Abstract
The aim of the present study was to examine three different Galium species from the native population of Estonia, Galium verum, Galium aparine, and Galium mollugo, to characterise their non-volatile and volatile phytochemical composition and antioxidant activity. The main groups of bioactive compounds in the plants were quantified by colorimetric tests, showing high concentrations of polyphenols (up to 27.2 ± 1.5 mg GAE/g), flavonoids (up to 7.3 ± 0.5 mg QE/g) and iridoids (up to 40.8 ± 2.9 mg AE/g). The species were compared using HPLC-DAD-MS/MS, revealing some key differences in the phytochemical makeup of the extracts. The most abundant compound in the extracts of Galium verum blossoms and herb was found to be asperuloside, in Galium aparine herb, asperulosidic acid, and in Galium mollugo herb, chlorogenic acid. Additionally, the composition of volatile compounds was analysed by SPME-GC-MS. The degree of variability between the samples was high, but three volatiles, hexanal, anethole, and β-caryophyllene, were quantified (≥1%) in all analysed samples. The antioxidative activity of all extracts was evaluated using the ORACFL method, demonstrating that the Galium species from Estonia all exhibit strong antioxidant capacity (up to 9.3 ± 1.2 mg TE/g). Out of the extracts studied, Galium verum blossoms contained the highest amounts of bioactives and had the strongest antioxidant capacity.
1. Introduction
Ethnomedicine has long used different plants for a variety of disease preventative and therapeutic purposes. Plants are a natural source of active compounds, phytochemicals, many of which with favourable bioactivity for humans. Analytical investigation allows for the determination of the phytochemical composition and confirmation of specific therapeutic properties of the plants already acknowledged for their potential by ethnomedicine [1,2,3,4,5]. The Galium L. genus, comprising about 667 species found worldwide, over a third of which can be found in Europe, includes several species that have been used in traditional medicine to alleviate a variety of ailments [6,7]. Galium verum has been used as a sedative, diuretic, and choleretic, as well as to treat gout, epilepsy, and spasms; Galium aparine has been used to reduce infection and inflammation, to treat wounds, burns, and skin diseases; Galium mollugo has been used as vulnerary, and to treat hysteria and epilepsy [7,8,9]. Many scientific studies have confirmed that the representatives of the Galium genus exhibit a wide range of biological activities, including antioxidant, anticancer, detoxicant, hepatoprotective, antihaemolytic, antibacterial, and antifungal effects [2,7,9,10,11]. Phytochemicals are typically extracted from plant materials using alcohols or acetone in varying proportions of water, depending on the properties of the compounds of interest [9]. Therefore, a comparative analysis of the earlier literature is complicated due to the use of varying extraction solvents. The most common analysis methods of plant extracts are HPLC or UHPLC, coupled with DAD and/or MS/MS, sometimes followed by identity confirmation with NMR [9,12,13]. Previously, Mitova et al. have analysed the iridoid patterns of the Bulgarian Galium species [12], and Vlase et al. have studied the polyphenolic content of Romanian Galium species [13]. As it is known that the geographic origin and growth environment of a plant play a large role in the phytochemical content of the extract [14], it is likely that the Galium species collected from different locations in Europe exhibit a different phytochemical profile. Therefore, it is important to assess the constituents of all medicinal plants in relation to their growth habitat. To the best of our knowledge, species of Galium native to a northern European country have not yet been thoroughly characterised. Additionally, when evaluating plant extracts as possible therapeutic agents, the assessment of antioxidative capacity is of clear importance. As excess free radicals are known to damage cellular components and thereby be involved in the development of several diseases, the free radical scavenging properties of antioxidants can alleviate or prevent illness [15].
Studies of medicinal plants have demonstrated that the health-promoting properties and therapeutically beneficial qualities can often be attributed to specific polyphenols or iridoids, two of the most prevalent groups of bioactives in many plant extracts, and in the representatives of the Galium genus [9,11,16,17]. Polyphenols are the most abundant secondary metabolites in plants, notably characterised by their potent antioxidant properties [18]. Polyphenols also modulate the activity of many enzyme and cell receptors, scavenge free radicals, regulate nitric oxide, decrease leukocyte immobilization, induce apoptosis, and inhibit cell proliferation and angiogenesis [18,19,20,21,22,23]. Therefore, polyphenols possess several mechanisms for preventing and treating illnesses. As deduced from epidemiologic data, polyphenols exert preventative effects over cardiovascular, neurodegenerative, and oxidative stress associated diseases, such as cancer [19,24,25,26,27]. Plant phenolics include phenolic acids, flavonoids, tannins, stilbenes and lignans. Phenolic acids include derivatives of benzoic acid such as gallic acid, and derivatives of cinnamic acid, such as coumaric, caffeic and ferulic acid. Flavonoids are the most prevalent polyphenols in the human diet [18], and possess a variety of pharmacological effects mainly tied to their free radical scavenging and antioxidative properties, such as hepatoprotective, antiatherosclerotic, anti-inflammatory, antithrombogenic, antitumor, antiosteoporotic, antibacterial, and antiviral effects [22,28,29,30]. Some of the most common flavonoids include quercetin, catechin, naringenin, kaempferol, rutin, cyanidin-glycoside, daidzein, genistein, and glycitein [19,31]. Prevalent in all land plants, the second major group of active compounds are iridoids, mainly found as conjugates, iridoid glycosides [32]. Derivatives of iridoids, secoiridoids, are present in about 57 plant families [33]. Specific iridoids can be used as chemical markers of several genera in different plant families, for example, asperuloside of Galium, aucubin of Plantago, and aucubin and harpagide of Scrophularia [12,34]. Iridoids are found in numerous ethnomedicinal plants that have been used as sedatives, hypotensives, antipyretics, and to treat diabetes, cough, wounds, skin disorders, and other inflammatory diseases [33,35,36]. Pharmacological research has confirmed that naturally occurring iridoids exhibit a variety of useful functionalities: neuroprotective, immunomodulatory, antidiabetic, cardioprotective, antihepatotoxic, hepatoprotective, choleretic, hypoglycemic and hypolipidemic, anti-inflammatory, wound healing, antispasmodic, antitumor, antiviral, antibacterial, and antifungal activities [32,33,36,37,38,39,40].
