Evaluation of Biological Activity of New 1,2,4-Triazole Derivatives Containing Propionic Acid Moiety
Abstract
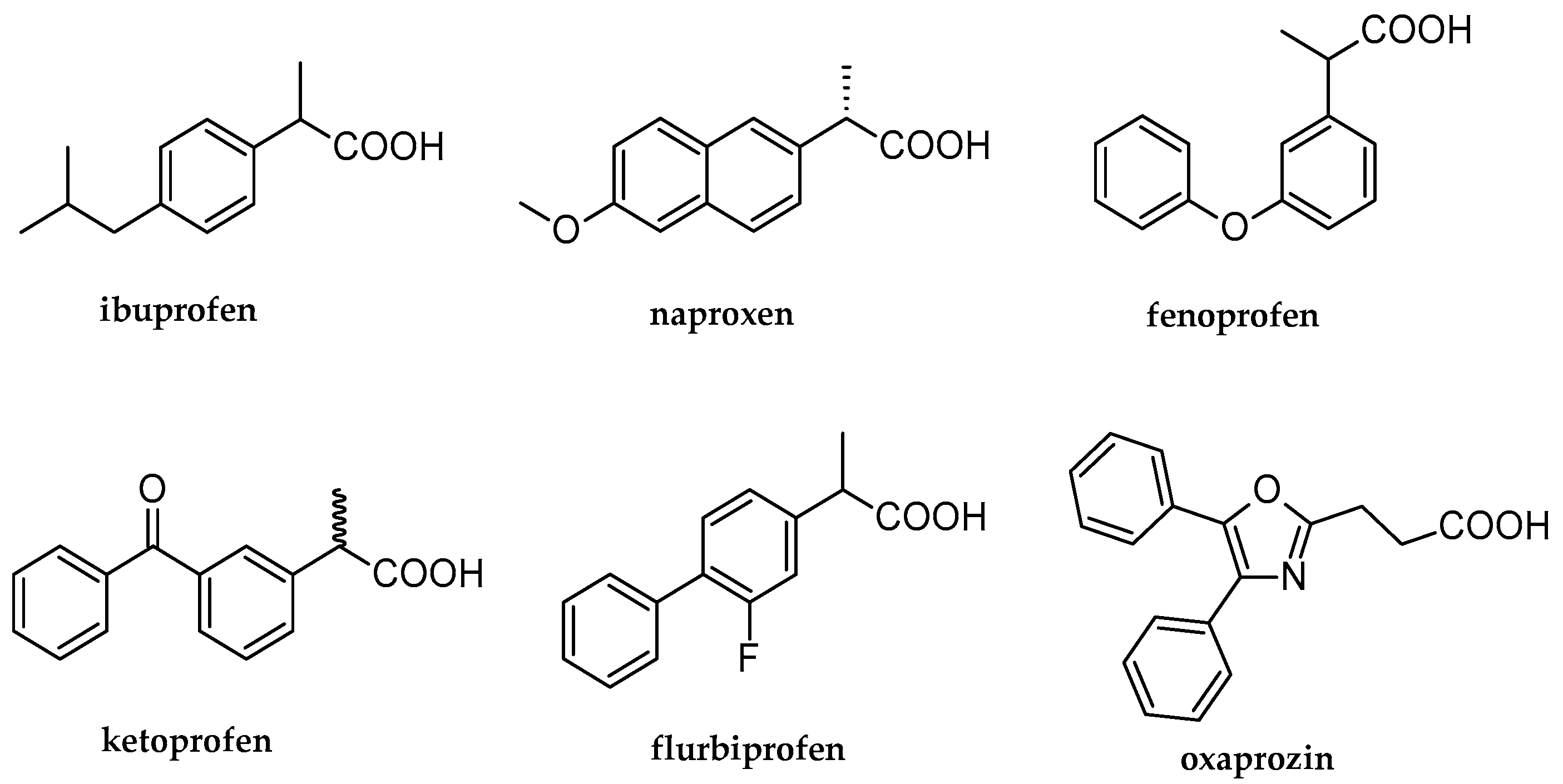
1. Introduction
2. Results
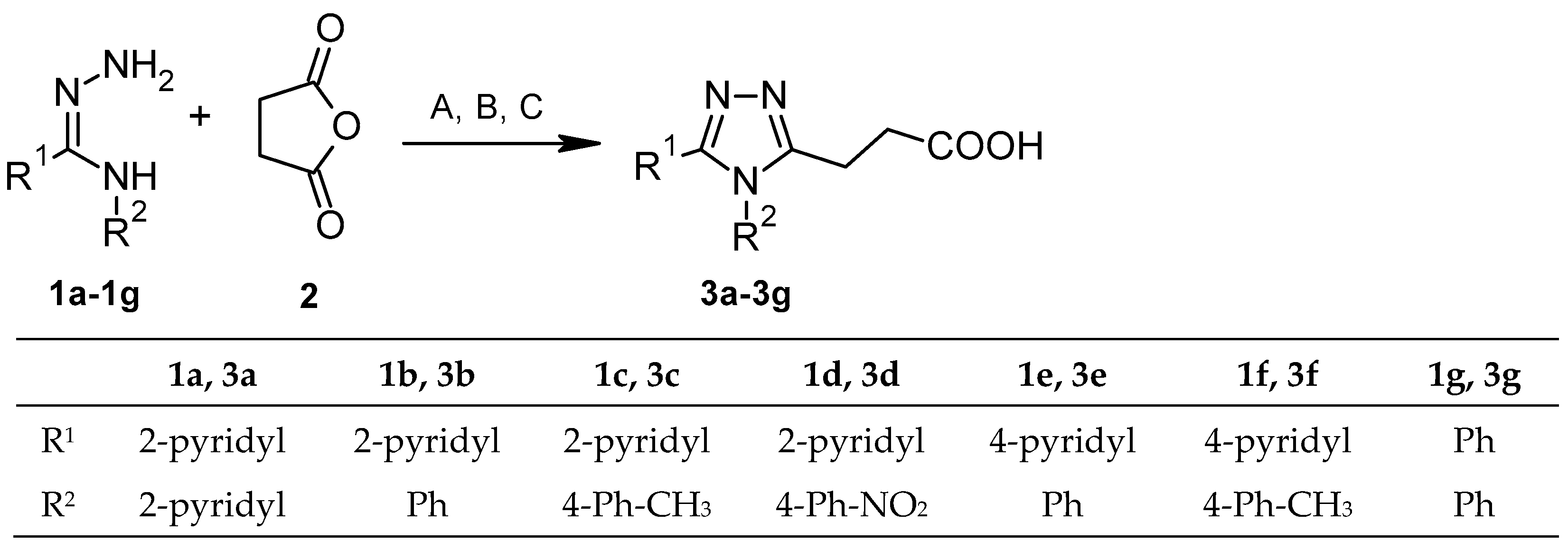
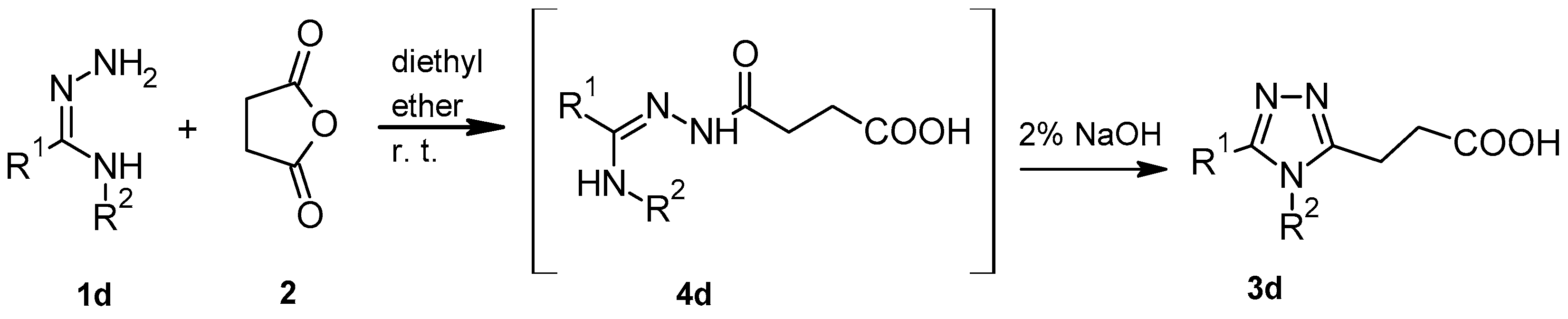
2.1. The Synthesis of Compounds 3a–3g
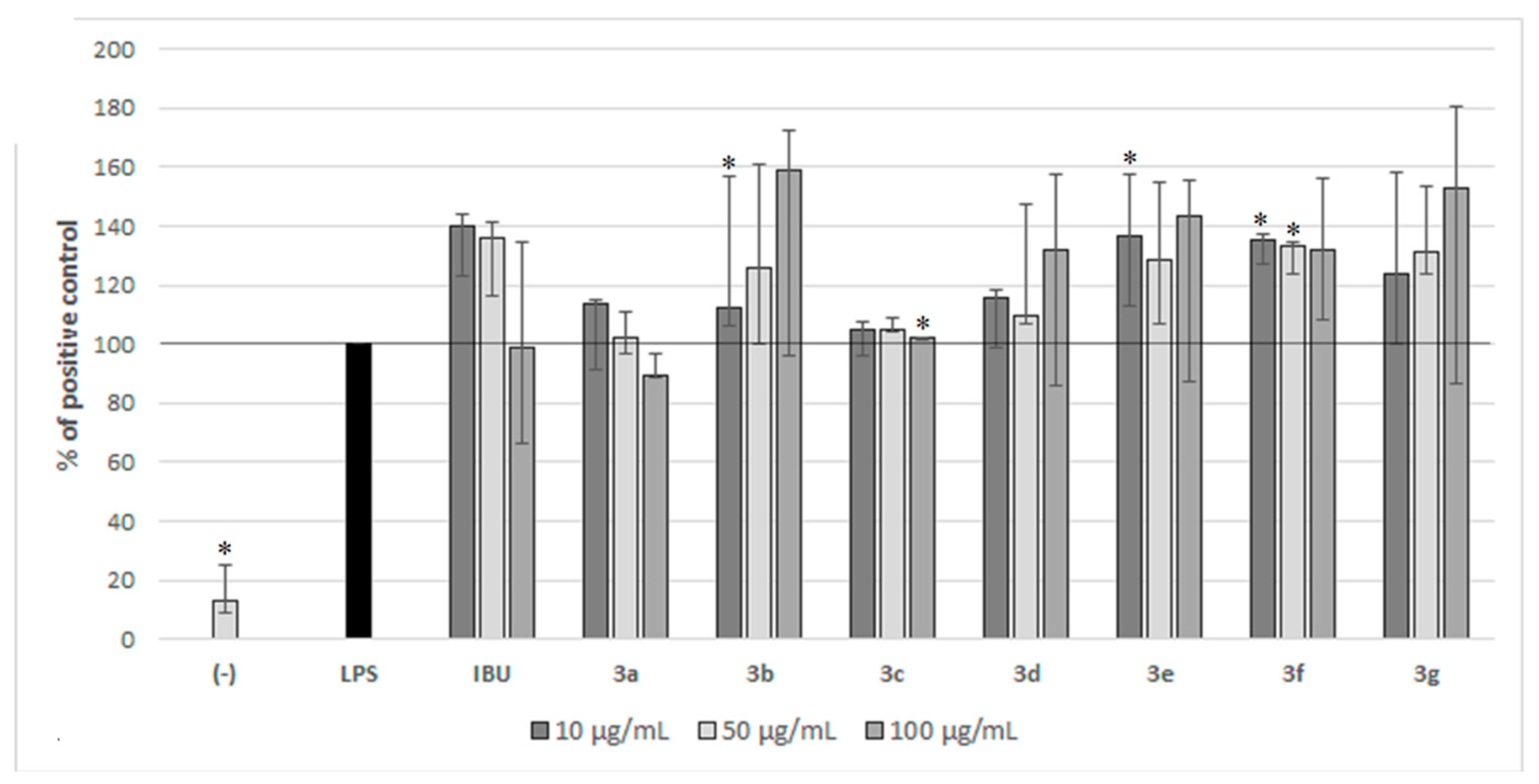
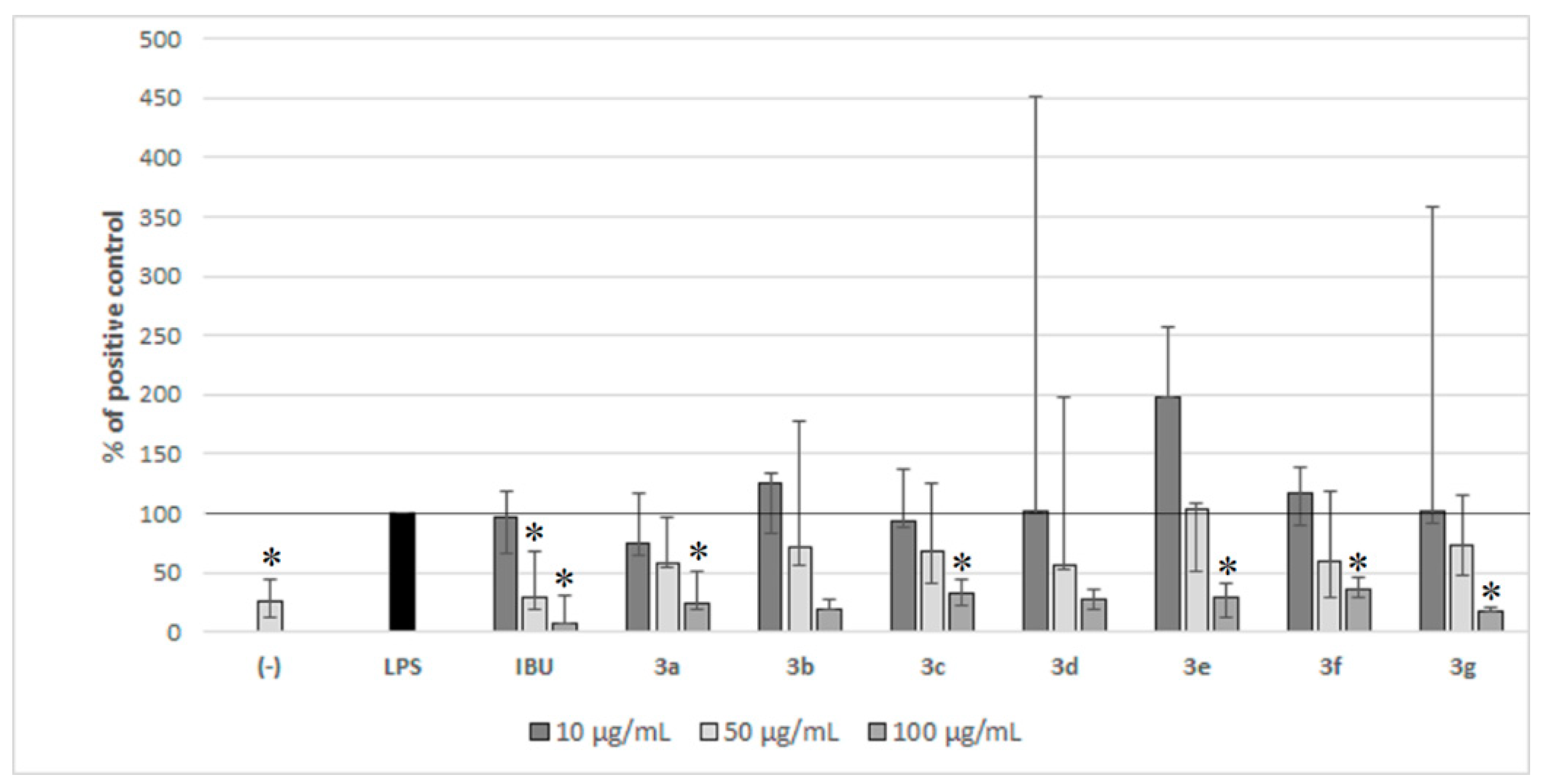
