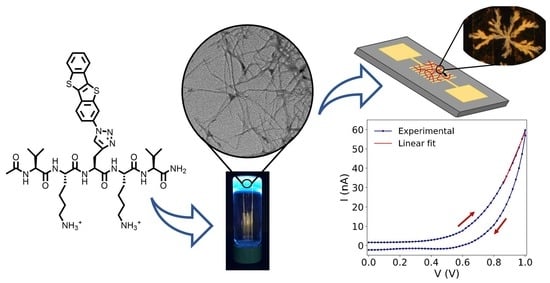
Self-Assembly and Electrical Conductivity of a New [1]benzothieno[3,2-b][1]-benzothiophene (BTBT)-Peptide Hydrogel
Abstract
1. Introduction
2. Results and Discussion
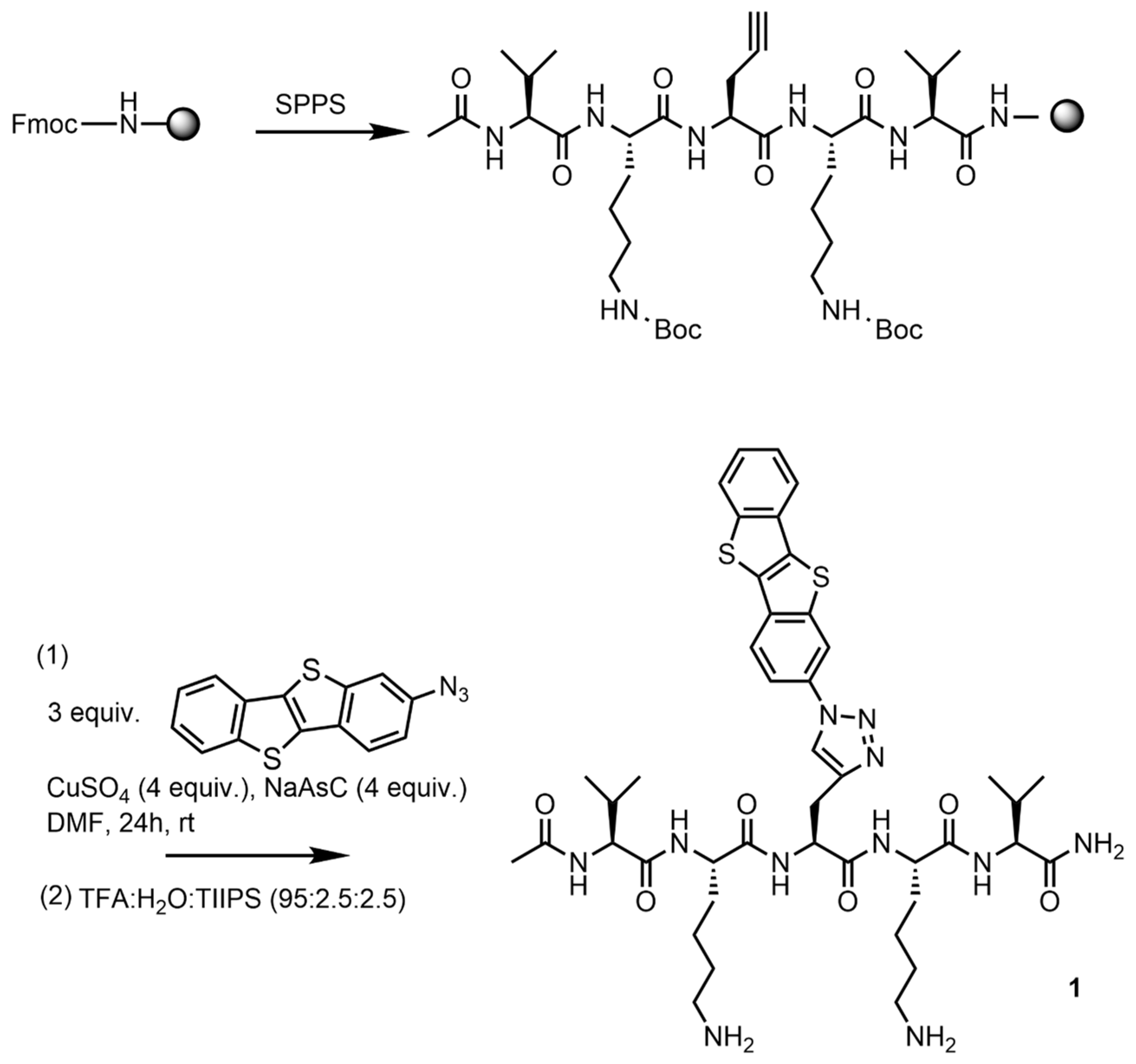
2.1. Design and Synthesis of the BTBT-Peptide Hybrid 1
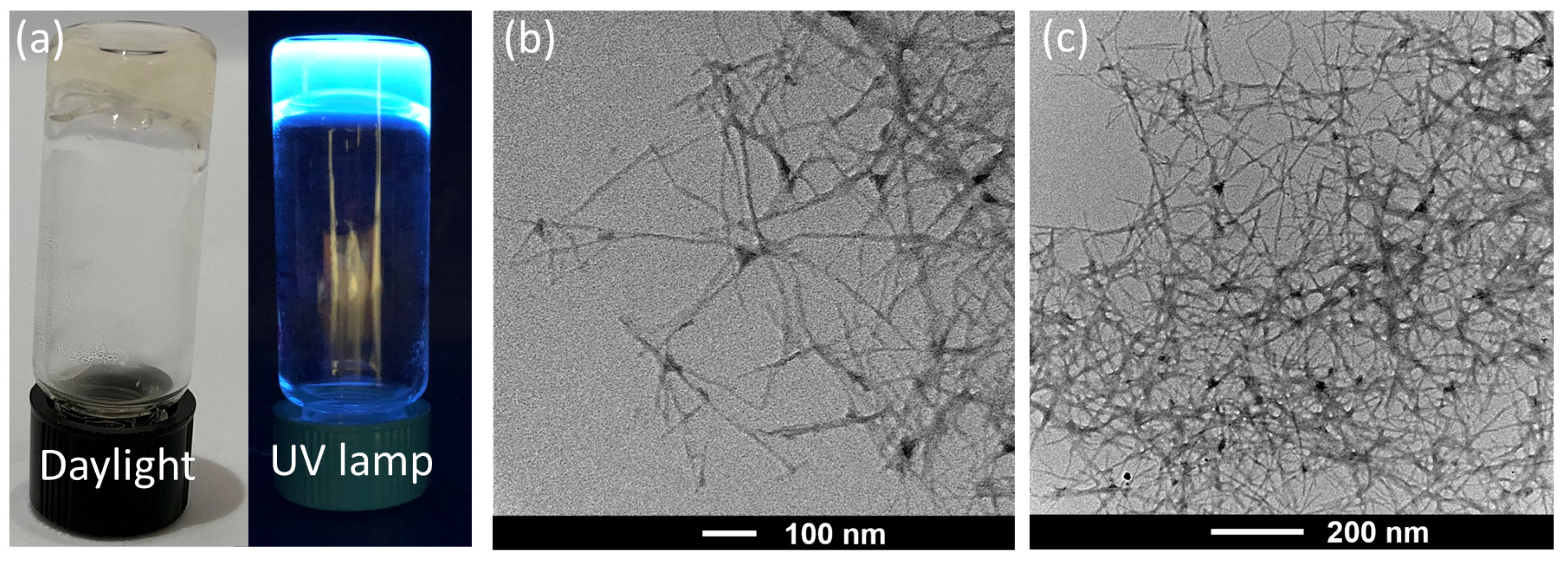
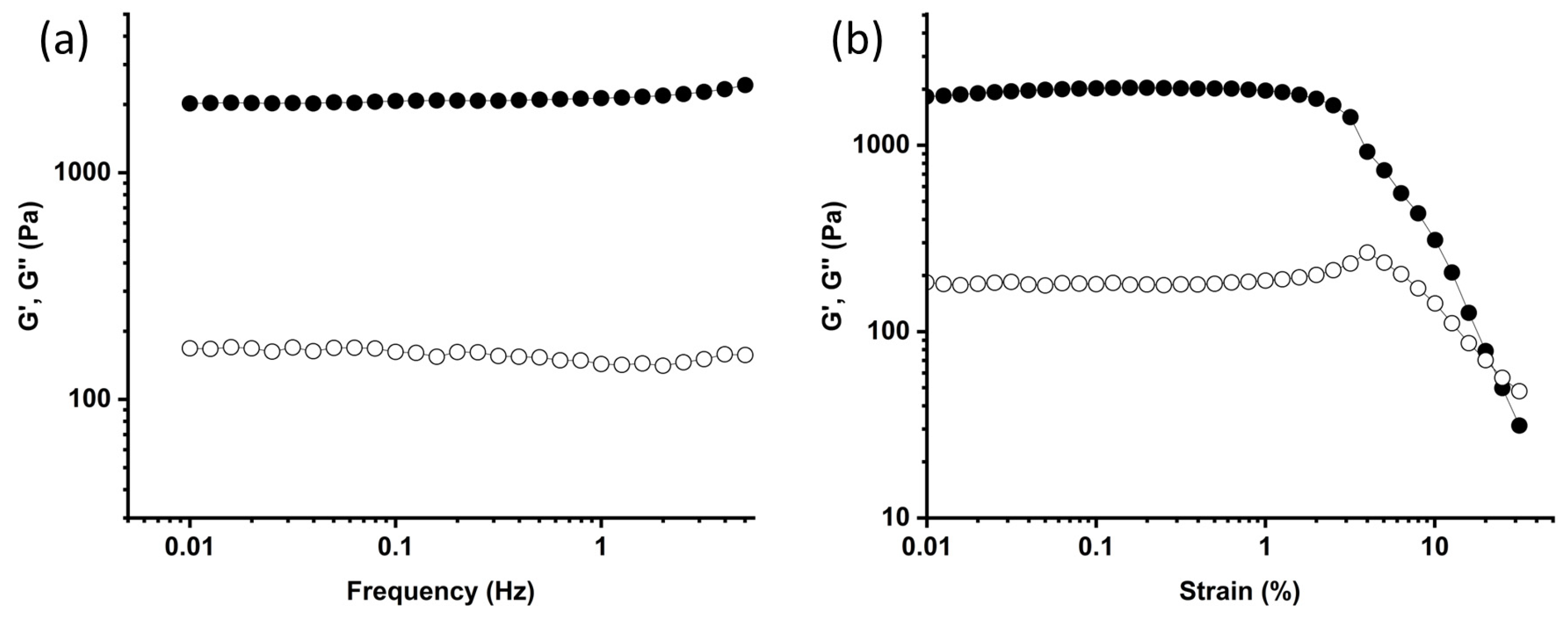
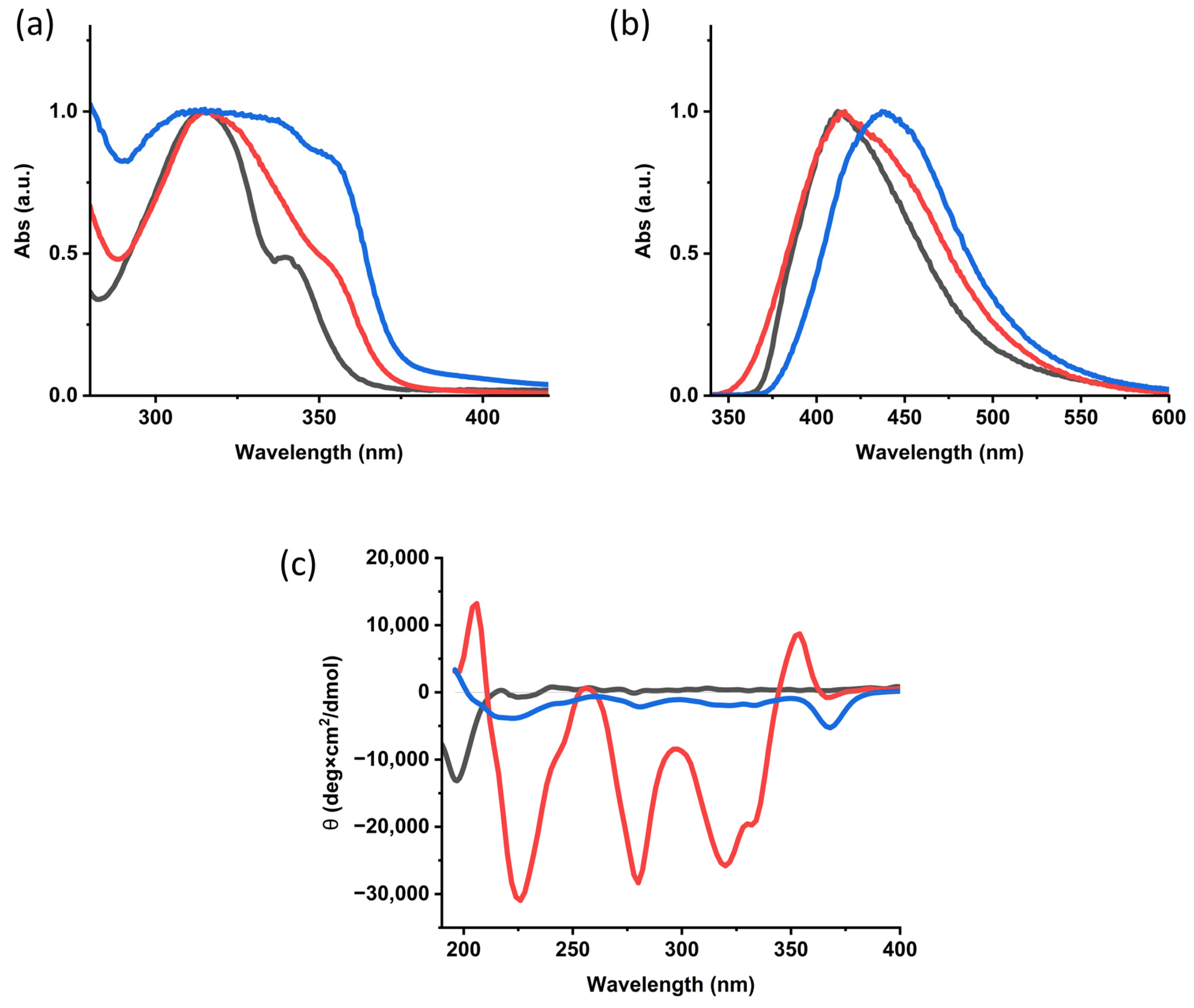
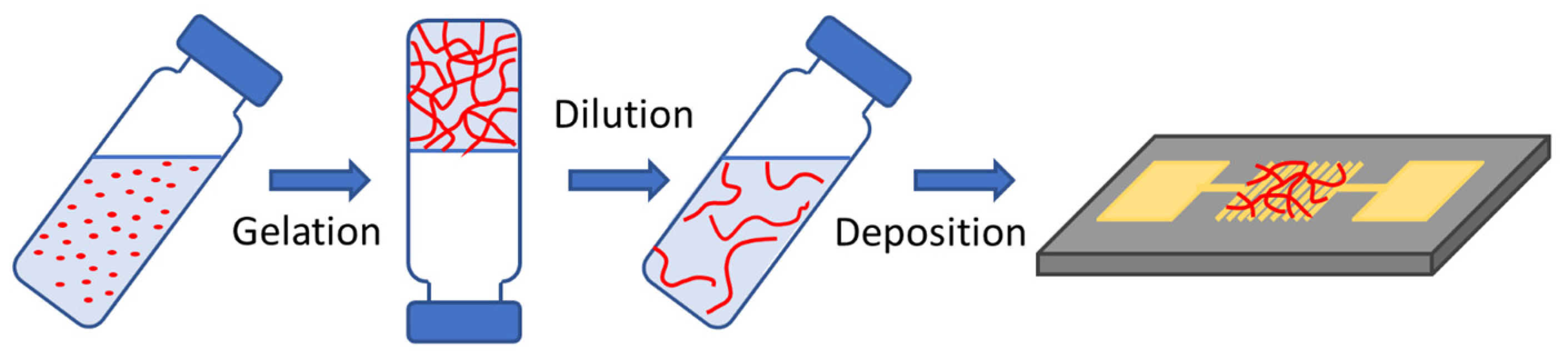
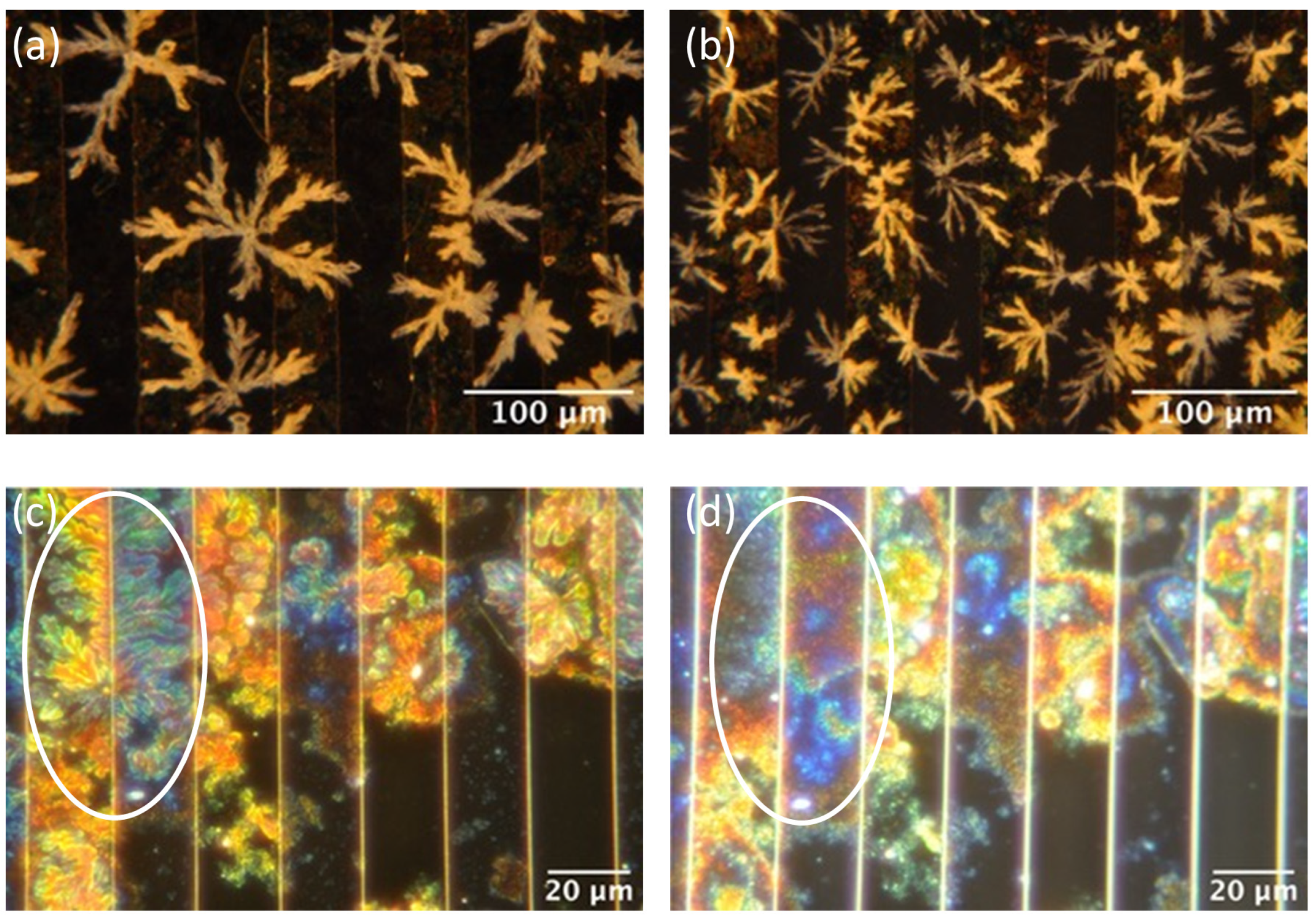
2.2. Gelation and Hydrogel Characterization
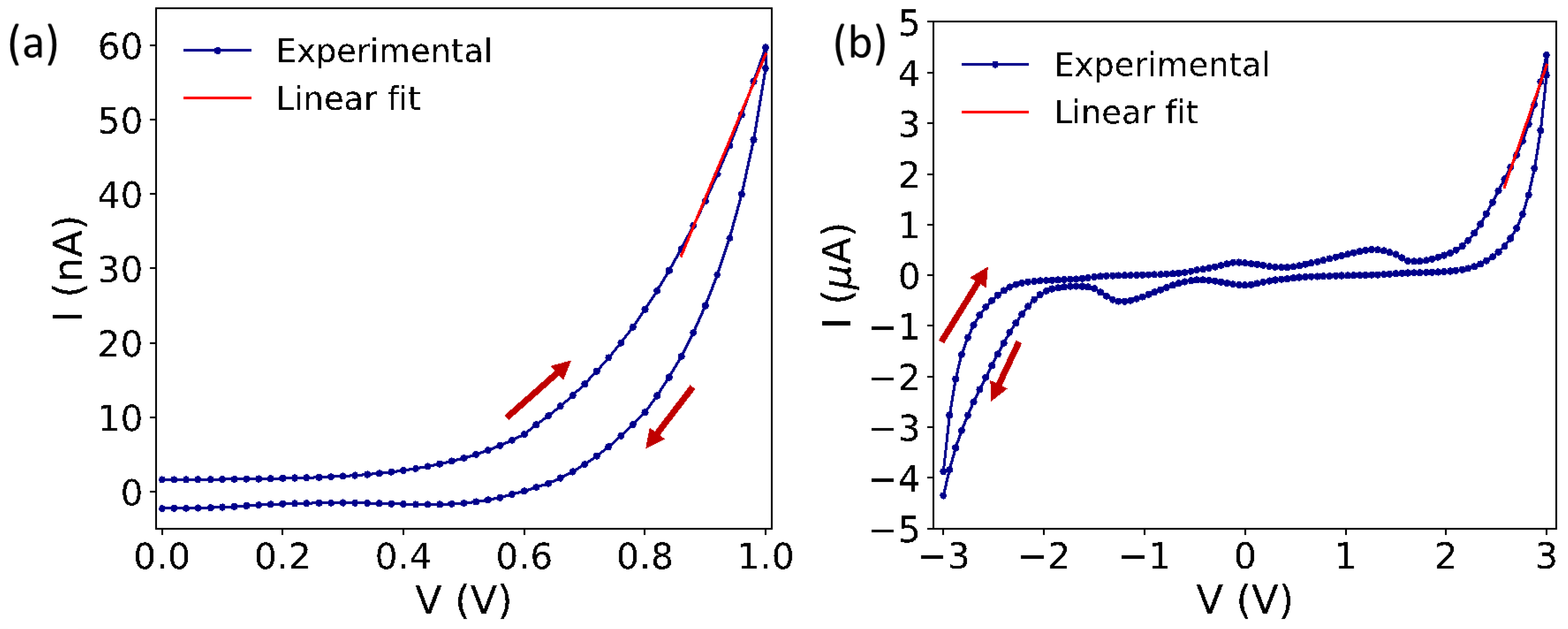
2.3. Electrical Characterization
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoeben, F.J.; Jonkheijm, P.; Meijer, E.; Schenning, A.P. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fraser Stoddart, J. From molecular to supramolecular electronics. Nat. Rev. Mater. 2021, 6, 804–828. [Google Scholar] [CrossRef]
- Leclere, P.; Surin, M.; Viville, P.; Lazzaroni, R.; Kilbinger, A.; Henze, O.; Feast, W.; Cavallini, M.; Biscarini, F.; Schenning, A. About oligothiophene self-assembly: From aggregation in solution to solid-state nanostructures. Chem. Mater. 2004, 16, 4452–4466. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Praveen, V.K. π-Organogels of self-assembled p-phenylenevinylenes: Soft materials with distinct size, shape, and functions. Acc. Chem. Res. 2007, 40, 644–656. [Google Scholar] [CrossRef]