In addition to non-volatile compounds, volatile phytochemicals are also known to possess many favourable biological activities, including antimicrobial, antioxidant, and anti-inflammatory effects [41,42]. There are a few studies on the comparison of chemical volatile profiles of Galium species [43,44,45,46]. Ciotlaus et al. analysed volatile compounds in Galium verum by SPME-GC-MS method using Carboxen/PDMS fibre coating and compared fresh and dried plant material [43]. The major compounds identified in the floral bouquet of dried flower were hexanal, Z-2-hexenal, 1-hexanol, eucalyptol, linalool, and camphor [43]. According to the Green Analytical Chemistry and Green Sample Preparation principles, SPME-GC-MS analysis procedure should be preferable compared to traditionally used essential oil extraction methods, such as steam- or hydrodistillation paired with GC-FID analysis [47,48,49]. The SPME-GC-MS analysis procedure is more sensitive, consumes less energy, involves no solvent use, and minimizes sample volume and waste production, but allows for a comparison of the composition of volatile compounds in different plant species [50]. The PDMS/DVB SPME fibre is coated with a mixed (bipolar) phase, which allows the analysis of both more polar and nonpolar compounds from the headspace and is often used for the analysis of volatiles from plant material [50].
The aim of this paper was to examine the hydroalcoholic and -acetonic extracts of the native Estonian Galium species (Galium verum blossoms, Galium verum herb, Galium aparine herb, Galium mollugo herb) to determine their non-volatile phytochemical profile and antioxidant potential dependent on the three different extraction solvents used. Moreover, a thorough profiling of the volatiles found in these plants helps to widen the reach of the potential therapeutic applications for the Estonian Galium species.
2. Results and Discussion
2.1. Colorimetric Assays and Antioxidativity
The antioxidant activities and the total polyphenolic, flavonoid, and iridoid content of the extracts are shown in Table 1. The Estonian Galium species, especially Galium verum, are an excellent source of polyphenols and iridoids. As discussed earlier, this shows the great therapeutic potential of these plants. The results suggest that extraction with 50% acetone yields the highest quantities of polyphenols (up to 25.1 ± 0.8 mg GAE/g) and flavonoids (up to 7.3 ± 0.5 mg QE/g), whereas 50% ethanol should be used to obtain extracts containing large amounts of iridoids (up to 36.6 ± 2.2 mg AE/g). Across the different extracts, both 50% acetone and 50% ethanol extracts are characterised by high antioxidant activities (up to 9.3 ± 1.2 and 7.2 ± 1.4 mg TE/g, respectively). The colorimetric analyses demonstrated that the extracts of Galium verum blossoms generally contained the highest amounts of bioactives and had the strongest antioxidant properties. Additionally, the results from these experiments exemplify the linear correlation between the total polyphenolic content and the antioxidativity of the Galium extracts (correlation graph provided in Supplementary Materials, Figure S1), illustrated by the two extracts with the highest polyphenolic content also having the strongest antioxidative properties (namely, the 50% acetone extracts of Galium verum blossoms and Galium mollugo herb). Therefore, our results confirm the earlier reports of antioxidant properties being attributed to the polyphenolics the extracts contain. Regardless of the extraction solvent used, both Galium verum blossoms and Galium mollugo herb showed high polyphenolic content and strong antioxidant properties.

Table 1.
Antioxidant activity, and total polyphenolic, flavonoid, and iridoid content of the Galium extracts.
2.2. Identification and Quantification of Major Constituents in Extracts
The 27 non-volatile compounds that were quantified (Figure 1, Table 2 and Table 3) were chosen as the most representative to characterise both the similarities and differences amongst the Galium species. The results indicate a few key characteristics that clearly differentiate the chromatographic profile and the phytochemical content of the extracts. The specific phytochemicals in the analysed extracts were identified using reference standards and HPLC-DAD-MS/MS results, considering the UV spectra, molecular ion, and fragmentation patterns in comparison with the literature (Figure 1, Table 2). The phytochemicals were then quantified as described in the Materials and Methods section, according to the DAD signal and standard compound calibration curves (Table 3). The phytochemicals that were identified and quantified in all extracts were deacetylasperulosidic acid, asperulosidic acid, chlorogenic acid, asperuloside, rutin, and quercetin (compounds 1, 7, 9, 11, 14, 26). Neochlorogenic acid (comp. 6) was quantified in all extracts but the ones of Galium verum blossoms, showing the highest concentrations in Galium mollugo extracts. Additionally, the high content of diosmetin isomers (comp. 18, 19, 23) in Galium mollugo clearly sets apart this chromatographic profile from others. Galium aparine extracts are characterised by a comparatively large cryptochlorogenic acid (comp. 10) peak and a reduced asperuloside (comp. 11) peak, separating the profile of these extracts from other plants. Galium mollugo extracts also contain a significant amount of cryptochlorogenic acid (comp. 10), but the asperuloside (comp. 11) peak is similar in size to the extracts of both Galium verum blossoms and herb. The extracts of Galium verum blossoms contained significantly higher amounts of rutin (comp. 14) than other extracts and were also characterised by the largest isorhamnetin-3-O-rutinoside (comp. 17) peak. Whereas the general profile of the Galium verum herb extracts are like the ones of the Galium verum blossoms, the phytochemical content and the chromatographic peak sizes are lesser in the former. Aside from the great difference in the size of the rutin (comp. 14) peak, the appearance of the quercetin-3-rutinoside-7-glucoside (comp. 8) peak in the Galium verum blossoms extract also differentiates the chromatographic profiles of the two extracts of Galium verum. It is therefore possible to differentiate between the different species of Galium based on the general profile of HPLC-DAD analyses.
