Abstract
Sharpless asymmetric dihydroxylation is an important reaction in the enantioselective synthesis of chiral vicinal diols that involves the treatment of alkene with osmium tetroxide along with optically active quinine ligand. Sharpless introduced this methodology after considering the importance of enantioselectivity in the total synthesis of medicinally important compounds. Vicinal diols, produced as a result of this reaction, act as intermediates in the synthesis of different naturally occurring compounds. Hence, Sharpless asymmetric dihydroxylation plays an important role in synthetic organic chemistry due to its undeniable contribution to the synthesis of biologically active organic compounds. This review emphasizes the significance of Sharpless asymmetric dihydroxylation in the total synthesis of various natural products, published since 2020.
1. Introduction
Asymmetric synthesis plays an important role in the stereoisomeric and enantiomeric synthesis of drugs [1]. In this regard, Sharpless in the 1980s introduced Sharpless epoxidation, which is a facile methodology for the asymmetric synthesis of 2,3-epoxy alcohols by using primary and secondary allylic alcohols [2]. Considering the importance of enantioselective synthesis of Sharpless epoxidation, Sharpless asymmetric dihydroxylation was then employed by Sharpless to synthesize vicinal diols [3,4]. In this methodology, chiral vicinal diol moiety is obtained by the reaction of an alkene with osmium tetroxide in the presence of an optically active quinine ligand [5,6].
The presence of a chiral ligand is the primary requirement for osmium catalyzed bishydroxylation [7]. Since the inception of this protocol, various co-oxidant ligands have been employed by different researchers. However, it was inferred that low yields of diol were obtained by employing stoichiometric oxidants, i.e., H2O2 and NaClO3 or KClO3 [8,9]. Improvements in the yield of target molecules were observed by employing Upjohn dihydroxylation in the presence of N-methylmorpholine N-oxide and by using alkaline tert-butyl hydroperoxide (t-BHP) [10]. Moreover, potassium hexacyanoferrate (III) has been found to be the most effective in recent times. Similarly, Sharpless also utilized this optically active terminal oxidant for the bishydroxylation of alkenes [11]. As time passed, Sharpless and his coworkers devoted their attention to the use of cinchona alkaloids as chiral ligands, which gave tremendous yields with high enantioselectivity. Later on, pyrimidine and phthalazine incorporated dimeric alkaloids were employed as asymmetric ligands for the efficient synthesis of vicinal diols. K2OsO2(OH)4, K2CO3, (DHQD)2PHAL, (DHQ)2PHAL, and K3Fe(CN)6 were found to be mandatory chemical substances for carrying out Sharpless asymmetric dihydroxylation. The mixture of these four reagents is referred to as “AD-mix”. The (DHQ)2PHAL-containing mixture is termed “AD-mix-α”, while “AD-mix-β” includes (DHQD)2PHAL as ligand [11,12]. The general scheme for osmium-catalyzed asymmetric dihydroxylation is given below [12] (Scheme 1).
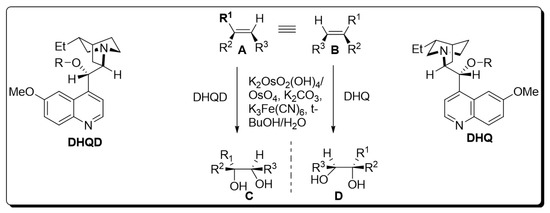
Scheme 1.
Osmium-catalyzed asymmetric dihydroxylation.
Sharpless asymmetric dihydroxylation has found tremendous applications in the synthesis of a variety of naturally occurring biologically active compounds [13,14]. Total synthesis of various natural products such as alkaloids, lactones, amino acids, flavones, polyketides, macrolides, glycosides, terpenes, etc., involves Sharpless asymmetric dihydroxylation as an integral step [15,16,17,18]. This protocol results in the high enantioselectivity of vicinal diols, which are then reacted further in the presence of required reagents to synthesize the desired medicinally important target molecules [19,20]. The following figure illustrates the structure of a few biologically important natural products, whose total synthesis is achieved by employing Sharpless asymmetric dihydroxylation as an essential step (Figure 1) [21,22].
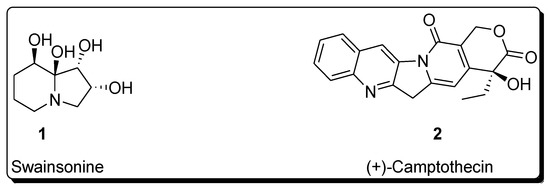
Figure 1.
Structure of biologically important organic compounds employing Sharpless asymmetric dihydroxylation in their total synthesis.
There are a huge number of other applications of Sharpless asymmetric dihydroxylation. For example, Sharpless AD has been found to be the main step in the total synthesis of Codonopsinine, Synargenotolides, Noneneolide and many more, which are isolated from different plant extracts and play a vital role against a number of bacterial diseases, in combating the spread of tumor cells and exhibiting high efficacy against malarial infections, respectively [23,24,25]. Our review highlights the recent applications of Sharpless asymmetric dihydroxylation for the synthesis of various natural products.
2. Review of the Literature
2.1. Synthesis of Alkaloid-Based Natural Products
2.1.1. Lycorine-Type Alkaloids
Zephyranthine belongs to the class of lycorine alkaloids, which are known for their medicinal usage [26]. Members of this class have been found to interrupt the acetylcholine activity and uncontrolled division of cancer cells [27]. Zhao et al. [28] in 2021 reported the efficient synthesis of (-)-zephyranthine by utilizing two one-pot reactions. Evans’ nickel (II) catalyst 9 initiated the synthesis by reacting unsaturated β-ketoeaster 5 and nitro olefin 8 via a series of Michael addition reactions. Both reactants 5 and 8 were prepared individually. In this regard, starting material 2,2,6-trimethyl-[1,3]dioxin-4-one 3 was reacted with diethylchlorophosphite and LiHMDS followed by oxidation in the presence of hydrogen peroxide, thereby giving compound 4 in 90% yield, which was then reacted with methanol and sodium hydride in the presence of tetrahydrofuran to give compound 5 in 87% yield. Then, substituted carbaldehyde 6 was reacted with p-TSA and ethylene glycol followed by treatment with toluene and diethylether, which resulted in the synthesis of compound 7 in 90% yield. The compound 7 was then reacted with MeNO2 and trifluoroacetic acid to obtain compound 8 in 84% yield. For the first Michael addition, use of toluene and Triton B were considered to be effective after optimizing the various reaction conditions. This reaction resulted in 85% yield of penta-substituted cyclohexane 10, which is highly enantioselective (90% ee) and (>20:1) diastereoselective in nature. In order to prevent the undesirable aldol reaction, the enol moiety in 10 was protected by treating with benzoylchloride (BzCl) in the presence of pyridine, which resulted in 97% yield of benzoate 11 with more than 99% enantioselectivity. The compound 11 was cyclized through one-pot reaction on treatment with HBr and acetic acid in the presence of tetrahydrofuran followed by reaction with zinc powder and hydrochloric acid, obtaining intermediate 12 in 57% yield. The resulting intermediate was treated with Comins’ reagent and LiHMDS in the presence of tetrahydrofuran, resulting in the synthesis of triflate, which was further treated with palladium acetate, formic acid and triphenylphosphine to synthesize compound 13 in 85% yield. In the last step, enantioselective synthesis of zephyranthine 14 in quantitative yield (67%) with 7.2:1 diastereoselectivity ratio was achieved by Sharpless asymmetric dihydroxylation on reaction with AD-mix-β in the presence of tetrahydrofuran or water (Scheme 2).
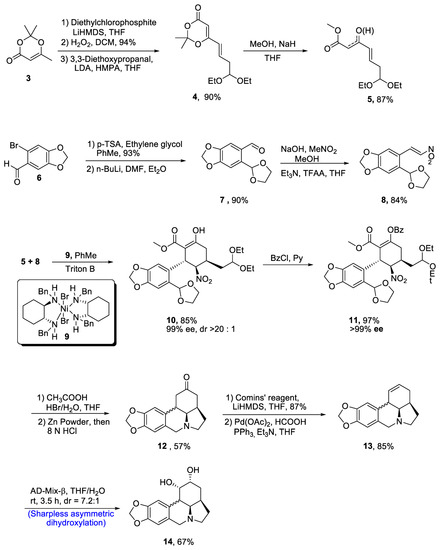
Scheme 2.
Synthesis of (−)-zephyranthine 14.
2.1.2. Muscarine Alkaloid
There are several tetrahydrofurans containing natural products including various alkaloids such as muscarine, epi-muscarine and allo-muscarine, which are extracted from Amanita muscaria, i.e., a mushroom species [29]. Muscarine and its derivatives are highly potent pharmacological agents that also act as acetylcholine inhibitors [30]. Owing to their wide biological importance, Gehlawat et al. [31] in 2020 reported the total synthesis of epi-mucarine alkaloid by using various reactions including Sharpless asymmetric dihydroxylation, cyclization by bimolecular nucleophilic substitution reaction, and cleavage of epoxide ring. In the first step, (p-methoxybenzyl) glycidyl ether 15 was treated with propyl lithium in the presence of boron triflouride diethyl etherate followed by reduction with lithium aluminum hydride, resulting in the synthesis of compound 16 in 86% yield. The compound 16 was further treated with tosyl chloride in the presence of dimethyl aminopyridine and triethylamine followed by reaction with osmium tetroxide and potassium hexacyanoferrate (III) via Sharpless asymmetric dihydroxylation to obtain tosyl protected diol intermediate 17. This newly synthesized intermediate was subjected to base catalyzed reaction to prepare compound 18 in 90% yield. Compound 18 was further made to react with cerium ammonium nitrate in the presence of acetonitrile followed by treatment with imidazole, pyrrolidine and toluene. Synthesis of epi-muscarine 19 was achieved by treatment with trimethyl amine and methanol in 95% yield (Scheme 3).
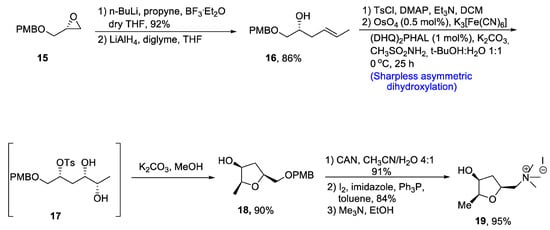
Scheme 3.
Synthesis of epi-muscarine alkaloid 19.
2.1.3. Monoterpenoid Indole Alkaloid
A major class of natural products comprises monoterpenoid indole alkaloids (MIAs), which are effective against a wide range of bacterial, viral and neurological disorders [32]. These are also useful against tumor-causing agents [33,34]. Depending upon the positioning of atoms, MIAs are divided into three categories, most of which are derived from different parts of Alstonia scholaris. Zhang et al. in 2020 [35] proposed the total synthesis of two important MIAs, i.e., alstolarines A and B. They stated that the total synthesis could be initiated with the forerunner difforlemenine 20, which could be subjected to reduction followed by ring opening reaction, leading to the generation of compound 21. Then, nucleophilic substitution could result in the synthesis of compound 22, a common precursor in the synthesis of alstolarines A and B. For the synthesis of alstolarine A, compound 22 could be subjected to dehydration followed by Sharpless asymmetric dihydroxylation to obtain compound 23, which could synthesize target molecule 24 via cyclization of acetal. Similarly, oxidation and removal of carboxylic acid could synthesize alstolarine B 25 (Scheme 4). Both compounds were tested for their acetylcholinase inhibitory potential, and molecule 25 showed average inhibitory potential with an IC50 value = 19.3 µM.
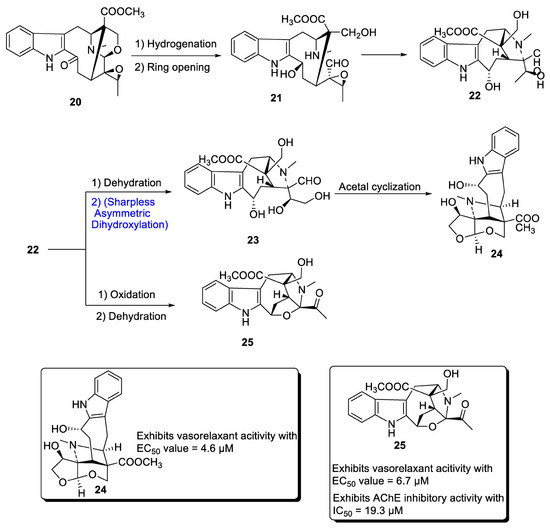
Scheme 4.
Synthesis of alstolarines A 24 and B 25.
2.1.4. Glyphaeaside Alkaloids
The glyphaeaside alkaloids are a class of iminosugars that are found in nature, as they are derived from plants, i.e., Glyphaea brevis [36]. These natural products belong to the family of carbohydrates whose structures are composed of many hydroxyl groups along with phenylalkyl side-chains [37]. On the basis of the arrangement of he iminosugar center, the glyphaeasides have been categorized into three classes, named A, B and C. Glyphaeaside C, which was formerly referred to as 1-deoxynojirimycin, has been found to exhibit highly efficient inhibitory activity against β-glucosidase and snail β-mannosidase. However, it was determined that the inhibitory activity of glyphaeasides is dependent on the role of side chains. Moreover, the total synthesis of glyphaeaside C revealed that its structure closely resembles that of 2,5-dideoxy-2,5-imino-D-mannitol (D-DMDP). In 2021, Byatt et al. [38] reported the multi-step facile synthesis of glyphaeaside C. The total synthesis of our target molecule initiated with the synthesis of pyrrolidine fragments from readily available starting reagent, i.e., 2,3,5-tri-O-benzyl-β-D-arabinofuranose 26. The fragments were then reacted to give oxazolidinone a and b. In the next step, commonly available 1-allly-4-methoxy-benzene 28 was treated with boron tribromide followed by reaction with benzyl bromide, leading to the protection of hydroxyl group. Then, Grubb’s first-generation catalyst was employed to treat pyrrolidine fragment 27 with benzyl group-protected 4-allylphenol, leading to the synthesis of a racemic mixture of compound 29 in 66–76% yield. Compound 29 was then subjected to α and β type Sharpless asymmetric dihydroxylation one by one, thereby giving 30 and 31 in 87% and 90% yields, respectively. Compounds 30 and 31 were further subjected to deprotection, leading to the synthesis of four diastereomers of our target molecule. In the end, RP-HPLC was used to separate the glyphaeaside C (Scheme 5).
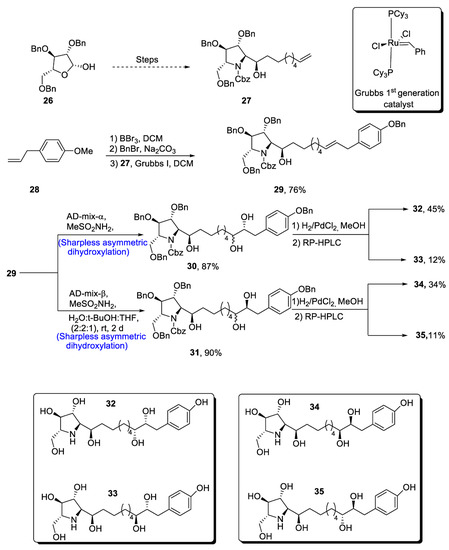
Scheme 5.
Synthesis of glyphaeaside C.
2.2. Synthesis of Terpene-Based Natural Products
2.2.1. Sesquiterpenoids
Englerin is isolated from Phyllanthus engleri, and it has been found to exhibit significant cytotoxic potential against cancer cells of kidney [39]. Moreover, englerin A is responsible for activation of protein kinase, hence regulating the glucose level [40]. Its structure is composed of seven chiral carbons along with epoxyguaine framework. Considering the significance of englerin A, various researchers have attempted to report its total synthesis. In 2020, Mou et al. [41] described an efficient synthetic route for the total synthesis of englerin A. For this purpose, 36 was oxidized in the presence of m-chloroperoxybenzoic acid, sodium bicarbonate and dichloromethane followed by reaction with PhSiH3 in the presence of acetone and Co catalyst to synthesize compound 37 in 87% yield. Compound 37 was then subjected to Sharpless asymmetric dihydroxylation in the presence of K2OsO4, (DHQ)2PHAL and methane sulfonamide to obtain compound 38 in 59% yield. Compound 38 was further used in the synthesis of englerin A 39 (99% yield) (Scheme 6).
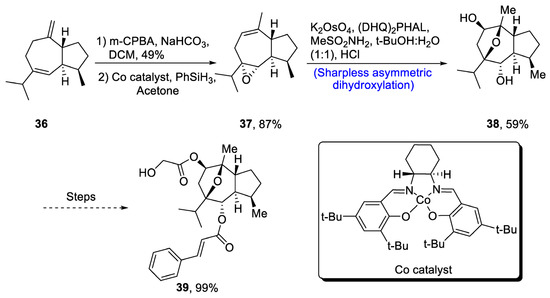
Scheme 6.
Synthesis of (−)-englerin A 39.
Goyazensolide is a biologically active, naturally occurring furanoheliangolide sesquiterpenoid that plays an effective role in combating tumor cells [42]. It is isolated from plants, and its derivatives have been found to be more active against cancer cell lines, e.g., 15-deoxygoyazensolide [43]. Considering the biologically active potential of goyazensolide, Liu et al.. [44] in 2021 reported the total synthesis of this natural product. In the first step of the total synthesis, compound 40 was reacted with Dess–Martin periodinane followed by treatment with trimethylsilylacetylene via Sonogashira coupling, which yielded compound 41 in 72% yield. Compound 41 was then subjected to Sharpless asymmetric dihydroxylation via (DHQD)2Pyr followed by protection with tert-butyldiphenylsilyl group in the presence of imidazole and dimethylaminopyridine, which gave compound 42 in 80% yield. Compound 42 then underwent a number of steps leading to the synthesis of goyazensolide 43 in 41% yield (Scheme 7).
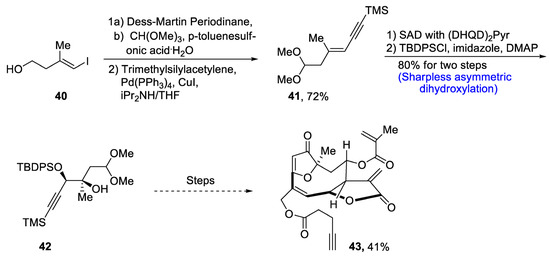
Scheme 7.
Synthesis of goyazensolide 43.
