Abstract
In this study, silver nanoparticles were synthesized using Cucumis melo L. leaf extract via a green synthesis approach and their potential against diabetes and coccidiosis was tested under in vitro conditions. The phytochemical components in the leaf extract reacted with silver nitrate in solution and yielded C. melo-silver nanoparticles (Cm-AgNPs). The synthesis of AgNPs was confirmed via UV–visible spectroscopy by obtaining a peak at 440 nm. The nanoparticles were characterized by their morphology, crystallinity, and the presence of functional groups. In vitro α-amylase and α-glucosidase inhibition assays were carried out at different concentrations in the range of 20 to 100 μg/mL of Cm-AgNPs. The Cm-AgNPs exhibited enzyme inhibitory activity in a concentration-dependent manner. As the concentration of Cm-AgNPs increased the inhibitory activities were also increased linearly and the highest inhibition was observed at 100 μg/mL. The effectiveness of Cm-AgNPs against Eimeria tenalla was assessed by an in vitro 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay using Madin–Darby bovine kidney (MDBK) cell lines. The results revealed that the viability of the oocysts and further sporulation were decreased with the increased concentration of Cm-AgNPs. The AgNPs synthesized from the C. melo leaf extract have shown promising potential against diabetes and coccidiosis, and they could be used in biomedical applications.
1. Introduction
The current innovations in the field of nanotechnology bring new dimensions to medical science, research and development, and our day-to-day life. The nanoparticles, either organic (carbon, C) or inorganic metals (silver, Ag, and gold, Au), play a significant role as biomedical agents and pharmaceutical products. Among the metallic nanoparticles, Ag-based nanoparticles (AgNPs) are small in size, ranging from 10–100 nm with specific physicochemical characteristics such as size, shape, electrical conductivity, optical density, and high reactive surface area [1]. These unique characteristics allow control over the different characteristics of drugs or other biomedical agents such as alteration in solubility and blood pool retention time, controlled release over short or long durations, environmentally triggered controlled release, or highly specific site-targeted delivery [2]. The AgNPs synthesized were proven to have potential therapeutic applications against many diseases, such as cancers [3,4], and antimicrobial, antifungal, and antioxidant effects [5,6,7,8].
Type 2 diabetes mellitus is one of the most commonly prevalent non-communicable and life-threatening diseases among populations around the world [1]. In normal conditions, intestinal enzymes, such as α-amylase and α-glucosidase, degrade the starch and oligosaccharides found in food to glucose, and the insulin hormone secreted by the pancreas helps glucose enter into cells to be used for body energy [9,10]. Sometimes the pancreas does not make enough insulin or the body does not use the secreted insulin properly. Thus, glucose stays in the blood and, over time, it causes the development of chronic hyperglycemia. The combination of insulin resistance and the inhibition of insulin secretion results in type 2 diabetic conditions, influenced by genetic determinants, the over-intake of food, a sedentary lifestyle, and aging [10]. Clinical studies have shown that currently available treatments like insulin injection and antihyperglycemic drugs can cause several side effects in patients when the drugs are used in the long term and slow down therapeutic responses [11]. Today, the role of biosynthesized nanoparticles is attracting the interest of the pharmaceutical industry to create safe antidiabetic drugs to be used among patients.
Coccidiosis disease is caused by the protozoan parasites of the genus Eimeria developing within the intestine of most domestic and wild animals and birds. Live attenuated and non-attenuated vaccines are available to curtail the risks of infection, but they are not cost-effective. Another disadvantage is that live vaccines need host cells to replicate and to instigate active immunity, which causes them to result in subclinical coccidiosis [12]. The control of coccidiosis is difficult due to the emergence of drug resistance and the limitations of available anticoccidial vaccines lead to frequent disease outbreaks. Therefore, alternative approaches, such as the use of AgNPs and plant extracts as anticoccidial agents are being considered and are now the focus of many researchers. The infection of chicken intestines begins with the ingestion of sporulated oocysts releasing sporocysts, which in turn release sporozoites. The discharge of sporozoites from infected oocysts/sporocysts, otherwise known as the excystation process, is a critical step in the intracellular development of parasites inside the host cells [13].
Generally, AgNPs are prepared by employing different chemical reactions, such as the reduction in Ag ions in aqueous solutions with or without stabilizing agents [14], thermal decomposition of inorganic solvents [15], chemical reduction and photoreduction in reverse-micelles processes [16,17], and microwave-assisted irradiation [18]. However, these chemical methods have certain disadvantages, such as the involvement of toxic and hazardous chemicals during the processes, being economically not viable, being unsafe to the environment, and the resultant product being biologically unsafe or incompatible with living entities [19,20]. Thus, there is a growing need to develop nontoxic, biocompatible, eco-friendly, and efficient methods to synthesize AgNPs for their use in medical and pharmaceutical products. This has caused researchers turn their focus to living organisms.
In recent times, many studies have been conducted by exploring the different kinds of organisms, such as bacteria [21], fungi [22], algae [23], and plants [24,25], to create cost-effective, safe, and environmentally compatible AgNPs. Many plants and plant extracts have been reported for the successful production of stable nanoparticles, particularly Cantharanthus roseus leaf extracts [26] and Clitorea ternatea and Solanum nigrum [27]. Kesharwani et al. fabricated high-density stable nanoparticles of size 16–40 nm using the leaf extracts of Datura metal [28]. Black tea leaf extracts were also used in the formation of AgNPs [29]. It is assumed that the leaf extracts contain a large number of active metabolites, such as alcoholic components, alkaloids, proteins, amino acids, and enzymes that aided in the reduction in Ag ions and led to the formation of AgNPs [28].
Cucumis melo L. is commonly known as musk melon, and it belongs to the family of Cucurbitaceae. It is a climbing annual herb. It grows well in a well-drained deep soil and it has long been cultivated mainly for its edible fruits; however, it is found as a weed in cultivated fields, natural open areas, grasslands, and wastelands [30]. Cucumis melo leaves are simple and alternate, approximately rounded and pubescent with cordate bases on long petioles, and with 3 to 7 shallow or irregular lobes [31]. The leaves have medicinal importance, owing to the presence of a group of phytoconstituents [32]. It has been found that the leaf extracts of the Cucurbitaceae family have antibacterial, antifungal, wound-healing, antiviral, antidiabetic (type 2), antidiarrheal, and anti-inflammatory activities [33,34].