- Jatsch, A.; Schillinger, E.-K.; Schmid, S.; Bäuerle, P. Biomolecule assisted self-assembly of π-conjugated oligomers. J. Mater. Chem. 2010, 20, 3563–3578. [Google Scholar] [CrossRef]
- Gong, C.; Sun, S.; Zhang, Y.; Sun, L.; Su, Z.; Wu, A.; Wei, G. Hierarchical nanomaterials via biomolecular self-assembly and bioinspiration for energy and environmental applications. Nanoscale 2019, 11, 4147–4182. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, C.; Yuan, X.; Wei, G. Controlling the Self-Assembly of Biomolecules into Functional Nanomaterials through Internal Interactions and External Stimulations: A Review. Nanomaterials 2019, 9, 285. [Google Scholar] [CrossRef]
- Ekiz, M.S.; Cinar, G.; Khalily, M.A.; Guler, M.O. Self-assembled peptide nanostructures for functional materials. Nanotechnology 2016, 27, 402002. [Google Scholar] [CrossRef]
- Hu, X.; Liao, M.; Gong, H.; Zhang, L.; Cox, H.; Waigh, T.A.; Lu, J.R. Recent advances in short peptide self-assembly: From rational design to novel applications. Curr. Opin. Colloid Interface Sci. 2020, 45, 1–13. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef]
- Bowerman, C.J.; Nilsson, B.L. Review self-assembly of amphipathic β-sheet peptides: Insights and applications. Pept. Sci. 2012, 98, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective Differentiation of