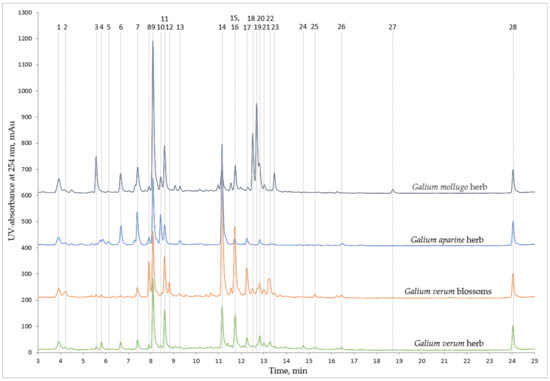
Figure 1.
All quantified phytochemicals on chromatograms of 50% ethanol extracts of analysed Galium species.

Table 2.
Identification of all quantified compounds in the Galium extracts.

Table 3.
Quantification of phytochemicals in Galium extracts (mg/L).
The study of the Bulgarian Galium species conducted by Mitova et al. revealed that Galium verum and mollugo share a similar phenolic profile, one of the similarities being the involvement of diosmetin. Even though this could be stated to be true for Estonian species, the abundance of diosmetin isomers in our extracts distinctly differentiates the two chromatographic profiles as the corresponding peaks are much lesser in size for Galium verum (Figure 1). Mitova et al. also analysed the iridoid patterns of the Bulgarian Galium species and found that Galium verum, aparine and mollugo all contain asperulosidic acid [12], and this was confirmed by our data to also be true for the Estonian species (comp. 7). When studying the polyphenolic content of 70% ethanol extracts of Romanian Galium species, Vlase et al. found that Galim verum, aparine and mollugo all contained chlorogenic acid, rutin, and quercetin [13]. Our research demonstrates that 50% acetone, 50% ethanol, and 80% ethanol extracts of these species all contain these polyphenols also (comp. 9, 14, 26). It is important to note that the abundance of these polyphenolics between the different species is varied, and, in general, the use of a less polar solvent (50% acetone) helps enhance the quantities of polyphenolics in the extracts (Table 2). Interestingly, kaempferol-7-O-glucoside (comp. 16) was identified in both Galium verum and Galium mollugo, whereas in the study of Bulgarian Galium species by Mitova et al., only Galium verum was shown to contain kaempferol glycosides [12]. This finding demonstrates how the phytochemical makeup of a species can vary based on the geographic origin.
Our research also demonstrates the differences in phytochemical content dependant on the extraction solvent used (and its polarity), as 50% acetone, 50% ethanol, and 80% ethanol extracts showed varied yields of phytochemicals quantified (Table 3). The extracts of Galium verum blossoms contained the highest amounts of the same two compounds, asperuloside and rutin, regardless of the extraction solvent used. Thirdly, either dicaffeoylquinic acid isomer (comp. 22; in the 50% acetone and 80% ethanol extract), or chlorogenic acid (in the 50% ethanol extract), was found. In the Galium verum herb extract, the phytochemical in the highest abundance was also asperuloside, followed by chlorogenic acid, and thirdly, either deacetylasperulosidic acid (50% ethanol extract) or asperulosidic acid (50% acetone and 80% ethanol extracts). In Galium aparine extracts, the most abundant phytochemical was either asperulosidic acid (50% acetone and 50% ethanol extracts) or asperuloside (80% ethanol extract). The second and third compound were similar in hydroethanolic extracts, chlorogenic acid and rutin, and differed in the hydroacetonic extract, rutin and asperuloside. Finally, in the Galium mollugo extracts, the three most common phytochemicals were similar, but found in differing amounts for each extraction solvent: these compounds were chlorogenic acid (in highest abundance in 50% acetone); asperuloside (in highest abundance in 80% ethanol); and diosmetin isomer, comp. 19 (in highest abundance in 50% ethanol). In conclusion, the most abundant compound in the extracts of Galium verum blossoms and herb was found to be asperuloside, in Galium aparine herb, asperulosidic acid, and in Galium mollugo herb, chlorogenic acid. Asperuloside has shown to have a wide array of therapeutically beneficial qualities [51]. Hence, it is important to note that the species with the highest abundance of asperuloside is Galium verum, with the extracts of Galium verum blossoms showing higher amounts of asperuloside compared to the extracts of the whole aerial part of the plant. Additionally, a less polar solvent should be preferred when extracting asperuloside from Galium species, as the yields were shown to be more favourable with hydroacetonic extracts compared to the more polar, hydroethanolic extracts. On the other hand, it is noteworthy that the yields were not significantly less in the hydroethanolic extracts. This is an advantage for future biological applications, as acetonic extracts would not be suitable for such purposes. Extracts of Estonian Galium species are also rich in polyphenolics, especially chlorogenic acid and rutin. As polyphenols are strong antioxidants, these extracts could also be used in therapeutic research against oxidative stress induced illnesses.
