How Closely Does Induced Agarwood’s Biological Activity Resemble That of Wild Agarwood?
Abstract
1. Introduction
2. Results
2.1. Chemical Composition Analysis
2.1.1. Volatile Chemical Composition Analysis
2.1.2. Total Chromone Content
2.2. Bioactivity Analysis
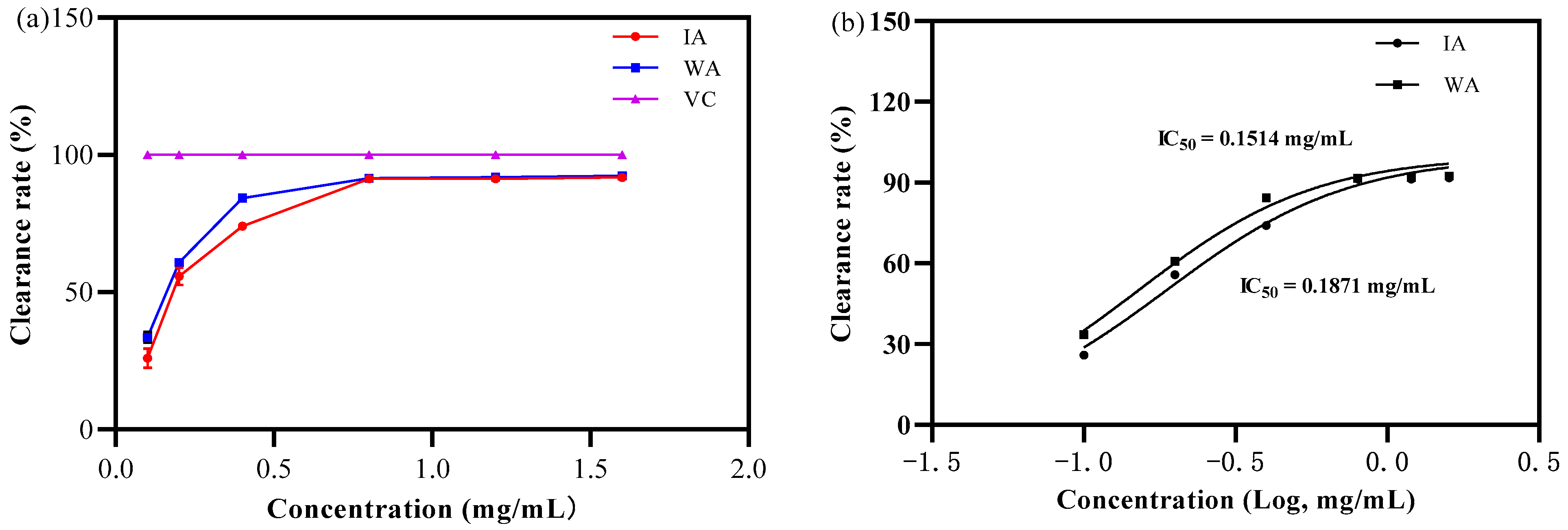
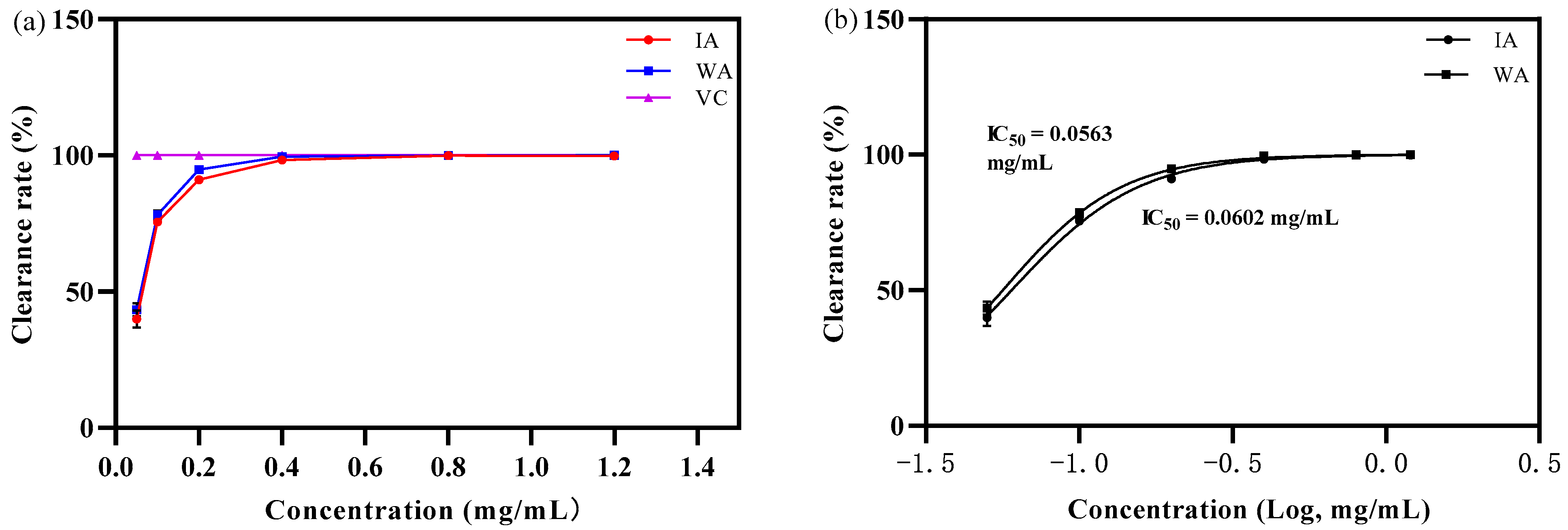
2.2.1. Antioxidant Activity Analysis
- DPPH free radical scavenging capacity
- ABTS+ free radical scavenging capacity
2.2.2. Anti-Acetylcholinesterase Activity Capacity
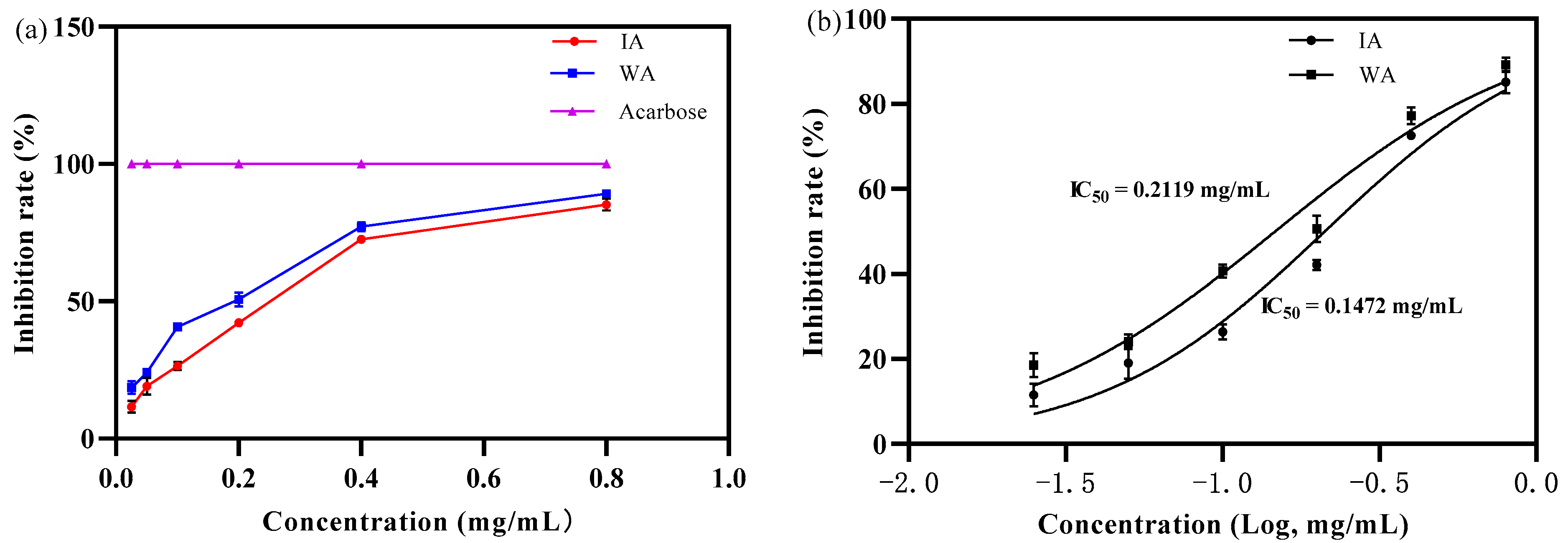
2.2.3. Anti-α-Glucosidase Activity Capacity
3. Discussion
3.1. Chemical Composition of Induced Agarwood and Wild Agarwood
3.2. Biological Activity of Induced Agarwood and Wild Agarwood
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Chemical Composition Determination
- Volatile chemical composition determination
- Total chromone content
4.2.2. Bioactivity Measurements
- Preparation of alcohol-soluble extract
- Determination of antioxidant capacity
- Determination of acetylcholinesterase activity inhibition
- Determination of anti-α-glucosidase activity inhibition
- Data processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B


References
- Azren, P.D.; Azren, P.D.; Lee, S.Y.; Lee, S.Y.; Emang, D.; Emang, D.; Mohamed, R.; Mohamed, R. History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: A review. J. For. Res. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Naziz, P.S.; Das, R.; Sen, S. The scent of stress: Evidence from the unique fragrance of agarwood. Front. Plant Sci. 2019, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Abdin, J. The agar wood industry: Yet to utilize in Bangladesh. Int. J. Econ. Manag. Sci. 2014, 3. [Google Scholar] [CrossRef]
- López-Sampson, A.; Page, T. History of use and trade of agarwood. Econ. Bot 2018, 72, 107–129. [Google Scholar] [CrossRef]
