New Insights into Adsorption Properties of the Tubular Au26 from AIMD Simulations and Electronic Interactions
Abstract
1. Introduction
2. Results and Discussion
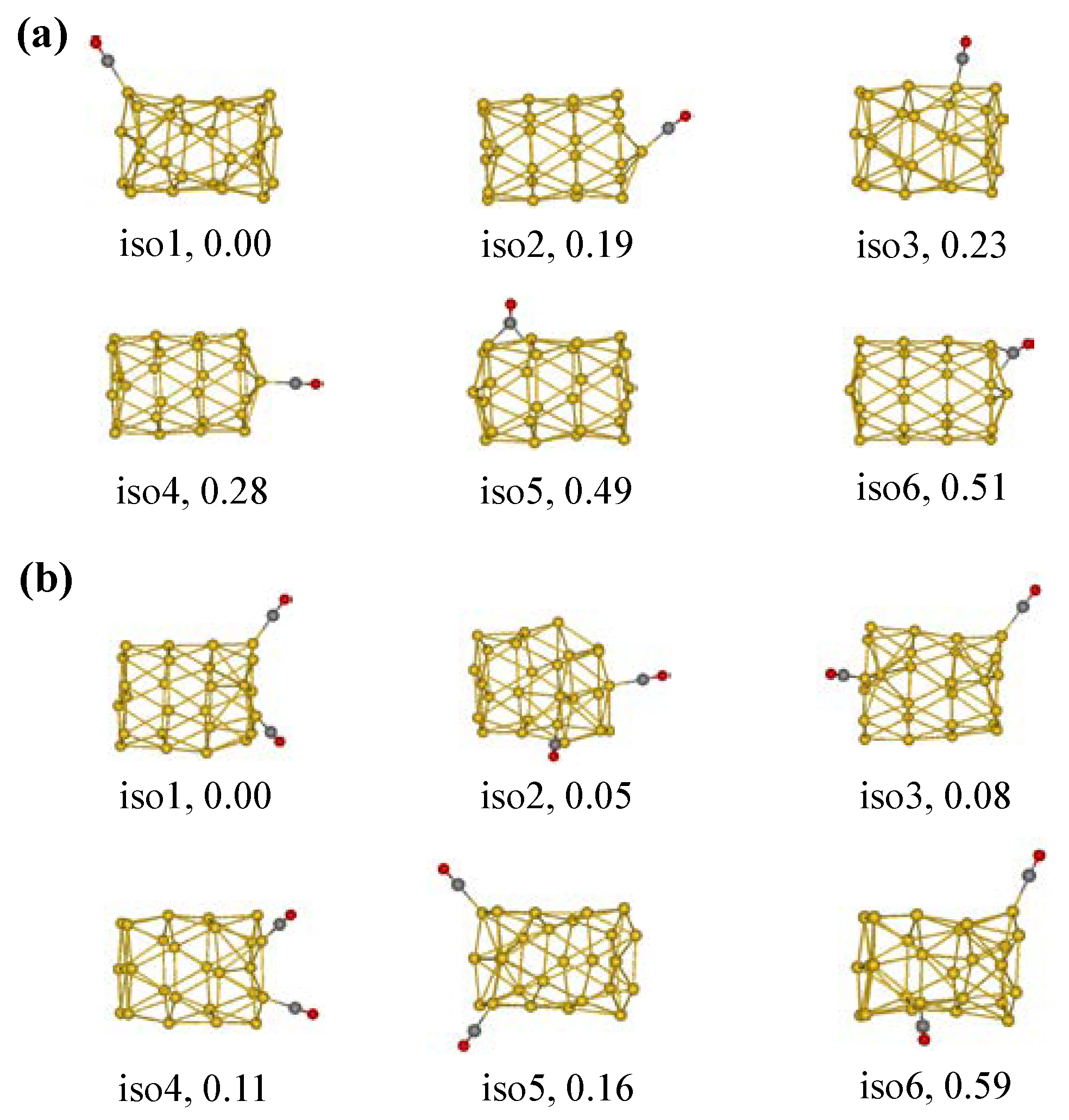
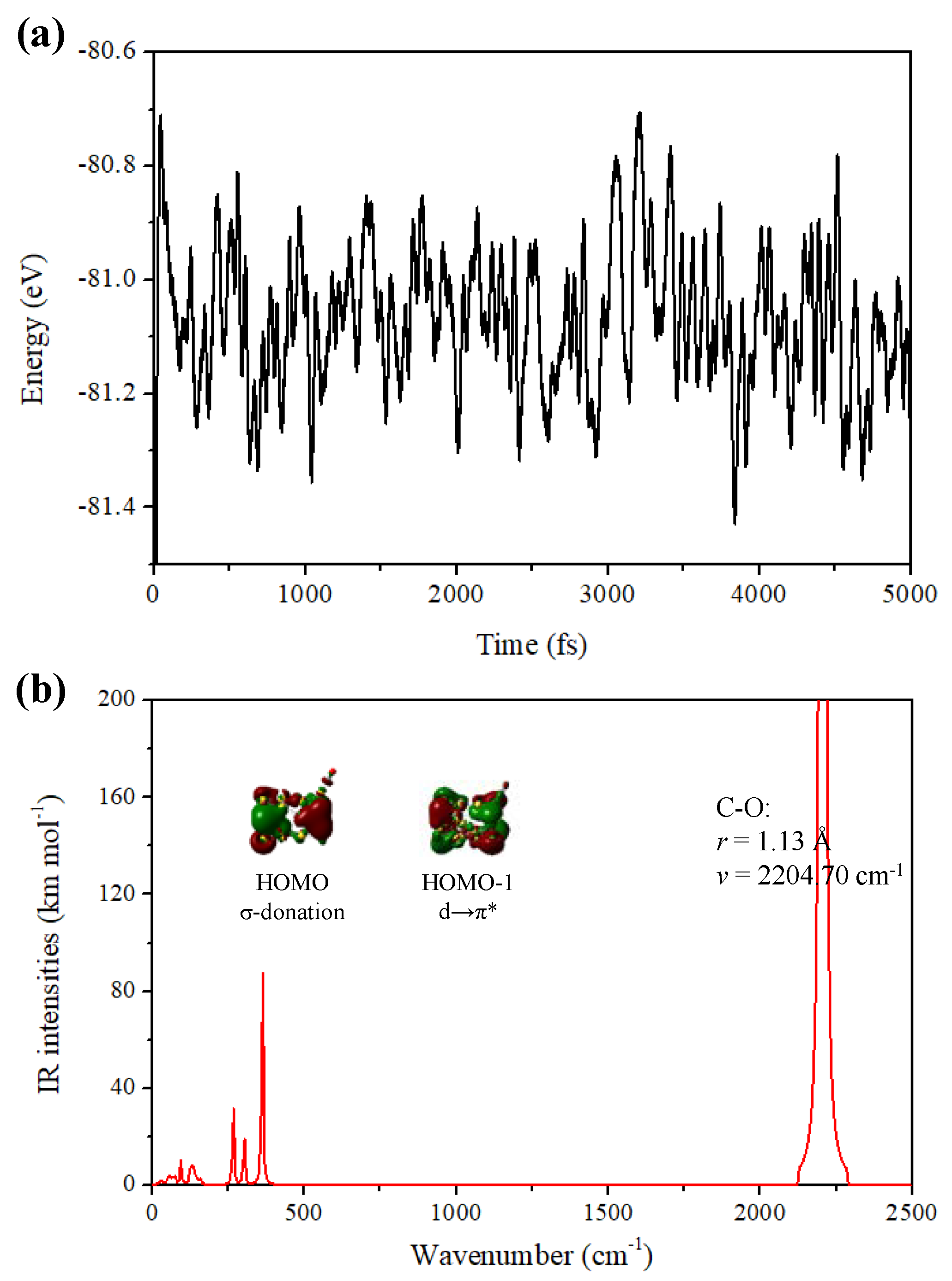
2.1. Adsorption Properties of the Tubular Au26 Cluster with CO Molecules
2.2. Adsorption Properties of the Tubular Au26 Clusters with Hydrogens
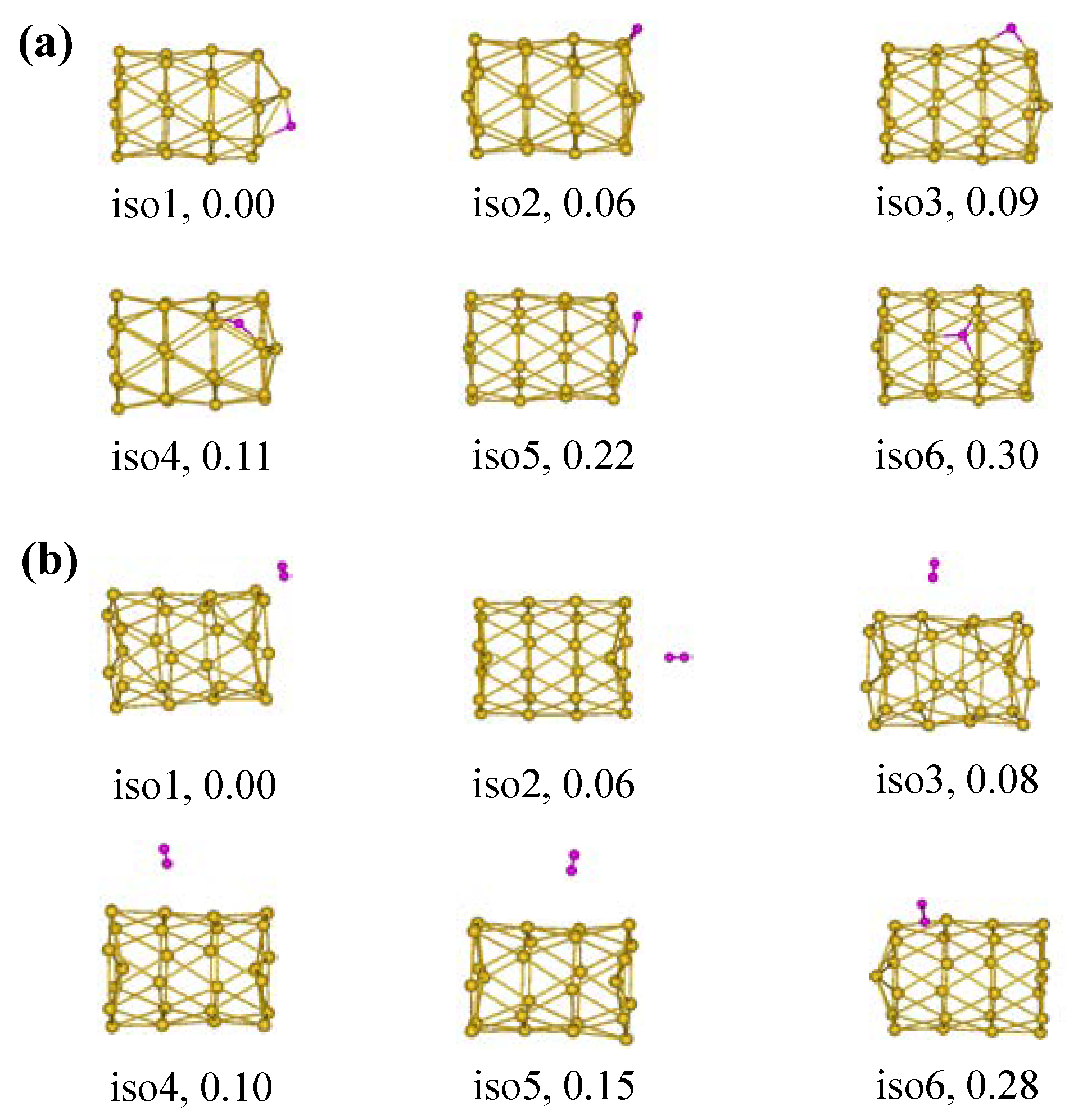
2.3. Adsorption Properties of the Tubular Au26 Clusters with a BH3 Molecule
3. Computational Methods and Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.-M.; Dornfeld, M.; Frauenheim, T.; Ganz, E. Glitter in a 2D monolayer. Phys. Chem. Chem. Phys. 2015, 17, 26036–26042. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Lu, K.-Q.; Tang, Z.; Chen, H.M.; Xu, Y.-J. Stabilizing ultrasmall Au clusters for enhanced photoredox catalysis. Nat. Commun. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.C.; Goodman, D.W. The influence of metal cluster size on adsorption energies: CO adsorbed on Au clusters supported on TiO2. J. Am. Chem. Soc. 2004, 126, 1892–1899. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, X.; Zhou, W.; Gao, R.; Xu, W.; Ye, Y.; Lin, L.; Wen, X.; Liu, P.; Chen, B.; et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction. Science 2017, 357, 389–393. [Google Scholar] [CrossRef]
- Tan, X.P.; Zhang, Z.; Cao, T.; Zeng, W.J.; Huang, T.; Zhao, G.F. Control assembly of pillar[6]arene-modified Ag nanoparticles on covalent organic framework surface for enhanced sensing performance toward paraquat. ACS Sustain. Chem. Eng. 2019, 7, 20051–20059. [Google Scholar] [CrossRef]
- Tan, X.P.; Liu, X.; Zeng, W.J.; Zhao, G.F.; Zhang, Z.; Huang, T.; Yang, L. Control assembly of Au nanoparticles on macrocyclic host molecule cationic pillar[5]arene functionalized MoS2 surface for enhanced sensing activity towards p-dinitrobenzene. Anal. Chim. Acta 2019, 1078, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, S.; Koyasu, K.; Tsukuda, T. Nonscalable oxidation catalysis of gold clusters. Acc. Chem. Res. 2014, 47, 816–824. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.-Y.T.; Schouteden, K.; Picot, T.; Liao, T.-W.; Seliverstov, A.; Van Haesendonck, C.; Pacchioni, G.; Janssens, E.; Lievens, P. Unraveling the atomic structure, ripening behavior, and electronic structure of supported Au20 clusters. Sci. Adv. 2020, 6, eaay4289. [Google Scholar] [CrossRef]
- Walter, M.; Moseler, M. Ligand-protected gold alloy clusters: Doping the superatom. J. Phys. Chem. C 2009, 113, 15834–15837. [Google Scholar] [CrossRef]
- Pilli, V.V.N. Synthesis, structural characterization and transformation of an eight-electron superatomic alloy,[Au@Ag19{S2P(OPr)2}12]. Nanoscale 2018, 10, 6855–6860. [Google Scholar]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Zhai, H.-J.; Wang, L.-S. Au20: A Tetrahedral Cluster. Science 2003, 299, 864–867. [Google Scholar] [CrossRef]
- Ji, M.; Gu, X.; Li, X.; Gong, X.; Li, J.; Wang, L.-S. Experimental and Theoretical Investigation of the Electronic and Geometrical Structures of the Au32 Cluster. Angew. Chem. Int. Ed. 2005, 44, 7119–7123. [Google Scholar] [CrossRef]
- Pande, S.; Huang, W.; Shao, N.; Wang, L.-M.; Khetrapal, N.; Mei, W.-N.; Jian, T.; Wang, L.-S.; Zeng, X.C. Structural Evolution of Core–Shell Gold Nanoclusters: Aun– (n = 42–50). ACS Nano 2016, 10, 10013–10022. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, J. Communication: New insight into electronic shells of metal clusters: Analogues of simple molecules. J. Chem. Phys. 2013, 138, 141101. [Google Scholar]
- Luo, Z.; Castleman, A.W. Special and General Superatoms. Acc. Chem. Res. 2014, 47, 2931–2940. [Google Scholar] [CrossRef]