2.3. Analysis of Volatiles by SPME-GC-MS
The volatiles of Galium verum blossoms, herb, Galium aparine herb, and Galium mollugo herb were quantified and are shown in Table 4, which includes only the compounds that amount to more than 1.0% of total volatiles in each sample. Significant quantitative and qualitative differences were observed between the different Galium species. There were three volatile compounds, hexanal, anethole, β-caryophyllene, that were identified in all analysed samples (out of all compounds that made up more than 1.0% of total volatiles in a specific sample). According to the previous studies, these are all compounds that should be present in Galium species [43,44,45,46]. It has previously been proven that anethole is effective against yeast, bacterial and fungal strains [52,53], and caryophyllene-rich oil has been reported to have high inhibitory activity against some fungi and bacteria [54].

Table 4.
Identified volatile compounds in the samples of the Galium species.
In general, the volatiles in Galium verum blossom and herb samples were similar. However, the herb sample contained a larger variety of volatile compounds, for example, β-terpinen, β-pinene, copaene, linalool, estragole and dihydroactinidiolide did not occur in the blossom sample.
Table 4 gives a comprehensive overview of the volatile compounds that were identified in the different Galium species and demonstrates the high diversity present in the chemical composition of the samples. The highly variable content of volatile compounds certainly contributes to the potential and widespread use of these species, e.g., in the cosmetics and pharmaceutical industry.
3. Materials and Methods
3.1. Plant Material and Preparation of Plant Extracts
Galium verum herb was purchased from Kubja Ürt OÜ (Tallinn, Estonia). Galium verum blossoms were purchased from Norman Ravimtaimed OÜ (Karepa, Estonia). Galium aparine and Galium mollugo herbs were obtained from a private garden in Saaremaa, Estonia. All the plant materials studied were previously air-dried and kept at an ambient temperature in a dark space before being subjected to the extraction procedure.
The plant material was ground finely using a coffee bean grinder Bomann KSW 445 CB (China). The solvent used for the extraction of plant material was either 50% ethanol, 80% ethanol, or 50% acetone (v/v in ultrapure water), the ratio of plant material to solvent 1:20 (w/v). The procedure included 30 min of shaking using OrbitalShaker DOS-20M (Latvia) at 250 r/min (with a direction change after 99 r), then sonication at 640 W (350 kHz) in Sonorex™ Digital 10P bath (Bandelin, Germany) for 30 min at 35 °C. The extract was then vacuum filtered through a SartoriusTM 3 h filter (70 mm, 65 g/m3) (Sartorius, France). All the following qualitative and quantitative analyses were performed in triplicate for all the four different plant samples using three different extraction solvents, for each replicate the extract was prepared by a different member of the research group.
3.2. Chemicals
Ultrapure water (≥18 MΩcm) produced within the laboratory with a Milli-Q water purification system (Merck KGaA, Darmstadt, Germany) was used to prepare all aqueous solutions. Extraction solvent ethanol (96.7%) was obtained from Sigma-Aldrich (Munich, Germany). Acetonitrile (≥99.9%), an HPLC eluent, and acetone (≥99.9%), an extraction solvent, were purchased from Honeywell (Seelze, Germany). Formic acid (≥99.0%) used in HPLC eluents was purchased from Fisher Chemical (Czech Republic). Total polyphenolic content of the extracts was evaluated using the 2 M Folin–Ciocalteu reagent, purchased from Sigma-Aldrich (Switzerland), and water-free sodium carbonate, purchased from Sigma-Aldrich (Germany). Standard solutions used were prepared from gallic acid monohydrate (Sigma-Aldrich, China) and 96.7% ethanol (Sigma-Aldrich, Germany). For the detection of total flavonoid content, aluminium chloride, obtained from Fluka (Switzerland), was used. Standard solutions were prepared from quercetin (≥99.0%, Lachema/Chemapol) and methanol (≥99.9%, Honeywell, Charlotte, NC, USA). Total iridoid content was determined using the Trim–Hill reagent, prepared from water-free acetic acid (≥99.0%, Sigma-Aldrich, Germany), 37% hydrochloric acid (Honeywell/Fluka, Austria), and copper sulfate pentahydrate (Sigma-Aldrich, Munich, Germany). Standard solutions were prepared using asperuloside purchased from MedChemExpress. Fluorescein sodium salt (≥98.5%) and AAPH (2,2′-azobis(2-methyl-propionamidine)dihydrochloride, 97%) used in the antioxidativity studies, were purchased from Fluka (Switzerland) and Sigma-Aldrich (Germany), respectively. Standard solutions were prepared from Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, 97%), purchased from Sigma-Aldrich (Germany). Standard compounds used for phytochemical quantification, asperuloside (≥95%), neochlorogenic acid (≥98%), chlorogenic acid (≥95%), rutin (≥95%), and quercetin (≥95%), and internal standard, bicalutamide (≥99.8%), were all purchased from Sigma-Aldrich (Germany).
3.3. Colorimetric Analyses
The main groups of bioactive compounds in the plants were quantified by colorimetric tests: total flavonoids by the AlCl3, total iridoids by the Trim–Hill, and total polyphenols by the Folin–Ciocalteu method [55,56,57]. All colorimetric analyses were conducted on the Varian Cary 50 Bio UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CO, USA), using 1.5 mL single-use semi-micro cuvettes (Nerbe Plus GmbH & Co. KG, Winsen (Luhe), Germany). Total polyphenolic content of the extracts was determined using calibration solutions of 10, 25, 50, 75, and 100 mg/L of gallic acid in ethanol, prepared from a 5 g/L stock solution. The calibration solutions for the quantification of flavonoid contents were prepared in concentrations of 2, 5, 10, 20, and 40 mg/L of quercetin in methanol, prepared from a 2 g/L stock solution. The calibration solutions for iridoid content measurements were prepared from a 1 g/L stock solution of asperuloside in a solvent corresponding to the extraction solvent. The concentrations for calibration solutions were 100, 200, 400, 800, and 1000 mg/L. All samples were measured in triplicate and the results given in mg of either mean gallic acid, quercetin, or asperuloside equivalents per g of plant material (mg GAE/QE/AE/g) ± standard deviation (Table 1).