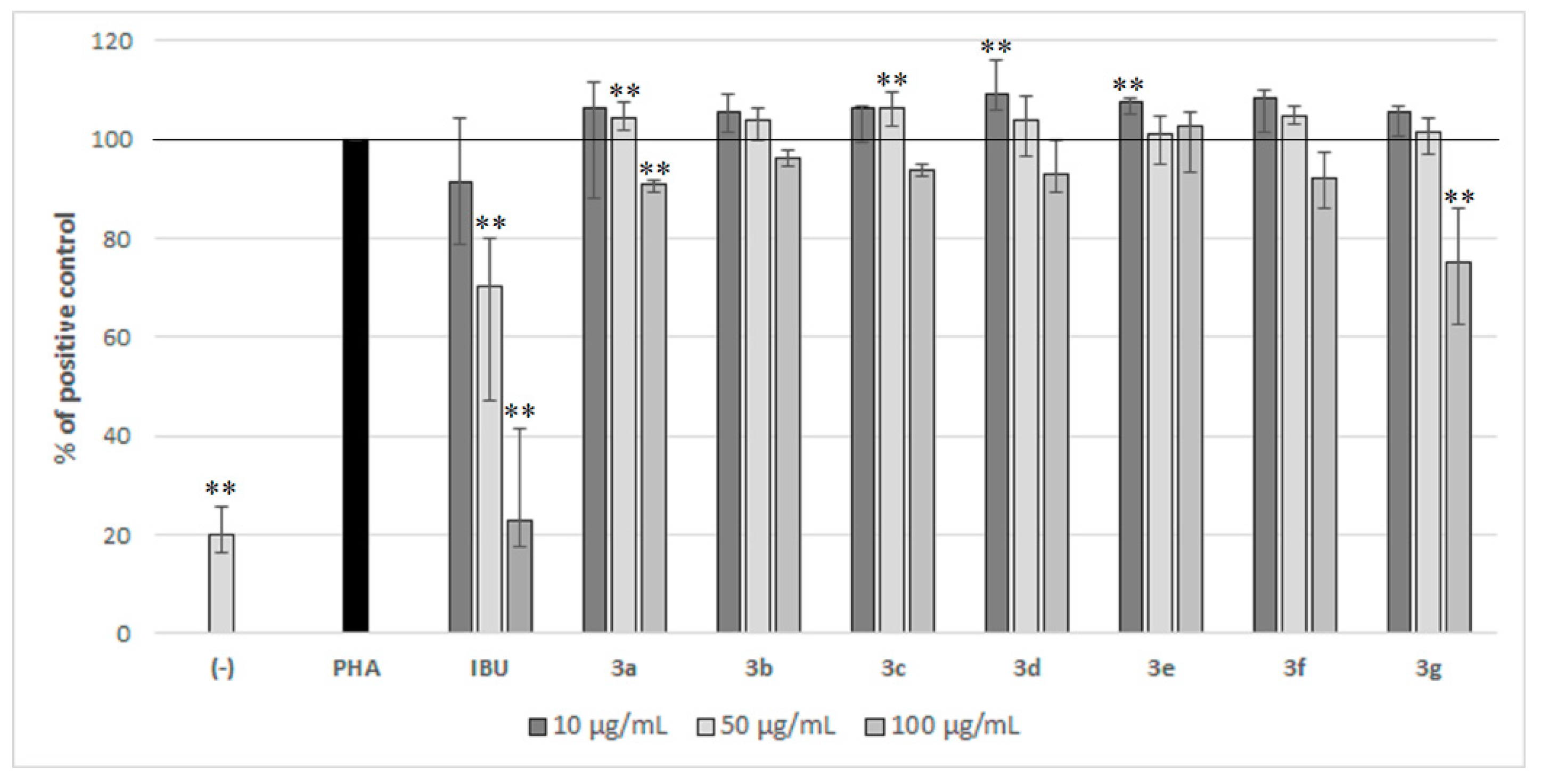
2.2. Biological Activity of Compounds 3a–3g in PBMC
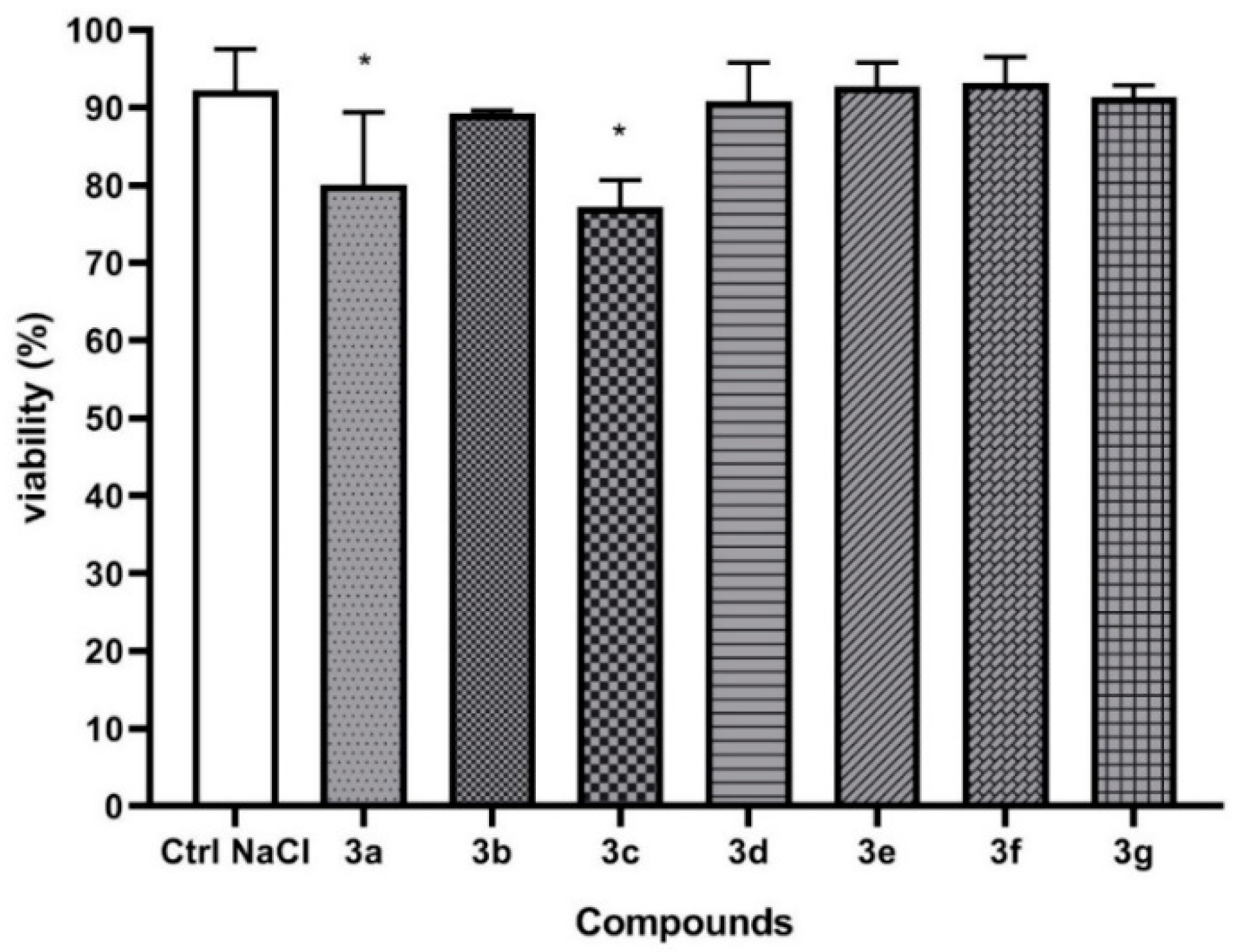
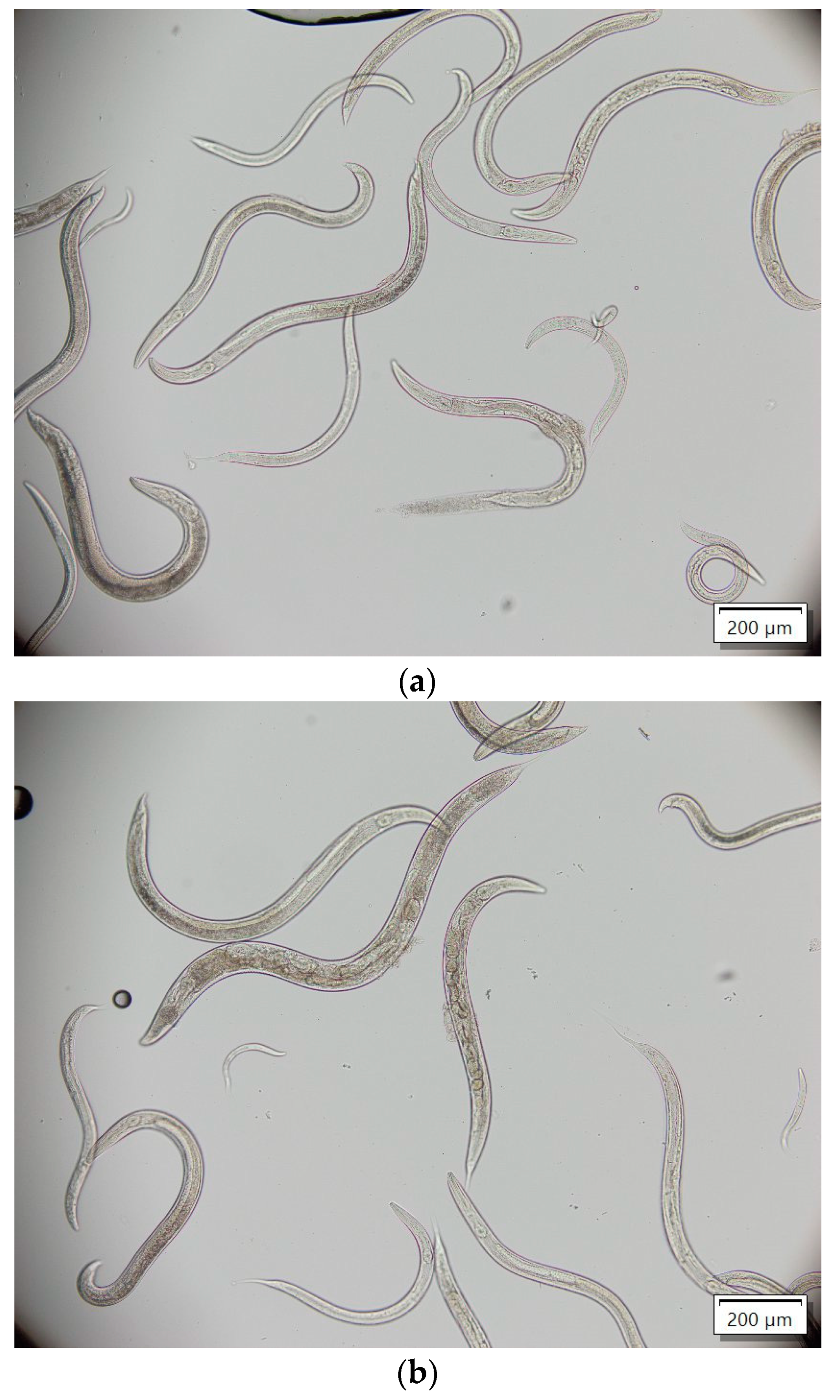
2.3. Anthelmintic Activity
2.4. Antibacterial Activity
3. Discussion
- The presence of 2-pyridyl substituents in R1 position increased TNF-α inhibitory activity of compounds 3a and 3c;
- The presence of two 2-pyridyne substituents (3a), 4-pyridyl substituent in R1, and phenyl ring in R2 (3e) or two phenyl rings in R1 and R2 (3g) improved the ability to decrease IFN-γ levels. Observations for the last two types of substitutions are consistent with the previously described observations for methacrylic acid derivatives [25];
- The presence of 4-pyridyne substituent in R1 and phenyl ring in R2 positions in 3e derivative had beneficial influence on the level of IL-10 cytokine;
- The presence of two phenyl or two 2-pyridyl substituents on R1 and R2 positions seemed to increase the antiproliferative activity of 3g and 3a derivatives;
- The presence of two 2-pyridyne substituents in R1 and R2 or 2-pyridyne substituent in R1 and 4-methylphenyl substituent in R2 seemed to increase the anthelmintic potential of compounds 3a and 3c;
- The presence of two 2-pyridyl substituents or 4-pyridyl and phenyl substituents in R1 and R2 positions increased the antituberculosis activity;
- The presence of the propionic acid group reduced the antibacterial activity of compounds 3a–3g compared to analogous 1,2,4-triazole derivatives substituted with methacrylic acid [32].
