Advanced Fabrication and Multi-Properties of Aluminum-Based Aerogels from Aluminum Waste for Thermal Insulation and Oil Absorption Applications
Abstract
1. Introduction
2. Results and Discussion
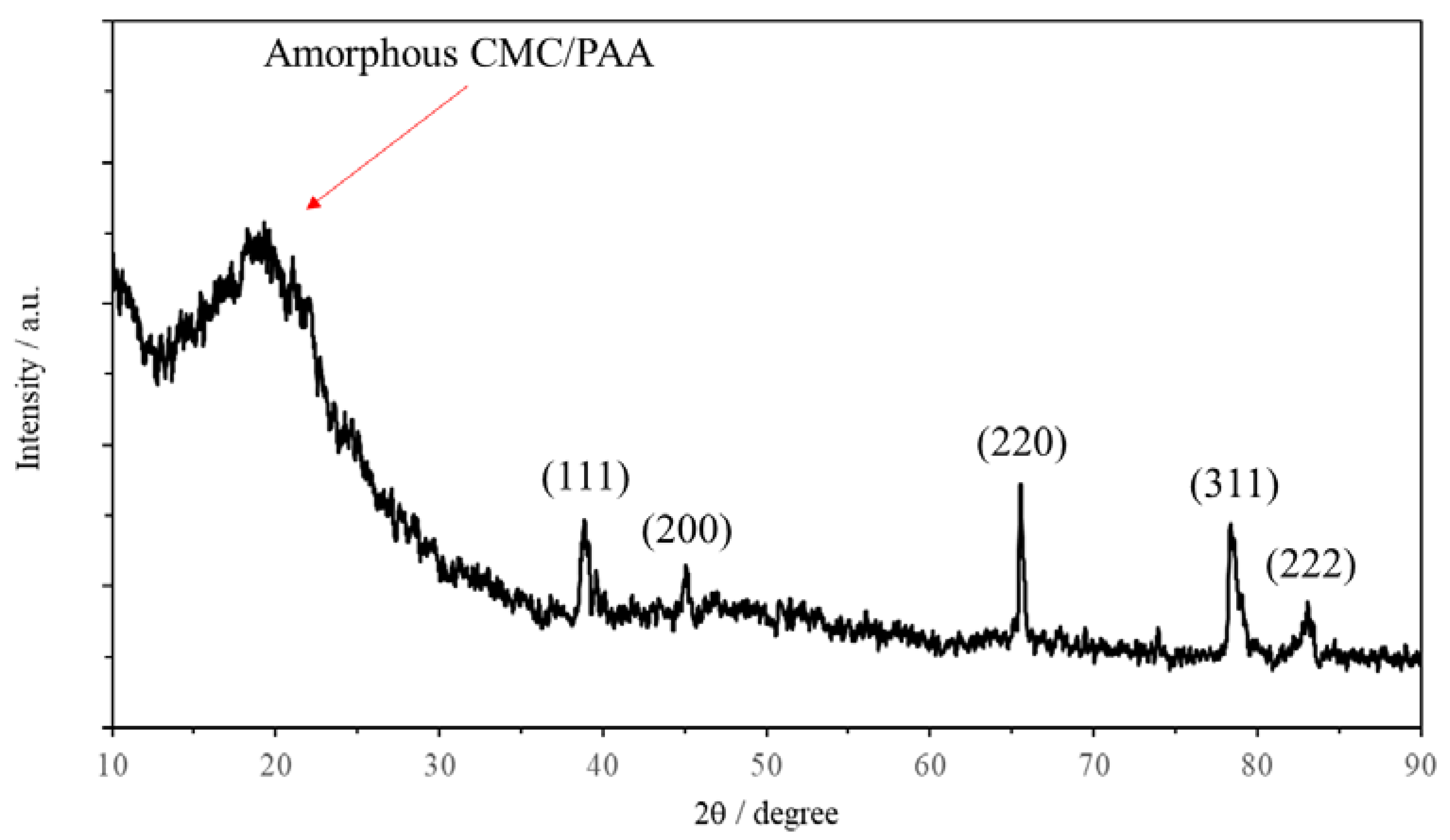
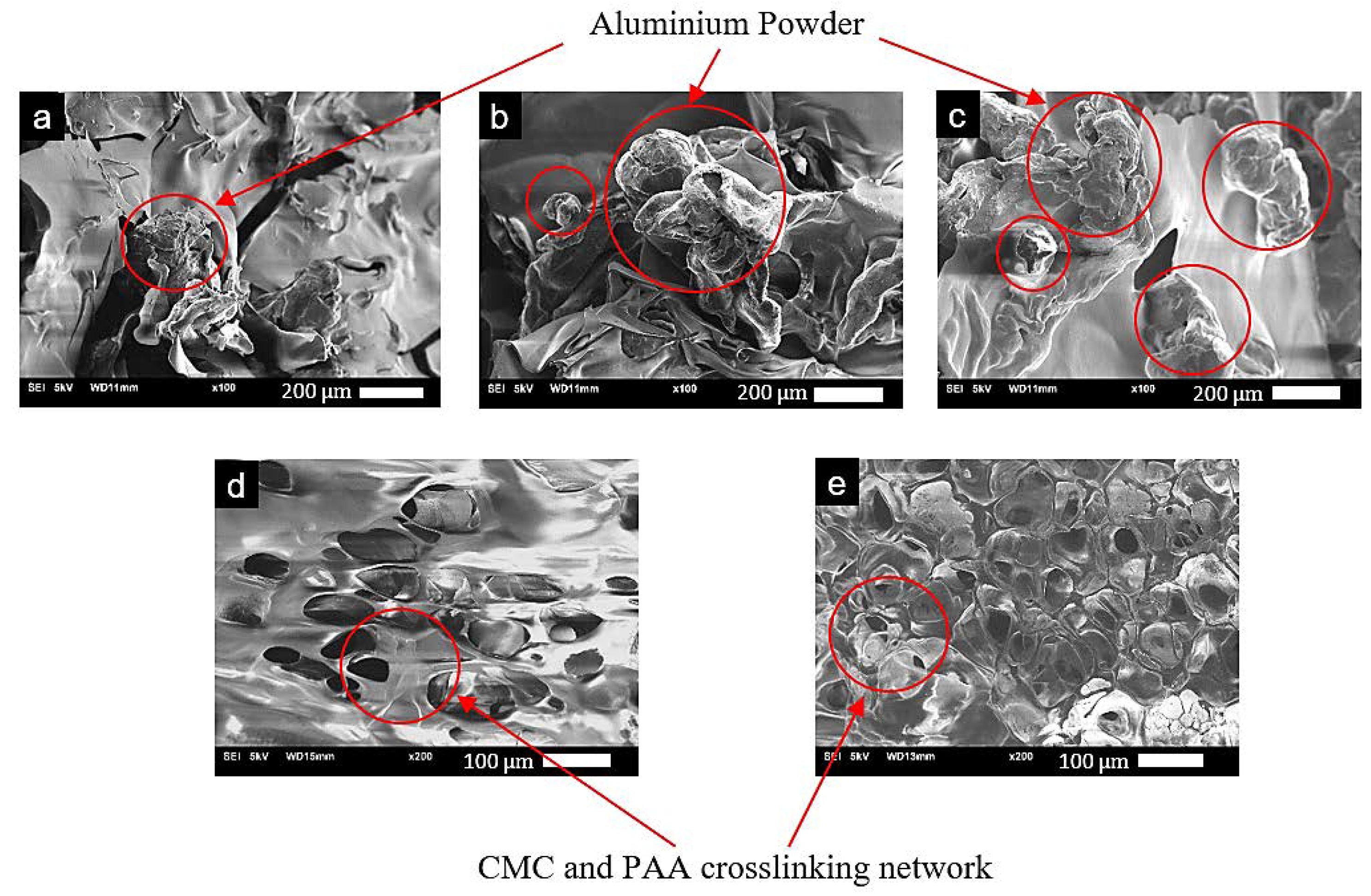
2.1. Morphology and Structure of the AAs
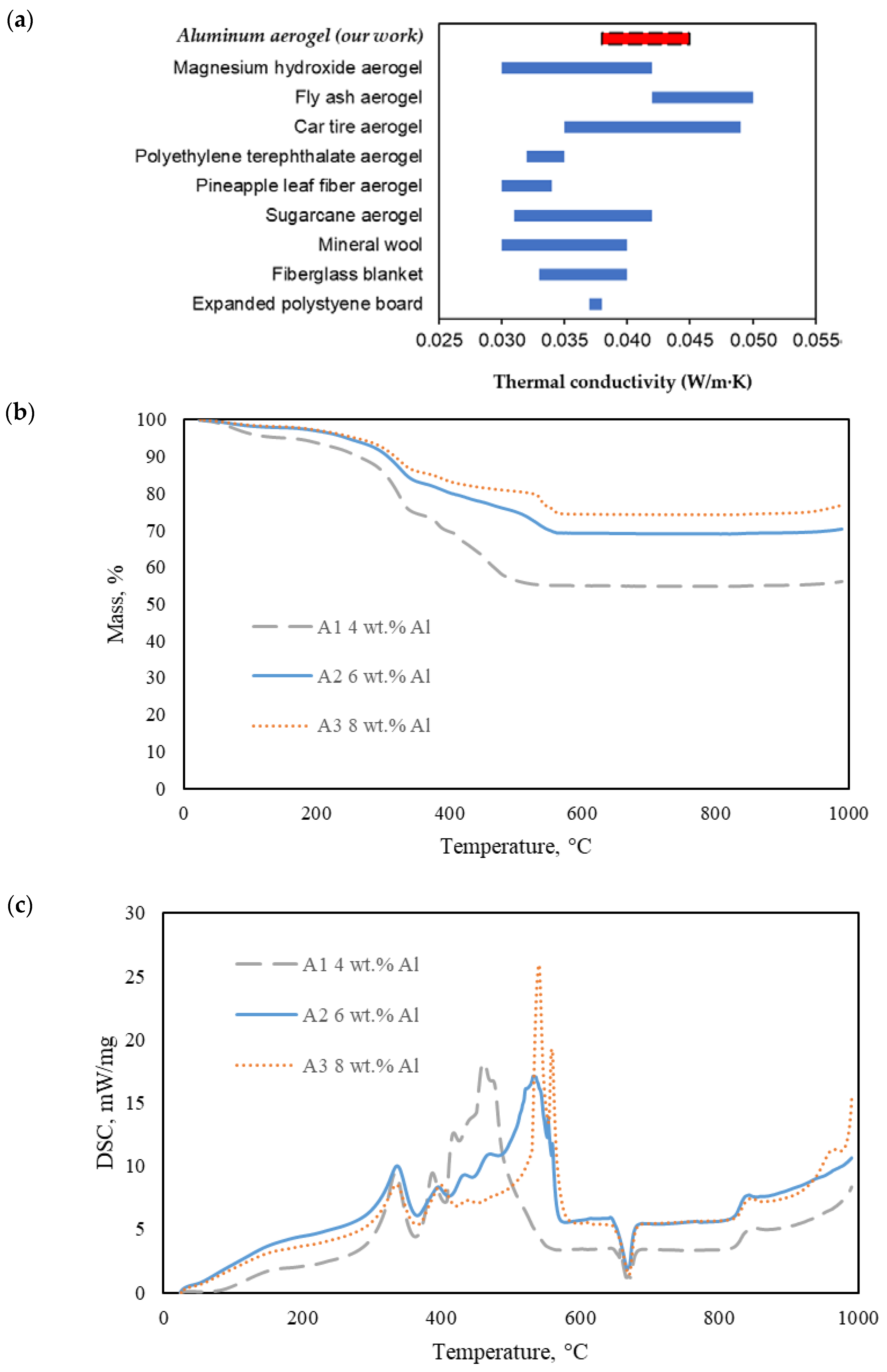
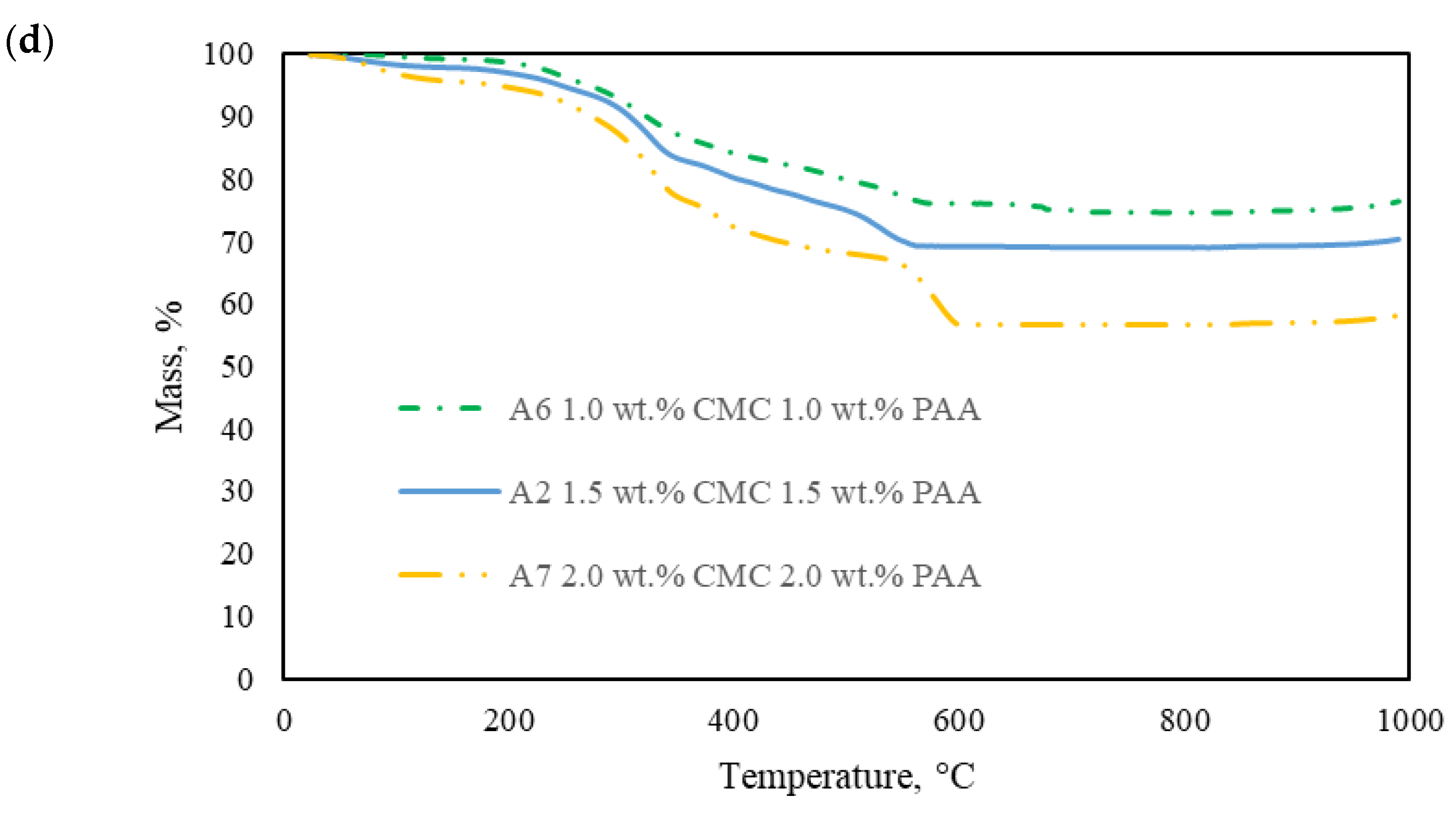
2.2. Thermal Property of the AAs
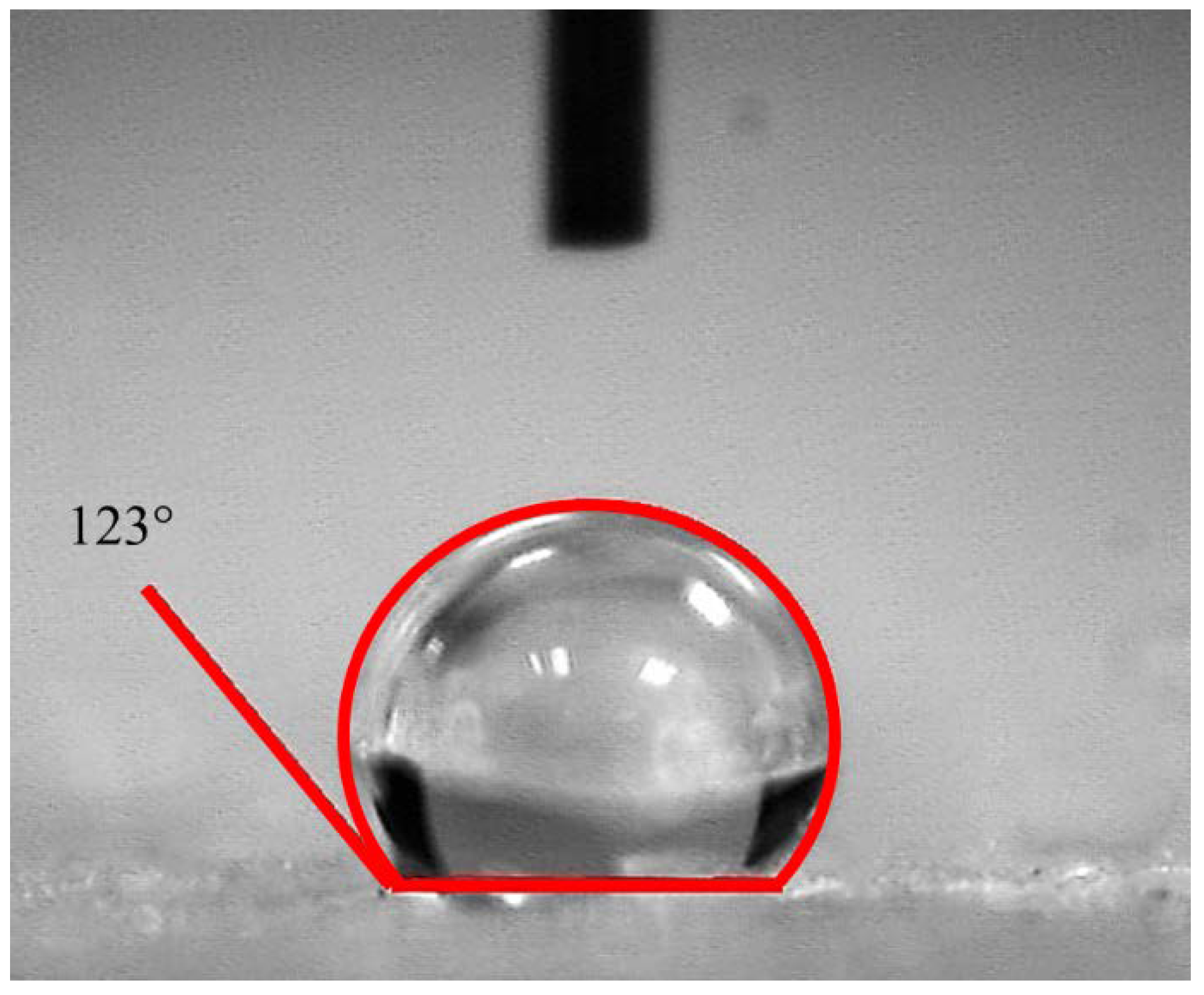