The present study to synthesize AgNPs from the leaf extracts of C. melo has proven its excellent antidiabetic potential and anticoccidial efficacy under in vitro conditions.
2. Results
2.1. Synthesis and Characterization of Silver Nanoparticles Using C. melo
2.1.1. UV–Visible Spectroscopy
The synthesis of AgNPs using C. melo leaf extract was successful, as observed visually via a shift in color from pale yellow to dark brown, and a resulting relatively stable solution in Figure 1. The color change is directly attributed to the interaction of metabolites present in the leaf extract of C. melo with added AgNO3, which leads to the reduction in Ag+ ions to Ag0 nanoparticles.
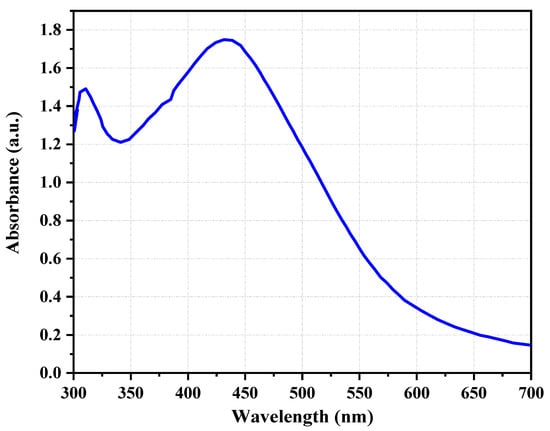
Figure 1.
The UV–visible absorption spectrum of synthesized silver nanoparticles (AgNPs) from the leaf aqueous extract of C. melo.
2.1.2. Zeta Potential Distribution
The stability of the synthesized Cm-AgNPs was confirmed by aqueous solution via zeta potential analysis. The zeta potential values of AgNPs were measured—12.8 mV ± 1.0 mV in Figure 2. The negatively charged surface value confirms the formation of the nanoparticles without aggregation.
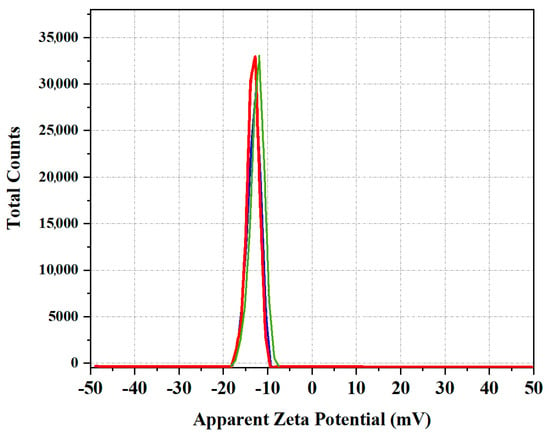
Figure 2.
Zeta potential distribution value of synthesized Cm-AgNPs.
2.1.3. High Resolution Scanning Electron Microscopy (HR-SEM) Analysis
The surface morphology of biosynthesized AgNPs was analyzed using an SEM FEI-Quanta FEG 250. The surface morphology and particle size were studied from the SEM micrographs. The SEM images showed the uniform distribution of spherical shape AgNPs. The normal range of SEM observation is from 1 nm to 100 nm. The SEM micrographs of synthesized Cm-AgNPs magnified at 8.3 mm × 40.0 k, 8.3 mm × 25.0 k, and 8.3 mm × 90.0 k are shown in Figure 3 respectively. These different magnified images show well-defined spherical, porous, and smooth Cm-AgNPs. The observed range for Cm-AgNPs was 66.7 to 92.3 nm (Figure 3A).
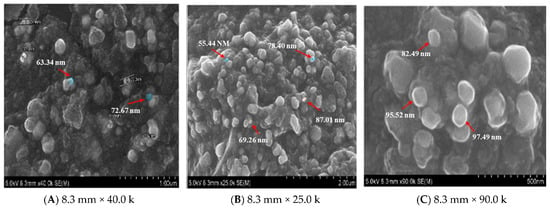
Figure 3.
SEM images of biosynthesized silver nanoparticles (AgNPs) using C. melo leaf extract.
2.1.4. X-ray Diffraction Method
The XRD pattern with diffraction peaks showed the crystallinity nature of silver nanoparticles. The diffraction patterns were recorded for a 2θ scan angle from 0 to 80°. The XRD pattern of biosynthesized Cm-AgNPs was confirmed by the characteristic peak observed in XRD image (Figure 4). In the XRD pattern, six distinct diffraction peaks were observed at 2θ scan angles of 30°, 32.7°, 40.0°, 44°, 64.2°, and 79.4°, which were indexed at (101), (104), (111), (200), (220), and (311) of the cubic and face-centered structure of metallic silver, respectively. The peaks observed in the pattern were correlated with pure silver available from the Joint Committee on Powdered Diffraction Standards (JCPDS No. 04-0783). In a comparison of biosynthesized AgNPs with the standard XRD pattern, the XRD pattern of C. melo-mediated AgNPs produced a high-quality nanocrystal with a different crystalline form.
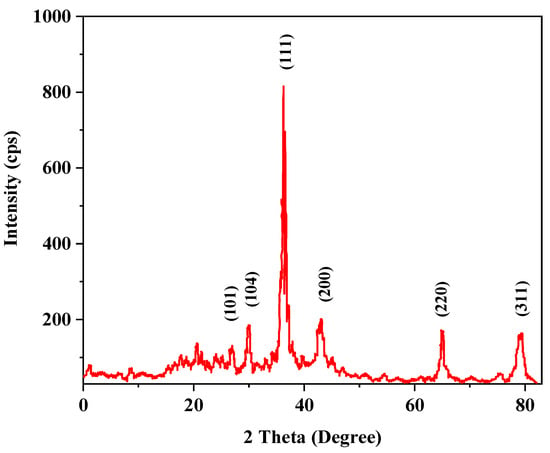
Figure 4.
XRD pattern of C. melo-mediated silver nanoparticles (AgNPs).