4. Materials and Methods
4.1. General Information
4.2. General Procedures of Synthesis
4.2.1. Method A (Synthesis of Compounds 3a–3c and 3e–3g)
4.2.2. Method B (Synthesis of Compounds 3a–3g)
4.2.3. Method C (Synthesis of Compounds 3a–3c and 3e)
4.2.4. Method D (Compound 3d)
- 3-(4,5-di(pyridin-2-yl)-4H-1,2,4-triazol-3-yl)propanoic acid (3a): yield 55.39%, m.p. 197–199 °C. 1H NMR (700 MHz, DMSO-d6, δ ppm): 12.25 (sb, 1H, COOH), 8.56 (d, 1H, J = 4.0 Hz), 8.23, (d, 1H, J = 4.0 Hz), 8.07–7.89 (m, 3H), 7.58–7.50 (m, 2H), 7.38–7.33 (m, 1H), 2.85–2.67 (m, 4H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 173.6, 155.3, 152.8, 149.7, 149.3, 148.9, 146.8, 139.6, 137.8, 125.0, 124.8, 123.6, 122.8, 30.8, 20.9. HR-MS m/z: 296.1150 [M+ + 1] (calculated for C15H14N5O2: 296.1147). Rf = 0.53. Elem. Anal. for C15H13N5O2 calculated: C, 61.01; H, 4.44; N, 23.72%; obtained C, 61.18; H, 4.67; N, 23.63%.
- 3-(4-phenyl-5-(pyridin-2-yl)-4H-1,2,4-triazol-3-yl)propanoic acid (3b): yield 85.76%, m.p. 178–180 °C. 1H NMR (400 MHz, DMSO-d6, δ ppm): 12.26 (sb, 1H, COOH), 8.30–8.28, (m, 1H), 7.97–7.87 (m, 2H), 7.52–7.47 (m, 3H), 7.37–7.32 (m, 3H), 2.72 (s, 4H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 173.6, 155.7, 152.9, 149.4, 147.2, 137.6, 135.5, 129.8 (2×), 129.5, 127.8 (2x), 124.6, 124.1, 30.8, 20.8. HR-MS m/z: 29.1197 [M+ + 1] (calculated for C16H15N4O2: 295.1195). Rf = 0.41. Elem. anal. for C16H14N4O2 * H2O calculated: C, 61.54; H, 5.13; N, 17.95%; obtained C, 61.89; H, 5.08; N, 17.72%.
- 3-(5-(pyridin-2-yl)-4-p-tolyl-4H-1,2,4-triazol-3-yl)propanoic acid (3c): yield 73.53%, m.p. 157–160 °C. 1H NMR (700 MHz, DMSO-d6, δ ppm): 12.24 (sb, 1H, COOH), 8.32 (d, 1H, J = 4.2 Hz), 7.92 (d, 1H, J = 7.7 Hz), 7.89–7.86 (m, 1H), 7.35–7.32 (m, 1H), 7.28 (d, 2H, J = 8.4 Hz), 7.22 (d, 2H, J = 8.4 Hz), 2.72–2.67 (m, 4H), 2.36 (s, 3H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 173.6, 155.7, 153.0, 149.5, 147.3, 139.0, 137.5, 132.9, 130.3 (2×), 127.6 (2×), 124.6, 124.2, 30.8, 21.2, 20.8. HR-MS m/z: 309.1357 [M+ + 1] (calculated for C17H17N4O2: 309.1352). Rf = 0.32. Elem. Anal. for C17H16N4O2 calculated: C, 66.22; H, 5.23; N, 18.17%; obtained C, 66.56; H, 5.25; N, 18.17%.
- 3-(4-(4-nitrophenyl)-5-(pyridin-2-yl)-4H-1,2,4-triazol-3-yl)propanoic acid (3d): yield 32.83%, m.p. 189–191 °C. 1H NMR (400 MHz, DMSO-d6, δ ppm): 12.29 (sb, 1H, COOH), 8.36 (d, 2H, J = 9.2 Hz), 8.26 (d, 1H, J = 4.4 Hz), 8.09 (d, 1H, J = 8.0 Hz), 7.93 (td, 1H, J1 = 8 Hz, J2 = 1.6 Hz), 7.75–7.69 (m, 2H), 7.39–7.34 (m, 1H), 2.75 (s, 4H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 173.3. 155.2, 152.2, 148.9, 147.5, 146.4, 141.1, 137.5, 129.2 (2x), 124.7 (2×), 124.5, 123.4, 30.6, 20.5. HR-MS m/z: 340.1049 [M+ + 1] (calculated for C16H14N5O4: 340.1046). ESI—MS m/z: (M + 1): 340. Rf = 0.38. Elem. Anal. for C16H13N5O4 calculated: C, 56.64; H, 3.86; N, 20.64%; obtained C, 56.56; H, 3.92; N, 20.51%.
- 3-(4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)propanoic acid (3e): yield 80.82%, m.p. 235–237 °C. 1H NMR (700 MHz, DMSO-d6, δ ppm): 12.28 (sb, 1H, COOH), 8.54–8.55 (m, 2H), 7.59–7.63 (m, 3H), 7.47–7.52 (m, 2H), 7.25–7.29 (m, 2H), 2.73 (s, 4H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 173.6, 156.1, 151.5, 150.5 (2×), 134.8, 134.3, 130.7 (2×), 130.7, 128.0 (2×), 122.1 (2×), 30.7, 20.7. HR-MS m/z: 295.1196 [M+ + 1] (calculated for C16H15N4O2: 295.1195). Rf = 0.38. Elem. Anal. for C16H14N4O2 calculated: C, 61.54; H, 5.13; N, 17.95%; obtained C, 61.58; H, 5.12; N, 17.96%.