Neural Progenitor Cells by High-Epitope Density Nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.; Barman, R.; Sarkar, J.; Ghosh, S. pH-Responsive Biocompatible Supramolecular Peptide Hydrogel. J. Phys. Chem. B 2019, 123, 5909–5915. [Google Scholar] [CrossRef] [PubMed]
- Eakins, G.L.; Pandey, R.; Wojciechowski, J.P.; Zheng, H.Y.; Webb, J.E.A.; Valéry, C.; Thordarson, P.; Plank, N.O.V.; Gerrard, J.A.; Hodgkiss, J.M. Functional Organic Semiconductors Assembled via Natural Aggregating Peptides. Adv. Func. Mater. 2015, 25, 5640–5649. [Google Scholar] [CrossRef]
- Fichman, G.; Gazit, E. Self-assembly of short peptides to form hydrogels: Design of building blocks, physical properties and technological applications. Acta Biomater. 2014, 10, 1671–1682. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef]
- Dasgupta, A.; Mondal, J.H.; Das, D. Peptide hydrogels. RSC Adv. 2013, 3, 9117–9149. [Google Scholar] [CrossRef]
- Ardoña, H.A.M.; Tovar, J.D. Peptide π-Electron Conjugates: Organic Electronics for Biology? Bioconjug. Chem. 2015, 26, 2290–2302. [Google Scholar] [CrossRef]
- Bartocci, S.; Berrocal, J.A.; Guarracino, P.; Grillaud, M.; Franco, L.; Mba, M. Peptide-Driven Charge-Transfer Organogels Built from Synergetic Hydrogen Bonding and Pyrene–Naphthalenediimide Donor–Acceptor Interactions. Chem. Eur. J. 2018, 24, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Roy, S. Enzyme-Induced