- Wallace, W.T.; Whetten, R.L. Coadsorption of CO and O2 on Selected Gold Clusters: Evidence for Efficient Room-Temperature CO2 Generation. J. Am. Chem. Soc. 2002, 124, 7499–7505. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhai, H.-J.; Wang, L.-S. Probing the Interactions of O2 with Small Gold Cluster Anions (Aun−, n = 1−7): Chemisorption vs. Physisorption. J. Am. Chem. Soc. 2010, 132, 4344–4351. [Google Scholar] [CrossRef]
- Luo, Z.; Castleman, A.W.; Khanna, S.N. Reactivity of Metal Clusters. Chem. Rev. 2016, 116, 14456–14492. [Google Scholar] [CrossRef] [PubMed]
- Zielasek, V.; Jürgens, B.; Schulz, C.; Biener, J.; Biener, M.M.; Hamza, A.V.; Bäumer, M. Gold Catalysts: Nanoporous Gold Foams. Angew. Chem. Int. Ed. 2006, 45, 8241–8244. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, L.; Schatz, G.C.; Zhang, Y.; Guo, H. Mechanistic Insights into Photocatalyzed H2 Dissociation on Au Clusters. J. Am. Chem. Soc. 2020, 142, 13090–13101. [Google Scholar] [CrossRef]
- Yan, L.; Wang, F.; Meng, S. Quantum mode selectivity of plasmon-induced water splitting on gold nanoparticles. ACS Nano 2016, 10, 5452–5458. [Google Scholar] [CrossRef]
- Li, L.; Huang, S.; Song, J.; Yang, N.; Liu, J.; Chen, Y.; Sun, Y.; Jin, R.; Zhu, Y. Ultrasmall Au10 clusters anchored on pyramid-capped rectangular TiO2 for olefin oxidation. Nano Res. 2016, 9, 1182–1192. [Google Scholar] [CrossRef]
- Lim, E.; Heo, J.; Zhang, X.; Bowen, K.H.; Lee, S.H.; Kim, S.K. Anionic Activation of CO2 via (Mn–CO2)− Complex on Magic-Numbered Anionic Coinage Metal Clusters Mn– (M = Cu, Ag, Au). J. Phys. Chem. A 2021, 125, 2243–2248. [Google Scholar] [CrossRef]
- Sengupta, T.; Chung, J.S.; Kang, S.G. A mechanistic insight into rhodium-doped gold clusters as a better hydrogenation catalyst. Nanoscale 2020, 12, 5125–5138. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Zhang, D.; Hu, Y.; Zhu, Q.; Cheng, L. Adsorption properties of pyramidal superatomic molecules based on the structural framework of the Au20 cluster. Phys. Chem. Chem. Phys. 2022, 24, 12410–12418. [Google Scholar] [CrossRef]