Aromatic bisabolanes belong to the class of monocyclic sesquiterpenoids, and they are obtained from different living sources such as microorganisms, plants insects, etc. [45]. Various aromatic bisabolanes have been synthesized in their respective enantiomeric forms [46]. Yajima et al. [47] in 2021 reported the total synthesis of 1,3,5-bisabolatrien-7-ol, which involved the formation of a chiral center via Sharpless asymmetric dihydroxylation. In the first step of synthesis, compound 44 was treated with vinylmagnesium bromide followed by a reaction with methanesulfonic acid in the presence of THF and water, leading to the formation of compound 45 in 82% yield. After treatment with mercaptobenzothiazole, Sharpless asymmetric dihydroxylation was carried out, which gave chiral diol compound 47 in 98% yield. Compound 47 was further subjected to oxidation with meta-chloroperoxybenzoic acid followed by Smiles rearrangement, which gave compound 48 in 58% yield with er >99:1. Hydrogenation of compound 48 in the presence of platinum oxide led to the synthesis of S-Enantiomer 49 of 1,3,5-bisabolatrien-7-ol in 85% yield with an enantioselective ratio of more than 99:1. Similarly, R-Enantiomer 51 was achieved in an enantiomeric ratio of more than 99:1 by Sharpless asymmetric dihydroxylation of compound 46 along with similar steps (Scheme 8).
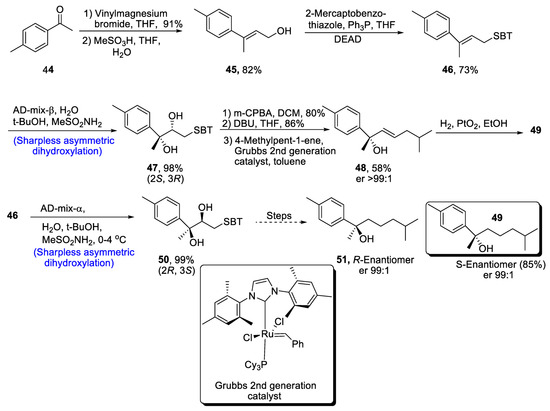
Scheme 8.
Synthesis of aromatic bisabolanes 49 and 51.
Through extensive research, it has been found that the compounds isolated from the plant Phythallus engleri possess mighty anti-cancerous potential [48]. Owing to being composed of a glycolic ester attachment, englerin A is a more promising anti-cancerous agent as compared to englerin B, which indicates that this functional group is responsible for increased cytotoxic potential [49]. There have been numerous reports covering the total synthesis of englerin-A. So, in 2021, Palli et al. [50] attempted to explain the total synthesis of englerin A by starting with commercially available limonene 52. Limonene 52 was transformed into compound 53 by using a number of previously reported steps, followed by its treatment with methanesulfonamide, thus carrying out Sharpless asymmetric dihydroxylation to give compound 54 in 80% yield with a more than 99% diastereoselectivity ratio. Compound 54 was then subjected to protection of diol groups on treatment with 2,2-DMP and CSA followed by hydrogenation, which gave compound 55 in 82% yield. Compound 55 then underwent a number of different steps to give a mixture of oxatricyclic alcohol 56a and 56b. In the next step, compound 56 was then subjected to acylation followed by elimination in the presence of Burgess reagent to obtain compound 57 in 86% yield. Compound 57 was further used to obtain target molecule 58 in 86% yield (Scheme 9).
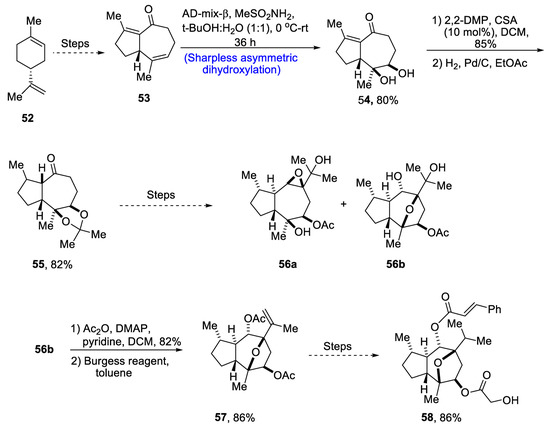
Scheme 9.
Synthesis of 4-epi-englerin A 58.
2.2.2. Neoclerodane Diterpene
Neoclerodane diterpene, (-)-salvinorin A is derived from Salvia divinorum, which is a medicinal plant [51]. This diterpene is used as a medicinal agent and employed as a treatment for stress, anxiety and pain. Owing to its wide medicinal importance, various research groups have reported different routes for its synthesis. Recently, in 2021, Zimdar et al. [52] devised an efficient strategy for the total synthesis of salvinorin. The total synthesis was initiated with the synthesis of lactone via a number of steps resulting in the generation of lactone 60 in 87% yield. Compound 60 was further reacted with osmium tetroxide and N-methylmorpholine N-oxide in the presence of acetone and water followed by its reaction with PIDA in the presence of dichloromethane, giving keto-aldehyde 61 in 88% yield. Compound 61 further underwent a number of steps to synthesize a racemic mixture of 62 and 63 in 44% and 14% yields, respectively, with more than 99% diastereoselectivity. Compound 61 was treated with chromium trichloride and iodoform in the presence of THF to obtain compound 64 in 58% yield. Compound 64 was then further subjected to a number of steps including Mitsunobu esterification and others to obtain compound 65 in 66% yield with 94% diastereoselectivity. Compound 65 was then subjected to Sharpless asymmetric dihydroxylation, leading to the synthesis of diol moiety 66 in 95% yield. Finally, the last step involved treatment with acetic acid and DBAD(di-tert-butyl azodicarboxylate), thereby giving salvinorin A 67 in 92% yield (Scheme 10).
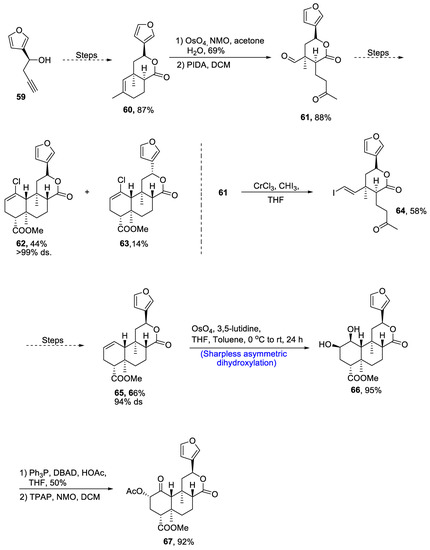
Scheme 10.
Synthesis of (−)-salvinorin A 67.
2.2.3. Nor-Triterpenoids
Plants that are members of Schisandra genus are the source of propindilactone G and its derivatives, which constitute a family of polycyclic natural products [53]. Most members of this family are abundantly utilized in several medicines in China due to their high bioactive potency [54]. Propindilactone G along with its other derivatives are obtained from Schisandra propinqua var. propinqua, and they are structurally composed of fused cyclic rings. Owing to their frequent utilization in medicines, several research groups have devoted attention to their total synthesis. Wang et al. [55] in 2020 reported an efficient strategy for the total synthesis of propindilactone G. Total synthesis began with the Mitsunobu reaction of 68 followed by a reaction with N-bromosuccinimide and perchloric acid to prepare compound 69. It was then transformed into stable chlorohydrin, which was then joined with it to prepare compound 70 via a few steps. Compound 70 was then allowed to react with mesityl chloride in the presence of triethyl amine and tert-butanol followed by its treatment with 1,2 dimethoxyethane in the presence of DBU. It was then treated with Et3SiH, Co(thd)2 in the presence of dichloroethane and oxygen, which resulted in compound 71 in high yield (75%). Starting reagent 68 was reacted via several steps, thereby giving compound 72 in 78% yield. Compound 72 was then treated with p-TSA in the presence of toluene followed by Sharpless asymmetric dihydroxylation and treatment with HF and acetonitrile to obtain compound 73 in 96% yield. Compound 73 was converted to 74 via different steps, which then underwent Sharpless asymmetric dihydroxylation to give the target molecule, i.e., propindilactone G 75 in 58% yield (Scheme 11).
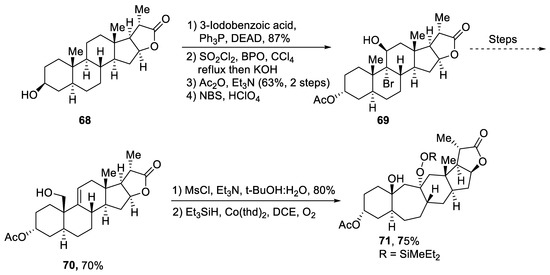
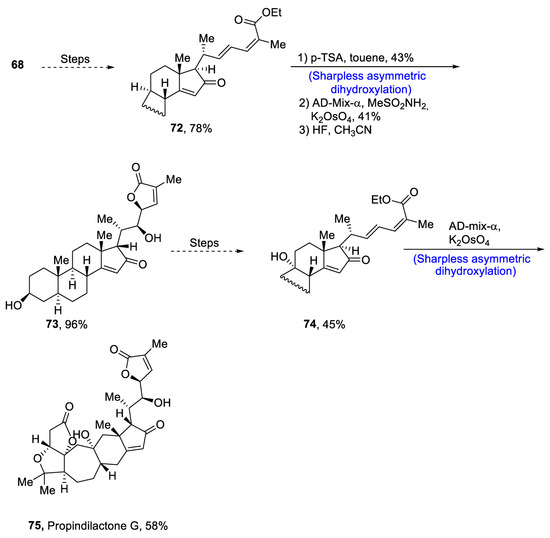
Scheme 11.
Synthesis of propindilactone G 75.
2.2.4. Monoterpenoids
Aromatic 3,5-dimethylorsellinic acid (DMOA) is a source of many fungal monoterpenoids that constitute a variety of structures [56]. DMOA-isolated monoterpenoids, i.e., berkeyleyone and preaustinoids 1, preaustinoid 2 and preaustinoid 3, are biologically active and are highly potent against a number of inflammatory diseases [57]. (–)Berkeyleyone A structure is composed of a nonane core based on four cyclic rings. Keeping in view the medicinal importance of DMOA-derived (-)berkeyleyone A and preaustiniod 1-3, Zhang et al. [58] in 2021 described an efficient synthetic route towards their total synthesis. In this regard, 2,4,6-trihydroxybenzoic acid hydrate 76 was passed through four different steps and gave compound 77, which was subjected to reduction followed by treatment with 78 (which was obtained by Sharpless asymmetric dihydroxylation of 85) and later on, passing through sequential Krapcho dealkoxycarbonylation to obtain compound 79. Compound 79 was then subjected to Wittig olefination followed by base catalyzed acylation. Then, Krapcho-type demethylation was carried out followed by protection with trimethylsilyl group, which was then allowed to undergo Mander’s reagent-catalyzed acylation. Finally, deprotection of the silyl group led to the synthesis of (-)berkeleyone A 80 in 94% yield. Berkeyleyone A underwent Dess–Martin periodinane-mediated oxidation to synthesize preaustinoid A1 81 in 88% yield, which was then treated with boron trifluoride diethyl etherate in the presence of MeCN to synthesize preaustinoid B 83 in 86% yield, which was converted to preaustinoid B2 84 by the addition of aqueous NaOH and absolute alcohol. Similarly, preaustinoid A 81 was subjected to oxidation in the presence of m-chloroperoxy benzoic acid to yield preaustinoid A1 82 in 43% yield (Scheme 12).
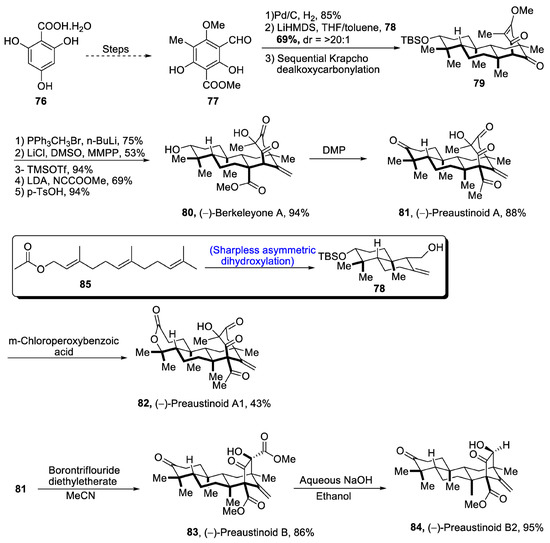
Scheme 12.
Synthesis of berkeleyone A 80 and preaustinoids 81, 82, 83 and 84.
2.2.5. Monoterpenoid Alcohol
The new monoterpene alcohol was first isolated from a Chinese herb, namely ‘Mentha haplocalyx’, by Liu and his co-researchers [59]. However, our recent studies do not correlate with their proposed structure, as it has been found to be incorrect via 13C and 1H-NMR spectroscopy. However, its structure was found to be quite similar to that of three other naturally occurring monoterpenes, namely asiasarinol, cosmosoxide B, and cis-p-menth-3-ene-1,2,8-triol [60]. This study [61] presented the total synthesis of trans-p-menth-3-ene-1,2,8-triol. The total synthesis began with the α and β-Sharpless asymmetric dihydroxylation, leading to the synthesis of compounds 87a and 87b in 40 and 43% yields with 33.8% and 1.2% enantiomeric excess, respectively, and similarly via route B, AD-mix-α and β leading to the synthesis of 90a and 90b in 76% and 91% yield with 54.5% ee and 59.4% enantiomeric excess, respectively. Compound 87a was further treated in the presence of methyl lithium and tetrahydrofuran followed by reaction with Dess–Martin periodinane and dichloromethane, thus finally converting to the synthesis of trans 88a (A-α) in 47% yield with 99.5% enantiomeric excess, by Luche reduction. On the other hand, other enantiomers of trans-monoterpenes were obtained by oxidation in the presence of manganese oxide followed by Luche reduction via both routes A and B. In this way, both enantiomers of the target molecule were obtained by using Sharpless asymmetric dihydroxylation (Scheme 13).
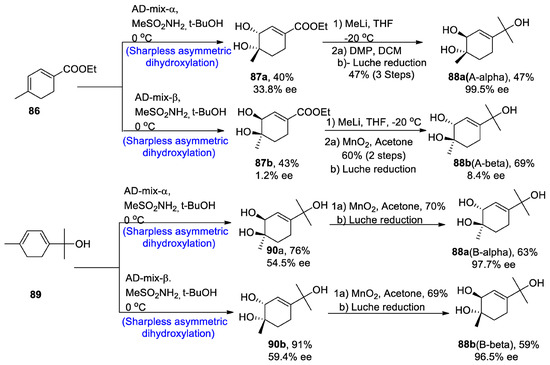
Scheme 13.
Synthesis of trans-p-menth-3-ene-1,2,8-triol 88.
2.3. Synthesis of Polyketide-Based Natural Products
Pladienolides A and B have been isolated from Streptomyces platensis, and they are known to be actively involved in the division of messenger ribonucleic acid (mRNA) [62]. Moreover, it has been discovered that pladienolide B is highly effective against the virus SARS-CoV-2 [63].Taking into account the wide pharmacological aspects of pladienolides, Rhoades et al. [64] in 2021 reported a facile and efficient route for their total synthesis. In the first step, starting reagent 91 was reacted with 3-buten-2-yl-acetate 92 in the presence of iridium catalyst and potassium phosphate to obtain compound 93 in 91% yield. Compound 93 was subjected to Sharpless asymmetric dihydroxylation, which gave compound 94 in 77% yield with 10:1 diastereoselectivity. Compound 94 was then reacted with NIS followed by its treatment with acetic anhydride, trimethylamine and DMAP to obtain compound 95 in 90% yield. Compound 96 (which was originally obtained via a number of steps) was made to react with 95 in the presence of Pd(dppf)Cl2, potassium phosphate and compound 97, leading to the synthesis of target molecule 98 in 87% yield (Scheme 14).
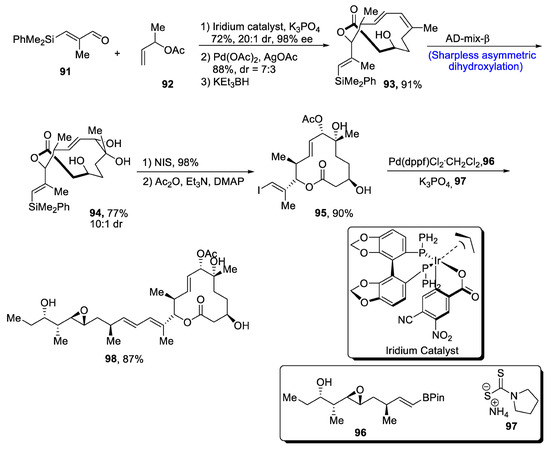
Scheme 14.
Synthesis of pladienolides 98.
Actinomycete streptomyces is a source of pharmacologically important alchivemycins A and B, which are polyketides comprising highly complicated structures [65]. These naturally occurring polyketides play essential roles against a number of bacterial diseases. Their structures consist of an oxazine heterocyclic ring and 17-membered polycyclic central ring along with five optically active carbon-containing C16-C25 fragments [66]. For the first time, Liao et al. [67] in 2021 attempted to report the total synthesis of alchivemycins A and B by describing a synthetic route to obtain C16–C25 moiety. Achmatowicz rearrangement and enantioselective dihydroxylation were the main steps of their synthetic scheme. The synthetic route started with the aldol condensation by using optically active Evans’ oxazolidinone in the presence of trimethylamine and dichloromethane. Later, compound 99 was obtained by treatment with t-butyl silyl chloride in the presence of imidazole, DMAP and DCM. Compound 99 was reacted further via different steps to obtain the alkene-containing compound 100 in 65% yield. Compound 100 was then treated with p-methoxy benzoyl chloride in the presence of other reagents, which gave compound 101 in 65% yield. Compound 101 was further subjected to Sharpless asymmetric dihydroxylation in the presence of t-butanol and water to give compound 102 in 66% yield. Compound 102 was then transformed into C16-C25 fragment 103 in 80% yield, which then could be used to synthesize alchivemycins A and B (Scheme 15).
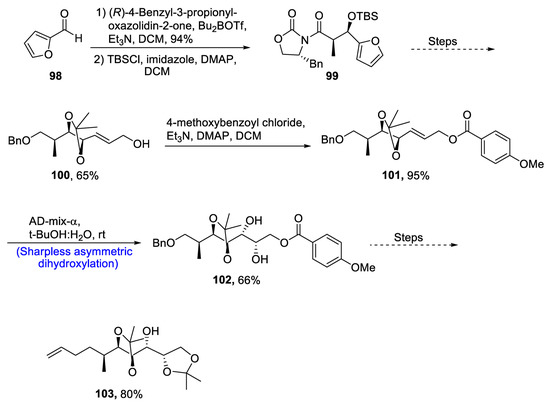
Scheme 15.
Synthesis of alchivemycins A and B 103.