Supramolecular Order in Pyrene Dipeptide Hydrogels for the Development of an Efficient Energy-Transfer Template. Biomacromolecules 2021, 22, 2393–2407. [Google Scholar] [CrossRef] [PubMed]
- Dibble, J.P.; Troyano-Valls, C.; Tovar, J.D. A Tale of Three Hydrophobicities: Impact of Constitutional Isomerism on Nanostructure Evolution and Electronic Communication in π-Conjugated Peptides. Macromolecules 2020, 53, 7263–7273. [Google Scholar] [CrossRef]
- Shao, H.; Parquette, J.R. A pi-conjugated hydrogel based on an Fmoc-dipeptide naphthalene diimide semiconductor. Chem. Commun. 2010, 46, 4285–4287. [Google Scholar] [CrossRef] [PubMed]
- Mba, M.; Moretto, A.; Armelao, L.; Crisma, M.; Toniolo, C.; Maggini, M. Synthesis and self-assembly of oligo(p-phenylenevinylene) peptide conjugates in water. Chem. Eur. J. 2011, 17, 2044–2047. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Kavianinia, I.; Hume, P.; De Leon-Rodriguez, L.M.; Kihara, S.; Williams, D.E.; McGillivray, D.J.; Plank, N.O.; Gerrard, J.; Hodgkiss, J.M. Directed self-assembly of peptide–diketopyrrolopyrrole conjugates–a platform for bio-organic thin film preparation. Soft Matter 2020, 16, 6563–6571. [Google Scholar] [CrossRef]
- Xie, P.; Liu, T.; Sun, J.; Yang, J. Structures, Properties, and Device Applications for [1]Benzothieno[3,2-b] Benzothiophene Derivatives. Adv. Funct. Mater. 2022, 32, 2200843. [Google Scholar] [CrossRef]