- 3-(5-(pyridin-4-yl)-4-p-tolyl-4H-1,2,4-triazol-3-yl)propanoic acid (3f): yield 64.30%, m.p. 208–211 °C. 1H NMR (300 MHz, DMSO-d6, δ ppm): 12.26 (sb, 1H, COOH), 8.56–8.52, (m, 2H), 7.41–7.24 (m, 6H), 2.70 (s, 4H), 2.39 (s, 3H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 173.6, 156.2, 151.5, 150.5 (2×), 140.5, 134.9, 131.7, 131.2 (2×), 127.8 (2×), 122.1 (2×), 30.8, 21.2, 20.7. Rf = 0.28. HR-MS m/z: 309.1355 [M+ + 1] (calculated for C17H17N4O2: 309.1352). Elem. Anal. for C17H16N4O2 * ½ H2O calculated: C, 64.40; H, 5.36; N, 17.68%; obtained C, 64.58; H, 5.47; N, 17.36%.
- 3-(4,5-diphenyl-4H-1,2,4-triazol-3-yl)propanoic acid (3g): yield 76.47%, m.p. 251–254 °C. 1H NMR (400 MHz, DMSO-d6, δ ppm): 12.23 (sb, 1H, COOH), 7.57–7.52 (m, 3H), 7.43–7.29 (m, 7H), 2.70 (s, 4H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 173.6, 155.0, 153.6, 134.8, 130.5 (2×), 130.2, 129.9, 129.0 (2×), 128.4 (2×), 128.1 (2×), 127.5, 30.8, 20.8. Rf = 0.31. HR-MS m/z: 294.1240 [M+ + 1] (calculated for C17H16N3O2: 294.1243). Elem. Anal. for C17H15N3O2 calculated: C, 69.61; H, 5.15; N, 14.33%; obtained C, 69.69; H, 5.26; N, 14.33%.
4.3. Biological Activity Evaluation for Compounds 3a–3g
4.3.1. Cell Culture Preparation and Toxicity of Compounds 3a–3g towards PBMC
4.3.2. The Influence of Compounds 3a–3g on Cytokines Production in PBMC
4.3.3. The Influence of Compounds 3a–3g on PBMC Proliferation
4.4. Anthelmintic Activity of Compounds 3a–3g
4.5. Antibacterial Activity of Compounds 3a–3g
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mohammed, A.; Kalle, A.M.; Reddanna, P. Managing SARS-CoV2 Infections Through Resolution of Inflammation by Eicosanoids: A Review. J. Inflamm. Res. 2022, 15, 4349–4358. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, M.; Rovati, G.E.; Diamant, Z.; Untersmayr, E.; Schwarze, J.; Lukasik, Z.; Sava, F.; Angelina, A.; Palomares, O.; Akdis, C.A.; et al. Effects of non-steroidal anti-inflammatory drugs and other eicosanoid pathway modifiers on antiviral and allergic responses: EAACI task force on eicosanoids consensus report in times of COVID-19. Allergy 2022, 77, 2337–2354. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Maddipati, K.R. Non-inflammatory Physiology of “Inflammatory” Mediators—Unalamation, a New Paradigm. Front. Immunol. 2020, 11, 580117. [Google Scholar] [CrossRef]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Jenny, M.; Klieber, M.; Zaknun, D.; Schroecksnadel, S.; Kurz, K.; Ledochowski, M.; Schennach, H.; Fuchs, D. In vitro testing for anti-inflammatory properties of compounds employing peripheral blood mononuclear cells freshly isolated from healthy donors. Inflamm. Res. 2011, 60, 127–135. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Clement, F.; Merabishvili, M.; Lavigne, R.; Vaneechoutte, M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci. Rep. 2017, 7, 8004. [Google Scholar] [CrossRef]
- Dargahi, N.; Johnson, J.C.; Apostolopoulos, V. Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes. Biologics 2021, 1, 396–415. [Google Scholar] [CrossRef]
- Dargahi, N.; Johnson, J.; Apostolopoulos, V. Streptococcus thermophilus alters the expression of genes associated with innate and adaptive immunity in human peripheral blood mononuclear cells. PLoS ONE 2020, 15, e0228531. [Google Scholar] [CrossRef]
- Mączyński, M.; Artym, J.; Kocięba, M.; Kochanowska, I.; Ryng, S.; Zimecki, M. Anti-inflammatory properties of an isoxazole derivative—MZO-2. Pharmacol. Rep. 2016, 68, 894–902. [Google Scholar] [CrossRef]
- Díaz, I. Rules of thumb to obtain, isolate, and preserve porcine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2022, 251, 110461. [Google Scholar] [CrossRef]
- Ertel, W.; Kremer, J.-P.; Kenney, J.; Steckholzer, U.; Jarrar, D.; Trentz, O.; Schildberg, F.W. Downregulation of Proinflammatory Cytokine Release in Whole Blood from Septic Patients. Blood 1995, 85, 1341–1347. [Google Scholar] [CrossRef]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Zhao, P. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef]
- Siedlik, J.A.; Deckert, J.A.; Benedict, S.H.; Bhatta, A.; Dunbar, A.J.; Vardiman, J.P.; Gallagher, P.M. T cell activation and proliferation following acute exercise in human subjects is altered by storage conditions and mitogen selection. J. Immunol. Methods 2017, 446, 7–14. [Google Scholar] [CrossRef]
- Lawlor, N.; Nehar-Belaid, D.; Grassmann, J.D.S.; Stoeckius, M.; Smibert, P.; Stitzel, M.L.; Pascual, V.; Banchereau, J.; Williams, A.; Ucar, D. Single Cell Analysis of Blood Mononuclear Cells Stimulated Through Either LPS or Anti-CD3 and Anti-CD28. Front. Immunol. 2021, 12, 636720. [Google Scholar] [CrossRef]
- Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Efrit, M.L.; Prim, D. Recent advances in the chemistry of 1,2,4-triazoles: Synthesis, reactivity and biological activities. Tetrahedron Lett. 2021, 86, 153518. [Google Scholar] [CrossRef]