2.1.5. Fourier Transform Infrared (FTIR) Spectroscopy
The absorption wavelength of electromagnetic radiation is characteristic of chemical bond stretching and bending vibrations. The characteristic absorption peaks were utilized for the quantitative analysis of chemical structural elucidation, functional groups present in the nanoparticles, and the possible interaction of functional groups. The FTIR spectrum of C. melo leaf extract alone in Figure 5a and silver nanoparticles (Cm-AgNPs) in Figure 5b was recorded using KBr pellets. The infrared light passed through the pellet, and the IR the absorption spectrum of secondary metabolites involved in the reduction in silver ions and the capping of the AgNPs is shown in Figure 5b. The functional groups present in the C. melo leaf extract were interrupted as follows: 3435.88 cm−1, 2925.14 cm−1, 1636.29 cm−1, 1439.68 cm−1, 1317.77 cm−1, 1034.47 cm−1, 796.44 cm−1, and 616.17 cm−1. The FTIR spectrum of Cm-AgNPs showed major absorption peaks at 3429.35 cm−1, 2426.62 cm−1, 1768.34 cm−1, 1630.52 cm−1, 1384.48 cm−1, 1097.15 cm−1, 832.97 cm−1, and 598.67 cm−1. The FTIR spectrum indicates the presence of various phytoconstituents in the leaf extract and is also similar in Cm- AgNPs. The of Cm-AgNP’s spectral features are slightly shifted as compared to C. melo extract. It can be concluded that these phytoconstituents may be involved in the silver ion reduction and capping processes.
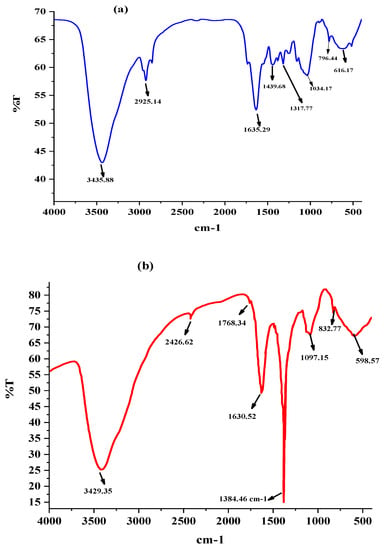
Figure 5.
(a) FTIR spectrum of C. melo leaf extract. (b) FTIR spectrum of synthesized silver nanoparticles (AgNPs) using C. melo leaf extract.
2.2. Biosynthesized Silver Nanoparticles as an Antidiabetic Agent
The antidiabetic activity of biosynthesized AgNPs from the aqueous leaf of extract of C. melo was assessed via α-amylase and α-Glucosidase inhibition assays. The in vitro enzyme inhibition assay was carried out with Cm-AgNPs at five different concentrations, namely 20, 40, 60, 80, and 100 μg/mL.
2.2.1. α-Amylase Inhibitory Activity
In the present study, Cm-AgNPs exhibited a linear increase in amylase inhibition activity with an increase in the concentrations of nanoparticles 20 to 100 μg/mL, as shown in Figure 6 (black line). The inhibition of α-amylase was 20 μg/mL for 17.6 ± 0.8, 40 μg/mL for 32.6 ± 1.1, 60 μg/mL for 42.1 μg/mL ± 0.6, 80 μg/mL for 50.7 ± 0.2, and 100 μg/mL for 65.5 ± 0.7. The highest inhibition activity of Cm-AgNPs was observed at the 100 μg/mL concentration.
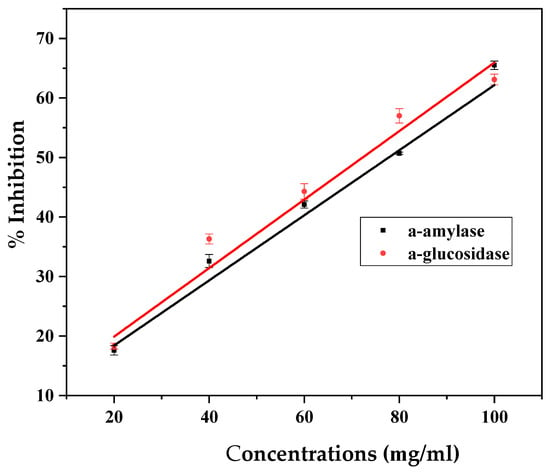
Figure 6.
(black line) Inhibition of α-amylase and (red line) α-glucosidase activities by different concentrations of silver nanoparticles (AgNPs) synthesized from C. melo leaf extract.
2.2.2. Alpha-Glucosidase Inhibitory Activity
The α-glucosidase inhibitory activity of Cm-AgNPs is presented in Figure 6 (red line). The concentrations of Cm-AgNPs 20 μg/mL for 18.3 ± 0.5, 40 μg/mL for 36.3 ± 0.8, 40 μg/mL for 44.3 μg/mL ± 1.3, 80 μg/mL for 57.0. ± 1.2, and 100 μg/mL for 63.1 ± 0.9. When the concentration of Cm-AgNPs increased, the enzyme inhibitory effect also increased in the range of 20 to 65%. The maximum inhibitory action of Cm-AgNPs towards the enzyme was observed at 100 μg/mL.
2.3. Biosynthesized Silver Nanoparticles as Anticoccidial Agent
Initially, the fecal matter of coccidiosis-infected chickens, containing oocysts of E. tenella, was collected from a poultry farm. The pathological examination of infected oocysts was carried out under a confocal microscope, and the results are presented in Figure 7. The fecal matter had both unsporulated and sporulated oocysts (Figure 6 (black line)). Unsporulated oocysts of E. tenella were ovoid in shape with a zygote inside that was surrounded by an oocyst wall (Figure 7b), whereas the sporulated oocysts displayed an ovoid shape with four sporocysts inside that were surrounded by an oocyst wall (Figure 7c).
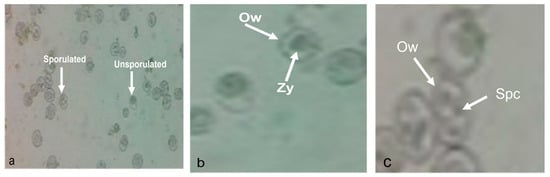
Figure 7.
Oocysts of E. tenella (a) both unsporulated and sporulated oocysts, (b) enlarged unsporulated oocysts containing oocyst wall (Ow) and zygote (Zy), and (c) enlarged sporulated oocyst containing oocyst wall (Ow) and four sporocysts (Spc).
