Porous Carbon with Alumina Coating Nanolayer Derived from Biomass and the Enhanced Electrochemical Performance as Stable Anode Materials
Abstract
1. Introduction
2. Results and Discussion
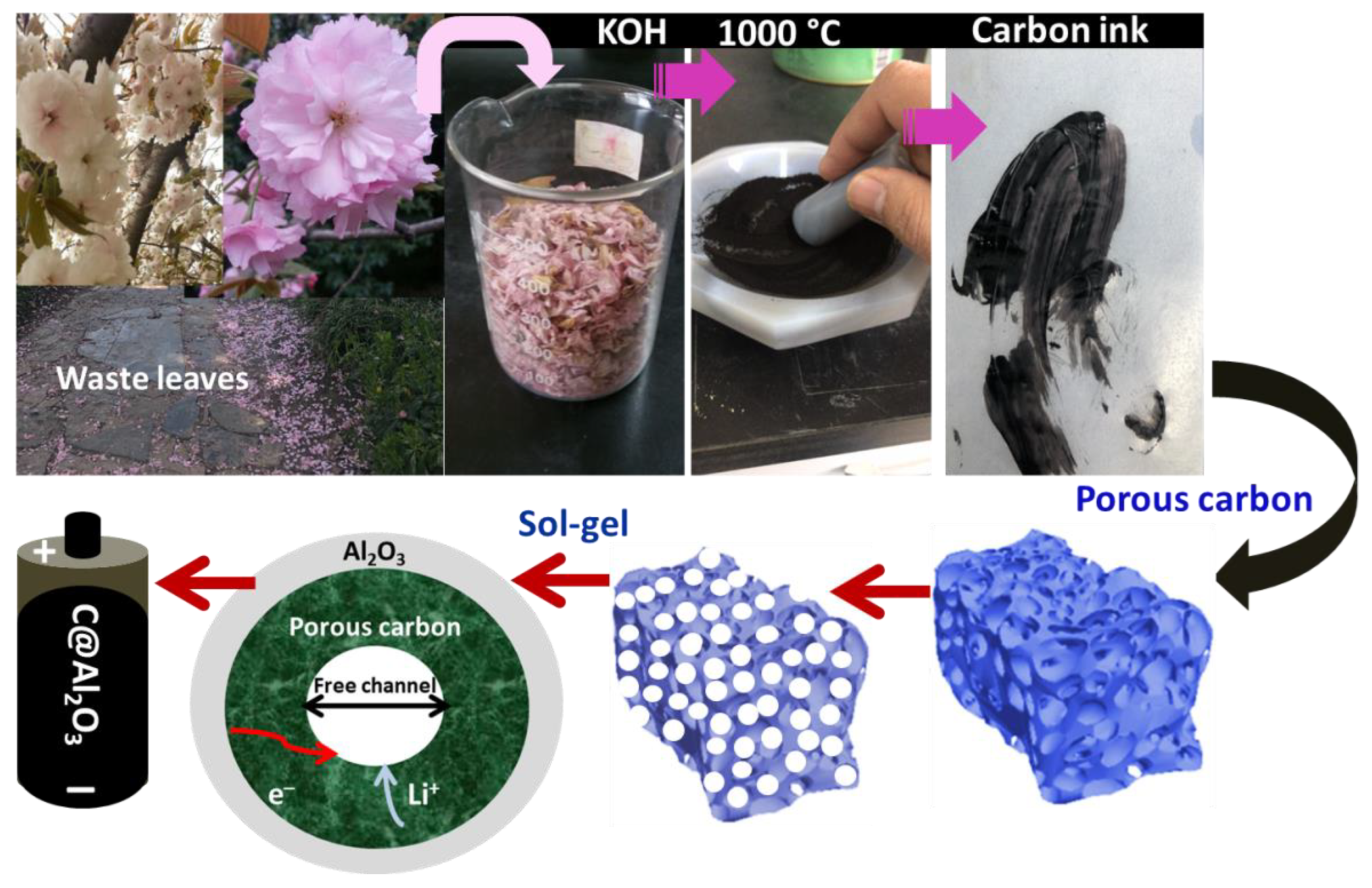
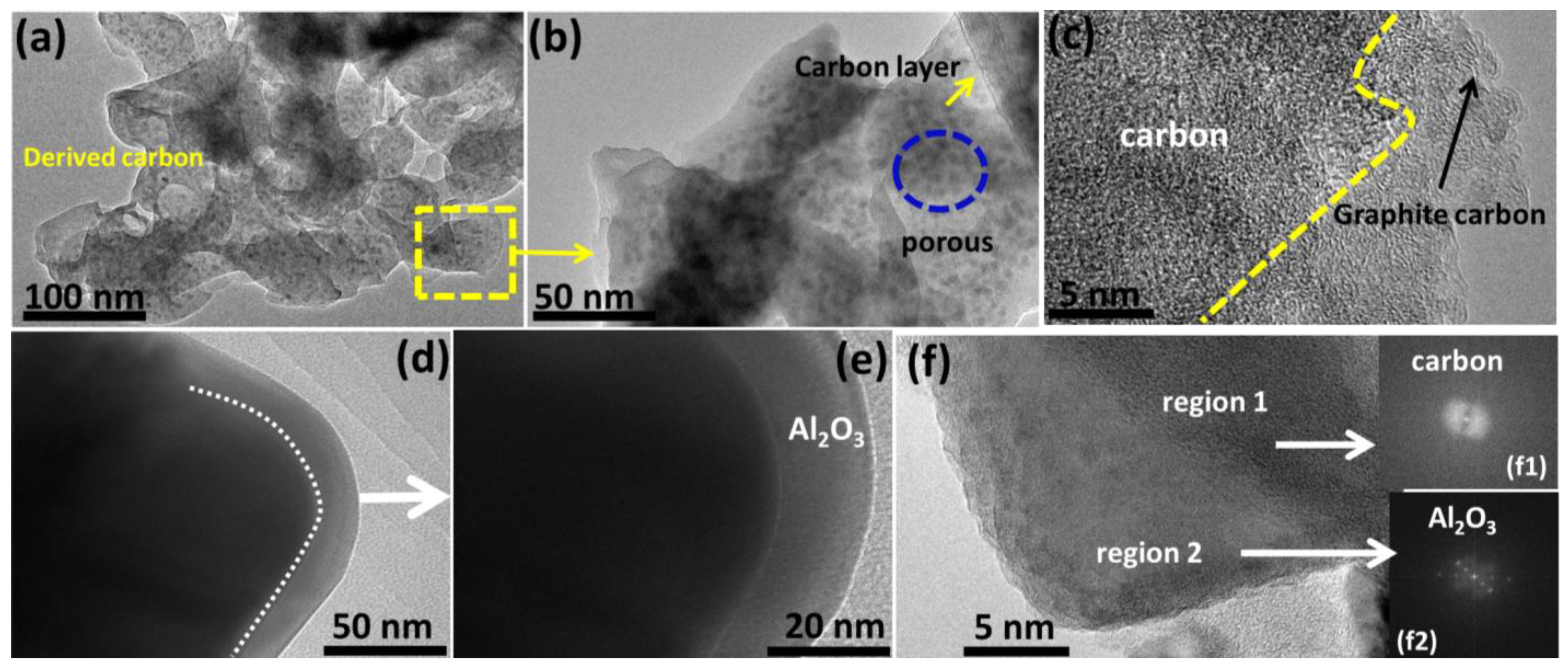
2.1. Structural Properties of Derived Porous Carbon and C@Al2O3
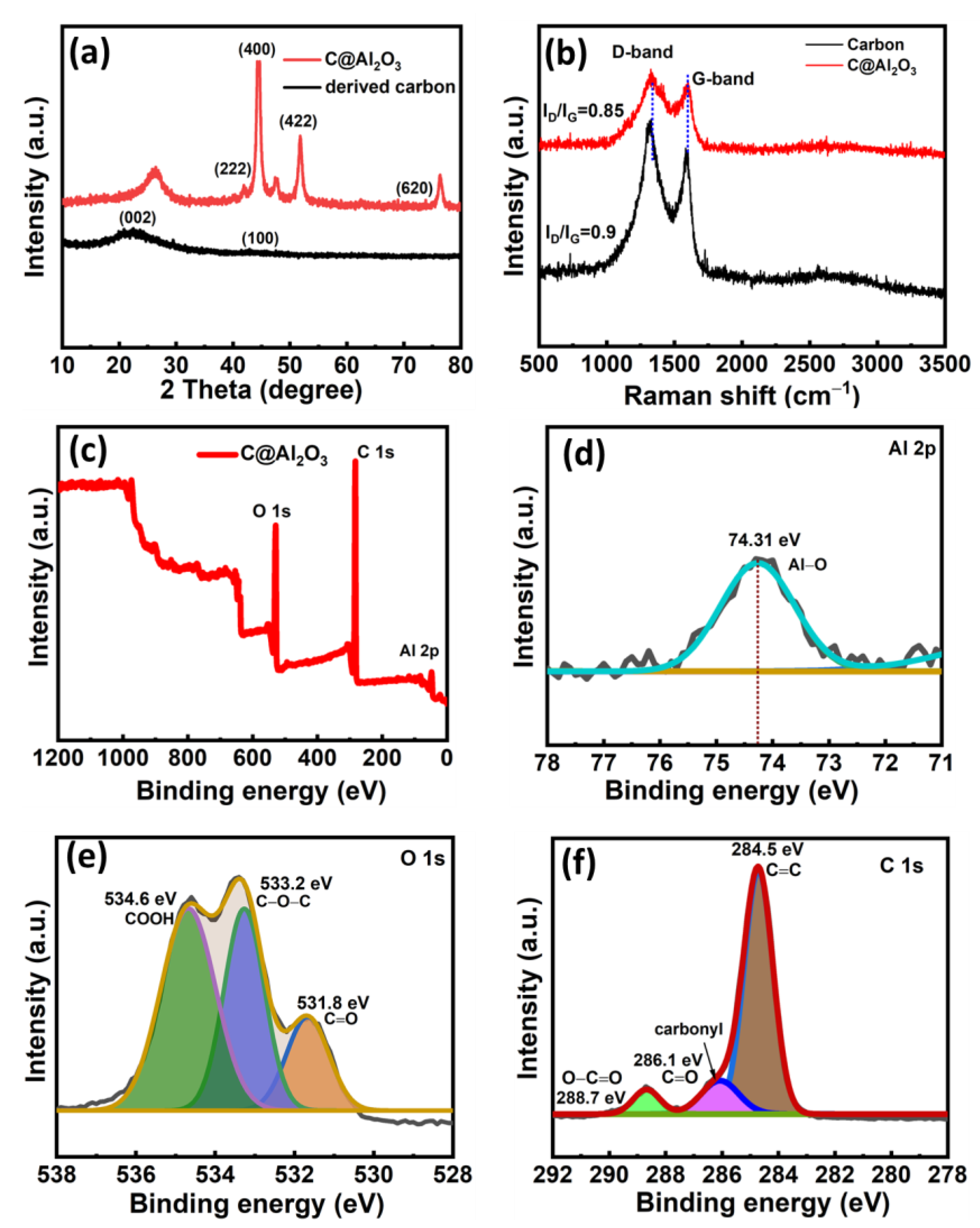
2.2. Material Structure Analysis
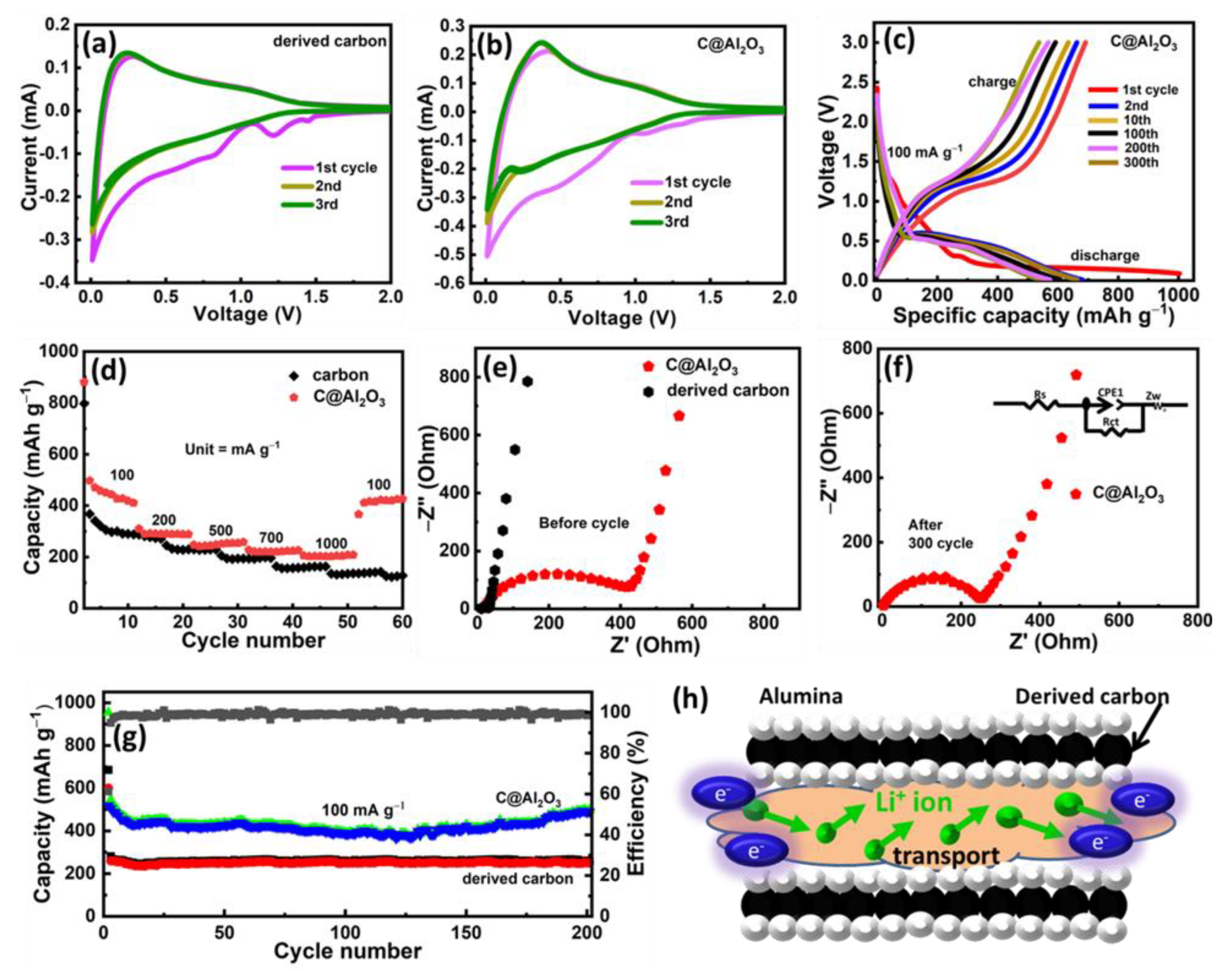
2.3. Electrochemical Performance of Porous Carbon and C@Al2O3
3. Experimental Section
3.1. Activation of Porous Carbon
3.2. Preparation of C@Al2O3
3.3. Material Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Guo, Z.; Yan, K.; Wan, S.; He, F.; Sun, B.; Wang, G. Towards high-energy-density lithium-ion batteries: Strategies for developing high-capacity lithium-rich cathode materials. Energy Storage Mater. 2021, 34, 716–734. [Google Scholar] [CrossRef]
- Qiao, Y.; Jiang, K.; Deng, H.; Zhou, H. A high-energy-density and long-life lithium-ion battery via reversible oxide–peroxide conversion. Nat. Catal. 2019, 2, 1035–1044. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J.; Cui, H.; Zhao, Y.; Cao, M. Carbon-based materials for fast charging lithium-ion batteries. Carbon 2021, 183, 721–734. [Google Scholar] [CrossRef]
- Jin, C.-B.; Shi, P.; Zhang, X.-Q.; Huang, J.-Q. Advances in carbon materials for stable lithium metal batteries. New Carbon Mater. 2022, 37, 1–24. [Google Scholar] [CrossRef]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors: A review. J. Energy Storage 2023, 61, 106716. [Google Scholar] [CrossRef]