3.4. Evaluation of Antioxidative Properties
The antioxidative activity of all extracts was evaluated using the ORACFL (oxygen radical absorbance capacity) method with minor modifications as described by Naguib [58]. The 24.25 mM fluorescein stock solution, 30 mM Trolox stock solution, and 600 mM AAPH solution were all prepared daily in a 100 mM phosphate buffer (pH = 7.4). The total volume of the reaction mixture was 3 mL, the mixture was composed of 2.7 mL of 24.25 nM fluorescein and 100 µL of sample/Trolox dilution, which was incubated at 37 °C for 3 min, and then 200 µL of 600 mM AAPH was added. The samples were measured by a Hitachi F-7000 Fluorescence Spectrophotometer (Chiyoda, Tokyo, Japan) at λex/em 495/520 nm, slits 5 nm, and the time scan was recorded for 3000 s once per s. The calibration solutions were prepared from a diluted, 300 µM Trolox solution, in concentrations of 0.5, 1, 2, 4, 6, 8, and 10 µM, and the calibration was given as area under curve change from 0 µM Trolox (blank sample). All extracts were analysed diluted 500-fold. Due to the matrix effects apparent in the diluted samples of 80% ethanol extracts, the calibration samples were prepared in 80% ethanol diluted 500-fold in phosphate buffer. For 50% ethanol and 50% acetone extracts, the matrix effects were negligible (<5%), and the Trolox calibration samples were prepared in phosphate buffer. All samples were measured in triplicate and the results given in mg of mean Trolox equivalents per g of plant material (mg TE/g) ± standard deviation (Table 1).
3.5. Phytochemical Screening and Quantification
The 1 mg/mL standard solutions of asperuloside, neochlorogenic acid, chlorogenic acid, rutin, and quercetin were prepared in ethanol, and diluted to create calibration curves for the quantitative HPLC-DAD analyses. The calibration curves were constructed based on the UV signal at 254 nm (slit 4 nm), using the ratio of the peak area of a standard compound to the peak area of the internal standard bicalutamide (2 g/L ethanolic solution was added at a final concentration of 40 mg/L into all the samples). All standard compounds exhibited a linear range between 5 to 250 mg/L. Other phytochemicals detected on the chromatograms were also quantitatively determined using these calibration curves, by matching these compounds with a standard compound based on the similarities in the respective UV spectra. The DAD spectra were measured from the analyses of 50% ethanol extracts in the range of 200 to 400 nm. These selections and respective results are given in Table 3 (all measured in triplicate and given as mean mg per L of extract ± standard deviation), and all UV spectra as Figures S2–S29 in the Supplementary Materials.
Before the HPLC-DAD-MS/MS analyses, the extracts were centrifuged, the sample then diluted in ultrapure water two-fold, and spiked with the internal standard bicalutamide at a final concentration of 40 mg/L. The sample volume injected was 5 µL. HPLC-DAD-MS/MS analyses were conducted using an Agilent 1260 Infinity II instrument (Agilent Technologies, Inc., USA) with an Agilent Poroshell 120 EC-C18 column, particle size 2.7 µm, measurements 4.6 mm × 100 mm (Agilent Technologies, Inc., USA) thermostated at 28 °C. The mobile phase consisted of ultrapure water (A) and acetonitrile (B), both acidified with 0.1% (v/v) formic acid. The elution procedure was a linear gradient increasing from 5% to 50% B (0–20 min), then from 50% to 95% B (20–25 min), isocratic 95% B (25–30 min), a linear gradient decreasing from 95% to 5% B (30–30.01 min), and isocratic 5% B (30.01–35 min). The flow rate was kept at 0.6 mL/min. The column was coupled with an Infinity 1260 DAD (Agilent Technologies, Inc., USA), the chromatograms were recorded at a UV absorbance wavelength of 254 nm (slit 4 nm), and DAD spectra in the range of 200 to 400 nm. Following the DAD analysis, the sample was analysed using the LC/MSD Trap XCT mass spectrometer (Agilent Technologies, Inc., USA) equipped with an electrospray ionization source. The mass spectra were recorded in negative-ion mode, in the m/z range from 100 to 1000. Nitrogen was used as the nebulizing and drying gas, and helium served as the collision gas. The MS/MS fragmentation patterns (generated using the automatic Agilent software MS/MS settings) were used to identify compounds for which there were no standard compounds available.
3.6. Analysis of Volatiles by SPME-GC-MS
Headspace solid phase microextraction (SPME) coupled with GC-MS allows to determine and compare volatile compounds composition in plant material. In the current study, the SPME procedure was performed on PDMS/DVB Stabile Flex fiber (polydimethylsiloxane/divinylbenzene coating thickness 65 μm, Supelco, Bellefonte, PA, USA) using a manual SPME fiber holder (Supelco-57330-U). SPME fiber was conditioned according to the manufacturer’s instructions prior to the first use. 50 mg of dried and powdered sample was placed into a 1.5 mL glass vial and closed. The vials were thermostated for 15 min at 50–55 °C to perform headspace extraction of volatiles from plant material. The fiber was then withdrawn from the needle and inserted into the GC injection port, where the analytes were thermally desorbed for the GC-MS analysis.