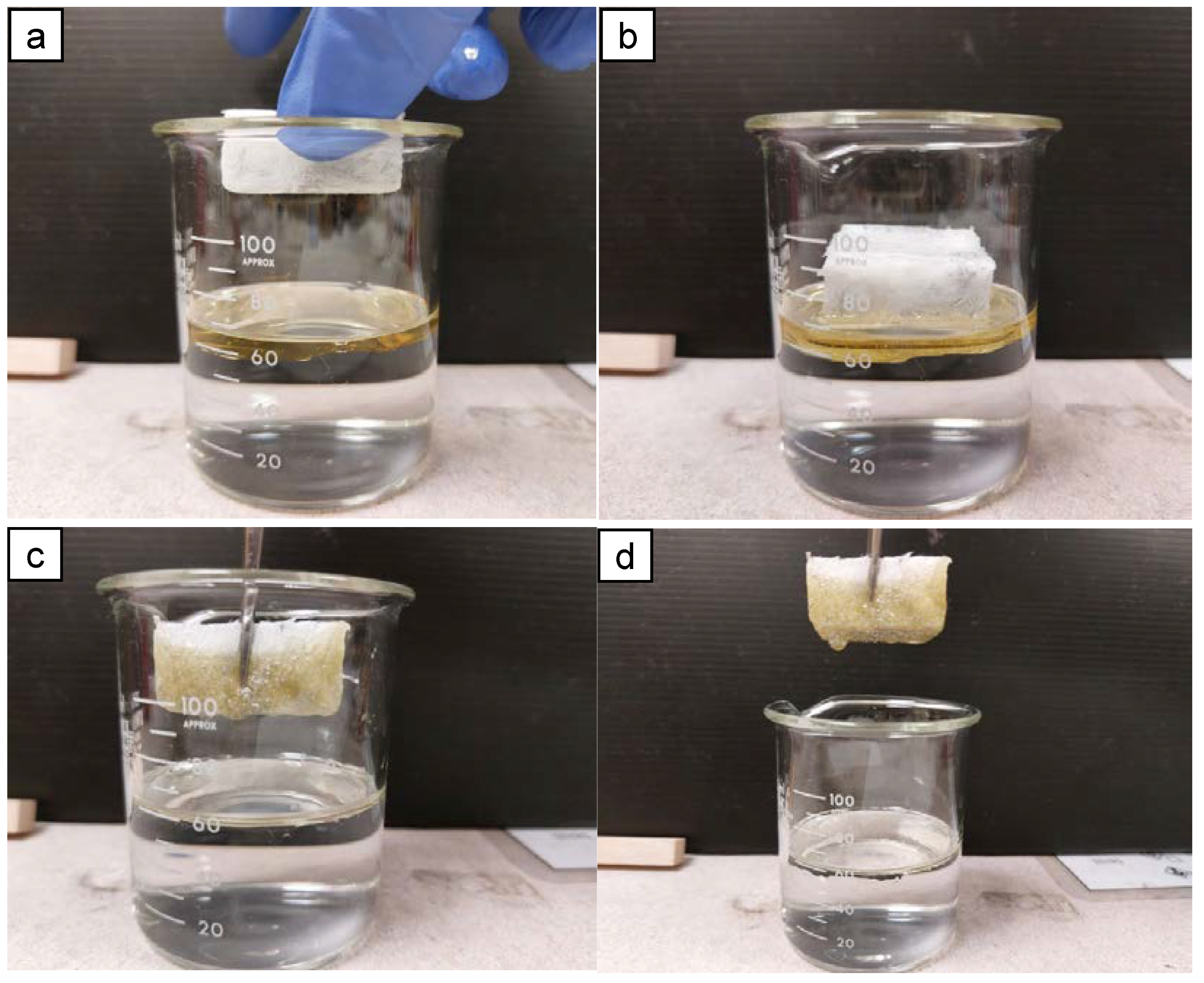
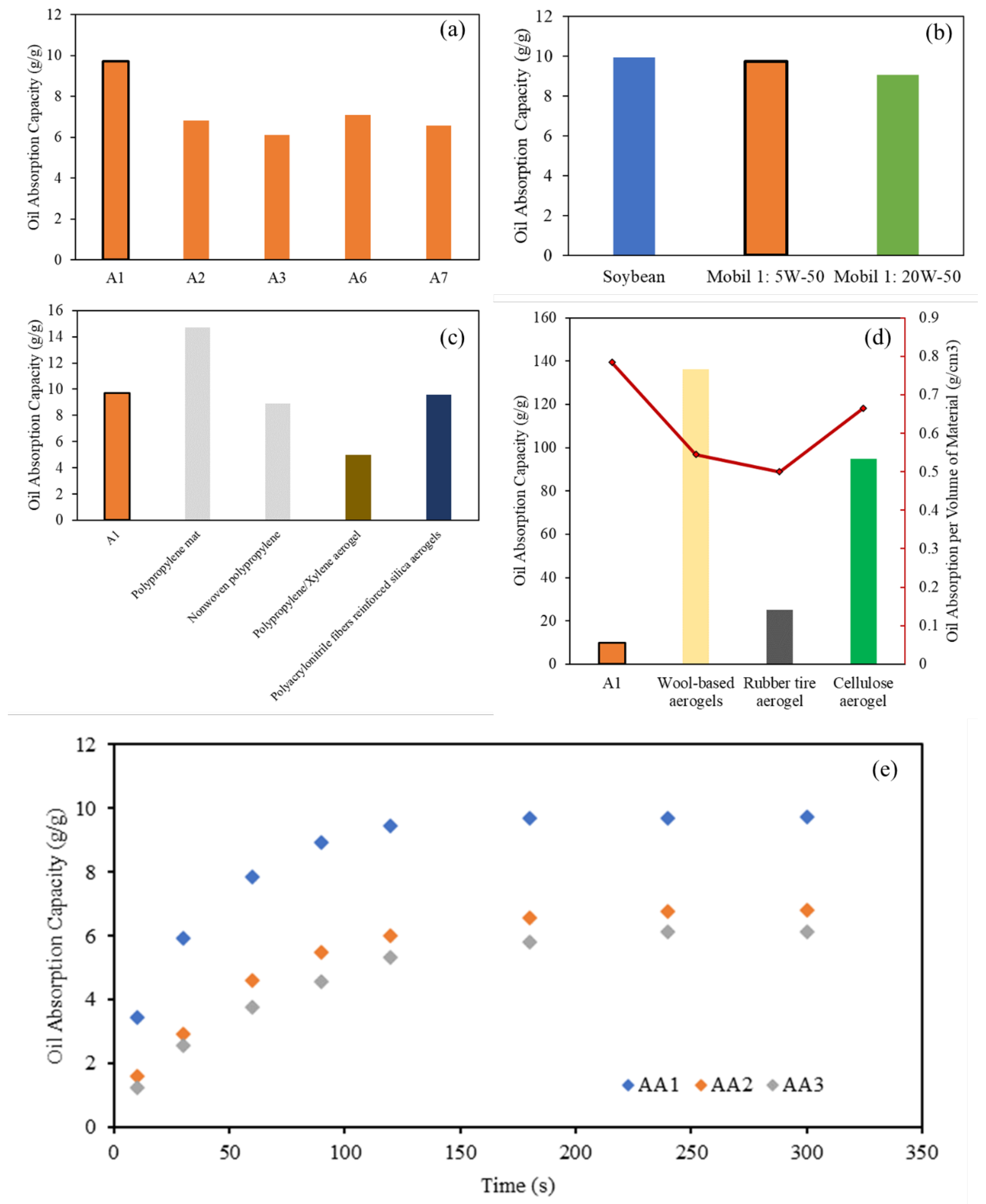
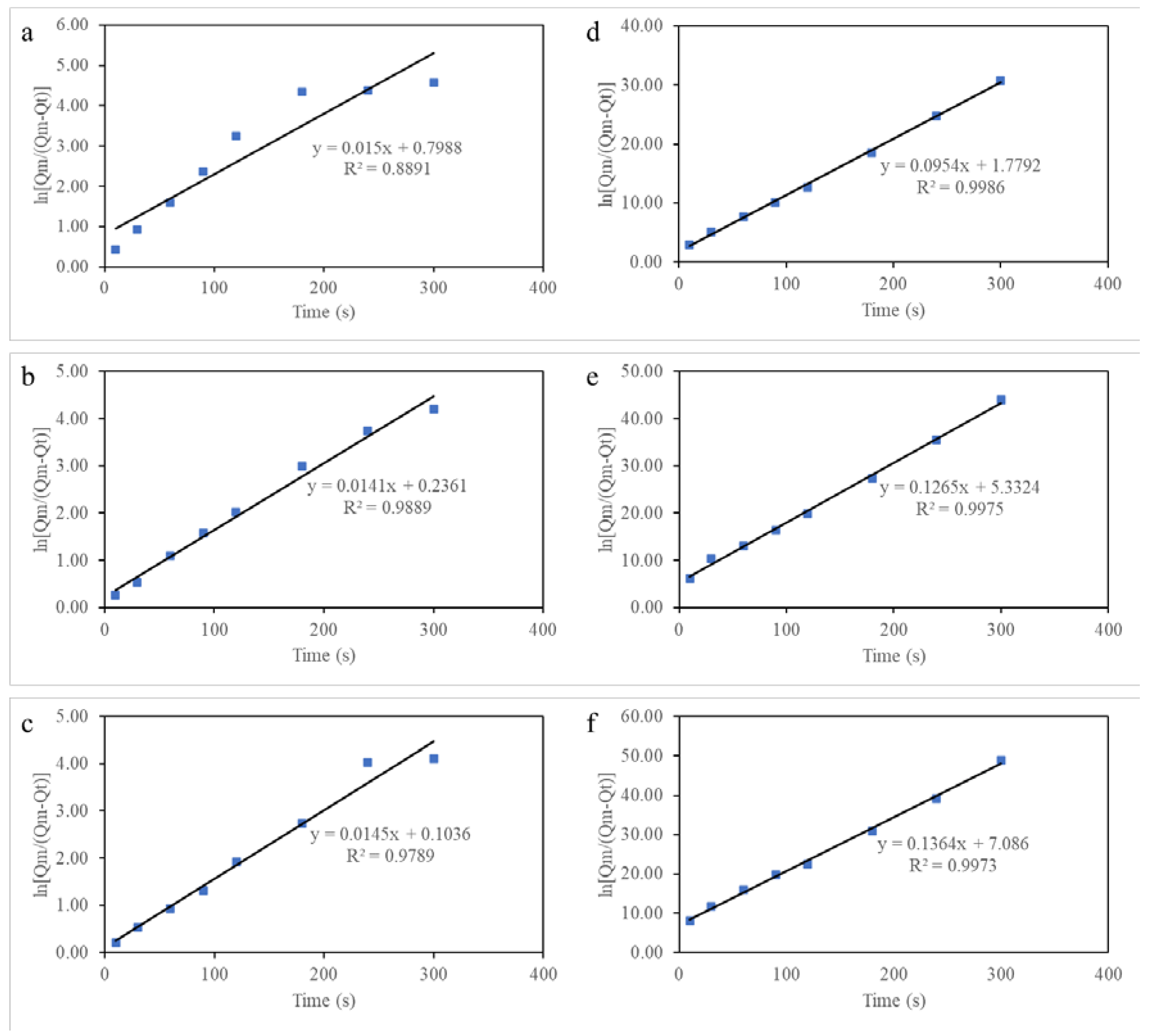
2.3. Oil Absorption Properties and Kinetics of AAs
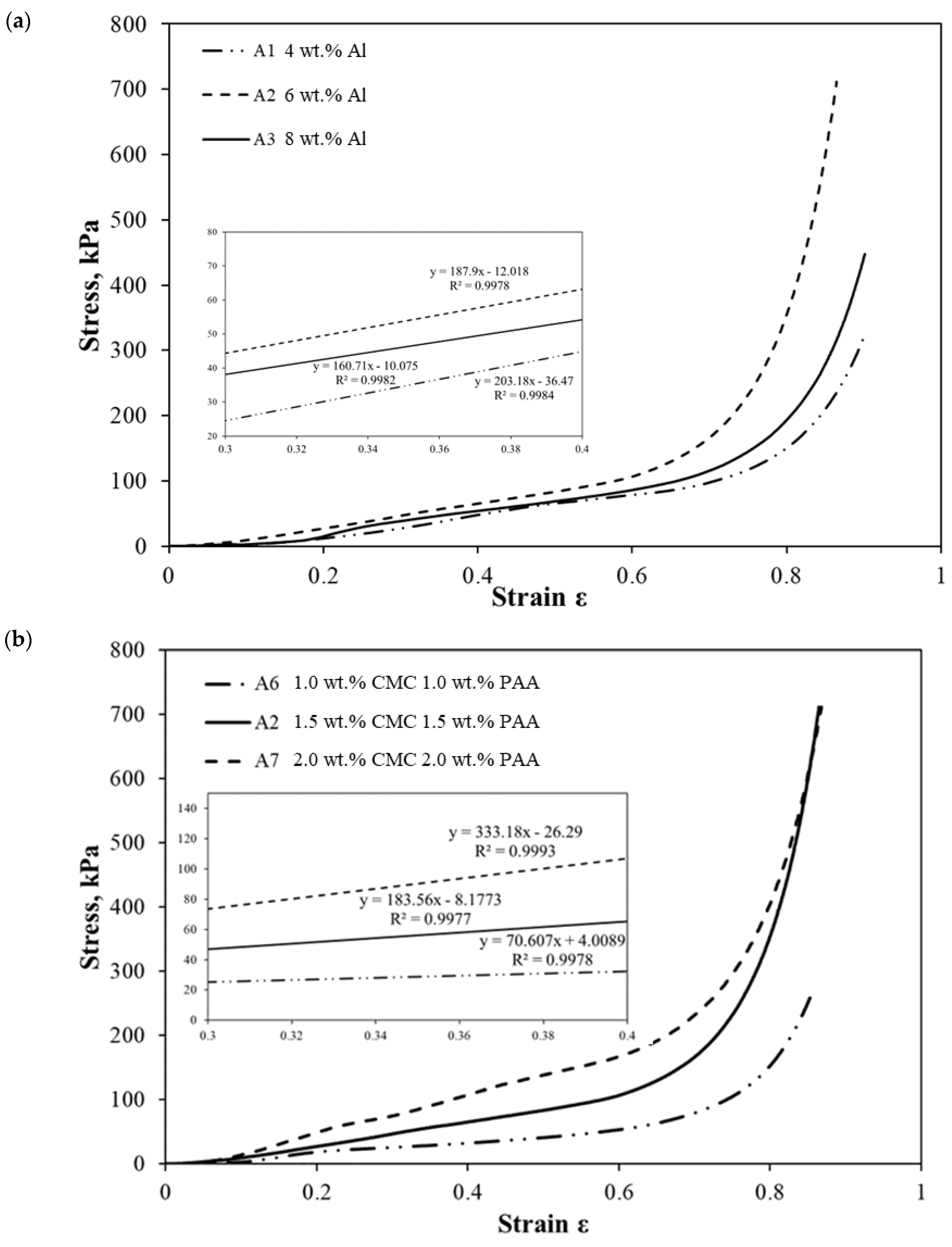
2.4. Mechanical Property of the AAs
3. Materials and Methods
3.1. Materials
3.2. Preparation of AAs
3.3. Surface Modification of AAs
3.4. Characterization of AAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Alghoul, M.A.; Mohammad, M.; Fudholi, A.; Akhtaruzzaman, M.; Amin, N.; Sopian, K. Biomass and industrial wastes as resource materials for aerogel preparation: Opportunities, challenges, and research directions. Ind. Eng. Chem. Res. 2019, 58, 17621–17645. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.-K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmüller, A. Noble Metal Aerogels—Synthesis, Characterization, and Application as Electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Yam, B.J.; Le, D.K.; Do, N.H.; Nguyen, P.T.; Thai, Q.B.; Phan-Thien, N.; Duong, H.M. Recycling of magnesium waste into magnesium hydroxide aerogels. J. Environ. Chem. Eng. 2020, 8, 104101. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Zhou, X.-C.; Fu, Z.-B.; Mi, R.; Wang, C.-Y. Freestanding monolithic Ni aerogel with large surface areas from cellulose aerogel templates. Mater. Lett. 2017, 196, 296–299. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wang, W.; Zou, L.; Lian, Y.; Zhang, Z.; Liu, B.; Zhang, F. Robust, highly thermally stable, core-shell nanostructured metal oxide aerogels as high-temperature thermal superinsulators, adsorbents, and catalysts. Chem. Mater. 2014, 26, 5761–5772. [Google Scholar] [CrossRef]
- Perez-Rangel, N.; Florez-Solano, E.; Palacio, L.H. Exploitation of chips and scrap aluminum through physical processes for the development of aluminum sheets and bars. J. Phys. Conf. Ser. 2020, 1587, 012027. [Google Scholar]
- United States Environmental Protection Agency (EPA). Aluminum: Material Specific Data. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/aluminum-material-specific-data (accessed on 27 January 2021).
- The International Aluminium Institute. Aluminium Recycling. 2020. Available online: https://recycling.world-aluminium.org/review/sustainability (accessed on 27 January 2021).