Fostriecin has been isolated from Streptomyces pulveraceus [68]. It is a naturally occurring polyketide that plays an effective role against different cancer lines such as lung, breast and ovarian cancer [69]. There have been various reports on the total synthesis of fostriecin since its discovery. In order to contribute to the facile synthesis of this naturally occurring polyketide, Dong et al. [70] in 2020 reported a facile synthetic route for its total synthesis. In their methodology, trienyne 104 was converted to compound 105 via several steps. Compound 105 was then subjected to olefination followed by Sharpless asymmetric dihydroxylation in the presence of osmium tetroxide and (DHQ)2PHAL, which resulted in the synthesis of compound 106 in 82% yield. Compound 106 was then treated with pyridine, DCM and (Cl3CO)2CO followed by reduction with formic acid to obtain compounds 107a and 107b in 34% and 31%, respectively. Compounds 107a and 107b were then subjected to Sharpless asymmetric dihydroxylation one by one followed by deprotection of the hydroxyl group in the presence of potassium carbonate and ethanol to obtain compounds 108 and 109 in 82% and 27% yield, respectively. Compound 108 was then transformed to fostriecin 110 after several steps (Scheme 16).
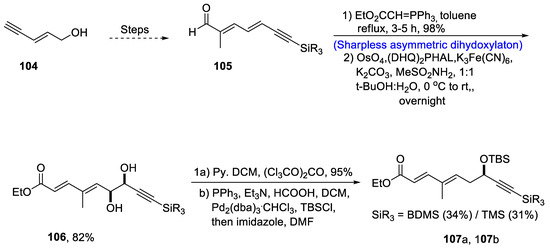
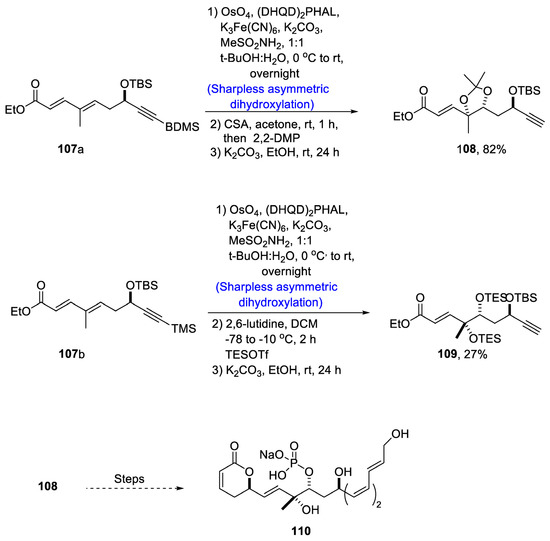
Scheme 16.
Synthesis of fostriecin 110.
Ascospiroketal B, which is a tricyclic core-containing polyketide, is isolated from a sea-water fungus, Ascochyta salicorniae [71]. Hara et al. [72] in 2020 reported the total synthesis of this natural product. The total synthesis began with the preparation of α, β-unsaturated ketone 111, which then underwent Sharpless asymmetric dihydroxylation to synthesize diol 112 in 65% yield. The diol was protected by 2,2-dimethoxypropane and toluene sulfonic acid, followed by reaction with (EtO)2P(O)CH2CO2Et via Horner–Wadsworth–Emmons reaction. It was followed by reduction with DIBAL-H, resulting in the synthesis of α,β-unsaturated ester 113 in 90% yield. Compound 113 was then reacted with disopropyl L-tartrate via Katsuki–Sharpless epoxidation to provide compound 114 in 89% yield. Over a few steps, compound 114 was converted into compound 115. Reaction of compound 115 with TESOTf and 2,6-lutidine in the presence of DCM followed by treatment with hydrated barium hydroxide in the presence of methanol resulted in γ- lactone 116 and 117 in 65% and 22% yield, respectively. Compound 116 was then reacted via several steps that resulted in 85% yield of targeted natural product 118 (Scheme 17).
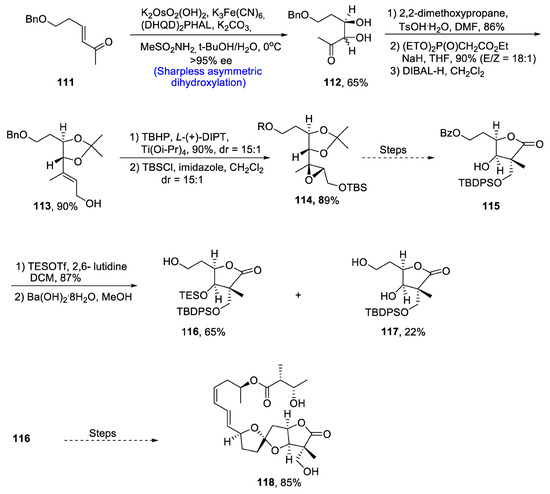
Scheme 17.
Synthesis of ascospiroketal B 118.
Bioinspired two-phase synthesis is described for the total synthesis of many natural products. Its first phase is referred to as the ‘cyclase phase’, which involve the production of natural products requiring the least oxidation [73]. The second one is referred to as the ‘oxidation phase’, which deals with the incorporation of oxidized functional groups leading to the synthesis of natural products. The synthesis of pharmacologically and medicinally important cytochalasins is also based on this two-phase synthesis strategy, which involves the formation of central moieties in the first stage via an integrated polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) [74]. They are composed of fused cyclic ring structures containing isoindolone moiety. Owing to their biologically active nature, efforts towards their total synthesis were inevitable. Gayraud et al. [75] in 2021 reported the total synthesis of these cyclic cytochalasin natural products, i.e., aspochalasins, leading to the synthesis of trichoderone. Their synthetic approach included Sharpless asymmetric dihydroxylation, Ireland–Claisen rearrangement, Diels–Alder reaction and Suzuki–Miyaura cross-coupling reaction as the main steps. The synthesis began with the enantioselective step, i.e., Sharpless asymmetric dihydroxylation in the presence of potassium osmate and (DHQD)2PHAL, providing vicinal diol 120 in 81% yield with a 90:10 enantioselective ratio, which was then subjected to Swern oxidation followed by introduction of enol 121 in 86% yield. Compound 120 was then subjected to Ireland-Claisen rearrangement in accordance with Suzuki–Miyaura cross-coupling reaction to obtain compound 124 in 71% yield. Compound 124 was then allowed to react with LDA, TBSCl and TPPA, thereby giving compound 125 in 71% yield. Compound 125 was then transformed over a number of steps into naturally occurring aspachalasan intermediate 126 in 87% yield. This intermediate then moved toward the total synthesis of trichoderone A 127 (Scheme 18).
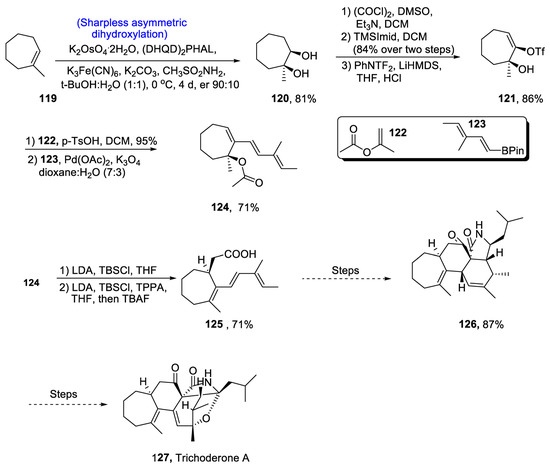
Scheme 18.
Synthesis of trichoderone A 127.
Amphirionin is a straight-chain polyketide with hexahydrofuro furan rings and alkene bonds attached to it. Amphirionin-2 was discovered from marine dinoflagellates Amphiridium sp. [76] and has been found to exhibit highly anti-cancerous potential [77]. It is used to inhibit the uncontrolled cell division in human colon, lungs and murine cells. Due to its wide pharmacological applications, various research groups have reported the total synthesis of amphirionin-2. Saha et al. [78] in 2020 reported the stereoselective synthesis of this 10 chiral center-containing amphirionin-2 by employing Sharpless asymmetric dihydroxylation, Julia–Kocienski olefination, cycloetherification, Crimmins propionate aldol reaction and Wittig olefination. For this purpose, aspartic acid 128 was converted into 129 after a number of steps and was then treated with TESCl and trimethylamine in the presence of DMAP and dichloromethane followed by Sharpless asymmetric dihydroxylation in the presence of osmium tetraoxide and NMO, and then subjected to oxidative cleavage in the presence of NaIO4 and sodium bicarbonate followed by reduction, leading to the synthesis of compound 130 in 76% yield. Compound 130 was then reacted further in the presence of p-toluenesulfonyl hadrazide and triphenyl phosophine followed by its reaction with m-CPBA, leading to the synthesis of compound 131 in 85% yield. Similarly, compound 132 was treated further via Sharpless asymmetric dihydroxylation to obtain compound 133 in 78% yield. Compounds 131 and 133 were then coupled via Julia–Kocienski olefination to obtain compounds 134 and 135. Compound 135 was further subjected to several steps, affording amphirionin-2 136 in 82% yield (Scheme 19).
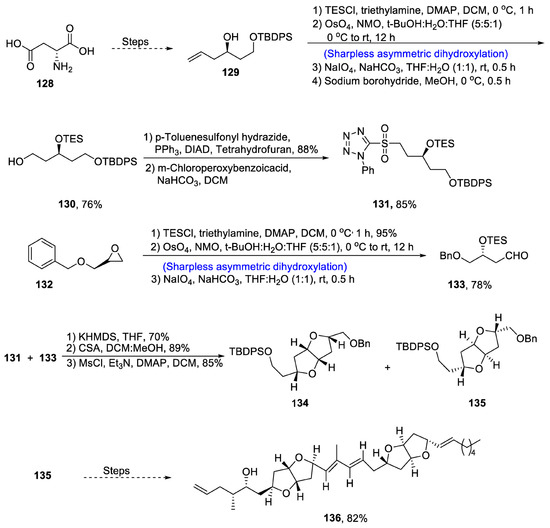
Scheme 19.
Synthesis of amphirionin-2 136.
Isolation of angucyclinone antibiotic (+)-PD-116740 from actinomycetes has been reported by different research groups [79]. It is effective against different cancer lines, i.e., mucus-producing glandular cells, lymph cancer cells, etc.
This antibiotic belongs to trans-9,10-dihydrophenanthrene-9,10-diol-containing natural products [80]. Owing to the biological significance of heterocycle-containing antibiotics, various research groups have reported different methodologies for their synthesis. However, Zheng et al. [81] in 2021 devised a facile route for the synthesis of PD-116740 involving Sharpless asymmetric dihydroxylation and oxidative cyclization. In the first step, quinone was subjected to sodium hydrosulfite followed by protection of the –OH group by benzyl bromide, which gave 138 in 91% yield. Component 139 was obtained by the addition of bromine followed by protection with MOMCl in 96% yield. For the synthesis of component 142, substituted benzyl alcohol 140 was treated with TBSOTf followed by reaction with n-BuLi in the presence of DMF to give compound 141 in 70% yield. Ohira–Bestmann reagent was treated with 141, followed by treatment with B(pin)2 and Cu(OTf)2 in the presence of acetonitrile, leading to the synthesis of compound 142 in 75% yield. Palladium catalyzed Suzuki–Miyama coupling reaction was carried out between 139 and 142 followed by asymmetric dihydroxylation in the presence of osmium tetroxide and (DHQ)2PHAL, which led to compound 143 in 97% yield. Protection of hydroxyl groups and Cu promoted oxidation over four steps, leading to the synthesis of target molecule 144 in 63% yield (Scheme 20).
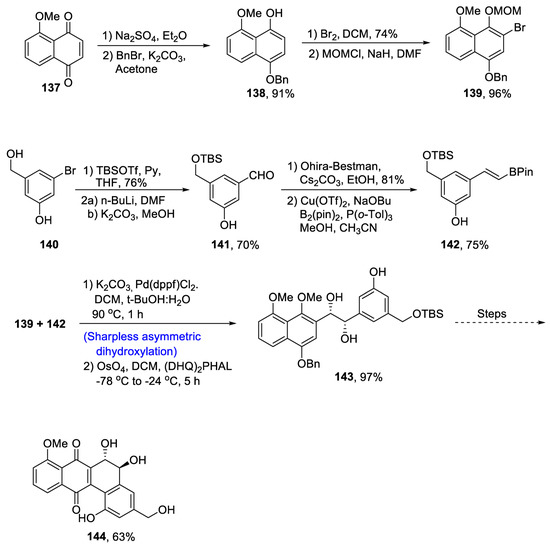
Scheme 20.
Synthesis of (+)-PD-116740 144.
2.4. Synthesis of Macrolide-Based Natural Products
Marine bugula neritina is the main source of a class of bryostatins composed of about 21 members that are known to be highly effective against cancer-causing agents. They are also responsible for neuron transmissions and also act as stimulators of protein Kinase C (PKC). Among the 21 members of this family, total synthesis of only nine bryostatins have been reported by some research groups. Bryostatin’s structure is very complicated and, therefore, it is very difficult to synthesize. To date, only one report on the total synthesis of bryostatin has been published. Keeping in view their wide biological applications, Trost et al. [82]. in 2020 took this challenging task to synthesize structurally complex bryostatin 3 via a relatively efficient and short synthetic route as compared to the one reported earlier. They utilized Sharpless asymmetric dihydroxylation, carbonylative esterification, Yamaguchi macrolactonization, epoxidation and endo-dig cyclization in their scheme. The first step of total synthesis involved the Sharpless asymmetric dihydroxylation of 145, yielding 146 in 84% yield. Compound 146 was then treated over a number of steps to obtain compound 147 in 76% yield. Compound 147 then underwent Sharpless asymmetric dihydroxylation in the presence of (DHQ)2PHAL, which gave compound 148 in 90% yield. 3-Methyl butyne 149 was treated with n-BuLi in the presence of TMEDA and diethyl ether followed by reaction with 1-bromo-2-ethoxy ethane 150 in the presence of dimethyl zinc and diethyl ether, which resulted in the synthesis of intermediate 151. The intermediate was later transformed via numerous steps into target molecule 152 in 60% yield (Scheme 21).
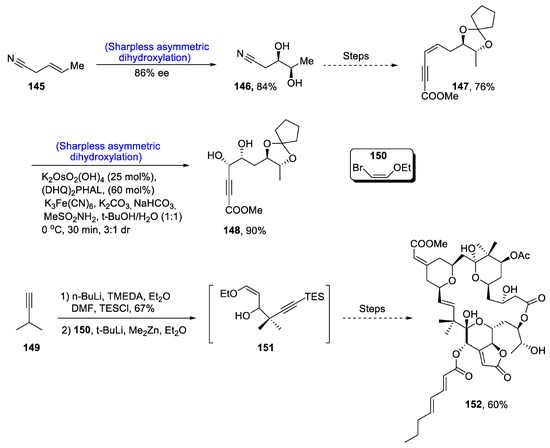
Scheme 21.
Synthesis of bryostatin 152.
Among recently used microtubule stabilizing agents (MSAs), zampanolide is found to be one of the most rare and effective macrolides that possesses high potential against cancer cells owing to its covalent bonding to β-tubulin [83]. Chen et al. [84] in 2020 reported the synthesis of two new zampanolide impersonators in a number of steps involving name reactions, i.e., Sharpless asymmetric dihydroxylation, Wittig reaction, Yamaguchi esterification and Horner–Wadsworth–Emmons reaction. The first step of total synthesis involved the Wittig reaction between 2-fluorobenzaldehyde 153 and ethyl 2-(triphenyl-phosphoranylidene)acetate 154, followed by reduction leading to the synthesis of compound 155. The chiral centers at position 17 and 18 were imported either via AD-mix-α or via AD-mix- β in the presence of respective ligand (DHQ)2PHAL/(DHDQ)2PHAL and osmium salt. Compound 156 was further made to react with MsCl followed by introduction of epoxide ring via Williamson ether synthesis. Methoxymethyl (MOM) was used as protecting group for alcohol moiety, which was further treated with iodo ether 158 to give a racemic mixture of compound 159 in 22–23% yield. Yamaguchi esterification was carried out to fuse the fragments 159 and 160, followed by treatment with the complex of hydrogen fluoride and pyridine, which resulted in the synthesis of compound 161. Compound 161 was made to react with Dess–Martin periodinane followed by Horner–Wadsworth–Emmons condensation in the presence of barium hydroxide catalyst. In the last step, HCl was used for the deprotection of alcohols, leading to the complete synthesis of zampanolide mimic 162 (Scheme 22).
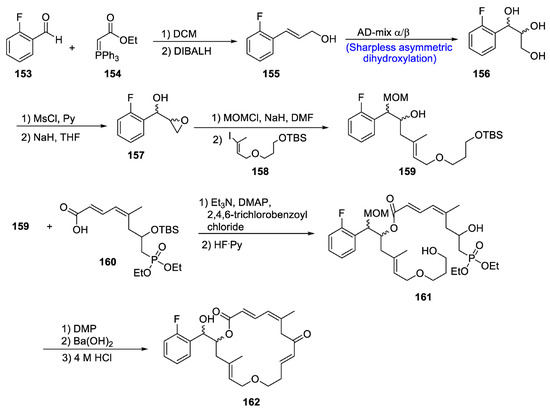
Scheme 22.
Synthesis of zampanolide mimics 162.
Carreira et al. [85] reported the total synthesis of formosalides A and B, which play an important role in combating cancer. These are macrolides that are isolated from the marine dinoflagellate Prorocentrum sp. Propargylation reaction, i.e., Krische propargylation between 163 and 164 afforded compound 165 in 56% yield with 95% ee and dr >20:1. Several steps were carried out to obtain compound 166 in 44% yield. In the next step, Sharpless asymmetric dihydroxylation and asymmetric ketene cycloaddition took place one after the other to give compound 167 in 46% yield. As a result of Weinreb ketone synthesis, Evans–Tishchenko reaction and ring-closing alkyne metathesis, compound 168 was obtained in 30% yield. In the last step, Stille coupling resulted in synthesis of two formosalides 169 (Scheme 23).
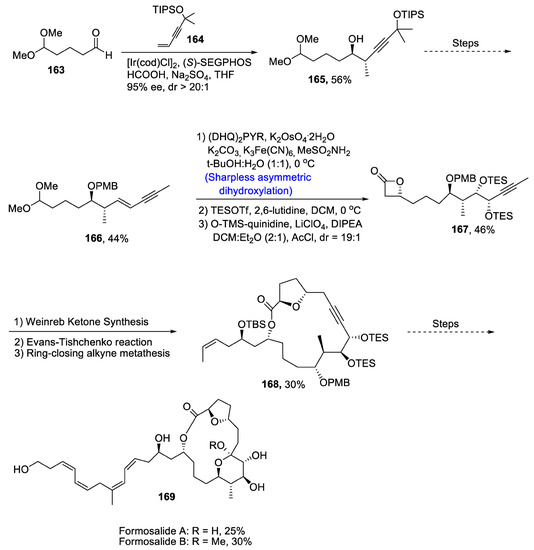
Scheme 23.
Synthesis of formosalides A and B 169.