- Fa, W.; Dong, J. Possible ground-state structure of Au26: A highly symmetric tubelike cage. J. Chem. Phys. 2006, 124, 114310. [Google Scholar] [CrossRef]
- Schaefer, B.; Pal, R.; Khetrapal, N.S.; Amsler, M.; Sadeghi, A.; Blum, V.; Zeng, X.C.; Goedecker, S.; Wang, L.-S. Isomerism and Structural Fluxionality in the Au26 and Au26–Nanoclusters. ACS Nano 2014, 8, 7413–7422. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Krishnamurty, S. Au26: A case of fluxionality/co-existence. Phys. Chem. Chem. Phys. 2018, 20, 8616–8623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, C.; Wu, X.; Cheng, L. Electronic shells of a tubular Au26 cluster: A cage–cage superatomic molecule based on spherical aromaticity. Nanoscale 2019, 11, 13227–13232. [Google Scholar] [CrossRef] [PubMed]
- Stenlid, J.H.; Brinck, T. Extending the σ-hole concept to metals: An electrostatic interpretation of the effects of nanostructure in gold and platinum catalysis. J. Am. Chem. Soc. 2017, 139, 11012–11015. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, X.; Xiao, K.; Zhu, Y.; Chen, M. Active-Site Tailoring of Gold Cluster Catalysts for Electrochemical CO2 Reduction. ACS Catal. 2021, 11, 11551–11560. [Google Scholar] [CrossRef]
- Ferrari, P.; Vanbuel, J.; Janssens, E.; Lievens, P. Tuning the reactivity of small metal clusters by heteroatom doping. Acc. Chem. Res. 2018, 51, 3174–3182. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shao, N.; Pei, Y.; Zeng, X.C. Icosahedral crown gold nanocluster Au43Cu12 with high catalytic activity. Nano Lett. 2010, 10, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.L.; Wang, W.L.; Sun, W.M. Polymeric tungsten carbide nanoclusters as potential non-noble metal catalysts for CO oxidation. Nanoscale 2022, 14, 18231–18240. [Google Scholar] [CrossRef]
- Reber, A.C.; Khanna, S.N. Superatoms: Electronic and geometric effects on reactivity. Acc. Chem. Res. 2017, 50, 255–263. [Google Scholar] [CrossRef]