- Yin, Y.; Jiao, L.; Dong, M.; Jiang, X.; Zhang, S. Wood Resources, Identification, and Utilization of Agarwood in China; Springer: Singapore, 2016; pp. 21–38. [Google Scholar] [CrossRef]
- Thanh, L.V.; Do, T.V.; Son, N.H.; Sato, T.; Kozan, O. Impacts of biological, chemical and mechanical treatments on sesquiterpene content in stems of planted Aquilaria crassna trees. Agrofor. Syst. 2015, 89, 973–981. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Dong, W.H.; Guo, Z.K.; Wang, H.; Mei, W.L.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives from chinese agarwood induced by artificial holing. Fitoterapia 2014, 98, 117–123. [Google Scholar] [CrossRef]
- Wang, S.L.; Tsai, Y.C.; Fu, S.L.; Cheng, M.J.; Chung, M.I.; Chen, J.J. 2-(2-phenylethyl)-4h-chromen-4-one derivatives from the resinous wood of Aquilaria sinensis with anti-inflammatory effects in lps-induced macrophages. Molecules 2018, 23, 289. [Google Scholar] [CrossRef]
- Mi, C.N.; Yuan, J.Z.; Zhu, M.M.; Yang, L.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives: Promising α-glucosidase inhibitors in agarwood from Aquilaria filaria. Phytochemistry 2021, 181, 112578. [Google Scholar] [CrossRef]
- Liao, G.; Mei, W.L.; Kong, F.D.; Li, W.; Yuan, J.Z.; Dai, H.F. 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones from artificial agarwood of Aquilaria sinensis and their inhibitory activity against acetylcholinesterase. Phytochemistry 2017, 139, 98–108. [Google Scholar] [CrossRef]
- Ma, S.; Fu, Y.; Li, Y.; Wei, P.; Liu, Z. The formation and quality evaluation of agarwood induced by the fungi in Aquilaria sinensis. Ind. Crops Prod. 2021, 173, 114129. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Xue, J.; Wei, J.; Zhang, Z.; Chen, H. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) Gilg trees. Molecules 2011, 16, 4884–4896. [Google Scholar] [CrossRef] [PubMed]
