An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products
Abstract
1. Introduction
2. The Active Substances in Cannabis, the Endocannabinoid System (ECS), and Metabolism
2.1. The Active Substances in Cannabis
2.2. The Endocannabinoid System (ECS)
2.3. Cannabinoid Receptors and Cannabinoids Effects
2.4. THC Metabolism
3. Acute Toxicity of Cannabis Use
3.1. Inhalation and Smoking
3.2. Ingestion
4. Chronic Toxicity of Cannabis Use
4.1. Inhalation
4.2. Ingestion
4.3. Exposure to Cannabis Regardless of Route
4.3.1. Cognitive Performance, Psychomotor Performance, and Psychopathology
4.3.2. Cardiovascular Systems
4.3.3. Hormone and Reproductive Systems
4.3.4. Diseases Related to Long-Term Cannabis Use
5. Individuality of Response
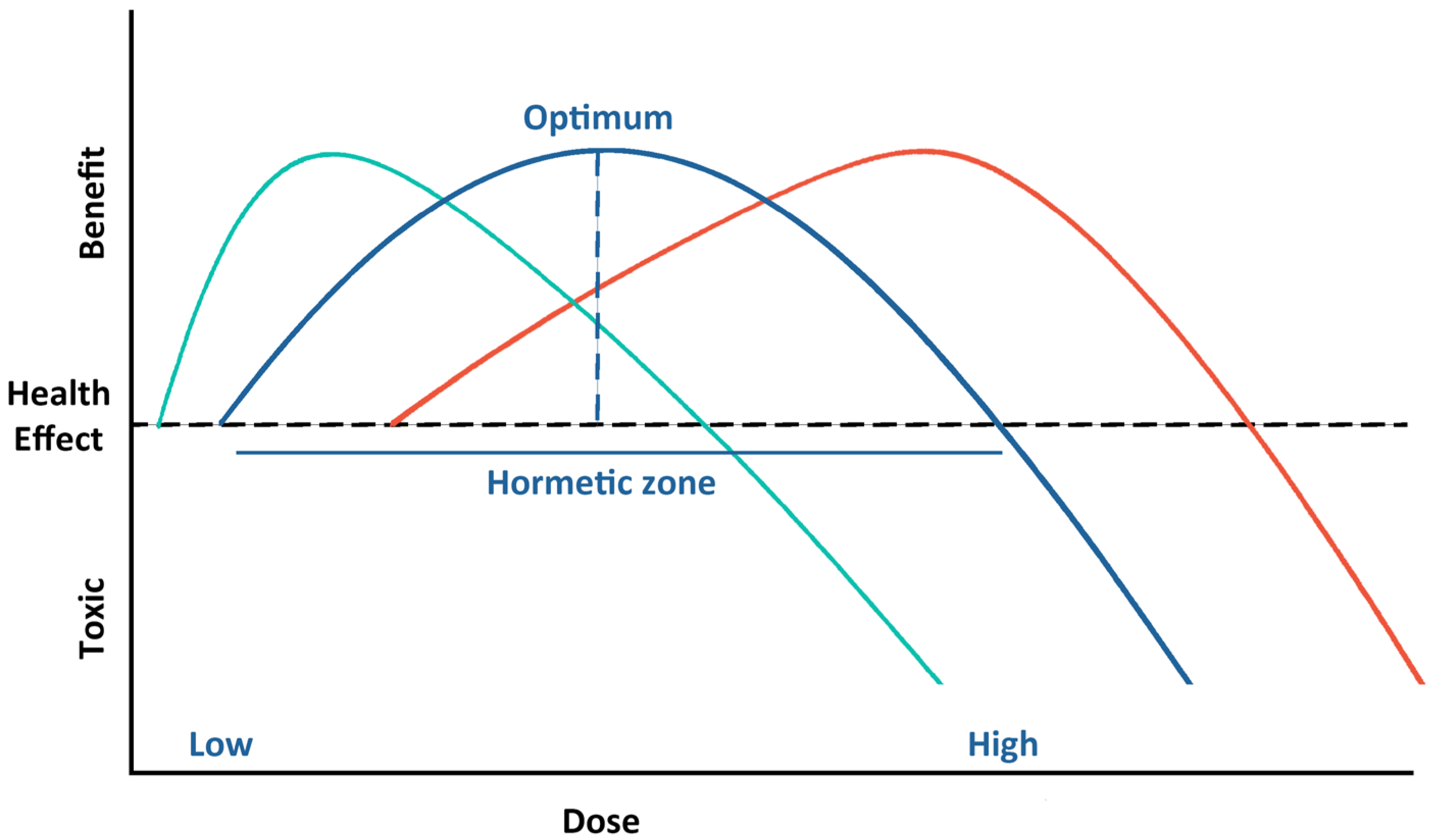
5.1. Biphasic Dose–Response
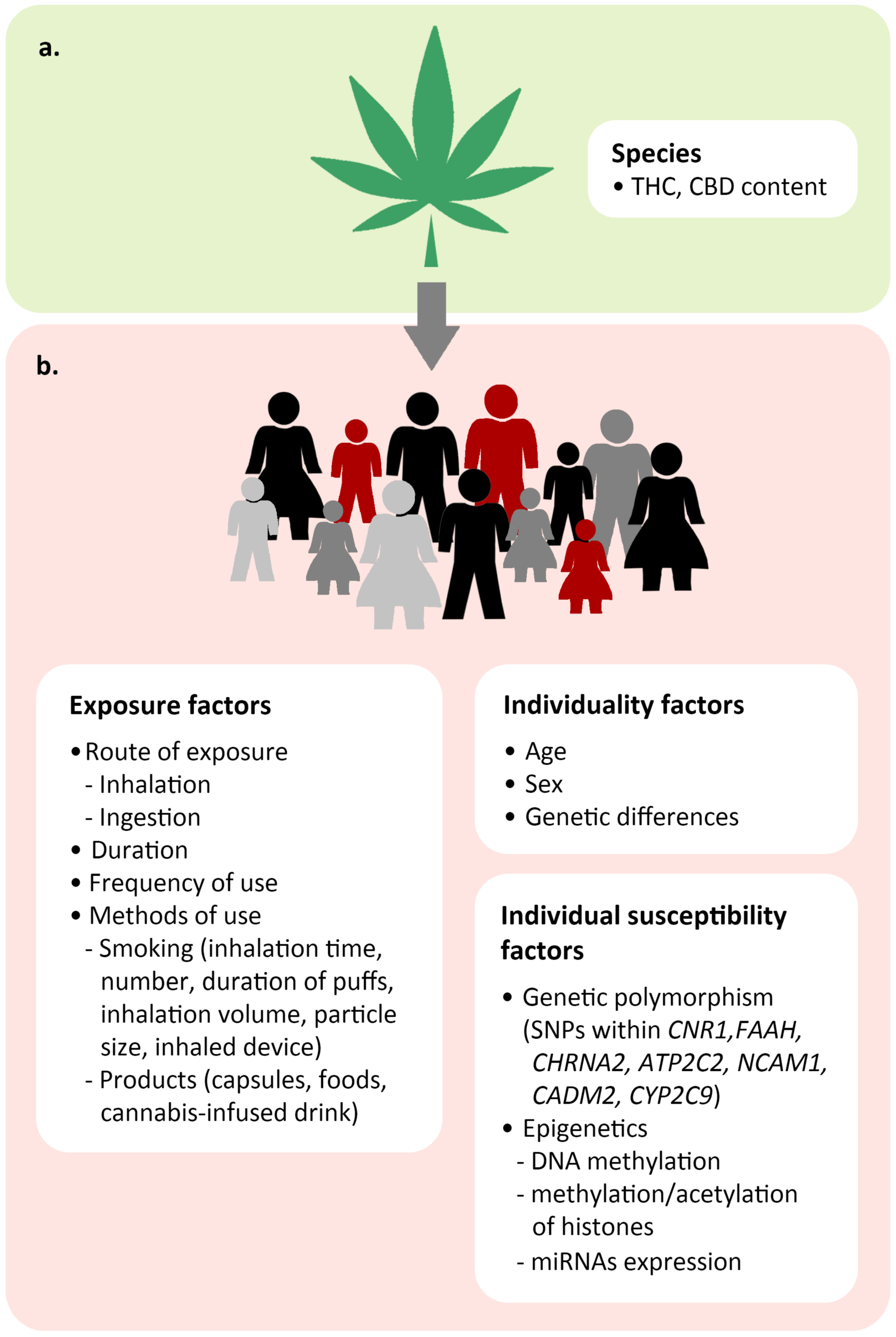
5.2. Factors Affecting Individual Response to Cannabinoids
5.2.1. Exposure Factors
5.2.2. Individual Factors
5.2.3. Individual Susceptibility Factors
Genetic Polymorphism
Epigenetic Regulation
6. Evidence-Based Clinical Safety and Efficacy of Cannabis Products
7. Challenges in Establishing Safe Doses of Dietary Cannabis Products
8. Conclusions and Future Perspectives
8.1. Conclusions
8.2. Future Questions to Be Addressed
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conway, J. Cannabis Market Worldwide—Statistics & Facts. Published on 23 March 2022. Available online: https://www.statista.com/topics/9159/global-cannabis-market/#dossierKeyfigures (accessed on 1 March 2023).
- World Health Organization. Cannabis. Available online: https://www.who.int/teams/mental-health-and-substance-use/alcohol-drugs-and-addictive-behaviours/drugs-psychoactive/cannabis (accessed on 9 November 2022).
- Statista. Legal Adult-Use Cannabis Sales Worldwide from 2020 to 2025. Available online: https://www.statista.com/statistics/1005176/global-legal-cannabis-market-size/ (accessed on 9 November 2022).
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. In 4, Therapeutic Effects of Cannabis and Cannabinoids; National Academies Press: Washington, DC, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK425767/ (accessed on 1 March 2023).
- Turner, A.R.; Spurling, B.C.; Agrawal, S. Marijuana Toxicity. [Updated 1 August 2022]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430823/ (accessed on 1 March 2023).
- Kelly, B.F.; Nappe, T.M. Cannabinoid Toxicity. [Updated 12 July 2022]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482175/ (accessed on 1 March 2023).