- Xie, L.; Tang, C.; Bi, Z.; Song, M.; Fan, Y.; Yan, C.; Li, X.; Su, F.; Zhang, Q.; Chen, C. Hard Carbon Anodes for Next-Generation Li-Ion Batteries: Review and Perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Li, W.; Bao, Z.; Wang, J.; Du, Q.; Jiao, K. Comparative simulation of thin-film and bulk-type all-solid-state batteries under adiabatic and isothermal conditions. Appl. Therm. Eng. 2023, 223, 119957. [Google Scholar] [CrossRef]
- Shin, J.; Kim, T.-H.; Lee, Y.; Cho, E. Key functional groups defining the formation of Si anode solid-electrolyte interphase towards high energy density Li-ion batteries. Energy Storage Mater. 2020, 25, 764–781. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Anyanwu, I.S.; Niu, Z.; Jin, S.; Jiao, K.; Gong, Z.; Liu, Z. Analysis of compression in uniform and non-uniform GDL microstructures on water transport. Int. J. Green Energy 2022, 19, 1389–1403. [Google Scholar] [CrossRef]
- Liu, A.; Liu, T.-F.; Yuan, H.-D.; Wang, Y.; Liu, Y.-J.; Luo, J.-M.; Nai, J.-W.; Tao, X.-Y. A review of biomass-derived carbon materials for lithium metal anodes. New Carbon Mater. 2022, 37, 658–674. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.-J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X.S. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef]
- Thompson, M.; Xia, Q.; Hu, Z.; Zhao, X.S. A review on biomass-derived hard carbon materials for sodium-ion batteries. Mater. Adv. 2021, 2, 5881–5905. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Z.; Ren, G.; Feng, C.; Cheng, F. Prolonged yield platform in bioinspired three dimensional carbon materials derived from crack deflection. Mater. Lett. 2020, 270, 127759. [Google Scholar] [CrossRef]