- Douglas, D.; Nei, P.; Aline, M. A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr. Opin. Green Sustain. 2018, 14, 60–66. [Google Scholar] [CrossRef]
- Turjaman, M.; Hidayat, A.; Santoso, E. Development of Agarwood Induction Technology Using Endophytic Fungi; Springer: Singapore, 2016; pp. 57–71. [Google Scholar] [CrossRef]
- Obara, K. Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in zinnia. Plant Physiol. 2001, 125, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.T. Programmed cell death in plant-pathogen interactions. Annu. Rev. Plant Biol. 1997, 48, 525–545. [Google Scholar] [CrossRef]
- Wang, X.H.; Gao, B.W.; Nakashima, Y.; Mori, T.; Zhang, Z.X.; Kodama, T.; Lee, Y.E.; Zhang, Z.K.; Wong, C.P.; Liu, Q.Q. Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl)chromones in agarwood. Nat. Commun. 2022, 13, 348. [Google Scholar] [CrossRef]
- Lv, F.; Yang, Y.; Sun, P.; Zhang, Y.; Liu, P.; Fan, X.; Xu, Y.; Wei, J. Comparative transcriptome analysis reveals different defence responses during the early stage of wounding stress in chi-nan germplasm and ordinary Aquilaria sinensis. BMC Plant Biol. 2022, 22, 464. [Google Scholar] [CrossRef]
- Liao, G.; Dong, W.H.; Yang, J.L.; Li, W.; Dai, H.F. Monitoring the chemical profile in agarwood formation within one year and speculating on the biosynthesis of 2-(2-phenylethyl)chromones. Molecules 2018, 23, 1261. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermat. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Zhou, P.; Zeng, Y.B.; Mei, W.L.; Dong, W.H.; Li, W.; Zhou, L.M.; Dai, H.F. Chemical constituents and bioactivities of Aquilariae Lignum Resinatum by artificial holing method. J. Chin. Med. Mater. 2017, 40, 2339–2343. (In Chinese) [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.; Pardal-Fernández, J.M.; García-Martínez, E.; Segura, T. Respiratory myoclonus, a side effect of galantamine. Farm Hosp. 2011, 35, 97–99. [Google Scholar] [CrossRef]