- Bortolato, M.; Bini, V.; Tambaro, S. Vulnerability Factors for the Psychiatric and Behavioral Effects of Cannabis. Pharmaceuticals 2010, 3, 2799–2820. [Google Scholar] [CrossRef]
- Procaccia, S.; Lewitus, G.M.; Lipson Feder, C.; Shapira, A.; Berman, P.; Meiri, D. Cannabis for Medical Use: Versatile Plant Rather Than a Single Drug. Front. Pharmacol. 2022, 13, 894960. [Google Scholar] [CrossRef]
- Jugl, S.; Sajdeya, R.; Morris, E.J.; Goodin, A.J.; Brown, J.D. Much Ado about Dosing: The Needs and Challenges of Defining a Standardized Cannabis Unit. Med. Cannabis Cannabinoids 2021, 4, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Schweikle, S.; Golombek, P.; Sproll, C.; Walch, S.G.; Lachenmeier, D.W. The Challenge of Risk Assessment of Tetrahydrocannabinol (THC) in Cannabidiol (CBD) Oils and Food Supplements: An Approach for Deriving Maximum Limits. Challenges 2022, 13, 32. [Google Scholar] [CrossRef]
- Kvamme, S.L.; Pedersen, M.M.; Rømer Thomsen, K.; Thylstrup, B. Exploring the use of cannabis as a substitute for prescription drugs in a convenience sample. Harm. Reduct. J. 2021, 18, 72. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Borchart, M.; Hasegawa, M.; Kochanski, J.; Orhurhu, V.; Viswanath, O. An Update of Current Cannabis-Based Pharmaceuticals in Pain Medicine. Pain Ther. 2019, 8, 41–51. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Bufo, S.A.; Karaman, R.; Scrano, L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins 2021, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Borah, R.; Sharma, B.; Pandhi, S.; Tripathi, V.; Yadav, H.S.; Devi, S.; Patil, U.; et al. Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: An overview. Phytother. Res. 2021, 35, 6010–6029. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, F.; Nanda, S.; Mohanty, S.; Dalai, A.K.; Kumar, V.; Ponnusamy, S.K.; Naik, S. Cannabis: Chemistry, extraction and therapeutic applications. Chemosphere 2022, 289, 133012. [Google Scholar] [CrossRef]
- Solymosi, K.; Köfalvi, A. Cannabis: A Treasure Trove or Pandora’s Box? Mini Rev. Med. Chem. 2017, 17, 1223–1291. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.-Y.; Li, S.-H.; Ma, W.; Wu, D.-T.; Li, H.-B.; Xiao, A.-P.; Liu, L.-L.; Zhu, F.; Gan, R.-Y. Cannabis sativa bioactive compounds and their extraction, separation, purification, and identification technologies: An updated review. TrAC Trends Anal. Chem. 2022, 149, 116554. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Brenneisen, R. Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents. In Marijuana and the Cannabinoids; ElSohly, M.A., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 17–49. [Google Scholar]
- Mostafaei Dehnavi, M.; Ebadi, A.; Peirovi, A.; Taylor, G.; Salami, S.A. THC and CBD Fingerprinting of an Elite Cannabis Collection from Iran: Quantifying Diversity to Underpin Future Cannabis Breeding. Plants 2022, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.-T.; Zhang, J.-C.; Zhang, H.; Liu, Q.-C.; Zhao, Y.; Hou, Y.-F.; Bai, L.; Zhang, L.; Liu, X.-Q.; Liu, X.-Y.; et al. Bioactive spirans and other constituents from the leaves of Cannabis sativa f. sativa. J. Asian Nat. Prod. Res. 2017, 19, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Baker, H.; Clancy, K.; Keepers, K.G.; Mendieta, J.P.; Pauli, C.S.; Tittes, S.B.; White, K.H.; Kane, N.C. Genetic and Genomic Tools for Cannabis sativa. Crit. Rev. Plant Sci. 2016, 35, 364–377. [Google Scholar] [CrossRef]
- Elzinga, S.; Fischedick, J.; Podkolinski, R.; Raber, J. Cannabinoids and terpenes as chemotaxonomic markers in cannabis. Nat. Prod. Chem. Res. 2015, 3, 2. [Google Scholar]
- Smith, C.J.; Vergara, D.; Keegan, B.; Jikomes, N. The phytochemical diversity of commercial Cannabis in the United States. PLoS ONE 2022, 17, e0267498. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011, 111, 5899–5921. [Google Scholar] [CrossRef]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Disc. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, I.; Krzysik-Walker, S.M.; Doyle, M.E.; Liu, Q.R.; Cimbro, R.; Santa-Cruz Calvo, S.; Ghosh, S.; Cieśla, Ł.; Moaddel, R.; Carlson, O.D.; et al. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci. Rep. 2016, 6, 33302. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar] [CrossRef]
- Tam, J.; Trembovler, V.; Di Marzo, V.; Petrosino, S.; Leo, G.; Alexandrovich, A.; Regev, E.; Casap, N.; Shteyer, A.; Ledent, C.; et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. Faseb. J. 2008, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Schacht, J.P.; Hutchison, K.E.; Filbey, F.M. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology 2012, 37, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Pan, C.H.; Hishimoto, A.; Li, C.Y.; Xi, Z.X.; Llorente-Berzal, A.; Viveros, M.P.; Ishiguro, H.; Arinami, T.; Onaivi, E.S.; et al. Species differences in cannabinoid receptor 2 (CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009, 8, 519–530. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharm. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Bardhi, K.; Coates, S.; Watson, C.J.W.; Lazarus, P. Cannabinoids and drug metabolizing enzymes: Potential for drug-drug interactions and implications for drug safety and efficacy. Expert Rev. Clin. Pharmacol. 2022, 15, 1443–1460. [Google Scholar] [CrossRef]