- Takimiya, K.; Osaka, I.; Mori, T.; Nakano, M. Organic semiconductors based on [1]benzothieno[3,2-b][1] benzothiophene substructure. Acc. Chem. Res. 2014, 47, 1493–1502. [Google Scholar] [CrossRef]
- Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T. Highly soluble [1]benzothieno[3, 2-b] benzothiophene (BTBT) derivatives for high-performance, solution-processed organic field-effect transistors. J. Am. Chem. Soc. 2007, 129, 15732–15733. [Google Scholar] [CrossRef]
- Mori, T.; Nishimura, T.; Yamamoto, T.; Doi, I.; Miyazaki, E.; Osaka, I.; Takimiya, K. Consecutive thiophene-annulation approach to pi-extended thienoacene-based organic semiconductors with [1]benzothieno[3,2-b][1]benzothiophene (BTBT) substructure. J. Am. Chem. Soc. 2013, 135, 13900–13913. [Google Scholar] [CrossRef]
- Minemawari, H.; Tanaka, M.; Tsuzuki, S.; Inoue, S.; Yamada, T.; Kumai, R.; Shimoi, Y.; Hasegawa, T. Enhanced layered-herringbone packing due to long alkyl chain substitution in solution-processable organic semiconductors. Chem. Mater. 2017, 29, 1245–1254. [Google Scholar] [CrossRef]
- Niimi, K.; Shinamura, S.; Osaka, I.; Miyazaki, E.; Takimiya, K. Dianthra[2,3-b:2’,3’-f]thieno[3,2-b]thiophene (DATT): Synthesis, characterization, and FET characteristics of new pi-extended heteroarene with eight fused aromatic rings. J. Am. Chem. Soc. 2011, 133, 8732–8739. [Google Scholar] [CrossRef]