In the present study, the examination of infected oocysts under a confocal microscope revealed that the first release of sporocysts from oocysts and the excystation of sporozoites were observed after 15–20 min of incubation in PBS solution (Figure 8). Depending on the sporulated oocyst’s age and excystation solution, 60–90% of excystation occurred.

Figure 8.
Release of sporocysts from sporulated oocysts of E. tenella (Ow—oocyst wall; Spc—sporocyst).
The effectiveness of Cm-AgNPs with different concentrations at 20 to 100 μg/mL against E. tenalla was assessed via an in vitro MTT assay using MDBK cell lines. The cell lines were used as host cells for E. tenella. These MDBK epithelial cells are commonly used in most in vitro studies on Eimeria. The cell lines were added with infected oocysts and synthesized Cm-AgNPs at different concentrations and the survival of the oocysts was monitored after 24 h of incubation in Figure 9. The results of the in vitro study demonstrated that Cm-AgNPs have anticoccidial potency against coccidiosis, as evidenced by cell shrinkage, and a decrease in the survival rate of oocysts as the concentrations of Cm-AgNPs increase. The survival rate of oocysts was evaluated using different concentrations, such as 20 μg/mL for 22.8 ± 0.8, 40 μg/mL for 18.1 ± 0.6, 60 μg/mL for 17.2 ± 1.7, 80 μg/mL 7.3 ± 1.1., and 100 μg/mL for 2.1 ± 0.7, respectively. The highest mortality of oocysts was observed at 2% at a concentration of 100 μg/mL Cm-AgNPs. Cm-AgNPs impaired the E.tenella in the host cells before the oocysts were completely formed and arrested the sporulation of oocysts.
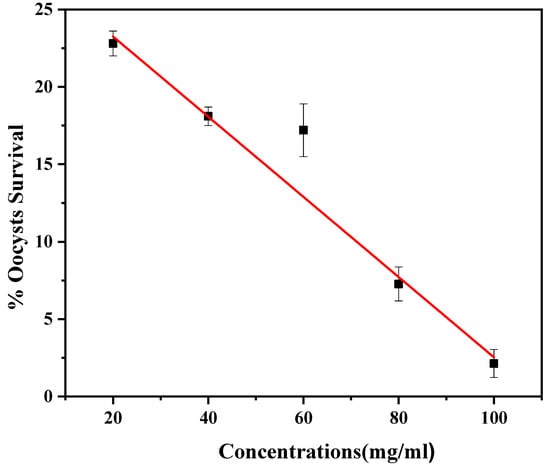
Figure 9.
Effect of silver nanoparticles (AgNPs) synthesized from C. melo against E. tenella oocysts survival rate (%).
3. Discussion
Nanotechnology has proven to be the greatest multidisciplinary field in recent years with its potential application in environmental remediation and the agriculture, chemical, and pharmaceutical industries. Despite the usefulness of metal nanoparticles, AgNPs are more appropriate in the clinical field due to their unique characteristics, such as electrical conductivity, antibacterial activity, chemical stability, etc. [35]. The chemical synthesis of AgNPs from toxic chemicals is hazardous to the environment and not suitable for biomedical applications. Therefore, the eco-friendly synthesis of AgNPs using plants, microorganisms, and biocompatible polymers [36,37] has attracted significant interest in recent years. Gopalasatheeskumar et al. reported that the total phenolic content in the leaves was 77.82 mg/g of extract while the total flavonoid content was 30.06 mg/g of extract [32]. Even though the mechanism of the bioreduction process has not been clearly ascertained, the presence of a group of phytochemical constituents, such as glycosides, alkaloids, flavonoids, terpenoid, steroids, saponin, tannin, reducing sugars, proteins, and amino acids, in the leaf extracts of C. melo reduced Ag+ ions to Ag0 and they, in turn, were oxidized to other species [38]. As shown by UV–visible absorption spectra in Figure 1, the AgNPs exhibited a well-defined absorption peak at 440 nm after 24 h of the reaction period. UV–visible spectroscopy is the most commonly used technique for the structural characterization of nanoparticles. The presence of a peak clearly indicates the biosynthesized AgNPs have strong surface plasmon resonance (SPR) electrons on the surface of nanoparticles [39]. A similar UV–visible spectrum at 440 nm was revealed by Baharara and co-workers while synthesizing the AgNPs using Salvia officinalis leaf extract [40], Moringa oleifera leaf extract [41], and the leaf extracts of Ocimum Sanctum [42].
The zeta potential value of Cm-AgNPs exhibited negatively charged and equally distributed particles in the aqueous medium, and similar results have been reported for Drosera spatulate [3]. These Cm-AgNP SEM results fall under the usual size range of silver nanoparticles. There were large-sized particles observed between the nanoparticles due to agglomeration. The morphology of AgNPs from the SEM results is in correlation with the previously reported AgNPs biosynthesized from C. melo [43]. Less than 100 nm of silver nanoparticles with spherical shapes are highly active in terms of sustainable silver release due to their vast surface region [44]. Silver nanoparticles of smaller sizes have proven antibacterial efficacy [45], antiviral properties, and other beneficial uses [46].
The IR peaks at 1640, 1748, and 3320 cm−1 are characteristic of AgNPs and are in close agreement with the previously synthesized AgNPs using plant extracts [47]. The IR peak at 3429.35 cm−1 was assigned to alcoholic O-H or N-H groups in the phenolic compound stretching vibration. A weak absorption peak at 1768.34 cm−1 corresponds to aldehyde, ketone, and carboxylic acid groups. From previous reports, it was predicted that the peak at 1768.34 cm−1 was involved (terpenoids) in the reduction process of silver ions to silver (ref). Terpenoids might have been oxidized to carboxyl groups and could have led to IR absorption in this position. A strong peak at 1630.52 cm−1 is suggested to be the capping of AgNPs. The peaks at 1384.48 cm−1, 1097.15 cm−1, 832.97 cm−1, and 598.67 cm−1 assigned to NO2, C-OH, aliphatic chloro compounds, and aliphatic bromo compounds were present in C. melo extract, respectively.