- Be Rnd Ibach, E.H. Acetylcholinesterase inhibition in Alzheimer’s disease. Curr. Pharm. Design. 2004, 10, 231–251. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Guo, Z.K.; Wang, H.; Zuo, W.J.; Dong, W.H.; Mei, W.L.; Dai, H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia 2015, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Mei, W.L.; Dong, W.H.; Li, W.; Wang, P.; Kong, F.D.; Gai, C.J.; Song, X.Q.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives in artificial agarwood from Aquilaria sinensis. Fitoterapia 2016, 110, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.; Chen, H.; Kong, F.; Cai, C.; Yang, L.; Mei, W.; Dai, H. Three new 2-(2-phenylethyl)chromone derivatives from artificial holing agarwood of Aquilaria sinensis. Phytochem. Lett. 2018, 26, 96–100. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y. Tcmsp: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Magliano, D.J. Idf diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Dabhi, A.S.; Bhatt, N.R.; Shah, M.J. Voglibose: An alpha glucosidase inhibitor. J. Clin. Diagn. Res. 2013, 7, 3023. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhao, D.G.; Zhou, A.Y.; Zhang, Y.; Du, Z.; Zhang, K. A-glucosidase inhibition and antihyperglycemic activity of phenolics from the flowers of Edgeworthia gardneri. J. Agric. Food Chem. 2015, 63, 8162. [Google Scholar] [CrossRef]
- Kang, K.X.; Dai, H.F.; Wang, P.; Kong, F.D.; Zhou, L.M.; Dong, W.H.; Zhu, G.P.; Mei, W.L. Sesquiterpenoids of agarwood from Aquilaria crassna. Chin. Tradit. Herb. Drugs 2017, 48, 4601–4607. (In Chinese) [Google Scholar] [CrossRef]