Patulolide C, a macrolide consisting of a lactone ring, is isolated from Penicillium urticae, along with patulolide A and patulolide B isomers [86]. Various research groups have evaluated their potential against a number of bacterial, fungal and viral diseases [87]. Owing to their vital pharmacological significance, there have been continuous efforts towards the total synthesis of patulolide C. However, previous methodologies reported by different research groups were linked with several strident steps, low yields and long reaction times. Paratapareddy et al. [88] in 2020 reported the facile total synthesis of patulolide C to troubleshoot the above difficulties. For this purpose, chiral epoxide 170 was treated with 7-bromohept-1-ene in the presence of magnesium followed by protection of –OH group by benzyl bromide, affording compound 171. Compound 171 was further subjected to Sharpless asymmetric dihydroxylation in the presence of methanesulfonamide and tert-butanol:water to obtain compound 172 in 75% yield. Compound 172 was further subjected to tosyl chloride and triethylamine followed by reduction with lithium aluminium hydride. The next step involved the treatment with tert-butyldimethylsilyl chloride to give compound 173 in 87% yield. Over two steps, diol was cleaved with NaIO4 followed by reaction with substituted triphenylphosphine and LiOH, which gave compound 174 in 80% yield. Further, deprotection of alcoholic group and Yamaguchi reaction led to the complete synthesis of patulolide C 175 in 68% yield (Scheme 24).
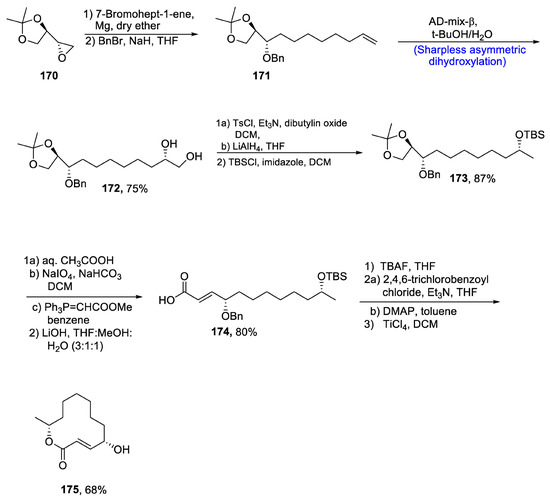
Scheme 24.
Synthesis of patulolide C 175.
2.5. Synthesis of Amino Acid-Based Natural Products
2.5.1. Tubulysins/Amino Acid
Due to developed resistance against many anti-cancerous drugs, there has been a constant urge to discover more potent anti-cancer agents [89]. Naturally occurring Tubulysins have been found to exhibit irresistible anti-cancerous activity due to their ability to interrupt tubulin division [90]. Tubulysins consist of four amino acid chains, i.e., one naturally occurring isoleucine and three other unofficial amino acids. One of them is tubuvaline (Tuv), whose facile synthesis was recently reported by Reddy et al. [91] in 2021. The key steps of this synthesis are Sharpless asymmetric dihydroxylation and aziridine ring opening reaction. The first step of synthesis involved the treatment of 176 with sodium borohydride and iodine followed by reaction with tosyl chloride in the presence of a base. Vinyl-substituted compound 178 was obtained by treatment with vinyl magnesium bromide followed by addition of di-tert-butyl dicarbonate. In the next step, compound 178 was subjected to Sharpless asymmetric dihydroxylation, which led to the efficient synthesis of optically active vicinal diols 179 in 88% yield. The alcohol moieties were protected step by step with the help of TBDPSCl and MOMCl, respectively. Compound 180 over a number of steps afforded tubuvaline fragment of tubulysins 181 in 83% yield (Scheme 25).
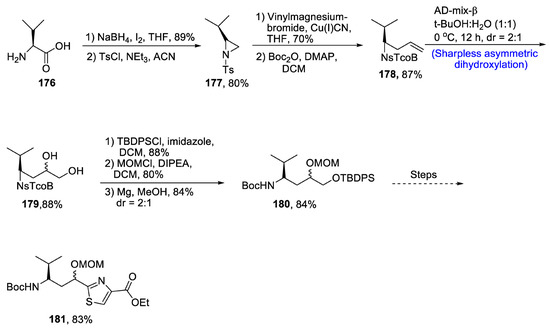
Scheme 25.
Synthesis of tubuvaline 181.
2.5.2. Amino Acid Derivative
Most of the biologically important metabolites are obtained from microorganisms. Microascus alveolaris strain PF1466 has been found to be a major source of new cyclodepsipeptide alveolarides A-C [92], which have been discovered to be highly effective against plant pathogens and thus are environmental friendly pest killers. They have been evaluated against a number deadly plant pests in which they exhibited efficient inhibitory activity [93]. The structure of alveolarides consists of 2,3-dihydroxy-4-methyltetradecanoic acid (DHMTDA) with a high number of carbon-containing cyclic rings. Keeping in view the eco-friendly nature of alveolarides, Saha et al. [94] in 2020 described the actual structure and total synthesis of alveolaride C by employing Sharpless asymmetric dihydroxylation, macrolactamization, Julia–Kocienski olefination, amide coupling reaction, etc. In the first step, 182 was treated with butyl lithium and NaIO4, which gave a diol moiety that was allowed to react with PivCl followed by protection of hydroxyl group by ter-butylsilyl group, and treatment with DIBAL-H resulted in the synthesis of alcohol 183 in 80% yield. Compound 183 was then transformed into acid derivative 184 in 72% yield by undergoing a number of steps that involved Julia–Kochienski olefination as well. Aldehyde-containing compound 185 was treated with thiazolidinethione 186 in the presence of titanium tetrachloride and DCM followed by treatment with lutidine and TBSOTf. It was further subjected to reduction that furnished 187 in 88% yield. Compound 187 was then made to react under different reagents, which led to the synthesis of another acid derivative 188 in 75% yield. Compound 189 was subjected to Swern oxidation followed by Wittig olefination, which gave 190 in 85% yield. Compound 190 then underwent Sharpless asymmetric dihydroxylation and was then reacted with 2′-DMP followed by treatment with LAH to give 191 in 90% yield. Compound 191 was then reacted further to obtain 192 in 85% yield. Compound 192 was then made to react in the presence of 6:1 of methanol:water, followed by treatment with hydrated lithium hydroxide in the presence of tetrahydrofuran:methanol:water (3:1:1) to obtain 193 in 75% yield. Similarly, 192 was reused and reacted via three different steps to obtain 194 in 84% yield. Compound 194 was then reacted over a few steps, giving alveolaride C 195 in 85 % yield (Scheme 26).
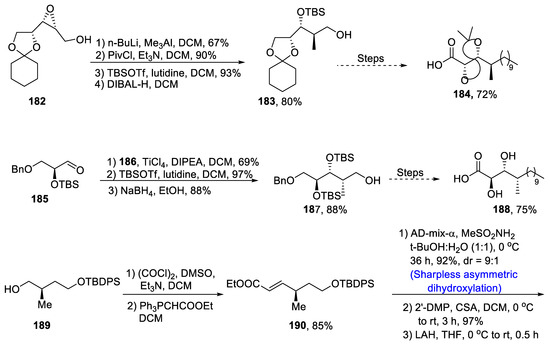
Scheme 26.
Synthesis of alveolaride C 195.
2.6. Synthesis of Flavonoid-Based Natural Products
2.6.1. DHPVs/Flavonols
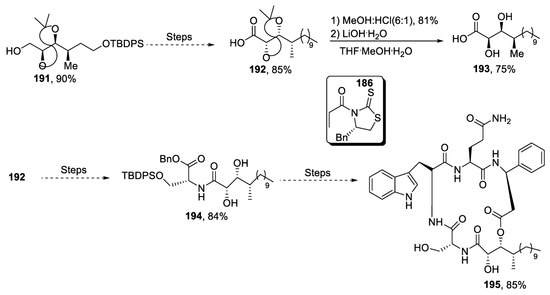
Walsura trifoliata is the source of a flavonol containing a phenylpropanoid unit [95]. Ramana et al. [96] in 2020 reported the facile, easily accessible and efficient synthetic methodology for its total synthesis via Sharpless asymmetric dihydroxylation, Grubb-2 catalyzed RCM reaction and Wittig reaction. Total synthesis began with easily available starting material 196, which was reacted with bromo methylbenzene in the presence of DMF and sodium hydride to obtain compound 197 in 70% yield. Compound 197 over several steps resulted in the synthesis of compound 198 in 55% yield. Compound 198 was then subjected to Sharpless asymmetric dihydroxylation to obtain diol 199 in 60% yield with 99% ee, which then afforded target molecule 200 in 85% yield over several steps (Scheme 27).
Scheme 27.
Synthesis of new phenolic constituent 200.
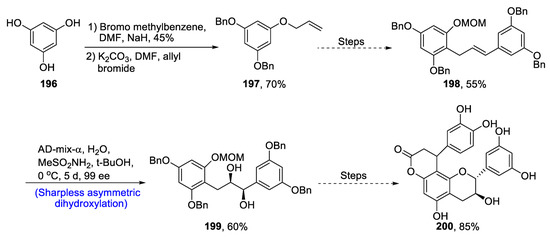
There are many advantages of microorganisms that live in the digestive tract of vertebrates, and there has been extensive research on these beneficial chemical compounds extracted from the intestines of vertebrates [97]. Owing to the anti-inflammatory effects of 5-(3,4′-dihydroxyphenyl)-γ-valerolactone) DHPV, Kim et al. [98] in 2020 reported the total synthesis of both enantiomers of DHPV via cross-metathesis (CM) and Sharpless asymmetric dihydroxylation. 3,4-Dimethoxyphenylpropene 201 and methyl-4-pentenoate 202 were treated in the presence of Grubbs 2nd generation catalyst and DCM via cross-metathesis to form compound 203 in 59% yield. Compound 203 was then subjected to Sharpless asymmetric dihydroxylation and AD-mix-α and β one by one followed by immediate lactonization, which led to the synthesis of compounds 204 and 205 in 62% and 55% yields, respectively. Compounds 204 and 205 were further treated separately with hydrogen molecule in the presence of Pd(OH)2, followed by treatment with BBr3 in the presence of DCM that afforded S- and R- enantiomers of DPHV 206 and 207 in moderate yields, i.e., 60% and 62%, respectively (Scheme 28).
Scheme 28.
Synthesis of DHPV 206 and 207.
2.6.2. Isoprenylated Chalcone Skeleton/Flavonoid
Various anti-cancerous drugs are isolated from natural products such as chalcones [99]. Sanjoseolide, a natural product containing an isoprenylated chalcone skeleton, obtained from Dalea frustecens, has wide pharmacological significance, as it has been found to be effective against inflammation, cancer and diabetes [100]. Owing to its huge medicinal importance, Tian et al. [101] in 2020 reported an efficient method for the synthesis of sanjoseolide by carrying out Sharpless asymmetric dihydroxylation, Stille coupling and Claisen–Schmidt condensation. In the first step, 2,4-dihydroxyacetophenone 208 was treated with I2 and KIO3 followed by protection with a methoxymethyl group, leading to the synthesis of compound 209 in 90% yield. Compound 209 was then reacted with Pd(PPh3)4 and 210 followed by Sharpless asymmetric dihydroxylation, giving compound 211 in 44% yield. Compound 211 was then treated with aldehyde in the presence of potassium hydroxide, and deprotection of hydroxyl groups was carried out in the presence of HCl to obtain sanjoseolide 212 in 49% yield (Scheme 29).
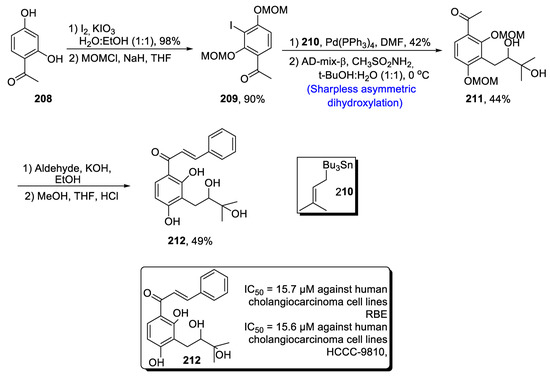
Scheme 29.
Synthesis of sanjoseolide 212.
Various natural products are composed of a homoisoflavonoid skeleton that is found to be effective against a range of diseases. Homoisoflavonoid fragments containing brazilin and other members of its family play an important role in combating different ailments. Brazilin is highly potent against bacterial, inflammatory and cancerous diseases [102]. It also plays a huge role in protection of the liver from toxicity and reduction in the tension of blood vessels. Considering the medicinal uses of brazilin, Huang et al. [103] in 2020 reported its synthetic route involving Sharpless asymmetric dihydroxylation and Prins/Friedel Craft reaction as the main steps. The total synthesis began with reaction of 4,bromo-1,2-dimethoxybenzene 213 and magnesium in the presence of iodine, thereby giving Grignard reagent, which then reacted with methyl (2-bromomethylacrylate) 214 in the presence of lithium chloride and copper iodide, leading to the synthesis of 215 in 89% yield. Compound 215 was then further treated with DIBAL-H and diisopropyl azodicarboxylate (DIAD), which gave compound 217 in 98% yield. Then, Sharpless asymmetric dihydroxylation was carried out by using K2OsO4.2H2O, potassium hexacyanoferrate (III), (DHQD)2PHAL and potassium carbonate in the presence of a 1:1 t-BuOH:H2O mixture at 0 °C, affording chiral diol containing compound 218 in 86% yield with 98% enantiomeric excess. Compound 218 was then subjected to Parikh–Doering oxidation in the presence of sulfur trioxide followed by treatment with trifluoroacetic acid to obtain compound 219 in 85% yield, which afforded targeted compounds 220 and 221 (Scheme 30).
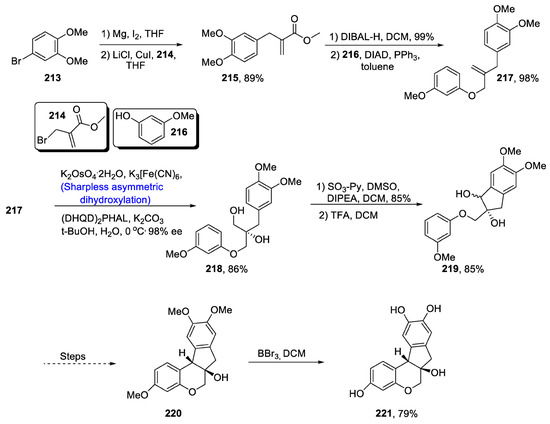
Scheme 30.
Synthesis of (+)-brazilin 220.
Cudrania tricuspidata belongs to the family Moraceae and is widely utilized as a medicinal and therapeutic drug in Asian countries. It is used in the treatment of a number of diseases. Cudrania tricuspidata is a major source of flavones that are very effective against cancer cell lines, liver infection and obesity [104]. Cudraisoflavone J is also derived from Cudrania tricuspidata and is found to be most effective against 6-hydroxydopamine, which is responsible for the death of cells in human neuroblastoma. Keeping in view the biological and medicinal potential of cudraisoflavone J, Lu et al. [105] in 2021 attempted to describe its total synthesis along with its enantiomers via Sharpless asymmetric dihydroxylation, Claisen rearrangement and Suzuki–Miyaura coupling reaction. The first step of total synthesis involved the reaction of 2′4′6′-trihydroxyacetophenone 222 with MOMCl in the presence of DIPEA and DCM followed by treatment with phenyl bromide in the presence of potassium carbonate and acetone, which gave compound 223 in 60% yield. Compound 223 was then subjected to para-Claisen rearrangement, which afforded compound 224 in 93% yield. Compound 224 was then allowed to react with benzyl bromide in the presence of potassium carbonate followed by Sharpless asymmetric dihydroxylation in the presence of methane sulfonamide, which gave compound 225 in 67% yield. Compound 225 was then treated with methane sulfonyl chloride in the presence of pyridine followed by reaction with potassium carbonate and methanol. It was then reacted with 10% palladium in the presence of charcoal, resulting in the synthesis of compound 226, which after several steps afforded curdaisoflavone J 227 in 15% overall yield (Scheme 31).
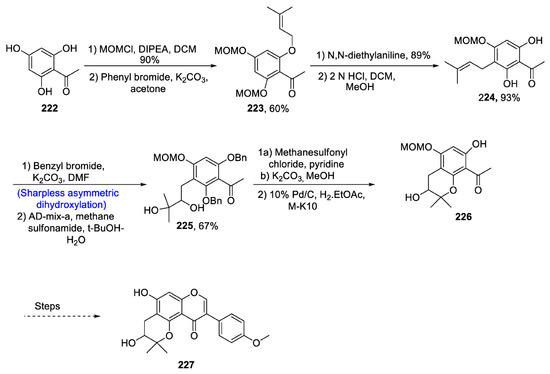
Scheme 31.
Synthesis of cudraisoflavone J 227.
2.7. Synthesis of Carbohydrate-Based Natural Products
FD-594 is a highly complicated polycyclic natural product that plays an effective role against tumor cells and a variety of bacterial diseases [106]. It is isolated from Streptomyces species TA-0256. The structure of FD-594 is composed of six heterocyclic fused rings containing trisaccharide units along with isochromanone and 9,10-dihydrophenanthrene-9,10-diol functionalities. Keeping in view the undeniable importance of these cyclic natural products in the pharmaceutical field, Xie et al. [107] for the first time described the total synthesis of FD-594 in 20% overall yield, which involved the usage of Sharpless asymmetric dihydroxylation and copper-catalyzed oxidative cyclization. The first step of total synthesis involved the diboration of 228 in the presence of platinum followed by its cross-coupling with 1-bromo-3,5-dimethoxy benzene 229 by using palladium diacetate and RuPhos; the and resulting product was then oxidized, which led to the synthesis of compound 230 in 70% yield with 91% enantiomeric excess. Compound 230 was then reacted further and over a few steps gave 231 in 87% yield. Xanthone fragments, i.e., 232 and 233, were treated in the presence of n-butyl lithium and tetrahydrofuran followed by oxidation in the presence of Dess–Martin periodinane, resulting in the synthesis of compound 234, which was then reacted under different conditions to afford compound 235 in 99% yield over five steps. Compounds 231 and 235 were then coupled via Suzuki–Miyaura coupling reaction followed by Sharpless asymmetric dihydroxylation in the presence of osmium tetroxide and dichloromethane, which gave diol 236 in 96% yield. It was then further reacted in the presence of TBSOTf and 2,6-lutidine followed by treatment with trifluoroacetic acid and dichloromethane, and the resulting product was allowed to undergo hydrogenation in the presence of palladium, which gave compound 237 in 96% yield. Compound 237 then underwent further reactions in six steps that led to the synthesis of target molecule 238 in 43% yield (Scheme 32).