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm J. 2020, 25, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodiver. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.U.; Baum, C.R. Acute Cannabis Toxicity. Pediatr. Emerg. Care 2019, 35, 799–804. [Google Scholar] [CrossRef]
- Martin, J.H.; Schneider, J.; Lucas, C.J.; Galettis, P. Exogenous Cannabinoid Efficacy: Merely a Pharmacokinetic Interaction? Clin. Pharmacokinet. 2018, 57, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb. Exp. Pharmacol. 2005, 168, 657–690. [Google Scholar] [CrossRef]
- Oberbarnscheidt, T.; Miller, N.S. Pharmacology of Marijuana. J. Addict. Res. Ther. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.H. Adverse effects of cannabis and cannabinoids. Br. J. Anaesth. 1999, 83, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Wang, T.; Shapiro, S.; Collet, J.-P.; Boulanger, A.; Esdaile, J.M.; Gordon, A.; Lynch, M.; Moulin, D.E.; O’Connell, C. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J. Pain 2015, 16, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.J.; Hedberg, K.; Hendrickson, R.G. Acute cannabis toxicity. Clin. Toxicol. 2019, 57, 735–742. [Google Scholar] [CrossRef]
- Richards, J.R.; Smith, N.E.; Moulin, A.K. Unintentional Cannabis Ingestion in Children: A Systematic Review. J. Pediatr. 2017, 190, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.H. Pharmacology and effects of cannabis: A brief review. Br. J. Psychiatry 2001, 178, 101–106. [Google Scholar] [CrossRef]
- Hall, W. The Health and Psychological Effects of Cannabis Use. Curr. Issues Crim. Justice 1994, 6, 208–220. [Google Scholar] [CrossRef]
- Reece, A.S. Chronic toxicology of cannabis. Clin. Toxicol. 2009, 47, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Roth, M.D. Pulmonary effects of inhaled cannabis smoke. Am. J. Drug Alcohol Abus. 2019, 45, 596–609. [Google Scholar] [CrossRef]
- Lee, M.H.; Hancox, R.J. Effects of smoking cannabis on lung function. Expert Rev. Respir. Med. 2011, 5, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Myung, S.K. Cannabis Smoking and Risk of Cancer: A Meta-Analysis of Observational Studies. J. Glob. Oncol. 2018, 4, 196s. [Google Scholar] [CrossRef]
- Marks, M.A.; Chaturvedi, A.K.; Kelsey, K.; Straif, K.; Berthiller, J.; Schwartz, S.M.; Smith, E.; Wyss, A.; Brennan, P.; Olshan, A.F.; et al. Association of marijuana smoking with oropharyngeal and oral tongue cancers: Pooled analysis from the INHANCE consortium. Cancer Epidemiol Biomark. Prev. 2014, 23, 160–171. [Google Scholar] [CrossRef]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids Induce Cancer Cell Proliferation via Tumor Necrosis Factor α-Converting Enzyme (TACE/ADAM17)-Mediated Transactivation of the Epidermal Growth Factor Receptor. Cancer Res. 2004, 64, 1943–1950. [Google Scholar] [CrossRef]
- Huber, G.L.; First, M.W.; Grubner, O. Marijuana and tobacco smoke gas-phase cytotoxins. Pharmacol. Biochem. Behav. 1991, 40, 629–636. [Google Scholar] [CrossRef]
- Roth, M.D.; Marques-Magallanes, J.A.; Yuan, M.; Sun, W.; Tashkin, D.P.; Hankinson, O. Induction and Regulation of the Carcinogen-Metabolizing Enzyme CYP1A1 by Marijuana Smoke and Δ9-Tetrahydrocannabinol. Am. J. Resp. Cell. Mol. Biol. 2001, 24, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Dziwenka, M.; Coppock, R.; Alexander, M.; Palumbo, E.; Ramirez, C.; Lermer, S. Safety Assessment of a Hemp Extract using Genotoxicity and Oral Repeat-Dose Toxicity Studies in Sprague-Dawley Rats. Toxicol. Rep. 2020, 7, 376–385. [Google Scholar] [CrossRef]
- Yassa, H.A.; Dawood, A.W.; Shehata, M.M.; Abdel-Hady, R.H.; Aal, K.M. Subchronic toxicity of cannabis leaves on male albino rats. Hum. Exp. Toxicol. 2010, 29, 37–47. [Google Scholar] [CrossRef]
- Block, R.I.; Ghoneim, M.M. Effects of chronic marijuana use on human cognition. Psychopharmacology 1993, 110, 219–228. [Google Scholar] [CrossRef]
- Pope, H.G., Jr.; Yurgelun-Todd, D. The residual cognitive effects of heavy marijuana use in college students. JAMA 1996, 275, 521–527. [Google Scholar] [CrossRef]
- Misner, D.L.; Sullivan, J.M. Mechanism of Cannabinoid Effects on Long-Term Potentiation and Depression in Hippocampal CA1 Neurons. J. Neurosci. 1999, 19, 6795–6805. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, D.M.; Horwood, L.J.; Swain-Campbell, N.R. Cannabis dependence and psychotic symptoms in young people. Psychol. Med. 2003, 33, 15–21. [Google Scholar] [CrossRef]
- Moore, T.H.M.; Zammit, S.; Lingford-Hughes, A.; Barnes, T.R.E.; Jones, P.B.; Burke, M.; Lewis, G. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet 2007, 370, 319–328. [Google Scholar] [CrossRef]
- Hartung, B.; Kauferstein, S.; Ritz-Timme, S.; Daldrup, T. Sudden unexpected death under acute influence of cannabis. Forensic Sci. Int. 2014, 237, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Patel, U.; Sharma, S.; Amin, P.; Bhuva, R.; Patel, M.S.; Sharma, N.; Shah, M.; Patel, S.; Savani, S.; et al. Recreational Marijuana Use and Acute Myocardial Infarction: Insights from Nationwide Inpatient Sample in the United States. Cureus 2017, 9, e1816. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Fergusson, D.; Chiaco, J.M.C. Mid-ventricular variant takotsubo cardiomyopathy associated with Cannabinoid Hyperemesis Syndrome: A case report. Hawaii J. Med. Public Health 2014, 73, 115–118. [Google Scholar]
- Khalid, S.; Khalid, A.; Maroo, P. Risk Factors and Management of Takotsubo Cardiomyopathy. Cureus 2018, 10, e2626. [Google Scholar] [CrossRef] [PubMed]