- Yuan, Y.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.; Chen, J.; Nordlund, D.; Toney, M.F.; Huang, J.; Bao, Z. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5, 3005. [Google Scholar] [CrossRef] [PubMed]
- Khalily, M.A.; Usta, H.; Ozdemir, M.; Bakan, G.; Dikecoglu, F.B.; Edwards-Gayle, C.; Hutchinson, J.A.; Hamley, I.W.; Dana, A.; Guler, M.O. The design and fabrication of supramolecular semiconductor nanowires formed by benzothienobenzothiophene (BTBT)-conjugated peptides. Nanoscale 2018, 10, 9987–9995. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A.; Sanzone, A.; Mattiello, S.; Beverina, L.; Mba, M. The pH-and salt-controlled self-assembly of [1]benzothieno[3,2-b][1]-benzothiophene–peptide conjugates in supramolecular hydrogels. New J. Chem. 2021, 45, 13389–13398. [Google Scholar] [CrossRef]
- Ardoña, H.A.M.; Besar, K.; Togninalli, M.; Katz, H.E.; Tovar, J.D. Sequence-dependent mechanical, photophysical and electrical properties of pi-conjugated peptide hydrogelators. J. Mater. Chem. C 2015, 3, 6505–6514. [Google Scholar] [CrossRef]
- Lehrman, J.A.; Cui, H.; Tsai, W.-W.; Moyer, T.J.; Stupp, S.I. Supramolecular control of self-assembling terthiophene–peptide conjugates through the amino acid side chain. Chem. Commun. 2012, 48, 9711–9713. [Google Scholar] [CrossRef]