- Ma, Q.; Dai, Y.; Wang, H.; Ma, G.; Guo, H.; Zeng, X.; Tu, N.; Wu, X.; Xiao, M. Directly conversion the biomass-waste to Si/C composite anode materials for advanced lithium ion batteries. Chin. Chem. Lett. 2021, 32, 5–8. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, X.; Liu, L.; Zhang, Z.; Teng, Y.; Yu, D.; Sui, J.; Wang, X. SiO2/C Composite Derived from Rice Husks with Enhanced Capacity as Anodes for Lithium-Ion Batteries. ChemistrySelect 2018, 3, 10338–10344. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Li, J.; Zhang, T.; Yang, M.; Guo, D.; Wu, N.; Wu, K.; Liu, X. Hierarchical N/O co-doped hard carbon derived from waste saccharomyces cerevisiae for lithium storage. J. Electroanal. Chem. 2022, 911, 116226. [Google Scholar] [CrossRef]
- Lu, X.; Xiang, K.; Wang, Y.; Zhou, W.; Zhu, Y.; Chen, W.; Chen, X.; Chen, H.; Cheng, H.; Lu, Z. Selective preparation of graphene- and rope-like NanoCarbons from camellia wastes as high performance electrode materials for energy storage. J. Alloys Compd. 2019, 811, 151616. [Google Scholar] [CrossRef]
- Seo, J.-C.; Umirov, N.; Park, S.B.; Lee, K.; Kim, S.-S. Microalgae-derived hollow carbon-MoS2 composite as anode for lithium-ion batteries. J. Ind. Eng. Chem. 2019, 79, 106–114. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Gao, X.; Li, H.; Tan, Q.; Zhong, Z.; Su, F. Enhancement of ZIF-8 derived N-doped carbon/silicon composites for anode in lithium ions batteries. J. Alloys Compd. 2021, 872, 159712. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, K.; Liu, S.; Song, H.; Zhou, J. Flexible Co0.85Se nanosheets/graphene composite film as binder-free anode with high Li- and Na-Ion storage performance. J. Alloys Compd. 2018, 731, 714–722. [Google Scholar] [CrossRef]
- Ling, H.Y.; Su, Z.; Chen, H.; Hencz, L.; Zhang, M.; Tang, Y.; Zhang, S. Biomass-Derived Poly(Furfuryl Alcohol)–Protected Aluminum Anode for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1800995. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Dai, H.; Yuan, X.; Peng, Y.; Huang, W.; Fu, L.; Zhu, Y.; Wu, Y.; Wang, X. A selenium-doped carbon anode of high performance for lithium ion batteries. J. Solid State Electrochem. 2021, 25, 457–464. [Google Scholar] [CrossRef]