- Tournebize, J.; Gibaja, V.; Puskarczyk, E.; Popovic, B.; Kahn, J.P. Myocarditis associated with cannabis use in a 15-year-old boy: A rare case report. Int. J. Cardiol. 2016, 203, 243–244. [Google Scholar] [CrossRef]
- Rettori, V.; Wenger, T.; Snyder, G.; Dalterio, S.; McCann, S.M. Hypothalamic Action of Delta-9-Tetrahydrocannabinol to Inhibit the Release of Prolactin and Growth Hormone in the Rat. Neuroendocrinology 1988, 47, 498–503. [Google Scholar] [CrossRef]
- Payne, K.S.; Mazur, D.J.; Hotaling, J.M.; Pastuszak, A.W. Cannabis and Male Fertility: A Systematic Review. J. Urol. 2019, 202, 674–681. [Google Scholar] [CrossRef]
- Somenath, G. Cannabis Effect on Female Reproductive Health. In Bioactive Compounds in Nutraceutical and Functional Food for Good Human Health; Kavita, S., Kanchan, M., Kula Kamal, S., Corina, D., Eds.; IntechOpen: Rijeka, Croatia, 2020; p. 17. [Google Scholar]
- Sims, E.D.; Anvari, S.; Lee, Y.; Samaan, Z.; Banfield, L.; Thabane, L.; Samaan, M.C. The effect of cannabis exposure on pubertal outcomes: A systematic review. Adolesc. Health Med. Ther. 2018, 9, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Karila, L.; Roux, P.; Rolland, B.; Benyamina, A.; Reynaud, M.; Aubin, H.J.; Lançon, C. Acute and long-term effects of cannabis use: A review. Curr. Pharm. Des. 2014, 20, 4112–4118. [Google Scholar] [CrossRef]
- Galli, J.A.; Sawaya, R.A.; Friedenberg, F.K. Cannabinoid hyperemesis syndrome. Curr. Drug Abuse Rev. 2011, 4, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, S.E.; Denysenko, L.; Mulcare, J.L.; Vito, J.P.; Chabon, B. Cannabinoid hyperemesis syndrome: A case series and review of previous reports. Psychosomatics 2012, 53, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Voirin, N.; Berthiller, J.; Benhaïm-Luzon, V.; Boniol, M.; Straif, K.; Ayoub, W.B.; Ayed, F.B.; Sasco, A.J. Risk of lung cancer and past use of cannabis in Tunisia. J. Thorac. Oncol. 2006, 1, 577–579. [Google Scholar] [CrossRef]
- Aldington, S.; Harwood, M.; Cox, B.; Weatherall, M.; Beckert, L.; Hansell, A.; Pritchard, A.; Robinson, G.; Beasley, R. Cannabis use and risk of lung cancer: A case-control study. Eur. Respir. J. 2008, 31, 280–286. [Google Scholar] [CrossRef]
- Berthiller, J.; Straif, K.; Boniol, M.; Voirin, N.; Benhaïm-Luzon, V.; Ayoub, W.B.; Dari, I.; Laouamri, S.; Hamdi-Cherif, M.; Bartal, M.; et al. Cannabis smoking and risk of lung cancer in men: A pooled analysis of three studies in Maghreb. J. Thorac. Oncol. 2008, 3, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Morgenstern, H.; Spitz, M.R.; Tashkin, D.P.; Yu, G.P.; Marshall, J.R.; Hsu, T.C.; Schantz, S.P. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomark. Prev. 1999, 8, 1071–1078. [Google Scholar]
- Hashibe, M.; Ford, D.E.; Zhang, Z.F. Marijuana smoking and head and neck cancer. J. Clin. Pharmacol. 2002, 42, 103s–107s. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Mandal, S.; Banerjee, S.; Mandal, G.K.; Bhowmick, A.K.; Murmu, N. Cannabis smoke can be a major risk factor for early-age laryngeal cancer—A molecular signaling-based approach. Tumour. Biol. 2015, 36, 6029–6036. [Google Scholar] [CrossRef] [PubMed]
- Sidney, S.; Quesenberry, C.P., Jr.; Friedman, G.D.; Tekawa, I.S. Marijuana use and cancer incidence (California, United States). Cancer Causes Control 1997, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Daling, J.R.; Doody, D.R.; Sun, X.; Trabert, B.L.; Weiss, N.S.; Chen, C.; Biggs, M.L.; Starr, J.R.; Dey, S.K.; Schwartz, S.M. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer 2009, 115, 1215–1223. [Google Scholar] [CrossRef]
- Efird, J.T.; Friedman, G.D.; Sidney, S.; Klatsky, A.; Habel, L.A.; Udaltsova, N.V.; Van den Eeden, S.; Nelson, L.M. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: Cigarette smoking and other lifestyle behaviors. J. Neurooncol. 2004, 68, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, E.C.; Daniels, J.; Pollock, B.H.; Olshan, A.F. Maternal Use of Recreational Drugs and Neuroblastoma in Offspring: A Report from the Children’s Oncology Group (United States). Cancer Causes Control 2006, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.L.; Buckley, J.D.; Daigle, A.E.; Wells, R.; Benjamin, D.; Arthur, D.C.; Hammond, G.D. Maternal drug use and risk of childhood nonlymphoblastic leukemia among offspring. An epidemiologic investigation implicating marijuana (a report from the Childrens Cancer Study Group). Cancer 1989, 63, 1904–1911. [Google Scholar] [CrossRef]
- Hashibe, M.; Straif, K.; Tashkin, D.P.; Morgenstern, H.; Greenland, S.; Zhang, Z.F. Epidemiologic review of marijuana use and cancer risk. Alcohol 2005, 35, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Sarne, Y. Beneficial and deleterious effects of cannabinoids in the brain: The case of ultra-low dose THC. Am. J. Drug Alcohol Abuse 2019, 45, 551–562. [Google Scholar] [CrossRef] [PubMed]
- DeVuono, M.V.; Parker, L.A. Cannabinoid Hyperemesis Syndrome: A Review of Potential Mechanisms. Cannabis Cannabinoid Res. 2020, 5, 132–144. [Google Scholar] [CrossRef]
- Tzavara, E.T.; Wade, M.; Nomikos, G.G. Biphasic Effects of Cannabinoids on Acetylcholine Release in the Hippocampus: Site and Mechanism of Action. J. Neurosci. 2003, 23, 9374. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Rubio-Casillas, A. Biphasic effects of THC in memory and cognition. Eur. J. Clin. Investig. 2018, 48, e12920. [Google Scholar] [CrossRef]