Pancreatic α-amylase is the key enzyme that breaks down the complex starch and carbohydrates, i.e., oligosaccharides, to disaccharides in the intestine. Hence, the inhibition activity of α-amylase offers an effective strategy to reduce the level of post-prandial hyperglycemia. Amylase inhibitors or starch blockers contain substances that prevent starch dietary components from being absorbed by the body. Therefore, the increase in blood sugar levels can be reduce through carbohydrate consumption [48]. Both C. melo leaf extracts [32] and biosynthesized AgNPs [48,49] have been reported as potential α-amylase inhibitors. The main role of α-glucosidase inhibitors is to inhibit the absorption of carbohydrates from the small intestine. α-glucosidase inhibitors are compared favorably to inhibitor enzymes that change complex non-absorbable into simple absorbable carbohydrates. These enzymes involved slow carbohydrate absorption and lead to a reduction in blood glucose level [50,51]. Similar dose-dependent amylase inhibition activity by Cassia auriculata-AgNPs was also reported by Thirumal and Sivakumar [48]. The results obtained in this study are in agreement with those of Kazeem et al., who mentioned that mild amylase inhibition is desirable compared to the excessive inhibition of amylase activity, since it leads to the abnormal bacterial fermentation of undigested starch in the colon [52]. They also inferred that the different biological components in the leaf extract might have greatly contributed to the inhibition of enzyme activity by competing with the substrate for binding to the active sites of α-amylase.
Our results closely match with the concentration-dependent inhibitory activity of biosynthesized AgNPS varied between 18.3% and 63.1% [48]. Kazeem et al. found that the phytochemicals present in leaves do not compete with the substrate for binding to the active sites on α-glucosidase; however, they compete for separate sites on enzymes and retard the cleavage of di- to monosaccharides and reduce the glucose level in the blood [52]. In line with previous reports [48,49], the AgNPs synthesized from the leaves of C. melo have also been shown to have strong intestinal α-glucosidase enzyme activity, which can effectively and safely be used against postprandial hyperglycemia.
Similar pathological observations were made by Kasem et al. [53]. Chickens can be infected with sporulated oocysts found in feed material, and these oocysts are the primary cause of coccidiosis spreading among chickens [53,54]. Additionally, E. tenella oocysts can remain viable and active for a period of nine months in poultry litter, making it a source for other poultry farms [55].
Many plant extracts-based nanoparticles have proven anticoccidial ability against Eimeria. Kasem et al. observed in vitro sporulation disruption with the chitosan-based nanoparticles from the ethanolic leaves extract of Rosmarinus officinalis in the oocysts of E.tenella [53]. Dkhil et al. reported that Ag-nanoparticles synthesized from the rhizome extracts of Zingiber officinale have anticoccidial ability against E. papillata [56]. Ismail et al. demonstrated that the leaves of C. melo are rich in phenolic compounds and exhibited high antioxidant potential [57]. Hence, it could be concluded that the secondary metabolites in the leaves of C. melo might have caused oxygen stress and disrupted the survival of the oocysts of E. tenella. Additionally, as suggested by Cedric et al., in extracts of Psidium guajava, the leaf extract might have penetrated the cell wall of oocysts and damaged the intracellular components [58].
4. Materials and Methods
4.1. Chemicals and Materials
Chemicals used in this study include silver nitrate (AgNO3), sodium phosphate buffer, α- amylase solution, dinitro salicylic acid, α-glucosidase solution, paranitrophenyl α-D-glucopyranoside, sodium carbonate (Na2CO3), phosphate-buffered saline (PBS) Solution, sodium hypochlorite, thiosulphate, sodium taurocholate, bovine trypsin, and Alsever’s solution. Instruments used in this study include a UV–visible spectrophotometer, an optical microscope, a confocal microscope, a scanning electron microscope, X-ray diffraction (XRD) analysis, and Fourier transform infrared (FTIR).
4.2. Synthesis and Characterization of Silver Nanoparticles
The fresh leaves of C. melo were collected from the Pallikaranai Wetland Region, Tamil Nadu, India. The collected samples were transferred to the laboratory and rinsed with distilled water before further processing to eliminate contamination. Then, the leaves were well dried under shade at room temperature (25 to 30 °C) and were pulverized into a fine coarse powder using a blender. Two grams of powdered leaves were mixed with 50 mL of distilled water and kept in a water bath with a temperature ranging from between 55 and 60 °C for 20 min. Then, the extract was filtered through Whatman No 1 filter paper and the filtrate was stored at 4 °C until further use.
All the synthesis process was carried out in the biosafety cabinet with a standard laboratory condition Scheme 1. The synthesized AgNPs from the leaf extract of C. melo were further characterized by their size, optical density, and morphology using different techniques, such as UV–visible spectrophotometry, SEM (JSM-6380LA (Tokyo, Japan), FTIR (Shimadzu Corporation, Kyoto, Japan), XRD (Ultima IV, Rigaku, Akishima, Japan), and Zeta Sizer (Malvern Instruments, Malvern, England).
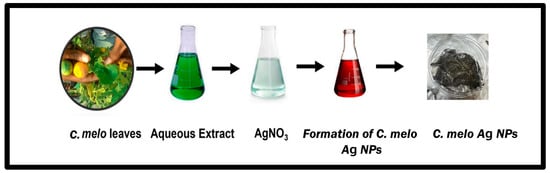
Scheme 1.
A total of 1 mL of aqueous leaf extract of C. melo was added to 9 mL of 1 mM silver nitrate (AgNO3) for AgNP synthesis. The mixture was then incubated in the dark for 24 h to achieve the bioreduction process at room temperature. The optimum condition for the formation of the silver nanoparticles was observed at near pH 7, which is a neutral condition.