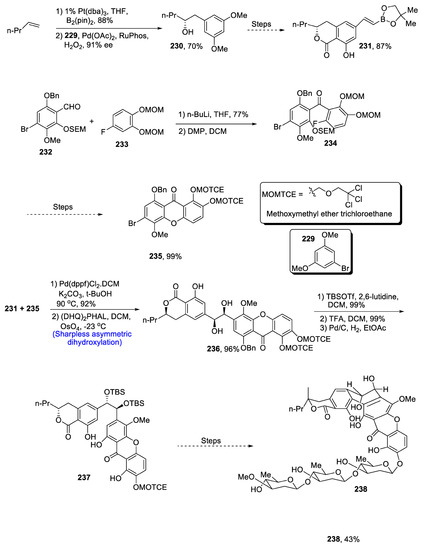
Scheme 32.
Synthesis of FD-594 238.
D-xylulose is a very crucial and sparce keto sugar that is indisputable in carrying out metabolic reactions in plant and animal cells [108]. Due to its wide importance and expensive market availability, there have been continuous efforts to devise the total synthesis of D-xylulose. Previously reported methodologies faced the problem of low yields; moreover, it is generally difficult to synthesize a moiety containing several hydroxyl groups, i.e., D-xylulose. Kalagara et al. [109] in 2020 described the total synthesis of D-xylulose by using specific synthetic approaches involving Sharpless asymmetric dihydroxylation and Wittig reaction. The total synthesis commenced with the incorporation of a protecting group followed by Swern oxidation to afford compound 240 in 76% yield. In the next step, compound 241 was treated with a protecting group followed by its reaction with bromine in the presence of carbon tetrachloride, which finally led to phosphonium bromide on treatment with triphenyl phosphine. Wittig reaction was then employed, thereby producing compound 243 in 47% yield. Compound 243 was reacted with KHCO3 and methanol to obtain compound 244 in 36% yield. Later on, Sharpless asymmetric dihydroxylation was carried out in the presence of osmium tetroxide, (DHQD)2PHAL, potassium hexacyanoferrate (III), methanesulfonamide, potassium carbonate and 1:1 t-butanol:water to obtain vicinal diols 245 in 78% yield. Finally, a deprotection strategy led to synthesis of target molecule 246 in 60% yield (Scheme 33).
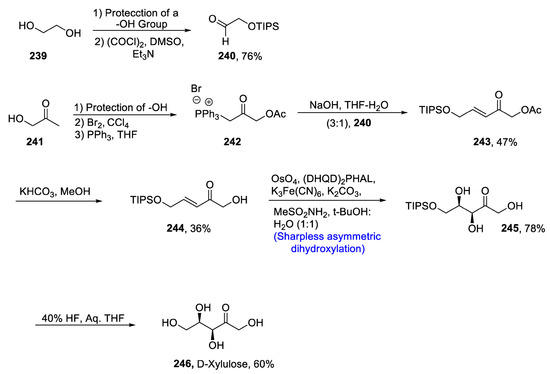
Scheme 33.
Synthesis of D-xylulose 246.
Iminosugars are structurally similar to carbohydrates in which oxygen is replaced by a nitrogen atom [110]. These iminosugars are abundantly found in nature and are isolated from various medicinal plants. Moreover, they are biologically active against several viral diseases, and they play an important role in glycosidase inhibition [111]. In 2020, Angelis et al. [112] devised the total synthesis of iminosugar 1,4-dideoxy-1,4-imino-D-iditol in 11% overall yield by employing Sharpless asymmetric dihydroxylation. In the first step of synthesis, compound 247 was treated with sodium hydride, tert-butyldiphenylsilyl chloride and tetrahydrofuran to obtain compound 248, which over several steps synthesized compound 249 in 74% yield. Compound 248 was also transformed into 250, which was reacted further through several steps to obtain compound 251 in 73% yield. Compounds 249 and 251 were then subjected to Sharpless asymmetric dihydroxylation in the presence of NMO and (DHQD)2AQN one by one, thereby giving 252, 253 and 254, 255, respectively. Compounds 252 and 253 were then reacted further via different steps to synthesize 1,4-dideoxy-1,4-imino-D-Iditol 256 and 1,4-dideoxy-1,4-imino-D-galacitol 257 in 81% and 85% yields, respectively (Scheme 34).
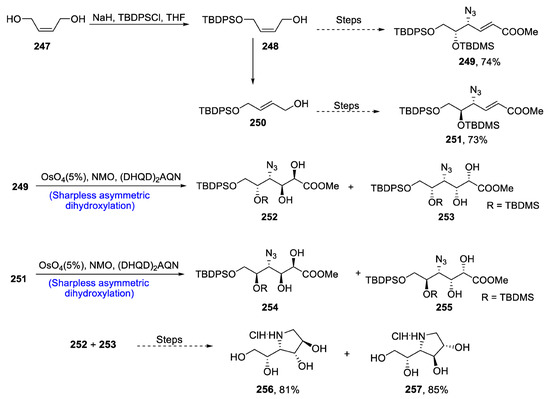
Scheme 34.
Synthesis of 1,4-dideoxy-1,4-imino-D-iditol 256 and 257.
2.8. Synthesis of Lactone-Based Natural Products
2.8.1. Naphthoquinonopyrano-γ-lactone
Naphthoquinonopyrano-γ-lactones are monomeric or dimeric natural products that have vast pharmacological applications. Crisamacin A, which is a dimeric naphthoquinonopyrano-γ-lactone isolated from soil bacterium Micromonospora purpureochromogenes. Different strategies have been proposed for the total synthesis of crisamacin due to its medicinal and biological applications [113]. Kopp et al. [114] in 2020 reported the total synthesis of crisamicin A by carrying out Sharpless asymmetric dihydroxylation, oxa-Pictet–Spengler cyclization, Heck coupling and Hartwig borylation. Total synthesis began with the generation of 262 by Heck coupling of 259 and 261, which were prepared independently from compounds 258 and 260. In the next step, compound 262 was subjected to Sharpless asymmetric dihydroxylation, resulting in the synthesis of compound 263 in 77% yield with more than 99% enantiomeric excess. The AD was followed by ‘simple’ oxa-Pictet–Spengler cyclization, resulting in the synthesis of compound 264 in 88% yield. Next, compound 264 was treated with compound 265 in the presence of [{Ir(µ-OMe)COD}2], pinacolborane and 4,4′-di-tert-butyl-2,2′-dipyridyl via Hartwig borylation to attain compound 266, which over a number of steps afforded target molecule 267 in 67% yield (Scheme 35).
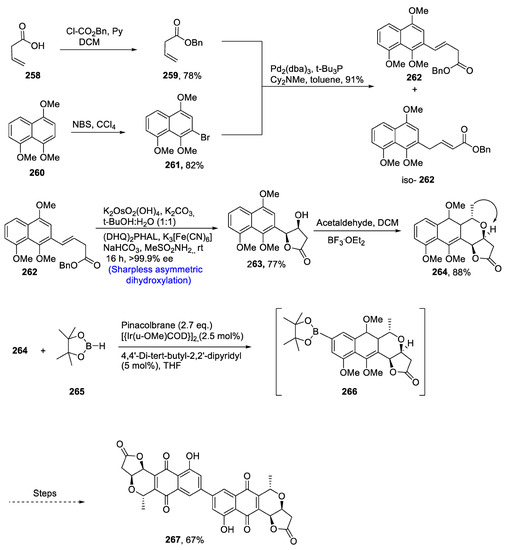
Scheme 35.
Synthesis of crisamicin 268.
2.8.2. Containing α,β-Unsaturated Lactone
A diverse variety of plants and animals have been found in Australian rainforests due to the favorable and alternating environment of that area [115]. Reddell and Gardon described the isolation of a family of natural products, i.e., EBC-23 and their derivatives, from the Cinnamonum laubati tree of the Lauraceae family. This family of natural products, and specifically EBC-23, has been discovered to play an effective role in cytotoxic activity,. EBC-23 is highly potent against a number of human cancer cell lines with minimum side effects, as normal cells are not affected by its action [116]. Due to its irrefutable cytotoxic potential, there have been numerous reports on the total synthesis of EBC-23. In 2021, Ghosh et al. [117] attempted to describe the enantioselective total synthesis of this biologically active natural product by utilizing Sharpless asymmetric dihydroxylation and Noyori asymmetric hydrogenation in 3.8% overall yield. For this purpose, easily available 1-tetradecanal 268 was converted to β-keto ester 269 via a reported methodology and was then subjected to Noyori asymmetric hydrogenation. It was then converted to Weinreb amide, which was made to react with allyl magnesium bromide in the presence of tetrahydrofuran followed by treatment with Et2BOMe and sodium borohydride to obtain diol, which was then protected to yield compound 270 in 87% yield. α, β-Unsaturated ester 271 was prepared by another protocol, which was described previously. Compound 271 was further subjected to Sharpless asymmetric dihydroxylation in the presence of methane sulfonamide to obtain vicinal diol 272 in 90% yield with 95% enantiomeric excess. Compound 272 was further reacted over a number of steps to obtain oxime 273 in 81% yield. Compounds 270 and 273 were then joined in the presence of oxone, potassium chloride and water followed by their reaction with iron powder, which resulted in the synthesis of a diasteromeric mixture of β-hydroxy ketone. Its isopropylidine protecting group was detached by using Montmorillonite K10 in the presence of dichloroethane, leading to the synthesis of target molecule EBC-23 274 in 42% yield (Scheme 36).
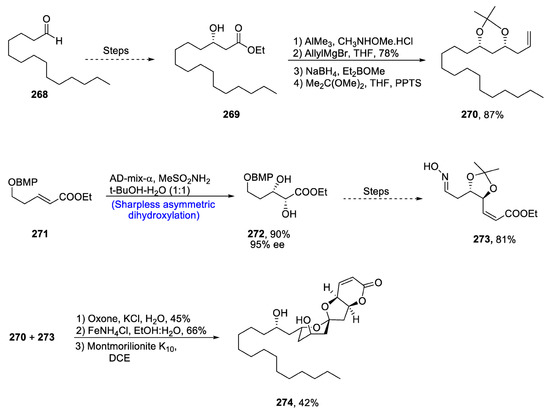
Scheme 36.
Synthesis of EBC-23 274.
2.8.3. δ-Lactone Ring Containing Natural Product/ Unsaturated Fatty Acids
Most of the compounds isolated from marine organisms have been discovered to be highly effective against a range of diseases, thus playing effective roles in pharmaceutical chemistry [118]. Ieodomycins are also obtained from a marine bacillus species, and they have been found to be highly potent against a variety of bacterial diseases caused by Gram-positive and Gram-negative bacteria. The chemical structure of ieodomycins consists of an alkene moiety along with hydroxyl groups. Considering the significance of such biologically active natural products, Choi et al. [119] in 2020 attempted to report the total synthesis of ieodomycins. Their synthetic approach involved Sharpless asymmetric dihydroxylation, Mukaiyama lactonization, Stille coupling, Keck asymmetric allylation and Wipf’s modification as the main steps. In this regard, firstly 4-pentyn-1-ol 275 was subjected to Swern oxidation followed by Keck asymmetric allylation in the presence of (S)/(R)-BINOL, which in turn was allowed to react in the presence of ZrCl2Cp2 278 and Me3Al via Wipf’s modification to obtain compounds 278 and 279 in 59% and 74% yields, respectively. Sharpless asymmetric dihydroxylation was then employed in the presence of (DHQD)2PYR, resulting in the synthesis of vicinal diol 280 in 52% yield. In the next step, ketal formation took place, followed by treatment with sodium cyanate, which gave compound 281 in 88% yield. Compound 281 was then further transformed to ieodomycin A 282 in 93% yield (Scheme 37).
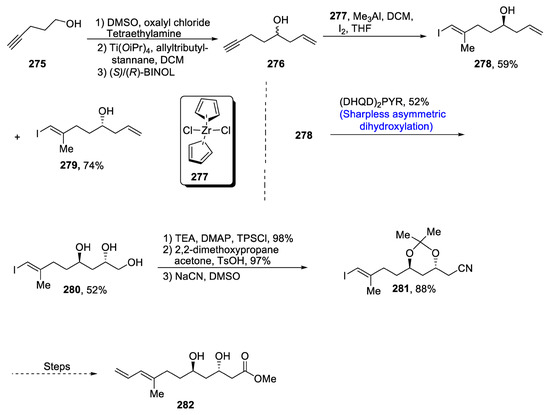
Scheme 37.
Synthesis of ieodomycins 282.
2.8.4. Styryllactone
Members of the Goniothalamus genus are of huge importance because of their unparalleled usage in the medicinal field, as they are found to be highly potent against a number of diseases such as malaria, cholera, fever, etc. Styryl lactone, i.e., cardiobutanolide, has been isolated from the parts of Goniyothalamus elegants [120,121]. Since its isolation, researchers have contributed to the total synthesis of this natural product, which is composed of five chiral centers. Sharpless asymmetric dihydroxylation and stereoselective olefination are the main steps of the synthetic scheme. Cardiobutanolide has been known to exhibit anti-cancerous activity. In 2021, Kovacevic et al. [122] reported the total synthesis of naturally occurring and medicinally important cardiobutanolide and 3-deoxycardiobutanolide in their work. In this regard, compound 283 was treated with tetrahydrofuran and zinc dust followed by cross-olefin metathesis in the presence of 2,2-dimethoxypropane to give compound 284 in 80% yield. Compound 284 was then subjected to Sharpless asymmetric dihydroxylation in the presence of osmium tetraoxide and NMO, which resulted in the excellent synthesis of compound 285 in 100% yield. Compound 285 was allowed to react with NaIO4, in the presence of a mixture of methanol and water followed by Wittig olefination and then treatment with 1:1 THF:H2O, which gave (Z) and (E)-isomers of compounds 286a and 286b in 88% and 66% yields, respectively. Compound 287 was subjected to Sharpless asymmetric dihydroxylation AD-mix-α and AD-mix-β followed by reaction with TFA/H2, which demonstrated that high yield was obtained with AD-mix-α. Compound 286 was subjected to Upjohn dihydroxylation in the presence of osmium tetroxide, butanol and NMO, which led to the synthesis of cardiobutanolide compound 293 in 51% yield along with a minimum amount of 35% of by-product compound 292 (Scheme 38).
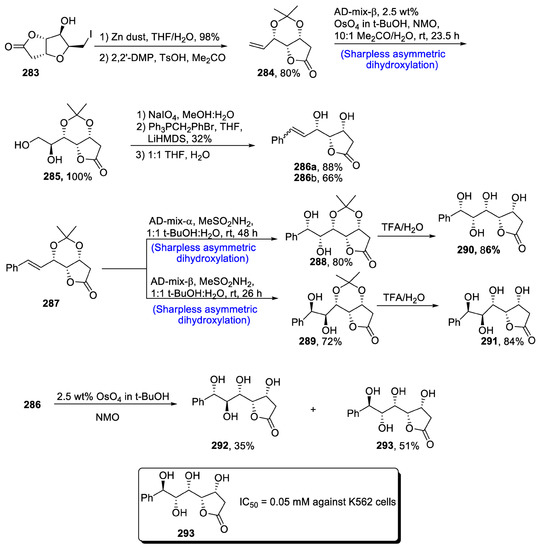
Scheme 38.
Synthesis of cardiobutanolide 293.
2.9. Synthesis of Tetrahydrofyran Ring-Based Natural Products
Artemisia caruifolia is the major source of highly effective caruifolin A, which is used in the treatment of a number of diseases and is a major component of the Chinese drug ‘Qing Hao’. Caruifolin A’s structure is composed of a carbon-based cyclic ring system along with THF. Fernandes A. and Bethi [123] in 2020 devised the synthesis of chiral centers and tetrahydrofuran rings in the structure of caruifolin A. For the total synthesis of our target molecule, ethyl lactate 294 was treated with protecting group followed by reduction in the presence of DIBAL-H. It was further reacted with allylbromide in the presence of zinc dust, leading to the synthesis of compounds 295 and 296 in 27% and 54% yields, respectively. Later on, compound 296 underwent ozonolysis followed by Wittig olefination in the presence of 297, thereby giving compound 298 in 79% yield. Compound 298 was then reacted with (DHQ)2PHAL and potassium hexacyanoferrate (III) via Sharpless asymmetric dihydroxylation, leading to the synthesis of a racemic mixture of vicinal diol 299. Furthermore, a nucleophilic substitution reaction was carried out followed by reduction and Wittig olefination, which resulted in the synthesis of compound 300 in 65% yield. A similar pathway was adopted to obtain diastereomer of tetrahydropyran ring 304 in 61% yield (Scheme 39).
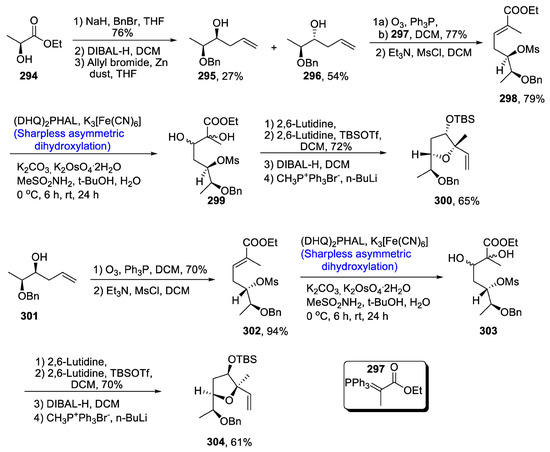
Scheme 39.
Synthesis of THF rings for caruifolin A 304.
Most of the medicinally important compounds have been isolated from marine dinoflagellates [124]. Similarly, formosalide A and B are obtained from marine dinoflagellates, and they have been found to be highly potent against cancer cells [125]. Their structure is composed of a 17-membered ring along with tetrahydropyran and tetrahydrofuran ring. Lu and his co-researchers reported the total synthesis of formosalide A in recent times. However, in 2020, Gajula et al. [126] reported the total synthesis of C1-C16 fragment of formosalide B. The synthesis began with the reduction of lactone 305 by using DIBAL-H in the presence of dichloromethane followed by Wittig olefination and treatment with mesylate chloride in the presence of trimethylamine, dimethylaminopyridine and dichloromethane, which gave mesylate ester 306 in 95% yield. Compound 306 was then subjected to Sharpless asymmetric dihydroxylation by using [(DHQD)2PHAL], potassium hexacyanoferrate (III), potassium carbonate, methane sulfonamide and osmium tetroxide to obtain tetrahyfuran-containing moiety 307 in 88% yield. Compound 307 was reacted further via several steps, leading to the synthesis of compound 308 in 90% yield. Pentane diol 309 over several steps afforded 310 in 89% yield. Compounds 308 and 310 were then reacted with lithium chloride, DIPEA and acetonitrile followed by Sharpless asymmetric dihydroxylation in the presence of [K3[Fe(CN)6], methane sulfonamide and tert-butanol to obtain diol 311 in 80% yield. Compound 311 was reacted further with PPTS and methanol to obtain target molecule 312 in 82% yield (Scheme 40).