Chromatographic separations were performed on an Agilent Technologies 7890A GC system equipped with an ultra-inert splitless liner (Agilent Technologies, type 5190-2293). The gas chromatograph was coupled to an Agilent 5975C mass spectrometer with an electron ionization source and a quadrupole mass analyser. The flow rate of carrier gas (helium 6.0, AGA, Estonia) was kept constant at 1.2 mL/min and compounds were separated in a ZB-5plus capillary column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, USA). The injector temperature was kept at 275 °C, injection was performed in the splitless mode for 2 min. The following oven temperature program was used: the initial temperature was 35 °C, then increased to 200 °C (5 °C min−1), and to 280 °C (20 °C min−1, held for 2 min). The total run time was 39 min starting from fiber introduction into the injection block. The analyte ionization was performed in electron ionization mode using the electron energy of 70 eV. The interface, ion source, and mass analyser temperatures were set at 280, 230, and 150 °C, respectively. Scan mode in the range of 20–500 m/z was used for monitoring all analytes. All samples were analysed thrice for confirmation. All the compounds were determined by the National Institute of Standards and Technology 17 (NIST 17) library and Agilent MassHunter Qualitative, Quantitative and Unknowns Analysis were used for data analysis.
4. Conclusions
This paper describes the phytochemical studies of three Galium species native to northern Europe. Our results show that the selected Estonian Galium species (Galium verum blossoms and herb, Galium aparine herb, and Galium mollugo herb) are a valuable source of phenolics with antioxidant properties, iridoids, and volatile phytochemicals. The quantitative studies showed that the most abundant non-volatile compound in the extracts of Galium verum blossoms and herb was asperuloside, in Galium aparine herb, asperulosidic acid, and in Galium mollugo herb, chlorogenic acid. The volatile compounds had a high degree of variability, but three, hexanal, anethole, and β-caryophyllene, were quantified (>1.0%) in all analysed samples. Additionally, it was found that some key differences in the chromatographic profiles of the extracts allow for a quick identification of an Estonian Galium plant of an unknown species. The Galium verum extracts exhibited a comparatively large rutin peak, whereas Galium aparine an enhanced cryptochlorogenic acid peak. The prominent peaks of diosmetin isomers set apart the Galium mollugo extract from the others. The effects of the polarity of an extraction solvent were characterised by the fact that extraction with 50% acetone yielded extracts rich in polyphenols (up to 25.1 ± 0.8 mg GAE/g) and flavonoids (up to 7.3 ± 0.5 mg QE/g), whereas 50% ethanol was preferable for iridoid extraction (up to 36.6 ± 2.2 mg AE/g). Both 50% ethanol and 50% acetone extracts were characterised by high antioxidant activities (up to 7.2 ± 1.4 and 9.3 ± 1.2 mg TE/g, respectively). As oxidative stress can lead to a variety of severe illnesses, the antioxidative properties of the Galium extracts contribute to their potential as future therapeutic agents. Out of the species studied, the Galium verum blossoms had the strongest antioxidant properties (up to 9.3 ± 1.2 mg TE/g). The therapeutic potential of several volatile and non-volatile phytochemicals found in the Galium species has already been demonstrated. Galium verum is rich in asperuloside, which has been shown to have anti-viral, anti-malarial, anti-protozoal, anti-tumorigenic, anti-hypertensive, anti-obesity, immunomodulatory, anti-inflammatory, and antioxidant properties. Estonian Galium species are rich in polyphenolics, mainly chlorogenic acid and rutin, known for their antioxidant properties. Volatile phytochemicals anethole and β-caryophyllene that were found in all extracts have previously shown potential as antibacterial and antifungal agents. In the future, the extraction procedure could be optimised to fully utilise the Estonian Galium verum (especially the blossoms) as a rich source for asperuloside. Though the hydroacetonic extract showed higher yields of asperuloside in the current study, the hydroethanolic yields were not significantly lesser. For potential future purposes, this presents an important advantage, as ethanolic extracts are suitable for biological applications. In conclusion, Galium species native to Estonia should be considered for future therapeutic approaches due to their many favourable properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28062867/s1. The graph illustrating the correlation of polyphenolic content and antioxidativity of the extracts (Figure S1) as well as the UV spectra of all quantified phytochemicals measured from the analyses of 50% ethanol extracts (Figures S2–S29) are provided as a separate file.
Author Contributions
Conceptualization, M.V.; methodology, P.S.-R., P.J., M.V.; formal analysis, P.-R.L., P.S.-R., P.J., O.B.; investigation, P.-R.L., P.S.-R., M.V.; data curation, P.-R.L., P.S.-R.; writing—original draft preparation, P.-R.L., P.J.; writing—review and editing, P.-R.L., P.S.-R., P.J., M.V.; visualization, P.-R.L.; supervision, P.S.-R., M.V.; project administration, M.V.; funding acquisition, M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Estonian Center of Analytical Chemistry (ECAC) funded by the Estonian Research Council (TT4) and the R&D project SS220044 “Evaluation of antioxidant and antibacterial activity of plant extracts” funded by Tallinn University of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank students Regina Drošnova and Kristin Düüna for the experimentation they conducted as a part of the formal analysis for this project.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AE—asperuloside equivalent; DAD—diode array detector; DVB—divinylbenzene; FID—flame ionisation detector; GAE—gallic acid equivalent; GC—gas chromatography; HPLC—high performance liquid chromatography; MS—mass spectrometry; NMR—nuclear magnetic resonance; ORAC—oxygen radical absorbance capacity; PDMS—polydimethylsiloxane; QE—quercetin equivalent; SPME—solid phase microextraction; TE—Trolox equivalent; UHPLC—ultra-high performance liquid chromatography; UV—ultraviolet (radiation).