- Wang, K.; Wang, M.; Huang, J. Natural-Cellulose-Derived Tin-Nanoparticle/Carbon-Nanofiber Composite as Anodic Material in Lithium-Ion Batteries. ChemNanoMat 2016, 2, 1040–1046. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, J.; Zeng, Y.; Chen, Y.; Guo, H.; Li, L.; Chen, X.; Zhang, Y. Two-dimensional ZnS@N-doped carbon nanoplates for complete lithium ion batteries. Nanotechnology 2021, 33, 065406. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Huang, C.-H.; Liu, W.-R. Co/ZnO/Nitrogen-Doped Carbon Composite Anode Derived from Metal Organic Frameworks for Lithium Ion Batteries. Polymers 2022, 14, 3085. [Google Scholar] [CrossRef]
- Wang, H.-H.; Jin, B.; Li, L.-L.; Lang, X.-Y.; Yang, C.-C.; Gao, W.; Zhu, Y.-F.; Wen, Z.; Jiang, Q. Lithium Storage in Carbon-coated Zinc Iron Oxides as Anode Materials for Lithium-Ion Batteries. Energy Technol. 2017, 5, 611–615. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Yokokura, T.J.; Rodriguez, J.R.; Pol, V.G. Waste Biomass-Derived Carbon Anode for Enhanced Lithium Storage. ACS Omega 2020, 5, 19715–19720. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, L.; Du, Q.; Jiao, K. Lithium ion transport in solid polymer electrolyte filled with alumina nanoparticles. Energy Adv. 2022, 1, 269–276. [Google Scholar] [CrossRef]
- Li, W.; Bao, Z.; Du, Q.; Xu, Y.; Jiao, K. Open-Source CFD Elucidating Mechanism of 3D Pillar Electrode in Improving All-Solid-State Battery Performance. Adv. Sci. 2022, 9, 2105454. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, C.; Fan, L.; Peng, S.; Xu, W.; Xu, J. A bacterial cellulose/Al2O3 nanofibrous composite membrane for a lithium-ion battery separator. Cellulose 2017, 24, 1889–1899. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Huang, J.; Li, K.; Zeng, J.; Yang, Y.; Li, J.; Wang, Y.; Wang, J.; Zhao, J. Investigation of the Reversible Intercalation/Deintercalation of Al into the Novel Li3VO4@C Microsphere Composite Cathode Material for Aluminum-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 28486–28494. [Google Scholar] [CrossRef]
- Edfouf, Z.; Sougrati, M.T.; Fariaut-Georges, C.; Cuevas, F.; Jumas, J.C.; Hézèque, T.; Jordy, C.; Caillon, G.; Latroche, M. Reactivity assessment of lithium with the different components of novel Si/Ni3.4Sn4/Al/C composite anode for Li-ion batteries. J. Power Sources 2013, 238, 210–217. [Google Scholar] [CrossRef]
- Vijaya Ramnath, B.; Parswajinan, C.; Dharmaseelan, R.; Thileepan, K.; Nithin Krishna, K. A review on aluminium metal matrix composites. Mater. Today Proc. 2021, 46, 4341–4343. [Google Scholar] [CrossRef]
- Yin, P.-S.; Peng, H.-T.; Xiao, Y.; Lin, T.-W.; Lin, J.-Y. Facile synthesis of an Al-doped carbon-coated Li4Ti5O12 anode for high-rate lithium-ion batteries. RSC Adv. 2016, 6, 77151–77160. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, X.; Liu, Y.; Lei, C.; Wei, T.; Guo, Z. Double-shell-structured Si@Al2O3@C nanoparticles as high-performance anode materials for lithium-ion batteries. J. Alloys Compd. 2022, 923, 166428. [Google Scholar] [CrossRef]
- Xiao, X.; Lu, P.; Ahn, D. Ultrathin Multifunctional Oxide Coatings for Lithium Ion Batteries. Adv. Mater. 2011, 23, 3911–3915. [Google Scholar] [CrossRef]
- Jung, S.C.; Han, Y.-K. How Do Li Atoms Pass through the Al2O3 Coating Layer during Lithiation in Li-ion Batteries? J. Phys. Chem. Lett. 2013, 4, 2681–2685. [Google Scholar] [CrossRef]