4.3. Antidiabetic Screening
4.3.1. α-Amylase Inhibition Assay
The α-amylase inhibition assay was performed using the DNSA method described elsewhere [59]. The assay mixture, which consisted of 500 μL of 0.02 M sodium phosphate buffer (containing 6 mM of NaCl, pH 6.9) and α-amylase solution (1 U/mL), was added with different concentrations of AgNPs (20–100 μg/mL). The assay mixture was pre-incubated at 37 °C for 20 min. After incubation, 250 μL of 1% starch solution in the aforementioned buffer was added to the tubes and incubated for 15 min at 37 °C. Finally, the catalytic reaction was terminated by adding 1 mL of di-nitrosalicylic acid reagent and then incubated in a boiling water bath (90 ± 5 °C) for 10 min. The tubes were cooled to room temperature and the absorbance was measured at 540 nm using a UV–visible spectrophotometer. The reference sample included all other reagents and enzymes except the test sample. The α-amylase inhibitory activity was expressed as a percentage inhibition, and it was calculated according to the equation below:
4.3.2. α-Glucosidase Inhibition Assay
The α-glucosidase inhibition assay was performed by following a modified method reported elsewhere [60]. The assay mixture, consisting of 150 μL of 0.1 M sodium phosphate buffer (containing 6 mM NaCl, pH 6.9) and 0.1 U of α-glucosidase, was added with different AgNP concentrations ranging from 20 to 100 μg/mL. The assay mixture was then pre-incubated at 37 °C for 10 min. Then, 50 μL of 2 mM paranitrophenyl α-D-glucopyranoside in 0.1 M sodium phosphate buffer was added to the mixture and incubated further to start the reaction. After 20 min of incubation at 37 °C, the reaction was terminated by adding 50 μL of 0.1 M Na2CO3. Then, the absorbance was measured at 405 nm using a UV–visible spectrophotometer. The tube with α-glucosidase but without AgNPs served as a control with 100% enzyme activity, and the antidiabetic drug acarbose was employed as a positive control. The percentage of the α-glucosidase inhibition activity was calculated as follows:
4.4. In Vitro Anticoccidial Activity Screening
The fecal matter of coccidiosis-infected chickens, containing oocysts of E. tenella, was collected from a poultry farm in Tamil Nadu, India, and the samples were preserved at 4 °C until further use. The pathological examination of infected oocysts was carried out under a confocal microscope.
The release of sporocysts from infected oocysts was carried out by following a previously described method. The infected oocysts were washed and suspended in cold PBS (pH 7.2) at a concentration of 108/3 mL. A total of 3 mL of 10.5% sodium hypochlorite was added to obtain a final concentration of 12% sodium hypochlorite. The mixture was stirred for 10 min, and the reaction was arrested by adding 1.05% thiosulphate. The suspension was centrifuged at 1500 rpm for 10 min at 4 °C. The oocysts pellets were re-suspended in cold PBS and re-centrifuged. The resuspension and centrifugation were repeated four times to achieve the complete removal of added sodium hypochlorite. The percentage of sporozoites excysting was increased by pre-incubating the oocysts in PBS for 1 h at 37 °C. Then, the sediment was mixed with pre-warmed excystation fluid consisting of 0.75% (w/v in PBS) sodium taurocholate (Aldrich) with 0.25% (w/v in PBS) bovine trypsin (Difco) and was incubated for 30–45 min at 37 °C in a warm water shaking bath. The excystation process was monitored microscopically.
4.5. An In Vitro MTT Assay on Anticoccidial Efficacy
The Madin–Darby bovine kidney (MDBK) cell lines were purchased from National Repository for cell lines in India. The (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay (MTT) was performed for anticoccidial activity on MDBK cell lines [61]. The in vitro assay was carried out by using a 12-well microtiter plate. Initially, MDBK cells (100 μL) were seeded in five wells and then C. melo aqueous extract was added at five different doses (20–100 μL). After that, 25% of infected sporulated oocysts in 2 mL of poultry fecal matter was inoculated in all five wells. The MDBK cell culture exposed to infection was incubated for 24 h. Then, the microtiter plate was checked for oocyst survival under a confocal microscope. A reference assay, consisting of uninfected MDBK cells, was also maintained throughout the experiment. The survival rate percentage of sporulated oocysts was calculated as follows:
5. Conclusions
In summary, we synthesized Cucumis melo leaf extract-mediated silver nanoparticles (Cm-AgNPs), which are important in clinical and therapeutic medication. Zeta potential Cm-AgNPs exhibited high stability with high negative zeta potential (−12.8 mV). The smaller size (less than 100 nm) of the biosynthesized Cm-AgNPs shows that more than 65% of α-amylase activity was inhibited at the concentration level of 100 μg/mL. The significant inhibition of α-glucosidase activity by Cm-AgNPs was also exhibited. The anticoccidial activity of Cm-AgNPs demonstrated the reduction in the survival of oocysts of E. tenella. However, the Cm-AgNPs proved to be efficient against diabetes and coccidiosis, and more in vivo work must be done for clinical field applications.
Author Contributions
Conceptualization, P.R., N.K. and S.D.; methodology, K.P. and N.A.; formal analysis and software, P.R. and M.S.A.; validation, investigation, resources, P.R., N.K. and S.D.; writing—original draft preparation, M.S.A., K.P. and M.N.; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
King Saud University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The corresponding author will provide the data on request.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education, Saudi Arabia, for funding this research (IFKSUOR3-180-1).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
On request.