References
- Raal, A.; Jaama, M.; Utt, M.; Püssa, T.; Žvikas, V.; Jakštas, V.; Koshovyi, O.; Nguyen, K.V.; Nguyen, H.T. The Phytochemical Profile and Anticancer Activity of Anthemis Tinctoria and Angelica Sylvestris Used in Estonian Ethnomedicine. Plants 2022, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Ilina, T.; Kashpur, N.; Granica, S.; Bazylko, A.; Shinkovenko, I.; Kovalyova, A.; Goryacha, O.; Koshovyi, O. Phytochemical Profiles and In Vitro Immunomodulatory Activity of Ethanolic Extracts from Galium aparine L. Plants 2019, 8, 541. [Google Scholar] [CrossRef] [PubMed]
- Saar-Reismaa, P.; Bragina, O.; Kuhtinskaja, M.; Reile, I.; Laanet, P.R.; Kulp, M.; Vaher, M. Extraction and Fractionation of Bioactives from Dipsacus fullonum L. Leaves and Evaluation of Their Anti-Borrelia Activity. Pharmaceuticals 2022, 15, 87. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokól-Lȩtowska, A.; Oszmiánski, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea Var. Kamtschatica sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Vaher, M.; Matso, K.; Levandi, T.; Helmja, K.; Kaljurand, M. Phenolic Compounds and the Antioxidant Activity of the Bran, Flour and Whole Grain of Different Wheat Varieties. Procedia Chem. 2010, 2, 76–82. [Google Scholar] [CrossRef]
- Yang, L.E.; Meng, Y.; Peng, D.L.; Nie, Z.L.; Sun, H. Molecular Phylogeny of Galium L. of the Tribe Rubieae (Rubiaceae)—Emphasis on Chinese Species and Recognition of a New Genus Pseudogalium. Mol. Phylogenet. Evol. 2018, 126, 221–232. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Trifan, A.; Blaga, A.C.; Tanasa, A.M.; Suteu, D. Antioxidants from Galium Verum as Ingredients for the Design of New Dermatocosmetic Products. Plants 2022, 11, 2454. [Google Scholar] [CrossRef]
- Hanganu, D.; Burtescu, R.F.; Petrescu, S.; Pripon Furtuna, F.R.; Chise, E.; Turcus, V.; Benedec, D.; Oniga, I.; Olah, N.K. Galium Species—Polyphenolic Content and Their Antioxidant Potential. Hop. Med. Plants 2018, 26, 84–93. [Google Scholar]
- Bradic, J.; Petkovic, A.; Tomovic, M. Phytochemical and Pharmacological Properties of Some Species of the Genus galium L. (Galium verum and Mollugo). Serb. J. Exp. Clin. Res. 2017, 1, 187–193. [Google Scholar] [CrossRef]
- Abdul, R.; Wang, M.R.; Zhong, C.J.; Liu, Y.Y.; Hou, W.; Xiong, H.R. An Updated Review on the Antimicrobial and Pharmacological Properties of Uncaria (Rubiaceae). J. Herb. Med. 2022, 34, 100573. [Google Scholar] [CrossRef]
- Kuhtinskaja, M.; Vaher, M. Extraction and Analysis of Bioactive Compounds from Dipsacus Fullonum and Galium Verum for Lyme Borreliosis Treatment. Biomed. J. Sci. Tech. Res. 2018, 11, 8614–8616. [Google Scholar] [CrossRef]
- Mitova, M.I.; Anchev, M.E.; Handjieva, N.V.; Popov, S.S. Iridoid Patterns in Galium L. and Some Phylogenetic Considerations. Z. Fur Naturforsch.-Sect. C. J. Biosci. 2002, 57, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Mocan, A.; Hanganu, D.; Benedec, D.; Gheldiu, A.; Crișan, G. Comparative Study of Polyphenolic Content, Antioxidant and Antimicrobial Activity of Four Galium Species (Rubiaceae). Dig. J. Nanomater. Biostruct. 2014, 9, 1085–1094. [Google Scholar]
- Kaškoniene, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraite-Surviliene, K.; Maruška, A.; Ragažinskiene, O.; Kornyšova, O.; Briedis, V.; et al. Evaluation of Phytochemical Composition of Fresh and Dried Raw Material of Introduced Chamerion angustifolium L. Using Chromatographic, Spectrophotometric and Chemometric Techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Kuhtinskaja, M.; Bragina, O.; Kulp, M.; Vaher, M. Anticancer Effect of the Iridoid Glycoside Fraction from Dipsacus fullonum L. Leaves. SAGE J. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Saar-Reismaa, P.; Koel, M.; Tarto, R.; Vaher, M. Extraction of Bioactive Compounds from Dipsacus fullonum Leaves Using Deep Eutectic Solvents. J. Chromatogr. A 2022, 1677, 463330. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollmann, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317–325. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.-T.; Newmark, H.L. Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-Oestrogens and Western Diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–774. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.E.; Frederiksen, H.; Krogholm, K.S.; Poulsen, L. Dietary Proanthocyanidins: Occurrence, Dietary Intake, Bioavailability, and Protection against Cardiovascular Disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Feskens, E.J.M.; Hollman, P.C.H.; Katan, M.B.; Kromhout, D. Dietary Flavonoids and Cancer Risk in the Zutphen Elderly Study. Nutr. Cancer 1994, 22, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Lim, G.P.; Yang, F.; Teter, B.; Begum, A.; Ma, Q.; Harris-White, M.E.; Frautschy, S.A. Prevention of Alzheimer’s Disease: Omega-3 Fatty Acid and Phenolic Anti-Oxidant Interventions. Neurobiol. Aging 2005, 26, 133–136. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tang, L.; He, J.W.; Li, J.; Wang, Y.Z. Ethnobotany, Phytochemistry and Pharmacological Properties of Eucommia Ulmoides: A Review. Am. J. Chin. Med. 2019, 47, 259–300. [Google Scholar] [CrossRef]