- Turkanis, S.A.; Karler, R. Excitatory and depressant effects of Δ9-tetrahydrocannabinol and cannabidiol on cortical evoked responses in the conscious rat. Psychopharmacology 1981, 75, 294–298. [Google Scholar] [CrossRef]
- Katsidoni, V.; Kastellakis, A.; Panagis, G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int. J. Neuropsychopharmacol. 2013, 16, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.A.; Purrio, M.; Viveros, M.-P.; Lutz, B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 2012, 37, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.E.; Bevins, R.A. Cannabinoid Conditioned Reward and Aversion: Behavioral and Neural Processes. ACS Chem. Neurosci. 2010, 1, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Lafenêtre, P.; Cannich, A.; Cota, D.; Puente, N.; Grandes, P.; Chaouloff, F.; Piazza, P.V.; Marsicano, G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010, 13, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Robinson, T.; Bullen, C.; Curran, V.; Jutras-Aswad, D.; Medina-Mora, M.E.; Pacula, R.L.; Rehm, J.; Room, R.; Brink, W.V.D.; et al. Lower-Risk Cannabis Use Guidelines (LRCUG) for reducing health harms from non-medical cannabis use: A comprehensive evidence and recommendations update. Int. J. Drug Policy 2022, 99, 103381. [Google Scholar] [CrossRef]
- Palrasu, M.; Wright, L.; Patel, M.; Leech, L.; Branch, S.; Harrelson, S.; Khan, S. Perspectives on Challenges in Cannabis Drug Delivery Systems: Where Are We? Med. Cannabis Cannabinoids 2022, 5, 102–119. [Google Scholar] [CrossRef]
- Kebede, L.; Masoomi Dezfooli, S.; Seyfoddin, A. Medicinal cannabis pharmacokinetics and potential methods of delivery. Pharm. Dev. Technol. 2022, 27, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, K.; Willette, S.; Lucker, B.F.; Kovar, S.E.; Holguin, F.O.; Guzman, I. The Effects of Food on Cannabidiol Bioaccessibility. Molecules 2021, 26, 3573. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar] [PubMed]
- Crockett, J.; Critchley, D.; Tayo, B.; Berwaerts, J.; Morrison, G. A phase 1, randomized, pharmacokinetic trial of the effect of different meal compositions, whole milk, and alcohol on cannabidiol exposure and safety in healthy subjects. Epilepsia 2020, 61, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Greaves, L.; Hemsing, N. Sex and Gender Interactions on the Use and Impact of Recreational Cannabis. Int. J. Environ. Res. Public Health 2020, 17, 509. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, C.; Mischley, L.K.; Sexton, M. Sex Differences in Cannabis Use and Effects: A Cross-Sectional Survey of Cannabis Users. Cannabis Cannabinoid Res. 2016, 1, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Sturman, D.A.; Moghaddam, B. The neurobiology of adolescence: Changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci. Biobehav. Rev. 2011, 35, 1704–1712. [Google Scholar] [CrossRef]
- Blest-Hopley, G.; Colizzi, M.; Giampietro, V.; Bhattacharyya, S. Is the Adolescent Brain at Greater Vulnerability to the Effects of Cannabis? A Narrative Review of the Evidence. Front. Psychiatry 2020, 11, 859. [Google Scholar] [CrossRef]
- Mustonen, A.; Niemelä, S.; Nordström, T.; Murray, G.K.; Mäki, P.; Jääskeläinen, E.; Miettunen, J. Adolescent cannabis use, baseline prodromal symptoms and the risk of psychosis. Br. J. Psychiatry 2018, 212, 227–233. [Google Scholar] [CrossRef]
- Stefanis, N.C.; Delespaul, P.; Henquet, C.; Bakoula, C.; Stefanis, C.N.; Van Os, J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction 2004, 99, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, B.J.; Ames, M.E.; Linden-Carmichael, A.N. Age-varying effects of cannabis use frequency and disorder on symptoms of psychosis, depression and anxiety in adolescents and adults. Addiction 2019, 114, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, H.H.A.; Talhat, M.A.; Khokhar, J.Y. High genes: Genetic underpinnings of cannabis use phenotypes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110164. [Google Scholar] [CrossRef]
- Murphy, T.; Matheson, J.; Mann, R.E.; Brands, B.; Wickens, C.M.; Tiwari, A.K.; Zai, C.C.; Kennedy, J.; Le Foll, B. Influence of cannabinoid receptor 1 genetic variants on the subjective effects of smoked cannabis. Int. J. Mol. Sci. 2021, 22, 7388. [Google Scholar] [CrossRef]
- Hill, S.Y.; Sharma, V.; Jones, B.L. Lifetime use of cannabis from longitudinal assessments, cannabinoid receptor (CNR1) variation, and reduced volume of the right anterior cingulate. Psychiatry Res. Neuroimaging 2016, 255, 24–34. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Schafer, G.; Gardner, C.; Bloomfield, M.A.P.; Bramon, E.; Morgan, C.J.A.; Curran, H.V. Acute effects of cannabinoids on addiction endophenotypes are moderated by genes encoding the CB1 receptor and FAAH enzyme. Addict. Biol. 2020, 25, e12762. [Google Scholar] [CrossRef]
- Hartman, C.A.; Hopfer, C.J.; Haberstick, B.; Rhee, S.H.; Crowley, T.J.; Corley, R.P.; Hewitt, J.K.; Ehringer, M.A. The association between cannabinoid receptor 1 gene (CNR1) and cannabis dependence symptoms in adolescents and young adults. Drug Alcohol Depend 2009, 104, 11–16. [Google Scholar] [CrossRef]