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Singh, S.K.; Singh, M. Green Synthesis of Silver Nanoparticles: Methods, Biological Applications, Delivery and Toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar]
- Gaddam, S.A.; Kotakadi, V.S.; Subramanyam, G.K.; Penchalaneni, J.; Challagundla, V.N.; Dvr, S.G.; Pasupuleti, V.R. Multifaceted Phytogenic Silver Nanoparticles by an Insectivorous Plant Drosera Spatulata Labill Var. Bakoensis and Its Potential Therapeutic Applications. Sci. Rep. 2021, 11, 21969. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, S.; Josephs, J.; Onani, M.O.; Meyer, M.; Madiehe, A.M. Biomedical Applications of Plant Extract-Synthesized Silver Nanoparticles. Biomedicines 2022, 10, 2792. [Google Scholar] [CrossRef]
- Binsalah, M.; Devanesan, S.; AlSalhi, M.S.; Nooh, A.; Alghamdi, O.; Nooh, N. Biomimetic Synthesis of Silver Nanoparticles Using Ethyl Acetate Extract of Urtica Diocia Leaves; Characterizations and Emerging Antimicrobial Activity. Microorganisms 2022, 10, 789. [Google Scholar] [CrossRef]
- Simon, S.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Madiehe, A.M.; du Preez, M.G. The Antimicrobial Activity of Biogenic Silver Nanoparticles Synthesized from Extracts of Red and Green European Pear Cultivars. Artif. Cells Nanomed. Biotechnol. 2021, 49, 613–624. [Google Scholar] [CrossRef]
- Renganathan, S.; Subramaniyan, S.; Karunanithi, N.; Vasanthakumar, P.; Kutzner, A.; Kim, P.-S.; Heese, K. Antibacterial, Antifungal, and Antioxidant Activities of Silver Nanoparticles Biosynthesized from Bauhinia Tomentosa Linn. Antioxidants 2021, 10, 1959. [Google Scholar] [CrossRef]
- Gibała, A.; Żeliszewska, P.; Gosiewski, T.; Krawczyk, A.; Duraczyńska, D.; Szaleniec, J.; Szaleniec, M.; Oćwieja, M. Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent. Biomolecules 2021, 11, 1481. [Google Scholar] [CrossRef]
- Das, M.; Rebecca, L.; Das, M. Characterization of Antidiabetic Activity of Silver Nanoparticles Using Aqueous Solution of Ficus Glomerata (Fig) Gum. Int. J. Pharm. Bio Sci. 2017, 8, 424–429. [Google Scholar]
- Wahab, M.; Bhatti, A.; John, P. Evaluation of Antidiabetic Activity of Biogenic Silver Nanoparticles Using Thymus Serpyllum on Streptozotocin-Induced Diabetic BALB/c Mice. Polymers 2022, 14, 3138. [Google Scholar] [CrossRef]
- Ratner, R.E. Glycemic Control in the Prevention of Diabetic Complications. Clin. Cornerstone 2001, 4, 24–37. [Google Scholar] [CrossRef] [PubMed]
- De Gussem, M. Coccidiosis in Poultry: Review on Diagnosis, Control, Prevention and Interaction with Overall Gut Health. In Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, France, 26–30 August 2007; pp. 253–261. [Google Scholar]
- Krücken, J.; Hosse, R.J.; Mouafo, A.N.; Entzeroth, R.; Bierbaum, S.; Marinovski, P.; Hain, K.; Greif, G.; Wunderlich, F. Excystation of Eimeria Tenella Sporozoites Impaired by Antibody Recognizing Gametocyte/Oocyst Antigens GAM22 and GAM56. Eukaryot. Cell 2008, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of Silver Nanoparticles by Chemical Reduction Method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Nakano, M.; Fujiwara, T.; Koga, N. Thermal Decomposition of Silver Acetate: Physico-Geometrical Kinetic Features and Formation of Silver Nanoparticles. J. Phys. Chem. C 2016, 120, 8841–8854. [Google Scholar] [CrossRef]
- Petit, C.; Lixon, P.; Pileni, M.P. In Situ Synthesis of Silver Nanocluster in AOT Reverse Micelles. J. Phys. Chem. 1993, 97, 12974–12983. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Atorngitjawat, P.; Meziani, M.J. Preparation of Silver Nanoparticles via Rapid Expansion of Water in Carbon Dioxide Microemulsion into Reductant Solution. Langmuir 2001, 17, 5707–5710. [Google Scholar] [CrossRef]
- Pal, A.; Shah, S.; Devi, S. Microwave-Assisted Synthesis of Silver Nanoparticles Using Ethanol as a Reducing Agent. Mater. Chem. Phys. 2009, 114, 530–532. [Google Scholar] [CrossRef]
- Bloch, K.; Pardesi, K.; Satriano, C.; Ghosh, S. Bacteriogenic Platinum Nanoparticles for Application in Nanomedicine. Front. Chem. 2021, 9, 624344. [Google Scholar] [CrossRef]
- Modan, E.; Schiopu, A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. Ann. Dunarea Jos Univ. Galati 2020, 43, 53. [Google Scholar] [CrossRef]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of Silver Nanoparticles from Phenerochaete Chrysosporium (MTCC-787) and Their Antibacterial Activity against Human Pathogenic Bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-Mediated Green Synthesis of Silver Nanoparticles Using Aspergillus Terreus. Int. J. Mol. Sci. 2011, 13, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green Synthesis of Silver Nanoparticles with Algae and the Importance of Capping Agents in the Process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef]
- Chung, I.-M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.A.; Maddux, B.L.; Hutchison, J.E. Toward Greener Nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [PubMed]
- Al-Shmgani, H.S.A.; Mohammed, W.H.; Sulaiman, G.M.; Saadoon, A.H. Biosynthesis of Silver Nanoparticles from Catharanthus Roseus Leaf Extract and Assessing Their Antioxidant, Antimicrobial, and Wound-Healing Activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Krithiga, N.; Rajalakshmi, A.; Jayachitra, A. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria Ternatea and Solanum Nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. J. Nanosci. 2015, 2015, 928204. [Google Scholar] [CrossRef]
- Kesharwani, J.; Yoon, K.-Y.; Hwang, J.; Rai, M. Phytofabrication of Silver Nanoparticles by Leaf Extract of Datura Metel: Hypothetical Mechanism Involved in Synthesis. J. Bionanoscience 2009, 3, 39–44. [Google Scholar] [CrossRef]
- Begum, N.A.; Mondal, S.; Basu, S.; Laskar, R.A.; Mandal, D. Biogenic Synthesis of Au and Ag Nanoparticles Using Aqueous Solutions of Black Tea Leaf Extracts. Colloids Surf. B Biointerfaces 2009, 71, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Su, W.; Zhang, D.; Sun, L.; Wang, H.; Xue, F.; Zhai, S.; Zou, Z.; Wu, R. Influence of Environmental Factors on Cucumis Melo L. Var. Agrestis Naud. Seed Germination and Seedling Emergence. PLoS ONE 2017, 12, e0178638. [Google Scholar] [CrossRef]
