix, D., & Antunes-Ricardo, M. (2023). Selenized Chickpea Sprouts Hydrolysates as a Potential Anti-Aging Ingredient. Molecules, 28(8), 3402. https://doi.org/10.3390/molecules28083402