- de Amorim, R.V.; Batista, K.E.; Nagurniak, G.R.; Orenha, R.P.; Parreira, R.L.; Piotrowski, M.J. CO, NO, and SO adsorption on Ni nanoclusters: A DFT investigation. Dalton Trans. 2020, 49, 6407–6417. [Google Scholar] [CrossRef]
- Abdulhussein, H.A.; Ferrari, P.; Vanbuel, J.; Heard, C.; Fielicke, A.; Lievens, P.; Janssens, E.; Johnston, R.L. Altering CO binding on gold cluster cations by Pd-doping. Nanoscale 2019, 11, 16130–16141. [Google Scholar] [CrossRef]
- Cesari, C.; Shon, J.-H.; Zacchini, S.; Berben, L.A. Metal carbonyl clusters of groups 8–10: Synthesis and catalysis. Chem. Soc. 2021, 50, 9503–9539. [Google Scholar] [CrossRef]
- Ehrlich, S.; Moellmann, J.; Grimme, S. Dispersion-corrected density functional theory for aromatic interactions in complex systems. Acc. Chem. Res. 2013, 46, 916–926. [Google Scholar] [CrossRef]
- Luna-Valenzuela, A.; Cabellos, J.L.; Posada-Amarillas, A. Effect of temperature on the structure of Pd8 and Pd7Au1 clusters: An Ab initio molecular dynamics approach. Theor. Chem. Acc. 2021, 140, 1–10. [Google Scholar] [CrossRef]
- Wu, J.Y.; Yu, D.H.; Liu, S.Y.; Rong, C.Y.; Zhong, A.G.; Chattaraj, P.K.; Liu, S.B. Is it possible to determine oxidation states for atoms in molecules using density-based quantities? An information-theoretic approach and conceptual density functional theory study. J. Phys. Chem. A 2019, 123, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.B.; Liu, S.Y.; Rong, C.Y.; Zhong, A.G.; Liu, S.B. Toward understanding the isomeric stability of fullerenes with density functional theory and the information-theoretic approach. ACS Omega 2018, 3, 17986–17990. [Google Scholar] [CrossRef] [PubMed]
- Buckart, S.; Ganteför, G.; Kim, Y.D.; Jena, P. Anomalous behavior of atomic hydrogen interacting with gold clusters. J. Am. Chem. Soc. 2003, 125, 14205–14209. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Hasegawa, S.; Suyama, M.; Tsukuda, T. Hydride doping of chemically modified gold-based superatoms. Acc. Chem. Res. 2018, 51, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-Y.; Hong, J.-H.; Becker, R.S. Hot hydrogen atoms: Initiators of reactions of interest in interstellar chemistry and evolution. Science 1974, 184, 984–987. [Google Scholar] [CrossRef]