- Tsakiridis, P. Aluminium salt slag characterization and utilization—A review. J. Hazard. Mater. 2012, 217, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sp, T.S.; Nguyen, P.T.; Do, N.H.; Le, D.K.; Thai, Q.B.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Advanced fabrication and multi-properties of aluminium hydroxide aerogels from aluminium wastes. J. Mater. Cycles Waste Manag. 2021, 23, 885–894. [Google Scholar] [CrossRef]
- Thai, Q.B.; Le, D.K.; Luu, T.P.; Hoang, N.; Nguyen, D.; Duong, H. Aerogels from wastes and their applications. JOJ Mater. Sci. 2019, 5, 555663. [Google Scholar]
- Nguyen, P.T.; Do, N.H.; Goh, X.Y.; Goh, C.J.; Ong, R.H.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Recent progresses in eco-friendly fabrication and applications of sustainable aerogels from various waste materials. Waste Biomass Valorization 2022, 13, 1825–1847. [Google Scholar] [CrossRef]
- Toledo, P.V.; Limeira, D.P.; Siqueira, N.C.; Petri, D.F. Carboxymethyl cellulose/poly (acrylic acid) interpenetrating polymer network hydrogels as multifunctional adsorbents. Cellulose 2019, 26, 597–615. [Google Scholar] [CrossRef]
- Abdel-Galil, A.; Ali, H.; Atta, A.; Balboul, M. Influence of nanostructured TiO2 additives on some physical characteristics of carboxymethyl cellulose (CMC). J. Radiat. Res. Appl. Sci. 2014, 7, 36–43. [Google Scholar] [CrossRef]
- Swain, S.K.; Prusty, K. Biomedical applications of acrylic-based nanohydrogels. J. Mater. Sci. 2018, 53, 2303–2325. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Zhang, B.; Chen, H.; Zhang, J.; Wang, T.; Zhang, K.; Zhang, J.; Huang, P. Effect of Mn content on microstructure and properties of 6000 series aluminum alloy. Appl. Phys. A 2019, 125, 490. [Google Scholar] [CrossRef]
- Ayieko, C.O.; Musembi, R.J.; Ogacho, A.A.; Aduda, B.O.; Muthoka, B.M.; Jain, P.K. Controlled texturing of aluminum sheet for solar energy applications. Adv. Mater. Phys. Chem. 2015, 5, 458–466. [Google Scholar] [CrossRef]
- Do, N.H.; Le, T.M.; Tran, H.Q.; Pham, N.Q.; Le, K.A.; Nguyen, P.T.; Duong, H.M.; Le, T.A.; Le, P.K. Green recycling of fly ash into heat and sound insulation composite aerogels reinforced by recycled polyethylene terephthalate fibers. J. Clean. Prod. 2021, 322, 129138. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Cui, S.; Wang, L.; Shen, X. Synthesis of a novel Al2O3-SiO2 composite aerogel with high specific surface area at elevated temperatures using inexpensive inorganic salt of aluminum. Ceram. Int. 2016, 42, 874–882. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.; Xu, H.; Zhong, Y.; Mao, Z. Preparation and characterization of thermal protective aluminum hydroxide aerogel/PSA fabric composites. J. Sol-Gel Sci. Technol. 2017, 82, 370–379. [Google Scholar] [CrossRef]
- Duong, H.M.; Ling, N.R.; Thai, Q.B.; Le, D.K.; Nguyen, P.T.; Goh, X.Y.; Phan-Thien, N. A novel aerogel from thermal power plant waste for thermal and acoustic insulation applications. Waste Manag. 2021, 124, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thai, Q.B.; Chong, R.O.; Nguyen, P.T.; Le, D.K.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Recycling of waste tire fibers into advanced aerogels for thermal insulation and sound absorption applications. J. Environ. Chem. Eng. 2020, 8, 104279. [Google Scholar] [CrossRef]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Karkar, Z.; Guyomard, D.; Roué, L.; Lestriez, B. A comparative study of polyacrylic acid (PAA) and carboxymethyl cellulose (CMC) binders for Si-based electrodes. Electrochim. Acta 2017, 258, 453–466. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, C.; Guo, R.; Lan, J.; Lin, S.; Jiang, S.; Lai, X.; Zhang, Y.; Xiao, H. Synthesis of carboxymethyl cellulose-reduced graphene oxide aerogel for efficient removal of organic liquids and dyes. J. Mater. Sci. 2019, 54, 1872–1883. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, L.; Kang, X.; Su, X.; Wang, F.; Wang, C.; Zhao, J.; Chen, Z. Preparation of the crosslinked GO/PAA aerogel and its adsorption properties for Pb (II) ions. Mater. Res. Express 2020, 7, 025514. [Google Scholar] [CrossRef]