- Jira, E.R.; Shmilovich, K.; Kale, T.S.; Ferguson, A.; Tovar, J.D.; Schroeder, C.M. Effect of Core Oligomer Length on the Phase Behavior and Assembly of pi-Conjugated Peptides. ACS Appl. Mater. Interfaces 2020, 12, 20722–20732. [Google Scholar] [CrossRef]
- Jones, C.W.; Morales, C.G.; Eltiste, S.L.; Yanchik-Slade, F.E.; Lee, N.R.; Nilsson, B.L. Capacity for increased surface area in the hydrophobic core of β-sheet peptide bilayer nanoribbons. J. Pept. Sci. 2021, 27, e3334. [Google Scholar] [CrossRef]
- Yilmaz, M.; Ozdemir, M.; Erdogan, H.; Tamer, U.; Sen, U.; Facchetti, A.; Usta, H.; Demirel, G. Micro-/Nanostructured Highly Crystalline Organic Semiconductor Films for Surface-Enhanced Raman Spectroscopy Applications. Adv. Func. Mater. 2015, 25, 5669–5676. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Sikorski, P. Electroconductive scaffolds for tissue engineering applications. Biomater. Sci. 2020, 8, 5583–5588. [Google Scholar] [CrossRef]
- Chow, E.; Herrmann, J.; Barton, C.S.; Raguse, B.; Wieczorek, L. Inkjet-printed gold nanoparticle chemiresistors: Influence of film morphology and ionic strength on the detection of organics dissolved in aqueous solution. Anal. Chim. Acta 2009, 632, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, H.; Liu, J.; Zhang, J.; Shi, X.; Shi, Y.; Li, C.; Liu, Z.; Li, T.; Jiang, L. Asymmetrical [1]Benzothieno [3,2-b][1] benzothiophene (BTBT) derivatives for organic thin-film and single-crystal transistors. Org. Electr. 2020, 77, 105537. [Google Scholar] [CrossRef]