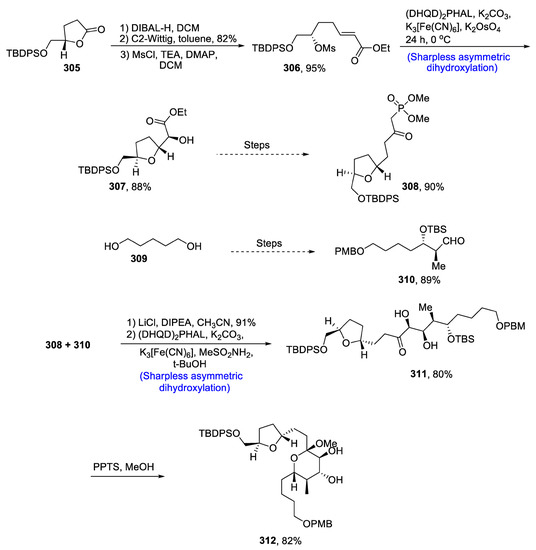
Scheme 40.
Synthesis of C1-C16 fragment of formosalide B 312.
There have been a number of reported and well-known HIV-protease inhibitor drugs, e.g., darunavir, etc. However, scientists are still trying to find more biologically active HIV-inhibitor drugs due to the threatening effects of HIV. For this purpose, Ghosh et al. [127] in 2020 reported the synthesis of tetrahydrofuran ring (THF) based aminobenzothiazole containing an HIV inhibitor by employing Sharpless asymmetric dihydroxylation as a key step. This was found to be more potent than other widely known drugs. In the first step of total synthesis, compound 313 was reduced in the presence of LiAlH4 followed by treatment with porcine pancreatic lipase to synthesize compound 314 in 82% yield. Compound 314 was then subjected to Swern oxidation followed by treatment with HC(OMe)3, Bu4NBr3 and methanol, which gave compound 315 in 84% yield. Compound 315 was then subjected to Sharpless asymmetric dihydroxylation, resulting in the mixture of diols 316 and 317, which over several steps afforded HIV-protease inhibitor drug 318 in 65% yield (Scheme 41).
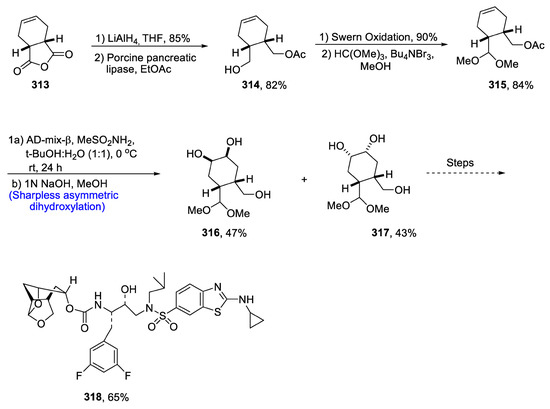
Scheme 41.
Synthesis of HIV-protease inhibitor 318.
Annonaceous acetogenins, which are obtained from the Annonaceae plant family, have gained huge importance in medicinal chemistry owing to their therapeutic nature [128]. Muconin is the leading anti-cancerous annonaceous acetogenin, whose structure consists of tetrahydropyran and tetrahydrofuran rings. Muconin plays a vital role in combating the uncontrolled cell division in human pancreatic and breast cells [129]. Considering the wide biological applications of muconin, Sugimoto et al. [130] in 2021 described its total synthesis by applying a number of reaction steps. Acrolein 319 was treated with lauryalmagnesium bromide 320, thereby providing 321 in 92% yield. Compound 321 was then treated with CH3C(OEt)2 and propionic acid, followed by reduction in the presence of lithium aluminium hydride and DIBAL-H, giving compound 322 in 68% yield. Compound 322 was then subjected to reaction with Ti(O-iPr)4 followed by Sharpless asymmetric dihydroxylation AD-mix-β in the presence of methanesulfonic acid to produce acetal 323 in 96% yield. It was then converted to epoxide 324 over six steps, in 82% yield. Compound 324 was made to react with butenyl magnesium bromide followed by cross-metathesis by using Grubb’s 2nd generation catalyst, producing compound 326 in 74% yield. Compound 326 was further allowed to react with Cl2Pd(MeCN)2, followed by its protection with MOMCl to obtain compound 327 in 96% yield. Compound 327 then underwent Sharpless asymmetric dihydroxylation, yielding vicinal diol 328. Over six steps, compound 328 was transformed to our target molecule muconin 329, in 82% yield (Scheme 42).
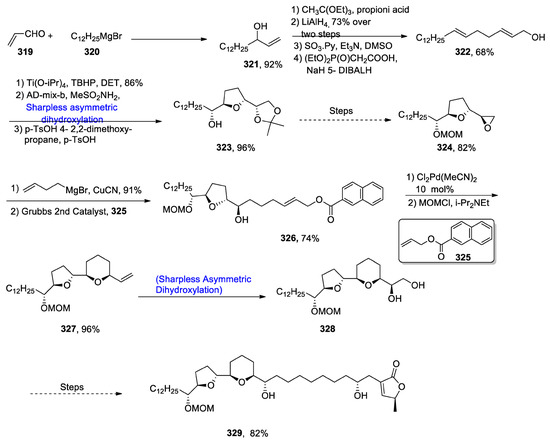
Scheme 42.
Synthesis of (+)-muconin 329.
Eribulin mesylate, also known as halaven, is a medicinal drug used in the treatment of breast cancer [131]. It is isolated from natural product halichondrin B, comprising 19 chiral centers incorporated in a complex molecule, i.e., polyether. It is indeed an arduous task to synthesize this compound composed of a long carbon chain (having 35 carbon atoms). Due to its wide application as an anti-cancerous agent, its total synthesis has been performed by different researchers using a number of strategies. Similarly, Senapati et al. [132] in 2021 reported the total synthesis of C14–C28, central rings of eribulin, by using Sharpless asymmetric dihydroxylation, gold-catalyzed alkynol cyclization and cross metathesis. Within this framework, tetrahydropyran and tetrahydrofuran rings are linked via a glycosidic bond. In their strategy, the first step involved the treatment of 330 with crotyl bromide in the presence of tin, leading to the synthesis of diastereoisomers. In the next step, a hydroxyl group was protected by benzyl group, thereby giving compound 333 in 76% yield. Alkyne moiety was introduced by employing the addition of hydrogen bromide, oxidation and Ohira–Bestmann reaction to give compound 334 in 95% yield. In this regard, allyl group was then introduced by reaction with allylbromide in the presence of Dess–Martin periodinane and sodium bicarbonate followed by treatment with OBr in the presence of methanol. Compound 334 was then subjected to gold catalyzed cyclization by utilizing AgSbF6 and Au(PPh3)Cl. It was succeeded by reduction in the presence of Et3SiH and boron triflouride diethyletherate, which resulted in the production of compound 335 in 96% yield after protection with tert-butyl silyl group. Compounds 335 and 336 were then joined through cross-metathesis followed by Sharpless asymmetric dihydroxylation in the presence of methane sulfonamide, leading to the synthesis of 337 in 71% yield. Compound 337 was then converted to our target molecule 338 in 97% yield by employing a cycloetherification strategy. Their scheme resulted in 7.2% overall yield of eribulin fragment (Scheme 43).
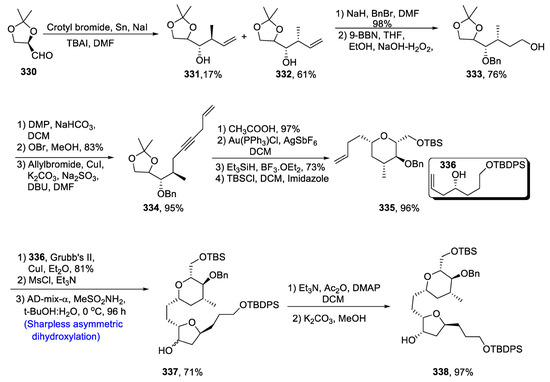
Scheme 43.
Synthesis of C14-C28 fragment of eribulin 338.
Numerous natural products that are obtained or isolated from several marine species are generally long-chained, high molecular weight molecules [133]. Obvious examples are amphidinol 3 (AM3), maitotoxin (MTX) and brevisulcenal-F (KBT-F). Amphidinol 3 (AM3) was obtained from dinoflagellate Amphidium krebsii. These are known to be involved in the destruction of red blood cells and are highly effective against various fungal diseases. Their structural formulas consist of multiple hydroxyl and alkene groups along with cyclic rings (i.e., bistetrahydropyran). In 2020, Oishi et al. [134] took a challenging job to report the total synthesis of 25 chiral centers containing amphidinol 3 for the very first time by employing Sharpless asymmetric dihydroxylation, cross-metathesis, Michael-Roush asymmetric crotylation and Mitsunobu reaction. Aldehyde-containing chain 343 was synthesized via coupling asymmetric reduction and cross-metathesis of iodoolefin 339, alkyne 340 and amide chain 341. Another fragment, (C21-C29) 346, was synthesized by joining acrolein 346 with terminal olefin 345 by utilizing a number of reactions including cross-metathesis and intramolecular-oxa Michael Roush asymmetric crotylation, hydroboration and Mitsunobu reaction. Furthermore, fragments 343 and 345 were combined through Julia–Kocienski olefination, Sharpless asymmetric dihydroxylation and Nishizawa–Grieco reaction to obtain the C1-C29 fragment of amphidinol 347. Compound 347 was then joined with C32-C52 fragment 348 and C53-C67 fragment 349 through five steps, thus finally leading to the synthesis of amphidinol 3, 350 (Scheme 44).
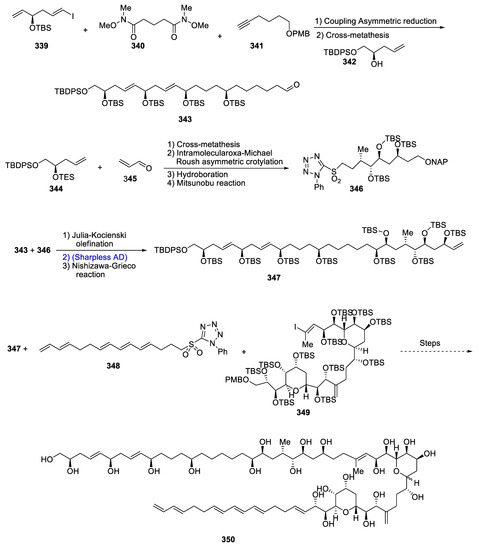
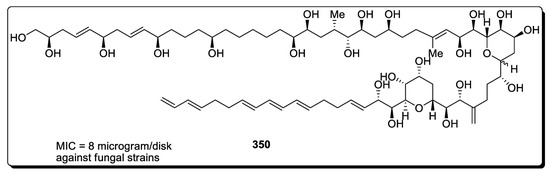
Scheme 44.
Synthesis of amphidinol 3 350.
2.10. Miscellaneous Natural Products
Alzheimer’s disease is one of the most dangerous disease of recent times. Drugs that inhibit the coagulation of amyloid-β (Aβ) and tau protein phosphorylation are considered effective in the treatment of Alzheimer’s disease, as the accumulation of these substances is a causative factor for this fatal disease [135]. Despite the utmost need for anti-Alzheimer’s drugs, there is a shortage of long-term, effective medicines against this disease. However, it has been discovered that 4,5-dihydroxypiperines, which are obtained from P. retrofractum, are effective against aluminum trichloride-induced dementia. Keeping in view the pharmacological aspects of 4,5-dihydroxypiperines, Luo et al. [136] in 2021 devised the total synthesis of their stereoisomers. For this purpose, piperine 351 was subjected to AD-mix-α and AD-mix-β one by one, thus producing 352 and 353 in 44.5% yields. Compounds 352 or 353 then underwent Mitsunobu reaction separately in the presence of NbzOH, triphenylphosphine and DEAD, thereby giving compounds 354 and 355 in 41.2 and 55% yields, respectively (Scheme 45).
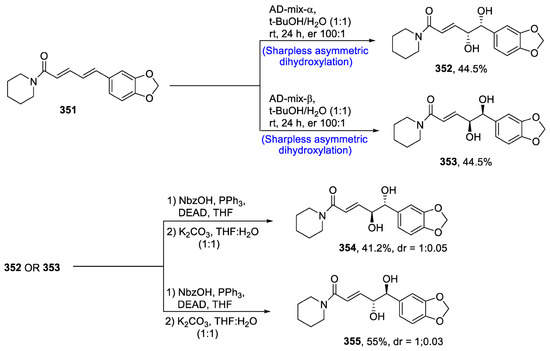
Scheme 45.
Synthesis of 4,5-dihydroxypiperines 354 and 355.
Penostatins A and C are isolated from a marine Penicillium species, i.e., Enteromorpha intestinalis [137]. These members of the penostatin family are highly effective in the treatment of cancer, as their IC50 values against protein tyrosine phosphatase 1B have been found to be 15.87 and 0.37µM, respectively. Keeping in view the biological importance of penostatins A and C, Wang et al. [138] in 2020 reported their total synthesis. Their synthetic scheme involved the use of Sharpless asymmetric dihydroxylation, Diels–Alder reaction and Ando–Horner–Wadsworth–Emmons olefination as key steps. The synthesis began with the formation of 6-alkyl-3-hydroxy-2-pyrone 357 in 17.6% yield, which was treated further with tert-butyl-di-phenyl silyl chloride followed by its reaction with Grubbs II generation catalyst and toluene to obtain a racemic mixture of endo and exo adduct 359a and 359b. These two compounds were then subjected to Sharpless asymmetric dihydroxylation to obtain a mixture of diol groups 360a and 360b in 85% yield. Compound 360b was protected via acetonide, followed by removal of silyl protecting group in the presence of HF and triethyl amine, and then subsequent reduction by using sodium borohydride and lithium hydride, affording compound 362 in 85% yield. Compound 362 was reacted via several steps, thus leading to the synthesis of penostatin A 363, which could be transformed to penostatin C 364 by dehydration in 83% yield (Scheme 46).
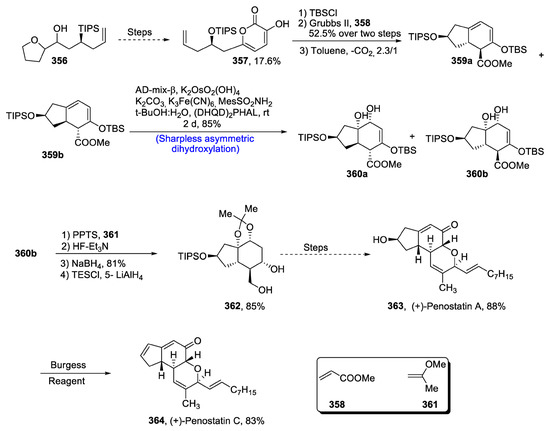
Scheme 46.
Synthesis of (+)-penostatins A 363 and C 364.
Most of the naturally occurring and medicinally important compounds contain optically active β-amino α-hydroxy acid functionality [139]. Similarly, the side chain of the widely known anti-cancer drug taxol contains chiral β-amino-α-hydroxy acid. Thus, various researchers have reported varied methodologies for its synthesis. Considering the vital importance of this moiety, Matsushima et al. [140] in 2021 reported the total synthesis of the taxol side chain. This side chain can be synthesized from demethoxy-4-epi-cytoxazone, which is a naturally occurring compound isolated from Streptomyces species. Cytoxazones are well known for their anti-cancer potential, as they act as cytokine inhibitors. Trichloroacetimidates were used for the incorporation of nitrogen functionality into the side chain of taxol. For this purpose, starting reagent 366 was introduced by Sharpless asymmetric dihydroxylation followed by its reaction with DBU and trichloroacetonitrile, and then cyclization was carried out to form oxazoline ring 367 in 74% yield. Oxazoline then was reacted further via several steps, leading to the synthesis of the target molecule, i.e., taxol side chain 368 in 39% yield (Scheme 47).
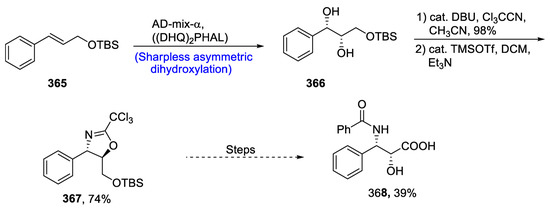
Scheme 47.
Synthesis of taxol side chain 368.
The resistance of deadly strains of TB against various drugs is of great concern nowadays [141]. It has been found that thuggacins are highly potent against various bacterial diseases including tuberculosis. The structure of thuggacins consists of a thiazole ring and α,β-unsaturated macrolide, and they are obtained from mycobacterium Sorangium cellulosum. In 2021, Tomohiro et al. [142] reported the total synthesis of medicinally important thuggacin cmc-A by employing Sharpless asymmetric dihydroxylation, Suzuki–Miyaura coupling, Sonogashira coupling and Shiina macrolactonization. The initial stage of total synthesis involved the transformation of malic acid into 370 in 75% yield. Compound 370 was then subjected to reduction followed by Swern oxidation and Wittig olefination to obtain 371 in 90% yield. Compound 371 was then treated via several steps leading to the synthesis of compound 372 in 50% yield. Optically active oxazolidinone 373 was then made to react with NaHMDS in the presence of tetrahydrofuran and toluene followed by further treatment via a known methodology to obtain compound 374 in 97% yield. Compound 374 was then subjected to Swern oxidation followed by Wittig olefination, which resulted in the synthesis of 375 in 79% yield. Next, Sharpless asymmetric dihydroxylation was carried out followed by replacement of protecting group and reduction to obtain compound 376 in 96% yield. Compound 376 was then reacted further through a number of steps to give compound 377 in 100% yield. Finally, compounds 372 and 377 were reacted over different steps, leading to the total synthesis of thuggacin cmc-A 378 in 45% yield (Scheme 48).
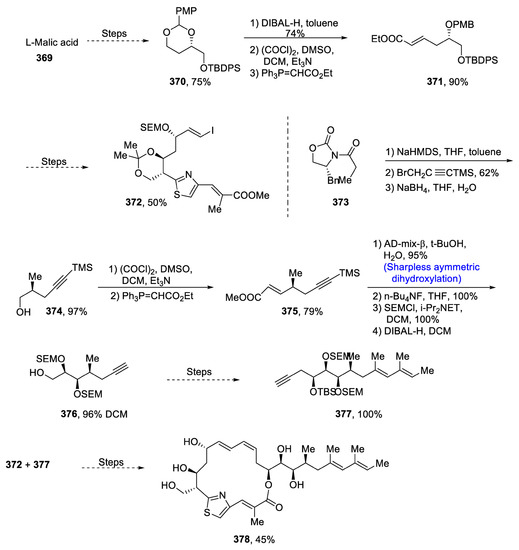
Scheme 48.
Synthesis of thuggacin cmc-A 378.