- Mei, W.; Yang, D.; Wang, H.; Yang, J.; Zeng, Y.; Guo, Z.; Dong, W.; Li, W.; Dai, H. Characterization and determination of 2-(2-phenylethyl) chromones in agarwood by GC-MS. Molecules 2013, 18, 12324–12345. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.D.L.; Ferreira, S.R.S.; Vitali, L.; Block, J.M. May the superfruit red guava and its processing waste be a potential ingredient in functional foods? Food Res. Int. 2019, 115, 451–459. [Google Scholar] [CrossRef]
- Wang, H.N.; Dong, W.H.; Huang, S.Z.; Li, W.; Kong, F.D.; Wang, H.; Wang, J.; Mei, W.L.; Dai, H.F. Three new sesquiterpenoids from agarwood of Aquilaria crassna. Fitoterapia 2016, 114, 7–11. [Google Scholar] [CrossRef]
- Hong, H.C.; Li, S.L.; Zhang, X.Q.; Ye, W.C.; Zhang, Q.W. Flavonoids with α-glucosidase inhibitory activities and their contents in the leaves of Morus atropurpurea. Chin. Med. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
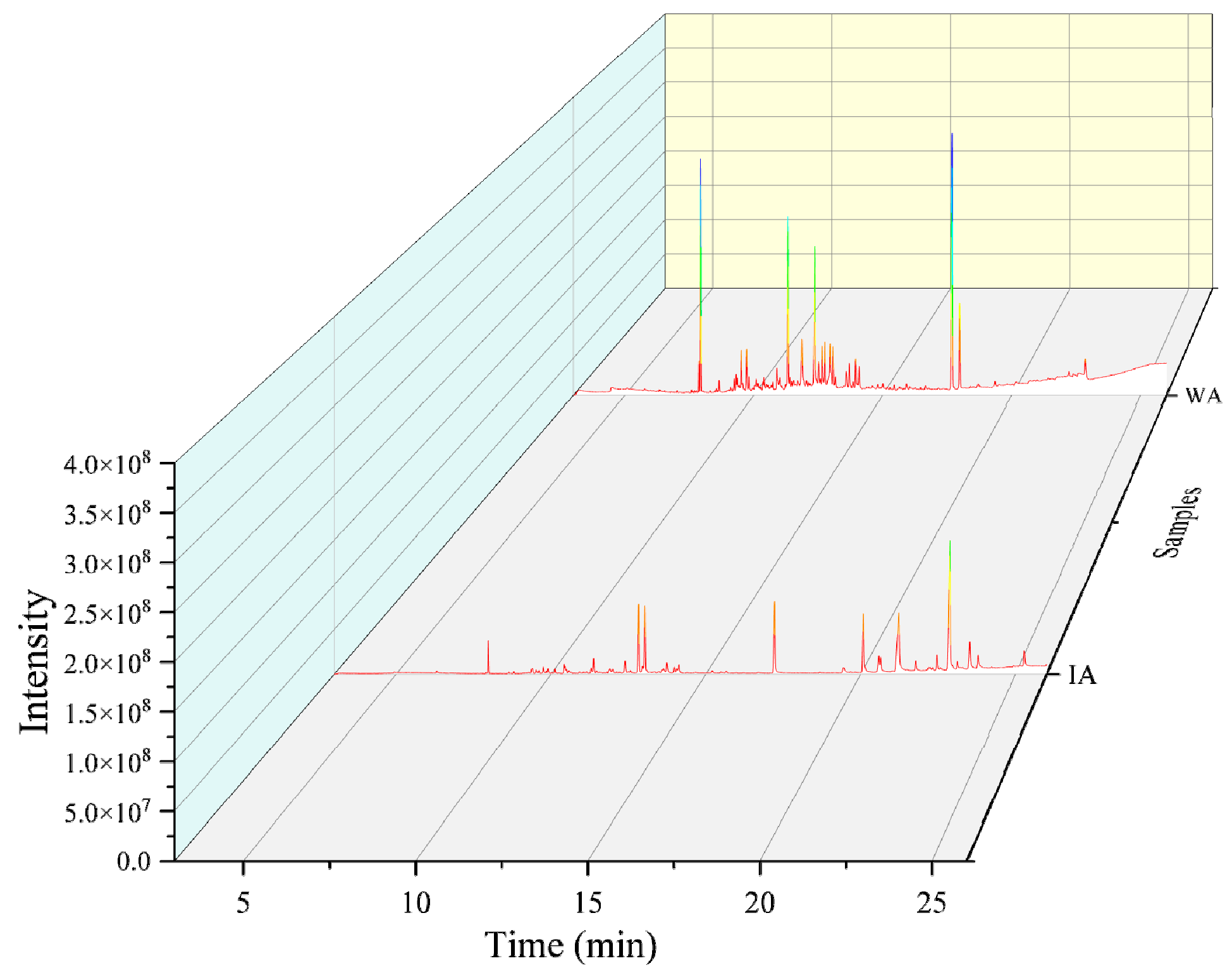



| IA | WA | ||||
|---|---|---|---|---|---|
| RT (min) | Component | Relative Content (%) | RT (min) | Component | Relative Content (%) |
| 6.309 | 4-phenyl-2-Butanone | 0.31 | 7.934 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl-, methylcarbamate | 8.11 |
| 9.518 | Calarene epoxide | 0.38 | 9.311 | 2-((2R,8R,8aS)-8,8a-Dimethyl-1,2,3,4,6,7,8,8a-octahydronaphthalen-2-yl)propan-2-ol | 0.76 |
| 9.612 | 4-(1,5-dimethyl-1,4-hexadienyl)-1-methyl-Cyclohexene | 0.30 | 9.354 | Azulene, 1,2,3,5,6,7,8,8a-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, [1S-(1.alpha.,7.alpha.,8a.beta.)]- | 0.48 |
| 9.754 | 2-(2R,4aR,8aR)-4a,8-Dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalen-2-yl)acrylaldehyde | 0.76 | 9.363 | Isolongifolen-5-one | 0.51 |
| 9.845 | 3,4,4a,5,6,7-hexahydro-4a,5-dimethyl-3-(1-methylethenyl)-, [3S-(3.alpha.,4a.alpha.,5.alpha.)]-1(2H)-Naphthalenone | 1.95 | 9.558 | Spiro[4.5]dec-8-en-7-ol, 4,8-dimethyl-1-(1-methylethyl)- | 0.28 |
| 9.902 | Humulene epoxide I | 0.55 | 9.724 | Acorenone B | 2.10 |