- Casino, S.; Heidrich, B.; Makvandi, A.; Beuse, T.; Gallasch, T.; Peterlechner, M.; Wilde, G.; Winter, M.; Niehoff, P. Al2O3 protective coating on silicon thin film electrodes and its effect on the aging mechanisms of lithium metal and lithium ion cells. J. Energy Storage 2021, 44, 103479. [Google Scholar] [CrossRef]
- Zhu, H.; Shiraz, M.H.A.; Liu, L.; Hu, Y.; Liu, J. A facile and low-cost Al2O3 coating as an artificial solid electrolyte interphase layer on graphite/silicon composites for lithium-ion batteries. Nanotechnology 2021, 32, 144001. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, A.; Li, Y.; Jiang, H.; Zhang, W.; Zhang, L.; Li, L.; Peng, S. Simple preparation of Si/N-doped carbon anodes from photovoltaic industry waste for lithium-ion batteries. J. Alloys Compd. 2022, 890, 161792. [Google Scholar] [CrossRef]
- Huang, C.; Feng, Z.; Pei, F.; Fu, A.; Qu, B.; Chen, X.; Fang, X.; Kang, H.; Cui, J. Understanding Protection Mechanisms of Graphene-Encapsulated Silicon Anodes with Operando Raman Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 35532–35541. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Roscow, J.; Chandrasekaran, S.; Deng, L.; Zhang, P.; He, T.; Wang, K.; Huang, L. Biomass-Derived Carbons for Sodium-Ion Batteries and Sodium-Ion Capacitors. ChemSusChem 2020, 13, 1275–1295. [Google Scholar] [CrossRef]
- Li, N.; Yi, Z.; Lin, N.; Qian, Y. An Al2O3 coating layer on mesoporous Si nanospheres for stable solid electrolyte interphase and high-rate capacity for lithium ion batteries. Nanoscale 2019, 11, 16781–16787. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Chen, H.; You, R.; Zheng, G.; Zhang, Q.; Wang, J.; Li, C.; Chen, S.; Yang, Y. Double-shelled microscale porous Si anodes for stable lithium-ion batteries. J. Power Sources 2019, 436, 226794. [Google Scholar] [CrossRef]