- Purseglove, J. The Origins and Migrations of Crops in Tropical Africa. Orig. Afr. Plant Domest. 1976, 291–310. [Google Scholar]
- Gopalasatheeskumar, K.; Kumar, G.A.; Sengottuvel, T.; Devan, V.S.; Srividhya, V. Quantification of Total Phenolic and Flavonoid Content in Leaves of Cucumis melo Var Agrestis Using UV-Spectrophotometer. Asian J. Res. Chem. 2019, 12, 335–337. [Google Scholar] [CrossRef]
- Pratama, O.; TUNJUNG, W.; SUTIKNO, S.; Daryono, B. Bioactive Compound Profile of Melon Leaf Extract (Cucumis melo L. ‘Hikapel’) Infected by Downy Mildew. Biodiversitas J. Biol. Divers. 2019, 20, d201143. [Google Scholar] [CrossRef]
- Saboo, S.S.; Thorat, P.K.; Tapadiya, G.G.; Khadabadi, S. Ancient and Recent Medicinal Uses of Cucurbitaceae Family. Int. J. Ther. Appl. 2013, 9, 11–19. [Google Scholar]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver Nanoparticles: Green Synthesis and Their Antimicrobial Activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Alfuraydi, A.A.; Devanesan, S.; Al-Ansari, M.; AlSalhi, M.S.; Ranjitsingh, A.J. Eco-Friendly Green Synthesis of Silver Nanoparticles from the Sesame Oil Cake and Its Potential Anticancer and Antimicrobial Activities. J. Photochem. Photobiol. B Biol. 2019, 192, 83–89. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.S. Green Synthesis of Silver Nanoparticles Using the Flower Extract of Abelmoschus Esculentus for Cytotoxicity and Antimicrobial Studies. Int. J. Nanomed. 2021, 16, 3343. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis Prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]
- Baharara, J.; Ramezani, T.; Mousavi, M.; Asadi-Samani, M. Antioxidant and Anti-Inflammatory Activity of Green Synthesized Silver Nanoparticles Using Salvia Officinalis Extract. Ann. Trop. Med. Public Health 2017, 10, 1265–1270. [Google Scholar]
- Prasad, T.; Elumalai, E. Biofabrication of Ag Nanoparticles Using Moringa Oleifera Leaf Extract and Their Antimicrobial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, 439–442. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Bai, Y.; Li, W.; Li, X.; Xing, X.; Wang, C.; Gao, L.; Yogi, M.; Swamy, M.K. Anticancer and Antibacterial Activities of Silver Nanoparticles (AgNPs) Synthesized from Cucumis melo L. J. Nanosci. Nanotechnol. 2020, 20, 4143–4151. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-Controlled Silver Nanoparticles Synthesized over the Range 5–100 Nm Using the Same Protocol and Their Antibacterial Efficacy. Rsc Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Ahmed, I.; Hassan, S.T.S.; Nawaz, M.Z.; Iqbal, H.M.N. Biogenic Nanoparticle–Chitosan Conjugates with Antimicrobial, Antibiofilm, and Anticancer Potentialities: Development and Characterization. Int. J. Environ. Res. Public Health 2019, 16, 598. [Google Scholar] [CrossRef]
- Thirumal, S.; Sivakumar, T. Synthesis of Silver Nanoparticles Using Cassia Auriculata Leaves Extracts and Their Potential Antidiabetic Activity. Int. j. botany stud. 2021.6, 35–38.
- Jini, D.; Sharmila, S. Green Synthesis of Silver Nanoparticles from Allium Cepa and Its in Vitro Antidiabetic Activity. Mater. Today Proc. 2020, 22, 432–438. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An Overview on the Role of Bioactive α-Glucosidase Inhibitors in Ameliorating Diabetic Complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Akmal, M.; Wadhwa, R. Alpha Glucosidase Inhibitors. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Kazeem, M.; Adamson, J.; Ogunwande, I. Modes of Inhibition of α-Amylase and α-Glucosidase by Aqueous Extract of Morinda Lucida Benth Leaf. BioMed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef]
- Kasem, S.M.; Mira, N.M.; Mahfouz, M.E.; Helal, I.B. In Vitro Study to Evaluate the Efficacy of Ultrasonicated Ethanolic Extract of Rosmarinus Officinalis and Its Chitosan-Based Nanoparticles Against Eimeria Tenella Oocysts of Chickens. AAPS PharmSciTech 2022, 23, 295. [Google Scholar] [CrossRef]
- Molan, A.-L.; Faraj, A.M. Effect of Selenium-Rich Green Tea Extract on the Course of Sporulation of Eimeria Oocysts. J. Dent. Med. Sci 2015, 14, 68–74. [Google Scholar]
- Mikail, H.; Yusuf, M.; Hussain, G. In Vitro Anticoccidial Activity of Methanolic Leaves Extract of Lannea Schimperi against Oocysts of Eimeria Tenella. IOSR J. Pharm. Biol. Sci. 2016, 11, 35–38. [Google Scholar]
- Dkhil, M.A.; Thagfan, F.A.; Morad, M.Y.; Al-Shaebi, E.M.; Elshanat, S.; Bauomy, A.A.; Mubaraki, M.; Hafiz, T.A.; Al-Quraishy, S.; Abdel-Gaber, R. Biosynthesized Silver Nanoparticles Have Anticoccidial and Jejunum-Protective Effects in Mice Infected with Eimeria Papillata. Environ. Sci. Pollut. Res. 2023, 30, 44566–44577. [Google Scholar] [CrossRef]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic Content and Antioxidant Activity of Cantaloupe (Cucumis melo) Methanolic Extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Cedric, Y.; Payne, V.; Nadia, N.; Kodjio, N.; Kollins, E.; Megwi, L.; Kuiate, J.-R.; Mbida, M. In Vitro Anticoccidial, Antioxidant Activities and Cytotoxicity of Psidium Guajava Extracts. Res. J. Parasitol. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.; Wickramaratne, D. In-Vitro Alpha Amylase Inhibitory Activity of the Leaf Extracts of Adenanthera Pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jeong, Y.-K.; Wang, M.-H.; Lee, W.-Y.; Rhee, H.-I. Inhibitory Effect of Pine Extract on α-Glucosidase Activity and Postprandial Hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Devanesan, S.; Alfuraydi, A.A.; Vishnubalaji, R.; Munusamy, M.A.; Murugan, K.; Nicoletti, M.; Benelli, G. Green Synthesis of Silver Nanoparticles Using Pimpinella Anisum Seeds: Antimicrobial Activity and Cytotoxicity on Human Neonatal Skin Stromal Cells and Colon Cancer Cells. Int. J. Nanomed. 2016, 11, 4439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).