| 10.124 | 2-(4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-2-yl)-prop-2-en-1-ol | 0.50 | 10.098 | Daucol | 0.60 |
| 10.426 | Ylangenal | 1.18 | 10.163 | Cyclohexanemethanol, 4-ethenyl-.alpha.,.alpha.,4-trimethyl-3-(1-methylethenyl)-, [1R-(1.alpha.,3.alpha.,4.beta.)]- | 0.31 |
| 11.908 | Arctiol | 0.69 | 10.356 | 3-epi-Cedrenal | 0.36 |
| 12.003 | 4,5,5a,6,6a,6b-hexahydro-4,4,6b-trimethyl-2-(1-methylethenyl)-2H-Cyclopropa[g]benzofuran | 0.57 | 10.9 | 7-(2-Hydroxypropan-2-yl)-1,4a-dimethyldecahydronaphthalen-1-ol | 1.25 |
| 12.392 | Valerenic acid | 1.64 | 11.006 | 2(3H)-Naphthalenone, 4,4a,5,6,7,8-hexahydro-4a,5-dimethyl-3-(1-methylethylidene)-, (4ar-cis)- | 0.64 |
| 12.567 | 4-(3,3-Dimethyl-but-1-ynyl)-4-hydroxy-3,5,5-trimethyl-cyclohex-2-enone | 0.29 | 11.237 | (2S,6R)-2,6-Dimethyl-2-(2-(4-methylfuran-3-yl)ethyl)cyclohexanone | 0.21 |
| 12.829 | 5,8-Dihydroxy-4a-methyl-4,4a,4b,5,6,7,8,8a,9,10-decahydro-2(3H)-phenanthrenone | 18.56 | 11.325 | 2,4,6,7,8,8a-hexahydro-3,8-dimethyl-4-(1-methylethylidene)-(8S-cis)-5(1H)-azulenone | 9.90 |
| 13.74 | 6-(1-Hydroxymethylvinyl)-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-1H-naphthalen-2-one | 1.64 | 11.405 | 1,8-dimethyl-8,9-epoxy-4-isopropyl-Spiro[4.5]decan-7-one | 0.58 |
| 13.981 | (2aS,3aR,5aS,9bR)-2a,5a,9-Trimethyl-2a,4,5,5a,6,7,8,9b-octahydro-2H-naphtho[1,2-b]oxireno[2,3-c]furan | 0.82 | 11.545 | 3.beta.,9.beta.-Dihydroxy-3,5.alpha.,8-trimethyltricyclo[6.3.1.0(1,5)]dodecane | 0.29 |
| 15.657 | 2-hydroxy-5-(3-methyl-2-butenyl)-4-(1-methylethenyl)-2,4,6-Cycloheptatrien-1-one | 0.29 | 11.869 | 1,1,4,7-Tetramethyldecahydro-1H-cyclopropa[e]azulene-4,7-diol | 4.27 |
| 17.216 | 2-(2-phenylethyl)chromone | 9.46 | 12.755 | 5,8-Dihydroxy-4a-methyl-4,4a,4b,5,6,7,8,8a,9,10-decahydro-2(3H)-phenanthrenone | 6.09 |