- Lin, L.; Lin, W.; Zhu, Y.X.; Zhao, B.Y.; Xie, Y.C.; Jia, G.Q.; Li, C. Uniformly Carbon-Covered Alumina and Its Surface Characteristics. Langmuir 2005, 21, 5040–5046. [Google Scholar] [CrossRef]
- Long, C.; Jiang, L.; Wu, X.; Jiang, Y.; Yang, D.; Wang, C.; Wei, T.; Fan, Z. Facile synthesis of functionalized porous carbon with three-dimensional interconnected pore structure for high volumetric performance supercapacitors. Carbon 2015, 93, 412–420. [Google Scholar] [CrossRef]
- Dong, X.; Jin, H.; Wang, R.; Zhang, J.; Feng, X.; Yan, C.; Chen, S.; Wang, S.; Wang, J.; Lu, J. High Volumetric Capacitance, Ultralong Life Supercapacitors Enabled by Waxberry-Derived Hierarchical Porous Carbon Materials. Adv. Energy Mater. 2018, 8, 1702695. [Google Scholar] [CrossRef]
- Plyuto, I.V.; Shpak, A.P.; Stoch, J.; Sharanda, L.F.; Plyuto, Y.V.; Babich, I.V.; Makkee, M.; Moulijn, J.A. XPS characterisation of carbon-coated alumina support. Surf. Interface Anal. 2006, 38, 917–921. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Kong, Q.; Zhang, C.; Pang, S.; Yue, L.; Wang, X.; Yao, J.; Cui, G. Renewable and Superior Thermal-Resistant Cellulose-Based Composite Nonwoven as Lithium-Ion Battery Separator. ACS Appl. Mater. Interfaces 2013, 5, 128–134. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]


| Sample | Specific Capacity (mAh g−1) | Cycles | Current (mA g−1) | References |
|---|---|---|---|---|
| Si/C | 420.7 | 150 | 3C | 2021 [17] |
| SiO2/C | 530 | 100 | 500 | 2018 [18] |
| N/O doped carbon | 307 | 500 | 1000 | 2022 [19] |
| Graphene- and rope-like nano carbons | 355 | 1000 | 1000 | 2019 [20] |
| Microalgae-derived hollow carbon-MoS2 | 300 | 880 | 5 | 2019 [21] |