- Filbey, F.M.; Schacht, J.P.; Myers, U.S.; Chavez, R.S.; Hutchison, K.E. Individual and Additive Effects of the CNR1 and FAAH Genes on Brain Response to Marijuana Cues. Neuropsychopharmacology 2010, 35, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Haughey, H.M.; Marshall, E.; Schacht, J.P.; Louis, A.; Hutchison, K.E. Marijuana withdrawal and craving: Influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction 2008, 103, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.H.C.; McGeary, J.E.; Knopik, V.S.; Bidwell, L.C.; Metrik, J.M. CNR1 and FAAH variation and affective states induced by marijuana smoking. Am. J. Drug Alcohol Abuse 2019, 45, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Ashenhurst, J.R.; Harden, K.P.; Mallard, T.T.; Corbin, W.R.; Fromme, K. Developmentally Specific Associations Between CNR1 Genotype and Cannabis Use Across Emerging Adulthood. J. Stud. Alcohol Drugs 2017, 78, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Schacht, J.P.; Selling, R.E.; Hutchison, K.E. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: An exploratory analysis. Psychopharmacology 2008, 203, 511. [Google Scholar] [CrossRef] [PubMed]
- Tyndale, R.F.; Payne, J.I.; Gerber, A.L.; Sipe, J.C. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: Studies of drug use and dependence in caucasians. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 660–666. [Google Scholar] [CrossRef]
- Thorpe, H.H.A.; Hamidullah, S.; Jenkins, B.W.; Khokhar, J.Y. Adolescent neurodevelopment and substance use: Receptor expression and behavioral consequences. Pharmacol. Ther. 2020, 206, 107431. [Google Scholar] [CrossRef]
- Wang, S.; van der Vaart, A.D.; Xu, Q.; Seneviratne, C.; Pomerleau, O.F.; Pomerleau, C.S.; Payne, T.J.; Ma, J.Z.; Li, M.D. Significant associations of CHRNA2 and CHRNA6 with nicotine dependence in European American and African American populations. Hum. Genet. 2014, 133, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Philibert, R.A.; Todorov, A.; Andersen, A.; Hollenbeck, N.; Gunter, T.; Heath, A.; Madden, P. Examination of the Nicotine Dependence (NICSNP) Consortium findings in the Iowa adoption studies population. Nicotine Tob. Res. 2009, 11, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Demontis, D.; Rajagopal, V.M.; Thorgeirsson, T.E.; Als, T.D.; Grove, J.; Leppälä, K.; Gudbjartsson, D.F.; Pallesen, J.; Hjorthøj, C.; Reginsson, G.W.; et al. Genome-wide association study implicates CHRNA2 in cannabis use disorder. Nat. Neurosci. 2019, 22, 1066–1074. [Google Scholar] [CrossRef]
- Minică, C.C.; Verweij, K.J.H.; van der Most, P.J.; Mbarek, H.; Bernard, M.; van Eijk, K.R.; Lind, P.A.; Liu, M.Z.; Maciejewski, D.F.; Palviainen, T.; et al. Genome-wide association meta-analysis of age at first cannabis use. Addiction 2018, 113, 2073–2086. [Google Scholar] [CrossRef]
- Stringer, S.; Minică, C.C.; Verweij, K.J.H.; Mbarek, H.; Bernard, M.; Derringer, J.; van Eijk, K.R.; Isen, J.D.; Loukola, A.; Maciejewski, D.F.; et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl. Psychiatry 2016, 6, e769. [Google Scholar] [CrossRef]
- Boutwell, B.; Hinds, D.; Me Research Team; Tielbeek, J.; Ong, K.K.; Day, F.R.; Perry, J.R.B. Replication and characterization of CADM2 and MSRA genes on human behavior. Heliyon 2017, 3, e00349. [Google Scholar] [CrossRef]
- Pasman, J.A.; Verweij, K.J.H.; Gerring, Z.; Stringer, S.; Sanchez-Roige, S.; Treur, J.L.; Abdellaoui, A.; Nivard, M.G.; Baselmans, B.M.L.; Ong, J.-S.; et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal effect of schizophrenia liability. Nat. Neurosci. 2018, 21, 1161–1170. [Google Scholar] [CrossRef]
- Gasse, A.; Vennemann, M.; Köhler, H.; Schürenkamp, J. Toxicogenetic analysis of Δ9-THC-metabolizing enzymes. Int. J. Legal. Med. 2020, 134, 2095–2103. [Google Scholar] [CrossRef]
- Brown, J.D. Potential Adverse Drug Events with Tetrahydrocannabinol (THC) Due to Drug-Drug Interactions. J. Clin. Med. 2020, 9, 919. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, J.; Huang, S.Q.; Su, H.H.; Zhou, S.F. Genetic polymorphism of the human cytochrome P450 2C9 gene and its clinical significance. Curr. Drug Met. 2009, 10, 781–834. [Google Scholar] [CrossRef]
- Stott, C.; White, L.; Wright, S.; Wilbraham, D.; Guy, G. A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus 2013, 2, 236. [Google Scholar] [CrossRef]
- Heinbockel, T.; Csoka, A.B. Epigenetic Effects of Drugs of Abuse. Int. J. Environ. Res. Public Health 2018, 15, 2098. [Google Scholar] [CrossRef] [PubMed]
- Szutorisz, H.; Hurd, Y.L. Epigenetic Effects of Cannabis Exposure. Biol. Psychiatry 2016, 79, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.J.; Pearson, J.F.; Noble, A.J.; Gemmell, N.J.; Horwood, L.J.; Boden, J.M.; Benton, M.C.; Macartney-Coxson, D.P.; Kennedy, M.A. Genome-wide DNA methylation analysis of heavy cannabis exposure in a New Zealand longitudinal cohort. Transl. Psychiatry 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, J.; Ehrlich, S.; Walton, E.; White, T.; Perrone-Bizzozero, N.; Bustillo, J.; Turner, J.A.; Calhoun, V.D. Methylation patterns in whole blood correlate with symptoms in schizophrenia patients. Schizophr. Bull. 2014, 40, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Van der Knaap, L.J.; Schaefer, J.M.; Franken, I.H.; Verhulst, F.C.; van Oort, F.V.; Riese, H. Catechol-O-methyltransferase gene methylation and substance use in adolescents: The TRAILS study. Genes Brain Behav. 2014, 13, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hegde, V.L.; Rao, R.; Zhang, J.; Nagarkatti, P.S.; Nagarkatti, M. Histone modifications are associated with Δ9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J. Biol. Chem. 2014, 289, 18707–18718. [Google Scholar] [CrossRef] [PubMed]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 2011, 70, 763–769. [Google Scholar] [CrossRef]