3. Conclusions
This review summarized the importance of Sharpless asymmetric dihydroxylation as a key step in the synthesis of different naturally occurring compounds. This methodology plays a significant role in the functionalization of olefins by reacting alkene with minute amounts of osmium tetroxide and potassium hexacyanoferrate (III), which is an optically active ligand. However, a high level of enantioselectivity is achieved by employing chiral nitrogenous ligands. The resulting chiral vicinal diols then act as synthetic intermediates in the synthesis of various medicinal and naturally occurring organic compounds. In this review, the use of Sharpless asymmetric dihydroxylation in the total synthesis of a wide range of natural products (alkaloids, lactones, terpenoids, amino acids, etc.), reported in recent years (since 2020) was described. We hope that our review will draw the attention of synthetic chemists and encourage them to make use of highly enantioselective Sharpless asymmetric dihydroxylation in their future efforts to synthesize biologically important natural products.
Author Contributions
Conceptualization, A.F.Z.; resources, A.F.Z., M.B., R.A., S.M.H. and A.I. data curation, A.I.; writing—original draft preparation, A.M.; writing—review and editing, A.F.Z., A.M., A.I., R.A., S.M.H., M.I., K.K.-M. and M.M.; supervision, A.F.Z.; project administration, A.F.Z. and M.M.; funding acquisition, K.K.-M. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work was partially financed from the funds of the project no. DS. 730 (Medical University of Lublin) and project no. UPH/WNSP/ICH/zadaniebadawcze/143/23/B (Siedlce University of Natural Sciences and Humanities).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is contained in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gnas, Y.; Glorius, F. Chiral auxiliaries—Principles and recent applications. Synthesis 2006, 12, 1899–1930. [Google Scholar] [CrossRef]
- Kitano, Y.; Matsumoto, T.; Sato, F. A highly efficient kinetic resolution of γ- and β-trimethylsilyl secondary allylic alcohols by the sharpless asymmetric epoxidation. Tetrahedron 1988, 44, 4073–4086. [Google Scholar] [CrossRef]
- Minato, M.; Yamamoto, K.; Tsuji, J. Osmium tetraoxide catalyzed vicinal hydroxylation of higher olefins by using hexacyanoferrate(III) ion as a cooxidant. J. Org. Chem. 1999, 55, 766–768. [Google Scholar] [CrossRef]
- Martin, V.S.; Woodard, S.S.; Katsuki, T.; Yamada, Y.; Ikeda, M.; Sharpless, K.B. Kinetic resolution of racemic allylic alcohols by enantioselective epoxidation. A route to substances of absolute enantiomeric purity? J. Am. Chem. Soc. 1981, 103, 6237–6240. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Amberg, W.; Bennani, Y.L.; Crispino, G.A.; Hartung, J.; Jeong, K.S.; Kwong, H.L.; Morikawa, K.; Wang, Z.M. The osmium-catalyzed asymmetric dihydroxylation: A new ligand class and a process improvement. J. Org. Chem. 1992, 57, 2768–2771. [Google Scholar] [CrossRef]
- Ogino, Y.; Chen, H.; Kwong, H.L.; Sharpless, K.B. On the timing of hydrolysis / reoxidation in the osmium-catalyzed asymmetric dihydroxylation of olefins using potassium ferricyanide as the reoxidant. Tetrahedron Lett. 1991, 32, 3965–3968. [Google Scholar] [CrossRef]
- Hofmann, K.A. Sauerstoff-übertragung durch Osmiumtetroxyd und Aktivierung von Chlorat-Lösungen. Ber. Dtsch. Chem. Ges. 1912, 45, 3329–3336. [Google Scholar] [CrossRef]
- Rheenen, V.V.; Kelly, R.C.; Cha, D.Y. An improved catalytic OsO4 oxidation of olefins to cis-1,2-glycols using tertiary amine oxides as the oxidant. Tetrahedron Lett. 1976, 17, 1973–1976. [Google Scholar] [CrossRef]
- Gao, Y. Osmium Tetroxide-t-Butyl Hydroperoxide. In Encyclopedia of Reagents for Organic Synthesis; John Wiley Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Mehltretter, G.M.; Döbler, C.; Sundermeier, U.; Beller, M. An improved version of the Sharpless asymmetric dihydroxylation. Tetrahedron Lett. 2000, 41, 8083–8087. [Google Scholar] [CrossRef]
- Hentges, S.G.; Sharpless, K.B. Asymmetric induction in the reaction of osmium tetroxide with olefins. J. Am. Chem. Soc. 1980, 102, 4263–4265. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Amberg, W.; Beller, M.; Chen, H.; Hartung, J.; Kawanami, Y.; Lubben, D.; Manoury, E.; Ogino, Y.; Shibata, T.; et al. New ligands double the scope of the catalytic asymmetric dihydroxylation of olefins. J. Org. Chem. 1991, 56, 4585–4588. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hajiabbasi, P. Recent advances in C-heteroatom bond forming by asymmetric Michael addition. Mol. Divers. 2014, 18, 411–439. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Asadi, S.; Lashkariani, B.M. Recent progress in asymmetric Biginelli reaction. Mol. Divers. 2013, 17, 389–407. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Recent advances in the application of the Oppolzer camphorsultam as a chiral auxiliary. Tetrahedron Asymmetry 2014, 25, 1061–1090. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hashemi, E. Recent applications of the Suzuki reaction in total synthesis. Tetrahedron 2012, 68, 9145–9178. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hashemi, E.; Azimian, F. Recent developments of the Stille reaction as a revolutionized method in total synthesis. Tetrahedron 2014, 70, 7–21. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hashemi, E.; Nzari, N. Negishi coupling: An easy progress for C–C bond construction in total synthesis. Mol. Divers. 2014, 18, 441–472. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hashemi, E.; Ghobadi, N. Development of Recent Total Syntheses Based on the Heck Reaction. Curr. Org. Syn. 2013, 17, 2192–2224. [Google Scholar] [CrossRef]
- Heravi, M.M.; Lashaki, T.B.; Poorahmad, N. Applications of Sharpless asymmetric epoxidation in total synthesis. Tetrahedron Asymmetry 2015, 26, 405–495. [Google Scholar] [CrossRef]
- Engler, E.M.; Andose, J.D.; Schleyer, V.R. Critical evaluation of molecular mechanics. J. Am. Chem. Soc. 1973, 95, 8005–8025. [Google Scholar] [CrossRef]
- Blagg, B.S.J.; Boger, D.L. Total synthesis of (+)-camptothecin. Tetrahedron 2002, 58, 6343–6349. [Google Scholar] [CrossRef]
- Reddy, J.S.; Rao, B.V. A Short, Efficient, and Stereoselective Total Synthesis of a Pyrrolidine Alkaloid: (−)-Codonopsinine. J. Org. Chem. 2007, 72, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Ramulu, U.; Rajaram, S.; Ramesh, D.; Babu, K.S. Total synthesis of synargentolide B. Tetrahedron Asymmetry 2015, 26, 928–934. [Google Scholar] [CrossRef]
- Das, T.; Mahapatra, T.; Nanda, S. Total synthesis of stagonolide B. Tetrahedron Lett. 2012, 53, 1186–1189. [Google Scholar] [CrossRef]
- Ghosal, S.; Saini, K.S.; Razdan, S. Crinum alkaloids: Their chemistry and biology. Phytochemistry 1985, 24, 2141–2156. [Google Scholar] [CrossRef]
- Pettit, G.; Orr, B.; Ducki, S. Anticancer, pancratistatin, phosphate prodrugs, phosphorylation. Anti-Cancer Drug Design 2000, 15, 389–395. Available online: https://www.ingentaconnect.com/contentone/cog/antcan/2000/00000015/00000006/art00001 (accessed on 12 March 2022). [PubMed]
- Zhao, Y.; Zhu, Y.; Ma, G.; Wei, Q.; Yang, S.; Zeng, X.; Zhang, H.; Chen, J. Short, Enantioselective, gram-scale synthesis of (−)-zephyranthine. Chem. Sci. 2021, 12, 9452–9457. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Menna, M.; Cai, Y.S.; Guo, Y.W. Polyacetylenes of Marine Origin: Chemistry and Bioactivity. Chem. Rev. 2015, 115, 1543–1596. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.; Kneuer, R. Synthesis of enantiopure (2R)-configured muscarine alkaloids via selective alkoxyl radical ring-closure reactions. Tetrahedron Asymmetry 2003, 14, 3019–3031. [Google Scholar] [CrossRef]
- Gehlawat, A.; Prakash, R.; Pandey, S.K. A Short and Efficient Enantioselective Synthesis of (+)-(2S,3S,5S)-epi-Muscarine. Chem. Sel. 2020, 5, 6373–6375. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.J.; Zhang, D.B.; Wen, J.; Zhao, X.W.; Li, Y.; Gao, K. An unusual indole alkaloid with anti-adenovirus and anti-HSV activities from Alstonia scholaris. Tetrahedron Lett. 2014, 55, 1815–1817. [Google Scholar] [CrossRef]
- Cai, Y.S.; Sarotti, A.M.; Zhou, T.L.; Huang, R.; Qiu, G.F.; Tian, C.K.; Miao, Z.H.; Mandi, A.; Kurtan, T.; Cao, S.G.; et al. Flabellipparicine, a flabelliformide-apparicine-type bisindol alkaloid from Tabernaemontana divaricate. J. Nat. Prod. 2018, 81, 1976–1983. [Google Scholar] [CrossRef]
- Pourroy, B.; Honore, S.; Pasquier, E.; Rey, B.V.; Kruczynski, A.; Briand, C.; Braguer, D. Antiangiogenic concentrations of vinflunine increase the interphase microtubule dynamics and decrease the motility of endothelial cells. Cancer Res. 2006, 66, 3256–3263. [Google Scholar] [CrossRef]
- Zhang, J.; Song, M.; Ao, Y.L.; Li, Y.; Zou, X.Y.; Xu, J.; Wang, Y.; Zhang, D.M.; Zhang, X.Q.; Ye, W.C. Alstolarines A and B, Two Unusual Monoterpenoid Indole Alkaloids with Acetal Moiety from Alstonia scholaris. Org. Chem. Front. 2020, 7, 3468–3473. [Google Scholar] [CrossRef]
- Gossan, D.P.A.; Alabdul Magid, A.; Yao, P.A.K.; Behr, J.B.; Ahibo, A.C.; Djakouré, L.A.; Harakat, D.; Nazabadioko, L.V. Glycosidase inhibitors from the roots of Glyphaea brevis. Phytochemistry 2015, 109, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Shibano, M.; Tsukamoto, D.; Kusano, G. Polyhydroxylated Alkaloids with Lipophilic Moieties as Glycosidase Inhibitors from Higher Plants. Heterocycles 2002, 57, 1539–1553. Available online: https://cir.nii.ac.jp/crid/1521417755858432768 (accessed on 12 March 2022).
- Byatt, B.J.; Kato, A.; Pyne, S.G. Synthesis and Structural Revision of Glyphaeaside C. Org. Lett. 2021, 23, 4029–4033. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, R.; Covell, D.; Ransom, T.T.; Gustafson, K.R.; Beutler, J.A. Englerin A, a Selective Inhibitor of Renal Cancer Cell Growth, from Phyllanthus engleri. Org. Lett. 2009, 11, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Sourbier, C.; Scroggins, B.T.; Ratnayake, R.; Prince, T.L.; Lee, S.; Lee, M.J.; Nagy, P.L.; Lee, Y.H.; Trepel, J.B.; Beutler, J.A.; et al. Englerin A Stimulates PKCθ to Inhibit Insulin Signaling and to Simultaneously Activate HSF1: Pharmacologically Induced Synthetic Lethality. Cancer Cell 2013, 23, 228–237. [Google Scholar] [CrossRef]
- Mou, S.B.; Xiao, W.; Wang, H.Q.; Wang, S.J.; Xiang, Z. Syntheses of Epoxyguaiane Sesquiterpenes (−)-Englerin A, (−)-Oxyphyllol, (+)-Orientalol E, and (+)-Orientalol F: A Synthetic Biology Approach. Org. Lett. 2020, 22, 1976–1979. [Google Scholar] [CrossRef]
- Vichnewski, W.; Sarti, S.J.; Gilbert, B.; Herz, W. Goyazensolide, a Schistosomicidal Heliangolide from Eremanthus-Goyazensis. Phytochemistry 1976, 15, 191–193. [Google Scholar] [CrossRef]
- Acuna, U.M.; Shen, Q.; Ren, Y.; Lantvit, D.D.; Wittwer, J.A.; Kinghorn, A.D.; Swanson, S.M.; Carcach de Blanco, E.J. Goyazensolide Induces Apoptosis in Cancer Cells in vitro and in vivo. Int. J. Cancer Res. 2013, 9, 36–53. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4303185/ (accessed on 12 March 2022). [CrossRef] [PubMed]
- Liu, W.; Patouret, R.; Barleunga, S.; Plank, M.; Loewith, R.; Winssinger, N. Identification of a Covalent Importin-5 Inhibitor, Goyazensolide, from a Collective Synthesis of Furanoheliangolides. ACS Cent. Sci. 2021, 7, 954–962. [Google Scholar] [CrossRef]
- Maurer, B.; Grieder, A. Sesquiterpenoids from Costus Root Oil (Saussurea lappa CLARKE). Helvetica 1977, 60, 2177–2190. [Google Scholar] [CrossRef]
- Seiichi, T.; Takumichi, S.; Kiyohiro, S.; Masashi, A.; Kunio, O. Enantiodivergent Route to the Aromatic Bisabolane Sesquiterpenes via a Chiral Acetylene Alcohol. Chem. Lett. 1989, 18, 1781–1784. [Google Scholar] [CrossRef]
- Yajima, A.; Shirakawa, I.; Shiotani, N.; Ueda, K.; Murakawa, H.; Saito, T.; Katsuta, R.; Ishigami, K. Practical synthesis of aromatic bisabolanes: Synthesis of 1,3,5-bisabolatrien-7-ol, peniciaculin A and B, and hydroxysydonic acid. Tetrahedron 2021, 92, 132253. [Google Scholar] [CrossRef]
- Ushakov, D.B.; Navickas, V.; Strobele, M.; Mossmer, C.M.; Sasse, F.; Maier, M.E. Total Synthesis and Biological Evaluation of (−)-9-Deoxy-englerin A. Org. Lett. 2011, 13, 2090–2093. [Google Scholar] [CrossRef]
- Peng, G.P.; Tian, G.; Huang, X.F.; Lou, F.C. Guaiane-type sesquiterpenoids from Alisma orientalis. Phytochemistry 2003, 63, 877–881. [Google Scholar] [CrossRef]
- Palli, K.K.; Anugu, R.R.; Chandrasekhar, S. Total Synthesis of (−)-4- epi -Englerin A. Eur. J. Chem. 2021, 22, 31990–33196. [Google Scholar] [CrossRef]
- Alvarado, R.B.H.; Mazon, A.M.; Ortega, A.; Mayorga, K.M. DARK Classics in Chemical Neuroscience: Salvinorin A. ACS Chem. Neurosci. 2020, 11, 3979–3992. [Google Scholar] [CrossRef]
- Zimdars, P.; Wang, Y.; Metz, P. A Protecting-Group-Free Synthesis of (-)Salvinorin A. Chem. A Eur. J. 2021, 27, 7968–7973. [Google Scholar] [CrossRef]
- Xiao, W.L.; Li, R.T.; Huang, S.X.; Pu, J.X.; Sun, H.D. Triterpenoids from the Schisandraceae family. Nat. Prod. Rep. 2008, 25, 871–891. [Google Scholar] [CrossRef]
- Li, X.; Cheong, P.H.Y.; Carter, R.G. Schinortriterpenoids: A case study in synthetic design. Angew. Chem. Int. Ed. 2017, 56, 1704–1718. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; He, X.; Gui, J. Bioinspired Synthesis of Nortriterpenoid Propindilactone G. J. Am. Chem. Soc. 2020, 142, 5007–5012. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.G.D.; Fo, S.R. Meroterpenes from Penicillium sp found in association with Melia azedarach. Phytochemistry 2002, 61, 907–912. [Google Scholar] [CrossRef]
- Guo, C.J.; Knox, B.P.; Chiang, Y.M.; Lo, H.C.; Sanchez, J.F.; Lee, K.H.; Okaley, B.R.; Bruno, K.S.; Wang, C.C.C. Molecular Genetic Characterization of a Cluster in A. terreus for Biosynthesis of the Meroterpenoid Terretonin. Org. Lett. 2012, 14, 5684–5687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, Y.; Franzoni, I.; Guo, C.; Jia, H.; Hong, B.; Li, H. Enantioselective Total Synthesis of Berkeleyone A and Preaustinoids. Angew. Chem. Int. Ed. 2021, 60, 14869–14874. [Google Scholar] [CrossRef]
- She, G.; Xu, C.; Liu, B.; Shi, R. Two new monoterpenes from Mentha haplocalyx Briq. Helv. Chim. Acta 2010, 93, 2495–2498. [Google Scholar] [CrossRef]
- Konishi, S.; Mori, N.; Takikawa, H. Synthesis guided structure revision of the monoterpene alcohol isolated from Mentha haplocalyx. Biosci. Biotechnol. Biochem. 2019, 83, 391–399. [Google Scholar] [CrossRef]
- Konishi, S.; Ogura, Y.; Takikawa, H.; Watanabe, H. Asymmetric synthesis of trans-p-menth-3-ene-1,2,8-triol, the monoterpene isolated from herbal plants. Biosci. Biotechnol. Biochem. 2020, 84, 37–42. [Google Scholar] [CrossRef]
- Yan, C.; Wan, R.; Shi, Y. Molecular Mechanisms of pre-mRNA Splicing through Structural Biology of the Spliceosome. Cold Spring Harb. Perspect. Biol. 2019, 14, a032409. Available online: https://cshperspectives.cshlp.org/content/11/1/a032409.short (accessed on 7 April 2022). [CrossRef]
- Sakai, T.; Sameshima, T.; Matsufuji, M.; Kawamura, N.; Dobashi, K.; Mizui, Y. Pladienolides, New Substances from Culture of Streptomyces Platensis Mer-11107. I. Taxonomy, Fermentation, Isolation and Screening. J. Antibiot. 2004, 57, 173–179. [Google Scholar] [CrossRef]