- Košata, B.; Kozmík, V.; Svoboda, J. Reactivity of [1]Benzothieno[3,2-b][1]benzothiophene—Electrophilic and Metallation Reactions. Collect. Czech. Chem. Commun. 2002, 67, 645–664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, A.; Hensel, R.C.; Casalini, S.; Mba, M. Self-Assembly and Electrical Conductivity of a New [1]benzothieno[3,2-b][1]-benzothiophene (BTBT)-Peptide Hydrogel. Molecules 2023, 28, 2917. https://doi.org/10.3390/molecules28072917
Fortunato A, Hensel RC, Casalini S, Mba M. Self-Assembly and Electrical Conductivity of a New [1]benzothieno[3,2-b][1]-benzothiophene (BTBT)-Peptide Hydrogel. Molecules. 2023; 28(7):2917. https://doi.org/10.3390/molecules28072917
Chicago/Turabian StyleFortunato, Anna, Rafael Cintra Hensel, Stefano Casalini, and Miriam Mba. 2023. "Self-Assembly and Electrical Conductivity of a New [1]benzothieno[3,2-b][1]-benzothiophene (BTBT)-Peptide Hydrogel" Molecules 28, no. 7: 2917. https://doi.org/10.3390/molecules28072917
APA StyleFortunato, A., Hensel, R. C., Casalini, S., & Mba, M. (2023). Self-Assembly and Electrical Conductivity of a New [1]benzothieno[3,2-b][1]-benzothiophene (BTBT)-Peptide Hydrogel. Molecules, 28(7), 2917. https://doi.org/10.3390/molecules28072917