| ZIF-8 derived N-doped carbon/silicon composites | 302 | 800 | 1 | 2021 [22] |
| Co0.85Se nanosheets/graphene | 522.7 | 500 | 2 | 2018 [23] |
| Biomass-derived poly(furfuryl alcohol)-protected aluminum | 400 | 25 | 2018 [24] | |
| Selenium-doped carbon | 450 | 580 | 0.5 | 2020 [25] |
| Tin–nanoparticle/carbon–nanofiber | 430 | 200 | 0.1 | 2016 [26] |
| ZnS@N-doped carbon nanoplates | 536 | 500 | 0.5 | 2021 [27] |
| Co/ZnO/nitrogen-doped carbon | 400 | 50 | 0.2 | 2022 [28] |
| ZnFe2O4/C | 579 | 100 | 0.1 | 2016 [29] |
| C@Al2O3 | 516 | 200 | 0.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, W.u.; Huang, H.; Yousaf, M.Z.; Aslam, F.; Wang, X.; Ghani, A. Porous Carbon with Alumina Coating Nanolayer Derived from Biomass and the Enhanced Electrochemical Performance as Stable Anode Materials. Molecules 2023, 28, 2792. https://doi.org/10.3390/molecules28062792
Rehman Wu, Huang H, Yousaf MZ, Aslam F, Wang X, Ghani A. Porous Carbon with Alumina Coating Nanolayer Derived from Biomass and the Enhanced Electrochemical Performance as Stable Anode Materials. Molecules. 2023; 28(6):2792. https://doi.org/10.3390/molecules28062792
Chicago/Turabian StyleRehman, Wasif ur, Haiming Huang, Muhammad Zain Yousaf, Farooq Aslam, Xueliang Wang, and Awais Ghani. 2023. "Porous Carbon with Alumina Coating Nanolayer Derived from Biomass and the Enhanced Electrochemical Performance as Stable Anode Materials" Molecules 28, no. 6: 2792. https://doi.org/10.3390/molecules28062792
APA StyleRehman, W. u., Huang, H., Yousaf, M. Z., Aslam, F., Wang, X., & Ghani, A. (2023). Porous Carbon with Alumina Coating Nanolayer Derived from Biomass and the Enhanced Electrochemical Performance as Stable Anode Materials. Molecules, 28(6), 2792. https://doi.org/10.3390/molecules28062792