- Rhoades, D.; Rheingold, A.L.; O’Malley, B.W.; Wang, J. Expedient Total Syntheses of Pladienolide-Derived Spliceosome Modulators. J. Am. Chem. Soc. 2021, 143, 4915–4920. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Kim, Y.; In, Y.; Ishida, T.; Kan, Y.; Fujita, T.; Iwashita, T.; Tabata, H.; Onaka, H.; Furumai, T. Alchivemycin A, a Bioactive Polycyclic Polyketide with an Unprecedented Skeleton from Streptomyces sp. Org. Lett. 2010, 12, 3402–3405. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liao, D.; Tian, X.; Lei, X. Access to the 2H-Tetrahydro-4,6-dioxo-1,2-oxazine Ring System from Nitrone via a Tandem Nucleophilic Addition and Transesterification Reaction. Org. Lett. 2016, 18, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Yang, S.; Ma, K.; Wang, X.; Lei, X. Stereoselective synthesis of the C16–C25 fragment of alchivemycins A and B. Tetrahedron Lett. 2021, 74, 153156. [Google Scholar] [CrossRef]
- Trost, B.M.; Knopf, J.D.; Brindle, C.S. Synthetic Strategies Employed for the Construction of Fostriecin and Related Natural Products. Chem. Rev. 2016, 116, 15035–15088. [Google Scholar] [CrossRef]
- Just, G.; O’Connor, B. Synthesis of the 5R,8R,9S,11R Dephosphorylated Derivative of CI-920, a Novel Antitumor Agent. Tetrahedron Lett. 1988, 29, 753–756. [Google Scholar] [CrossRef]
- Dong, G.; Li, B.; O’Doherty, G. Total and Formal Syntheses of Fostriecin. Org. Chem. Front. 2020, 7, 3608–3615. [Google Scholar] [CrossRef]
- Seibert, S.F.; Krick, A.; Eguereva, E.; Kehraus, S.; Konig, G.M. Ascospiroketals A and B, Unprecedented Cycloethers from the Marine-Derived Fungus Ascochyta salicorniae. Org. Lett. 2007, 9, 239–242. [Google Scholar] [CrossRef]
- Hara, Y.; Kamaike, K.; Ota, K.; Miyaoka, H. Total Synthesis of Ascospiroketal B. Synlett 2020, 31, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Baran, P.S. Total Synthesis of Eudesmane Terpenes by Site-Selective C−H Oxidations. Nature 2009, 459, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Wilde, N.C.; Isomura, M.; Mendoza, A.; Baran, P.S. Two-Phase Synthesis of (−)-Taxuyunnanine D. J. Am. Chem. Soc. 2014, 136, 4909–4912. [Google Scholar] [CrossRef] [PubMed]
- Gayraud, O.; Laroche, B.; Casaretto, N.; Nay, B. Synthesis of a Biomimetic Tetracyclic Precursor of Aspochalasins and Formal Synthesis of Trichoderone A. Org. Lett. 2021, 23, 5755–5760. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Minamida, M.; Akakabe, M.; Tsuda, M.; Konishi, Y.; Tominaga, A.; Tsuda, M.; Fukushi, E.; Kawabata, J. Amphirionin-2, a Novel Linear Polyketide with Potent Cytotoxic Activity from a Marine Dinoflagellate Amphidinium Species. Bioorg. Med. Chem. Lett. 2015, 25, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Mizukami, D.; Sugai, T.; Tsuda, M.; Fuwa, H. Total Synthesis and Complete Configurational Assignment of Amphirionin-2. Chem. Sci. 2021, 12, 872–879. [Google Scholar] [CrossRef]
- Saha, D.; Mandal, G.H.; Goswami, R.K. Asymmetric Total Synthesis of Amphirionin-2. J. Org. Chem. 2021, 86, 10006–10022. [Google Scholar] [CrossRef]
- Wilton, J.H.; Cheney, D.C.; Hokanson, G.C.; French, J.C.; Cunheng, H.; Clardy, J. A new dihydrobenz[a]anthraquinone antitumor antibiotic (PD 116,740). J. Org. Chem. 1985, 50, 3936–3938. [Google Scholar] [CrossRef]
- Rohr, J.; Thiericke, R. Angucycline Group Antibiotics. Nat. Prod. Rep. 1992, 9, 103–137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xie, T.; He, H.; Gao, S. Asymmetric Total Synthesis of PD-116740. Org. Lett. 2021, 23, 469–473. [Google Scholar] [CrossRef]
- Trost, B.M.; Wang, Y.; Buckl, A.K.; Huang, Z.; Nguyen, M.H.; Kuzmina, O. Total synthesis of bryostatin 3. Science 2020, 368, 1007–1011. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Kingston, D.G.I. Zampanolide and dactylolide: Cytotoxic tubulin-assembly agents and promising anticancer leads. Nat. Prod. Rep. 2014, 31, 1202–1226. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, Z.; Zhang, Q.; Wang, G.; Chen, Q.H. New Zampanolide Mimics: Design, Synthesis, and Antiproliferative Evaluation. Molecules 2020, 25, 362. [Google Scholar] [CrossRef]
- Carreira, E.M.; Pfaff, P. Total Synthesis of (+)-Formosalides A and B. Synfacts 2020, 16, 1387. [Google Scholar] [CrossRef]
- Sekiguchi, J.; Kuroda, H.; Yamada, Y.; Okada, H. Structure of patulolide a, a new macrolide from penicillium urticae mutants. Tetrahedron Lett. 1985, 26, 2341–2342. [Google Scholar] [CrossRef]
- Rodpha, D.; Sekiguchi, J.; Yamada, Y. New macrolides from Penicillium Urticae mutant S11R59. J. Antibiot. 1986, 39, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Paratapareddy, B.; Sreenevasulu, R.; Venkata, M.; Rao, B.; Raju, R.R. Stereoselective total synthesis of Patulolide C. Nat. Prod. Res. 2020, 34, 2760–2764. [Google Scholar] [CrossRef]
- Khalil, M.W.; Sasse, F.; Lunsdorf, H.; Elnakady, Y.A.; Reichenbach, H. Mechanism of Action of Tubulysin, an Antimitotic Peptide from Myxobacteria. Chembiochem 2006, 7, 678–683. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Erande, R.D.; Yin, J.; Vourloumis, D.; Aujay, M.; Sandoval, J.; Munneke, S.; Gavrilyuk, J. Improved Total Synthesis of Tubulysins and Design, Synthesis, and Biological Evaluation of New Tubulysins with Highly Potent Cytotoxicities against Cancer Cells as Potential Payloads for Antibody-Drug Conjugates. J. Am. Chem. Soc. 2018, 140, 3690–3711. [Google Scholar] [CrossRef]
- Reddy, R.B.; Krishnan, M.A.; Chelvam, V. Synthesis of tubuvaline (Tuv) fragment of tubulysin via diastereoselective diydroxylation of homoallylamine. Syn. Commun. 2021, 51, 797–809. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Rommert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Paul, D.; Goswami, R.K. Cyclodepsipeptide Alveolaride C: Total Synthesis and Structural Assignment. Chem. Sci. 2020, 11, 11259–11265. [Google Scholar] [CrossRef] [PubMed]
- Govindachari, T.R.; Kumari, K.G.N.; Suresh, G. Triterpenoids from Walsura piscidia. Phytochemistry 1995, 39, 167–170. [Google Scholar] [CrossRef]
- Ramana, L.V.; Apparao, K.M.C.; Rao, B.N.; Rao, S.A. Isolation & First Total Asymmetric Synthesis of New Phenolic Constituent: Isolation From Walsura Trifoliata. Eur. J. Pharm. Med. Res. 2021, 8, 616–621. [Google Scholar]
- Rinaldo, D.; Batista, J.M., Jr.; Rodrigues, J.; Benfatti, A.C.; Rodrigues, C.M.; Santos, L.C.D.; Furlan, M.; Vilegas, W. Determination of catechin diastereomers from the leaves of Byrsonima species using chiral HPLC-PAD-CD. Chirality 2010, 22, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chung, S.; Song, M.-Y.; Lim, S.; Shin, H.; Hur, J.; Kwon, H.; Suh, Y.-G.; Kim, E.-H.; Shin, D.; et al. Efficient and Divergent Enantioselective Syntheses of DHPVs and Anti-Inflammatory Effect on IEC-6 Cells. Molecules 2020, 25, 2215. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Shaffer, C.V.; Cai, S.X.; Peng, J.N.; Robles, A.J.; Hartley, R.M.; Powell, D.R.; Du, L.; Cichewicz, R.H.; Mooberry, S.L. Texas native plants yield compounds with cytotoxic activities against prostate cancer cells. J. Nat. Prod. 2016, 79, 531–540. [Google Scholar] [CrossRef]
- Tian, T.; Zhu, Z.; Ding, Y.; Li, G.; Li, N.; Shen, T. Synthesis and Cytotoxic Evaluation of Sanjoseolide and Representative Analogues. ACS Omega 2020, 5, 33478–33483. [Google Scholar] [CrossRef]
- Hikino, H.; Taguchi, T.; Fujimura, H.; Hiramatsu, Y. Antiinflammatory Principles of Caesalpinia Sappan Wood and of Haematoxylon Campechianum Wood. Plant Med. 1977, 31, 214–220. [Google Scholar] [CrossRef]
- Huang, S.; Ou, W.; Li, W.; Xiao, H.; Pang, Y.; Zhou, Y.; Wang, X.; Yang, X.; Wang, L. A total synthesis of (+)-brazilin. Tetrahedron Lett. 2020, 61, 152052. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Jo, Y.H.; Lee, K.Y.; Do, S.G.; Hwang, B.Y.; Lee, M.K. Optimization of pancreatic lipase inhibition by Cudrania tricuspidata fruits using response surface methodology. Bioorg. Med. Chem. Lett. 2014, 24, 2329–2333. [Google Scholar] [CrossRef]
- Lu, Q.; Harmalkar, D.S.; Quan, G.; Kwon, H.; Cho, J.; Choi, Y.; Lee, D.; Lee, K. Total Synthesis of the Neuroprotective Agent Cudraisoflavone J. J. Nat. Prod. 2021, 84, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Brase, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and Biology of Mycotoxins and Related Fungal Metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Zheng, C.; Chen, K.; He, H.; Gao, S. Asymmetric Total Synthesis of FD-594. Angew. Chem. Int. Ed. 2020, 132, 4390–4394. [Google Scholar] [CrossRef]
- Roman, G.N.; Jansen, N.B.; Hsiao, H.Y.; Tsao, G.T. Kinetic studies of the enzymatic isomerization of xylose. Enzym. Microb. Technol. 1985, 7, 129–133. [Google Scholar] [CrossRef]
- Kalagara, S.; Orozco, G.; Mito, S. The efficient synthesis of D-xylulose and formal synthesis of Syringolide 1. Tetrahedron Lett. 2020, 61, 152321. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R. Iminosugars: From Synthesis to Therapeutic Applications; Wiley: Chichester, UK, 2007. [Google Scholar] [CrossRef]
- Stocker, B.L.; Dangerfield, E.; Win-Mason, A.L.; Haslett, G.W.; Timmer, S.M. Recent Developments in the Synthesis of Pyrrolidine-Containing Iminosugars. Eur. J. Org. Chem. 2010, 9, 1615–1637. [Google Scholar] [CrossRef]
- Angelis, M.D.; Primitivo, L.; Lucarini, C.; Agostinelli, S.; Sappino, C.; Ricelli, A.; Righi, G. Stereocontrolled total synthesis of iminosugar 1,4-dideoxy-1,4-imino-D-iditol. Carbohydr. Res. 2020, 492, 108028. [Google Scholar] [CrossRef]
- Tatsuta, K.; Hosokawa, S. Total Synthesis of Selected Bioactive Natural Products: Illustration of Strategy and Design. Chem. Rev. 2005, 105, 4707–4729. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.; Hosokawa, S. Stereoselective Total Synthesis of the Dimeric Naphthoquinonopyrano-γ-lactone (−)-Crisamicin A: Introducing the Dimerization Site by a Late-Stage Hartwig Borylation. Org. Lett. 2020, 22, 3607–3612. [Google Scholar] [CrossRef]
- Dong, L.; Gordon, V.A.; Grange, R.L.; Johns, J.; Parsons, P.G.; Porzelle, A.; Reddell, P.; Schill, H.; Williams, C.M. Structure and Absolute Stereochemistry of the Anticancer Agent EBC-23 from the Australian Rainforest. J. Am. Chem. Soc. 2008, 130, 15262–15263. [Google Scholar] [CrossRef]
- Dong, L.; Schill, H.; Grange, R.L.; Porzelle, A.; Johns, J.P.; Parsons, P.G.; Gordon, V.A.; Reddell, P.W.; Williams, C.M. Anticancer Agents from the Australian Tropical Rainforest: Spiroacetals EBC-23, 24, 25, 72, 73, 75 and 76. Chem. A Eur. J. 2009, 15, 11307–11318. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Hsu, C.S. Enantioselective Total Synthesis of (+)-EBC-23, a Potent Anticancer Agent from the Australian Rainforest. J. Org. Chem. 2021, 86, 6351–6360. [Google Scholar] [CrossRef]
- Rao, N.N.; Meshram, H.M. Protection-free, short, and stereoselective synthesis of ieodomycin A and B. Tetrahedron Lett. 2013, 54, 4544–4546. [Google Scholar] [CrossRef]
- Choi, D.B.; Choi, H.; Lee, J.; Lee, Y.J.; Lee, H.S.; Joo, J.M.; Lee, J.S. Eantioselective Total Synthesis of (+)-Ieodomycin A, (+)-Ieodomycin B, and Their Three Stereoisomers. Org. Bio. Chem. 2020, 18, 9227–9230. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Jiang, M.M.; Feng, Y.F.; Gao, H.; Zhang, X.; Tang, J.S.; Yao, X.S. Three new bis-styryllactones from Goniothalamus cheliensis. Fitoterapia 2014, 82, 524–527. [Google Scholar] [CrossRef]
- Kovacevic, I.; Kesic, J.; Popsavin, M.; Francuz, J.; Kojic, V.; Jakimov, D.; Rodic, M.V.; Zelenovic, B.S.; Benedekovic, G.; Popsavin, V. Asymmetric synthesis and biological evaluation of (+)-cardiobutanolide, (−)-3-deoxycardiobutanolide and analogues as antiproliferative agents. Tetrahedron 2021, 97, 132408. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Bethi, V. A Concise Synthesis of the Key Tetrahydrofuran Moieties of Caruifolin A and EBC-342. Eur. J. Org. Chem. 2020, 2020, 6922–6928. [Google Scholar] [CrossRef]
- Kobayashi, J. Search for New Bioactive Marine Natural Products and Application to Drug Development. Chem. Pharm. Bull. 2016, 64, 1079–1083. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tsuda, M. Amphidinolides, bioactive macrolides from symbiotic marine dinoflagellates. Nat. Prod. Rep. 2004, 21, 77–93. [Google Scholar] [CrossRef]
- Gajula, S.; Reddy, A.V.V.; Reddy, D.P.; Yadav, J.S.; Mohapatra, D.K. Stereoselective Synthesis of the C1–C16 Fragment of the Purported Structure of Formosalide B. ACS Omega 2020, 5, 10217–10224. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Kovela, S.; Osswald, H.L.; Amano, M.; Aoki, M.; Agniswamy, J.; Wang, Y.F.; Weber, I.T.; Mitsuya, H. Synthesis of an HIV-1 Protease Inhibitor. J. Med. Chem. 2020, 63, 4867–4879. [Google Scholar] [CrossRef]
- Alali, F.Q.; Liu, X.-X.; McLaughlin, J.L. Annonaceous Aceotgenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef]
- Shi, G.; Kozlowski, J.F.; Schwedler, J.T.; Wood, K.V.; MacDougal, J.M.; MacLaughlin, J.L. Muconin and Mucoxin: Additional Nonclassical Bioactive Acetogenins from Rollinia mucosa. J. Org. Chem. 1996, 61, 7988–7989. [Google Scholar] [CrossRef]
- Sugimoto, M.; Nosho, S.; Hikosaka, G.; Hattori, Y.; Umezawa, K.; Kawamura, A.; Makabe, H. Synthesis of (+)-Muconin via Diastereoselective Oxypalladation. J. Org. Chem. 2021, 86, 4859–4866. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Seletsky, B.M.; Palme, M.H.; Lydon, P.J.; Singer, L.A.; Chage, C.E.; Lemelin, C.A.; Shen, Y.; Davis, H.; Tremblay, L.; et al. Macrocyclic ketone analogues of halichondrin B. Bioorg. Med. Chem. Lett. 2004, 14, 5551–5554. [Google Scholar] [CrossRef]
- Senapati, S.; Ramana, C.V. A Concise/catalytic approach for the construction of the C14–C28 fragment of eribulin. Org. Biomol. Chem. 2021, 19, 4542–4550. [Google Scholar] [CrossRef]
- Shimizu, Y. Microalgal metabolites. Chem. Rev. 1993, 93, 1685–1698. [Google Scholar] [CrossRef]
- Oishi, T. Structure Determination, Chemical Synthesis, and Evaluation of Biological Activity of Super Carbon Chain Natural Products. Bull. Chem. Soc. Jpn. 2020, 93, 1350–1360. [Google Scholar] [CrossRef]
- Becker, H.; Soler, M.A.; Sharpless, K.B. Selective Asymmetric Dihydroxylation of Polyenes. Tetrahedron 1995, 51, 1345–1376. [Google Scholar] [CrossRef]
- Luo, J.; Xiang, J.Y.; Yuan, H.Y.; Wu, J.Q.; Li, H.Z.; Shen, Y.H.; Xu, M. Isolation, synthesis and absolute configuration of 4,5-dihydroxypiperines improving behavioral disorder in AlCl3-induced dementia. Bioorg. Med. Chem. Lett. 2021, 42, 128057. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Numata, A.; Yamada, T.; Minoura, K.; Enomoto, S.; Konishi, K.; Nakai, M.; Matsuda, C.; Nomoto, K. Penostatins, novel cytotoxic metabolites from a Penicillium species separated from a green alga. Tetrahedron Lett. 1996, 37, 655–658. [Google Scholar] [CrossRef]
- Wang, J.; Cadena, M.A.M.; Tong, R. Asymmetric Total Syntheses of (+)-Penostatins A and C. Org. Lett. 2020, 22, 5074–5078. [Google Scholar] [CrossRef]
- Liu, M.; Sibi, F. Recent advances in the stereoselective synthesis of β-amino acids. Tetrahedron 2002, 58, 7991–8035. [Google Scholar] [CrossRef]
- Matsushima, Y.; Orita, M. Concise synthesis of the Taxol side chain and demethoxy-4-epicytoxazone via oxazoline formation through intramolecular benzylic substitution of a bis-trichloroacetimidate. Tetrahedron Lett. 2021, 73, 153095. [Google Scholar] [CrossRef]
- McKinney, J.D. In vivo veritas: The search for TB drug targets goes live. Nat. Med. 2000, 6, 1330–1333. Available online: https://www.nature.com/articles/nm1200_1330 (accessed on 7 April 2022). [CrossRef] [PubMed]
- Tsutsumi, T.; Matsumoto, M.; Iwasaki, H.; Tomisawa, K.; Komine, K.; Fukuda, H.; Eustache, J.; Jansen, R.; Hatakeyama, S.; Ishihara, J. Total Synthesis of Thuggacin cmc-A and Its Structure Determination. Org. Lett. 2021, 23, 5208–5212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).