- Takano, S.; Hirai, H.; Muramatsu, S.; Tsukuda, T. Hydride-doped gold superatom (Au9H)2+: Synthesis, structure, and transformation. J. Am. Chem. Soc. 2018, 140, 8380–8383. [Google Scholar] [CrossRef]
- Takano, S.; Hirai, H.; Muramatsu, S.; Tsukuda, T. Hydride-mediated controlled growth of a bimetallic (Pd@Au8)2+ superatom to a hydride-doped (HPd@Au10)3+ superatom. J. Am. Chem. Soc. 2018, 140, 12314–12317. [Google Scholar] [CrossRef]
- Yi, H.; Han, S.M.; Song, S.; Kim, M.; Sim, E.; Lee, D. Superatom-in-Superatom [RhH@Ag24 (SPhMe2)18]2− Nanocluster. Angew. Chem. Int. Ed. 2021, 60, 22293–22300. [Google Scholar] [CrossRef]
- Sun, C.; Teo, B.K.; Deng, C.; Lin, J.; Luo, G.-G.; Tung, C.-H.; Sun, D. Hydrido-coinage-metal clusters: Rational design, synthetic protocols and structural characteristics. Coord. Chem. Rev. 2021, 427, 213576. [Google Scholar] [CrossRef]
- Omoda, T.; Takano, S.; Tsukuda, T. Toward controlling the electronic structures of chemically modified superatoms of gold and silver. Small 2021, 17, 2001439. [Google Scholar] [CrossRef] [PubMed]
- Ishida, R.; Hayashi, S.; Yamazoe, S.; Kato, K.; Tsukuda, T. Hydrogen-mediated electron doping of gold clusters as revealed by in Situ X-ray and UV–vis absorption spectroscopy. J. Phys. Chem. Lett. 2017, 8, 2368–2372. [Google Scholar] [CrossRef]
- He, X.; Walter, M.; Jiang, D. Understanding Superatomic Ag Nanohydrides. Small 2021, 17, 2004808. [Google Scholar] [CrossRef] [PubMed]
- Kaldor, A.; Porter, R.F. Infrared spectra of the pyrolysis products of borane carbonyl in an argon matrix. J. Am. Chem. Soc. 1971, 93, 2140–2145. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Karkamkar, A.; Linehan, J.C.; Autrey, T. Synthesis of ammonia borane for hydrogen storage applications. Energy Environ. Sci. 2008, 1, 156–160. [Google Scholar] [CrossRef]