- Tomasiewicz, H.C.; Jacobs, M.M.; Wilkinson, M.B.; Wilson, S.P.; Nestler, E.J.; Hurd, Y.L. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol. Psychiatry 2012, 72, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Chandra, L.C.; Kumar, V.; Torben, W.; Stouwe, C.V.; Winsauer, P.; Amedee, A.; Molina, P.E.; Mohan, M. Chronic administration of Δ9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques. J. Virol. 2015, 89, 1168–1181. [Google Scholar] [CrossRef]
- Hegde, V.L.; Tomar, S.; Jackson, A.; Rao, R.; Yang, X.; Singh, U.P.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Δ9-tetrahydrocannabinol in vivo: Regulation of CCAAT/enhancer-binding protein α by microRNA-690. J. Biol. Chem. 2013, 288, 36810–36826. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, F.T.; Murthy, S.; Pinto, C.; Olson, K.L.; Ioannidis, J.P.; Mandl, K.D. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics 2012, 130, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Wilens, T.E. Medical Cannabinoids in Children and Adolescents: A Systematic Review. Pediatrics 2017, 140, e20171818. [Google Scholar] [CrossRef]
- Treves, N.; Mor, N.; Allegaert, K.; Bassalov, H.; Berkovitch, M.; Stolar, O.E.; Matok, I. Efficacy and safety of medical cannabinoids in children: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 23462. [Google Scholar] [CrossRef]
- Routledge, P.A.; O’Mahony, M.S.; Woodhouse, K.W. Adverse drug reactions in elderly patients. Brit. J. Clin. Pharmacol. 2004, 57, 121–126. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Van den Elsen, G.A.H.; Ahmed, A.I.A.; Lammers, M.; Kramers, C.; Verkes, R.J.; van der Marck, M.A.; Rikkert, M.G.M.O. Efficacy and safety of medical cannabinoids in older subjects: A systematic review. Ageing Res. Rev. 2014, 14, 56–64. [Google Scholar] [CrossRef]
- Velayudhan, L.; McGoohan, K.; Bhattacharyya, S. Safety and tolerability of natural and synthetic cannabinoids in adults aged over 50 years: A systematic review and meta-analysis. PLoS Med. 2021, 18, e1003524. [Google Scholar] [CrossRef]
- Suryadevara, U.; Bruijnzeel, D.M.; Nuthi, M.; Jagnarine, D.A.; Tandon, R.; Bruijnzeel, A.W. Pros and Cons of Medical Cannabis use by People with Chronic Brain Disorders. Curr. Neuropharmacol. 2017, 15, 800–814. [Google Scholar] [CrossRef]
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, F.; Hoehe, M.R. Cannabinoids: For better and for worse. Dialogues Clin. Neurosci. 2020, 22, 201–204. [Google Scholar] [CrossRef]
- Kanabus, J.; Bryła, M.; Roszko, M.; Modrzewska, M.; Pierzgalski, A. Cannabinoids-Characteristics and Potential for Use in Food Production. Molecules 2021, 26, 6723. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Public Health. Notification of the Ministry of Public Health. (No.427) B.E. 2564 (2021) Issued by Virtue of the Food Act B.E. 2522 Re: Food Products Containing Cannabis or Hemp Ingredients. Available online: https://food.fda.moph.go.th/law/data/announ_moph/P427.PDF (accessed on 1 March 2023).
- Ministry of Public Health, Thailand. Notification of the Ministry of Public Health. (429) B.E. 2564 (2021) Issued by Virtue of the Food Act B.E.2522 (1979) Re: Food Products Containing Cannabidiol Extract. Available online: https://food.fda.moph.go.th/law/data/announ_moph/P429.PDF (accessed on 1 March 2023).
- Ministry of Public Health, Thailand. Notification of the Ministry of Public Health. (428) B.E. 2564 (2021) Issued by Virtue of the Food Act B.E.2522 (1979) Re: Standard for Food Contaminating Tetrahydrocannabinol and Cannabidiol. Available online: https://food.fda.moph.go.th/law/data/announ_moph/P428.PDF (accessed on 1 March 2023).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin. EFSA J. 2015, 13, 4141. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Acute human exposure assessment to tetrahydrocannabinol (Δ9-THC). EFSA J. 2020, 18, e05953. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Cannabidiol Novel Food Evaluations on Hold Pending New Data. Published 7 June 2022. Available online: https://www.efsa.europa.eu/en/news/cannabidiol-novel-food-evaluations-hold-pending-new-data (accessed on 30 January 2023).
- Beitzke, B.; Pate, D.W. A broader view on deriving a reference dose for THC traces in foods. Crit. Rev. Toxicol. 2021, 51, 695–722. [Google Scholar] [CrossRef]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of Delta9-THCA-A from hemp and analytical aspects concerning the determination of Delta9-THC in cannabis products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.-H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern Med. 2018, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.; Stjepanović, D.; Caulkins, J.; Lynskey, M.; Leung, J.; Campbell, G.; Degenhardt, L. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet 2019, 394, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. https://doi.org/10.3390/molecules28062791
Kitdumrongthum S, Trachootham D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules. 2023; 28(6):2791. https://doi.org/10.3390/molecules28062791
Chicago/Turabian StyleKitdumrongthum, Sarunya, and Dunyaporn Trachootham. 2023. "An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products" Molecules 28, no. 6: 2791. https://doi.org/10.3390/molecules28062791
APA StyleKitdumrongthum, S., & Trachootham, D. (2023). An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules, 28(6), 2791. https://doi.org/10.3390/molecules28062791