- Wen, Y.; Lun, S.; Jiao, Y.; Zhang, W.; Hu, T.; Liu, T.; Yang, F.; Tang, J.; Zhang, B.; Bishai, W.R.; et al. Design, synthesis, and biological evaluation of 1,2,4-triazole derivatives as potent antitubercular agents. Chin. Chem. Lett. 2023, 108464, in press. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J. The Antibacterial Activity of 1,2,3-triazole- and 1,2,4-Triazole-containing Hybrids against Staphylococcus aureus: An Updated Review (2020-Present). Curr. Top. Med. Chem. 2022, 22, 41–63. [Google Scholar] [CrossRef]
- Paprocka, R.; Kołodziej, P.; Wiese-Szadkowska, M.; Helmin-Basa, A.; Bogucka-Kocka, A. Evaluation of Anthelmintic and Anti-Inflammatory Activity of 1,2,4-Triazole Derivatives. Molecules 2022, 27, 4488. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, R.; Wiese, M.; Eljaszewicz, A.; Helmin-Basa, A.; Gzella, A.; Modzelewska-Banachiewicz, B.; Michalkiewicz, J. Synthesis and anti-inflammatory activity of new 1,2,4-triazole derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 2664–2667. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, R.; Wiese-Szadkowska, M.; Kosmalski, T.; Frisch, D.; Ratajczak, M.; Modzelewska-Banachiewicz, B.; Studzińska, R. A Review of the Biological Activity of Amidrazone Derivatives. Pharmaceuticals 2022, 15, 1219. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese-Szadkowska, M.; Helmin-Basa, A.; Mazur, L.; Kutkowska, J.; Michałkiewicz, J.; Modzelewska-Banachiewicz, B.; Pazderski, L. Synthesis and evaluation of new amidrazone-derived hydrazides as a potential anti-inflammatory agents. Monatsh Chem. 2018, 149, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.; Pradhan, T.; Chawla, G. An updated review on diverse range of biological activities of 1,2,4-triazole derivatives: Insight into structure activity relationship. J. Mol. Struct. 2023, 1274, 134487. [Google Scholar] [CrossRef]
- Modzelewska, B.; Pyra, E. Synthesis of N3-substituted amidrazones. Ann. UMCS Sec. AA 1996, 50, 111–116. [Google Scholar]
- Modzelewska-Banachiewicz, B.; Paprocka, R.; Mazur, L.; Saczewski, J.; Kutkowska, J.; Stępień, D.K.; Cyrański, M. Experimental and theoretical study on the reaction of N3-phenyl-(pyridin-2-yl)carbohydrazonamide with itaconic anhydride. J. Mol. Struct. 2012, 1022, 211–219. [Google Scholar] [CrossRef]
- Sibi, J.M.; Mohan, V.; Munisankar, S.; Babu, S.; Aravindhan, V. Augmented Innate and Adaptive Immune Responses Under Conditions of Diabetes-Filariasis Comorbidity. Front. Immunol. 2021, 12, 716515. [Google Scholar] [CrossRef]
- Sayın, N.; Uygun, D.F.K.; Sallakçı, N.; Filiz, S.; Yeğin, O. Inhibitory Effects of Acetylsalicylic Acid and Ibuprofen on Interleukin-17 Production. Turk. J. Immunol. 2013, 1, 42–46. [Google Scholar] [CrossRef]
- Paprocka, R.; Modzelewska-Banachiewicz, B.; Kutkowska, J.; Pawłowski, K.; Piątkowska-Chmiel, I.; Jagiełło-Wójtowicz, E. Antibacterial and central nervous system activity of 4,5-diaryl-4H-1,2,4-triazol-3-yl)methylacrylic acid derivatives. Acta Pol. Pharm. 2017, 74, 289–292. [Google Scholar]
- Kutkowska, J.; Modzelewska-Banachiewicz, B.; Ziółkowska, G.; Rzesko, W.; Urbanik-Sypniewska, T.; Zwolska, Z.; Prus, M. Antimicrobial Activity of 3,4-disubstituted-1,2,4-triazole derivatives. Acta Pol. Pharm. 2005, 62, 303–306. [Google Scholar]
- Paprocka, R.; Pazderski, L.; Mazur, L.; Wiese-Szadkowska, M.; Kutkowska, J.; Nowak, M.; Helmin-Basa, A. Synthesis and Structural Study of Amidrazone Derived Pyrrole-2,5-Dione Derivatives: Potential Anti-Inflammatory Agents. Molecules 2022, 27, 2891. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprocka, R.; Wiese-Szadkowska, M.; Kołodziej, P.; Kutkowska, J.; Balcerowska, S.; Bogucka-Kocka, A. Evaluation of Biological Activity of New 1,2,4-Triazole Derivatives Containing Propionic Acid Moiety. Molecules 2023, 28, 3808. https://doi.org/10.3390/molecules28093808
Paprocka R, Wiese-Szadkowska M, Kołodziej P, Kutkowska J, Balcerowska S, Bogucka-Kocka A. Evaluation of Biological Activity of New 1,2,4-Triazole Derivatives Containing Propionic Acid Moiety. Molecules. 2023; 28(9):3808. https://doi.org/10.3390/molecules28093808
Chicago/Turabian StylePaprocka, Renata, Małgorzata Wiese-Szadkowska, Przemysław Kołodziej, Jolanta Kutkowska, Sara Balcerowska, and Anna Bogucka-Kocka. 2023. "Evaluation of Biological Activity of New 1,2,4-Triazole Derivatives Containing Propionic Acid Moiety" Molecules 28, no. 9: 3808. https://doi.org/10.3390/molecules28093808
APA StylePaprocka, R., Wiese-Szadkowska, M., Kołodziej, P., Kutkowska, J., Balcerowska, S., & Bogucka-Kocka, A. (2023). Evaluation of Biological Activity of New 1,2,4-Triazole Derivatives Containing Propionic Acid Moiety. Molecules, 28(9), 3808. https://doi.org/10.3390/molecules28093808