- Liu, G.; Fedik, N.; Martinez-Martinez, C.; Ciborowski, S.M.; Zhang, X.; Boldyrev, A.I.; Bowen, K.H. Realization of Lewis Basic Sodium Anion in the NaBH3− Cluster. Angew. Chem. Int. Ed. 2019, 58, 13789–13793. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Uchimaru, T. Accuracy of intermolecular interaction energies, particularly those of hetero-atom containing molecules obtained by DFT calculations with Grimme’s D2, D3 and D3BJ dispersion corrections. Phys. Chem. Chem. Phys. 2020, 22, 22508–22519. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision B. 01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Varetto, U. Molekel 5.4.0.8; Swiss National Supercomputing Centre: Manno, Switzerland, 2009. [Google Scholar]
- Tuckerman, M.E.; Ungar, P.J.; Von Rosenvinge, T.; Klein, M.L. Ab initio molecular dynamics simulations. J. Phys. Chem. 1996, 100, 12878–12887. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Liu, Q. New Insights into Adsorption Properties of the Tubular Au26 from AIMD Simulations and Electronic Interactions. Molecules 2023, 28, 2916. https://doi.org/10.3390/molecules28072916
Meng Y, Liu Q. New Insights into Adsorption Properties of the Tubular Au26 from AIMD Simulations and Electronic Interactions. Molecules. 2023; 28(7):2916. https://doi.org/10.3390/molecules28072916
Chicago/Turabian StyleMeng, Ying, and Qiman Liu. 2023. "New Insights into Adsorption Properties of the Tubular Au26 from AIMD Simulations and Electronic Interactions" Molecules 28, no. 7: 2916. https://doi.org/10.3390/molecules28072916
APA StyleMeng, Y., & Liu, Q. (2023). New Insights into Adsorption Properties of the Tubular Au26 from AIMD Simulations and Electronic Interactions. Molecules, 28(7), 2916. https://doi.org/10.3390/molecules28072916