| 19.43 | 7-Methoxy-3-(p-methoxyphenyl)chromone | 3.06 | 13.079 | (4aS,7R)-7-(2-Hydroxypropan-2-yl)-1,4a-dimethyl-4,4a,5,6,7,8-hexahydronaphthalen-2(3H)-one | 2.52 |
| 20.09 | 8-Methoxy-2-(2-phenylethyl)chromone | 8.39 | 13.59 | 6-(1-Hydroxymethylvinyl)-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-1H-naphthalen-2-one | 1.40 |
| 22.261 | Supraene | 0.35 | 13.705 | Valerenic acid | 1.64 |
| 22.469 | 6-Methoxy-2-(4-methoxyphenethyl)chromone | 2.15 | 13.952 | (2aS,3aR,5aS,9bR)-2a,5a,9-Trimethyl-2a,4,5,5a,6,7,8,9b-octahydro-2H-naphtho[1,2-b]oxireno[2,3-c]furan | 2.09 |
| 22.886 | 6,7-Dimethoxy-2-(2-phenethyl)chromone | 23.17 | 14.085 | 2,4,6-Cycloheptatrien-1-one, 2-hydroxy-5-(3-methyl-2-butenyl)-4-(1-methylethenyl)- | 1.32 |
| 25.286 | 6,7-Dimethoxy-2-(4-methoxyphenethyl)chromone | 2.53 | 17.993 | 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl-phenol | 4.54 |
| 22.197 | Supraene | 0.27 | |||
| 22.84 | 6,7-Dimethoxy-2-(2-phenethyl)chromone | 1.16 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Huang, M.; Fu, Y.; Qiao, M.; Li, Y. How Closely Does Induced Agarwood’s Biological Activity Resemble That of Wild Agarwood? Molecules 2023, 28, 2922. https://doi.org/10.3390/molecules28072922
Ma S, Huang M, Fu Y, Qiao M, Li Y. How Closely Does Induced Agarwood’s Biological Activity Resemble That of Wild Agarwood? Molecules. 2023; 28(7):2922. https://doi.org/10.3390/molecules28072922
Chicago/Turabian StyleMa, Sheng, Manqin Huang, Yunlin Fu, Mengji Qiao, and Yingjian Li. 2023. "How Closely Does Induced Agarwood’s Biological Activity Resemble That of Wild Agarwood?" Molecules 28, no. 7: 2922. https://doi.org/10.3390/molecules28072922
APA StyleMa, S., Huang, M., Fu, Y., Qiao, M., & Li, Y. (2023). How Closely Does Induced Agarwood’s Biological Activity Resemble That of Wild Agarwood? Molecules, 28(7), 2922. https://doi.org/10.3390/molecules28072922