- Huang, W.; Ding, L.; Zhang, N.; Li, W.; Koike, K.; Qiu, F. Flavonoids from Eucommia Ulmoides and Their in Vitro Hepatoprotective Activities. Nat. Prod. Res. 2021, 35, 3584–3591. [Google Scholar] [CrossRef]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Ruzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S. Flavonoid Intake and Long-Term Risk of Coronary Heart Disease and Cancer in the Seven Countries Study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, Dietary Sources and Bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Ilc, T.; Parage, C.; Boachon, B.; Navrot, N.; Werck-Reichhart, D. Monoterpenol Oxidative Metabolism: Role in Plant Adaptation and Potential Applications. Front. Plant. Sci. 2016, 7, 509. [Google Scholar] [CrossRef]
- Taskova, R.; Evstatieva, L.; Handjieva, N.; Popov, S. Iridoid Patterns of Genus plantago L. and Their Systematic Significance. Z. Fur. Naturforsch.-Sect. C. J. Biosci. 2002, 57, 42–50. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Hariyaga, Y. Naturally Occurring Iridoids. A Review, Part 1. Chem. Pharm. Bull. 2007, 55, 159–222. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Biological and Pharmacological Activity of Naturally Occurring Iridoids and Secoiridoids. Phytomedicine 1998, 5, 147–163. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Banik, R. Naturally Occurring Iridoids and Secoiridoids. An Updated Review, Part 4. Chem. Pharm. Bull. 2011, 59, 803–833. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Menichini, F.; Statti, G.; Menichini, F. Biological and Pharmacological Activities of Iridoids: Recent Developments. Mini-Reviews Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-Inflammatory Iridoids of Botanical Origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Merillon, J.M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kar, S.; Gupta, P.; Gupta, J. Essential Oils: Biological Activity Beyond Aromatherapy. Nat. Prod. Sci. 2018, 24, 139–147. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool Bioactive Properties and Potential Applicability in Drug Delivery Systems. Colloids Surfaces B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Ciotlaus, I.; Pojar-Fenesan, M.; Balea, A. Analysis of Volatile Organic Compounds from the Aerial Parts of Medicinal Plant, Galium Verum. Rev. Chim. 2020, 71, 136–144. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Ronga, D.; Avato, P. Identification of the Volatile Components of Galium verum L. and Cruciata Leavipes Opiz from the Western Italian Alps. Molecules 2020, 25, 2333. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Kırımer, N.; Deliorman, D.; Ergun, F. Composition of the Essential Oils of Galium aparine L. and Galium odoratum (L.) Scop. from Turkey. J. Essent. Oil Res. 2011, 16, 305–307. [Google Scholar] [CrossRef]
- Il’ina, T.V.; Kovaleva, A.M.; Goryachaya, O.V.; Aleksandrov, A.N. Essential Oil from Galium Verum Flowers. Chem. Nat. Compd. 2009, 45, 587–588. [Google Scholar] [CrossRef]
- Kokosa, J.M.; Przyjazny, A. Green Microextraction Methodologies for Sample Preparations. Green. Anal. Chem. 2022, 3, 100023. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The Ten Principles of Green Sample Preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacl, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Manzione, M.G.; Martorell, M.; Sharopov, F.; Bhat, N.G.; Kumar, N.V.A.; Fokou, P.V.T.; Pezzani, R. Phytochemical and Pharmacological Properties of Asperuloside, a Systematic Review. Eur. J. Pharmacol. 2020, 883, 173344. [Google Scholar] [CrossRef]
- De, M.; De, A.K.; Sen, P.; Banerjee, A.B. Antimicrobial Properties of Star Anise (Illicium Verum Hook F). Phyther. Res. 2002, 16, 94–95. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal Activity of the Essential Oil of Illicium Verum Fruit and Its Main Component Trans-Anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef]
- Sabulal, B.; Dan, M.; John, A.J.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-Rich Rhizome Oil of Zingiber Nimmonii from South India: Chemical Characterization and Antimicrobial Activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Jõul, P.; Kuhtinskaja, M.; Vaher, M.; Koel, M. Green Chemistry and Reconsidering Simple Analytical Methods. Chem. Today 2017, 35, 49–51. [Google Scholar]
- Aid, T.; Kaljurand, M.; Vaher, M. Colorimetric Determination of Total Phenolic Contents in Ionic Liquid Extracts by Paper Microzones and Digital Camera. Anal. Methods 2015, 7, 3193–3199. [Google Scholar] [CrossRef]
- Saar-Reismaa, P.; Kotkas, K.; Rosenberg, V.; Kulp, M.; Kuhtinskaja, M.; Vaher, M. Analysis of Total Phenols, Sugars, and Mineral Elements in Colored Tubers of Solanum tuberosum L. Foods 2020, 9, 1862. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. A Fluorometric Method for Measurement of Oxygen Radical-Scavenging Activity of Water-Soluble Antioxidants. Anal. Biochem. 2000, 284, 93–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).