- Yi, X.; Xu, Z.; Liu, Y.; Guo, X.; Ou, M.; Xu, X. Highly efficient removal of uranium (VI) from wastewater by polyacrylic acid hydrogels. RSC Adv. 2017, 7, 6278–6287. [Google Scholar] [CrossRef]
- Do, N.H.; Tran, V.T.; Tran, Q.B.; Le, K.A.; Thai, Q.B.; Nguyen, P.T.; Duong, H.M.; Le, P.K. Recycling of pineapple leaf and cotton waste fibers into heat-insulating and flexible cellulose aerogel composites. J. Polym. Environ. 2021, 29, 1112–1121. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Al-Homoud, M.S. Performance characteristics and practical applications of common building thermal insulation materials. Build. Environ. 2005, 40, 353–366. [Google Scholar] [CrossRef]
- Do, N.H.; Luu, T.P.; Thai, Q.B.; Le, D.K.; Chau, N.D.Q.; Nguyen, S.T.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Heat and sound insulation applications of pineapple aerogels from pineapple waste. Mater. Chem. Phys. 2020, 242, 122267. [Google Scholar] [CrossRef]
- Le, D.K.; Leung, R.I.H.; Er, A.S.R.; Zhang, X.; Tay, X.J.; Thai, Q.B.; Phan-Thien, N.; Duong, H.M. Applications of functionalized polyethylene terephthalate aerogels from plastic bottle waste. Waste Manag. 2019, 100, 296–305. [Google Scholar] [CrossRef]
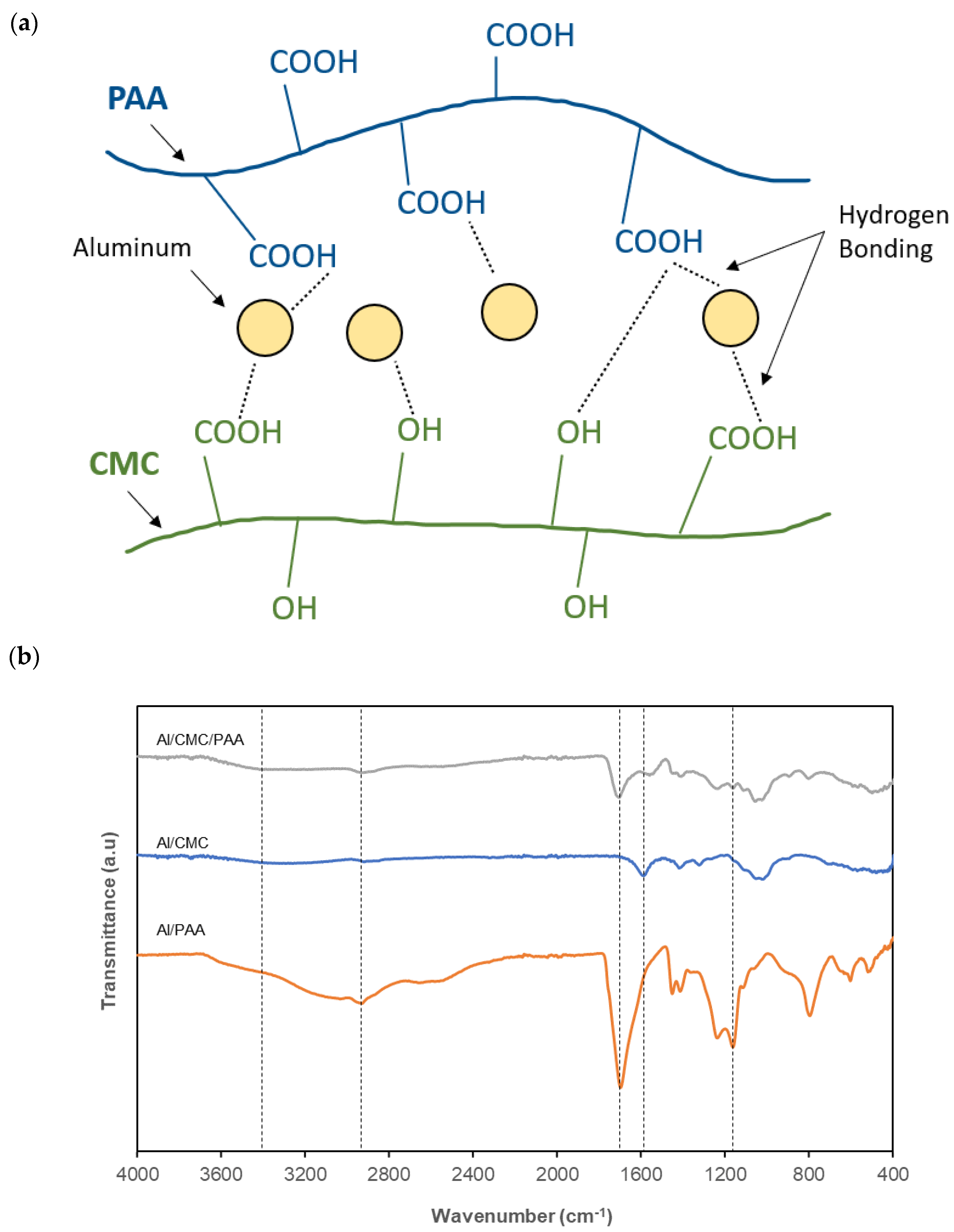
- Li, W.; Sun, B.; Wu, P. Study on hydrogen bonds of carboxymethyl cellulose sodium film with two-dimensional correlation infrared spectroscopy. Carbohydr. Polym. 2009, 78, 454–461. [Google Scholar] [CrossRef]
- Priya, G.; Narendrakumar, U.; Manjubala, I. Thermal behavior of carboxymethyl cellulose in the presence of polycarboxylic acid crosslinkers. J. Therm. Anal. Calorim. 2019, 138, 89–95. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, Y.; Liu, B. Polyacrylic acid functionalized nanographene as a nanocarrier for loading and controlled release of doxorubicin hydrochloride. J. Nanomater. 2013, 2013, 16. [Google Scholar] [CrossRef]
- Datsyuk, V.; Billon, L.; Guerret-Piécourt, C.; Dagréou, S.; Passade-Boupatt, N.; Bourrigaud, S.; Guerret, O.; Couvreur, L. In situ nitroxide-mediated polymerized poly (acrylic acid) as a stabilizer/compatibilizer carbon nanotube/polymer composites. J. Nanomater. 2007, 2007, 074769. [Google Scholar] [CrossRef]
- Lertsarawut, P.; Hemvichian, K.; Rattanawongwiboon, T.; Suwanmala, P. Dye adsorbent prepared by radiation-induced graft polymerization of acrylic acid onto carboxymethyl cellulose. In J. Phys. Conf. Ser.; 2019; Volume 1285, p. 012023. [Google Scholar]
- Laboureur, D.; Glabeke, G.; Gouriet, J. Aluminum nanoparticles oxidation by TGA/DSC. J. Therm. Anal. Calorim. 2019, 137, 1199–1210. [Google Scholar] [CrossRef]
- Do, N.H.; Luu, T.P.; Thai, Q.B.; Le, D.K.; Chau, N.D.Q.; Nguyen, S.T.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Advanced fabrication and application of pineapple aerogels from agricultural waste. Mater. Technol. 2020, 35, 807–814. [Google Scholar] [CrossRef]
- Koh, H.W.; Le, D.K.; Ng, G.N.; Zhang, X.; Phan-Thien, N.; Kureemun, U.; Duong, H.M. Advanced recycled polyethylene terephthalate aerogels from plastic waste for acoustic and thermal insulation applications. Gels 2018, 4, 43. [Google Scholar] [CrossRef]
- Thai, Q.B.; Siang, T.E.; Le, D.K.; Shah, W.A.; Phan-Thien, N.; Duong, H.M. Advanced fabrication and multi-properties of rubber aerogels from car tire waste. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 702–708. [Google Scholar] [CrossRef]
- Si, Y.; Fu, Q.; Wang, X.; Zhu, J.; Yu, J.; Sun, G.; Ding, B. Superelastic and superhydrophobic nanofiber-assembled cellular aerogels for effective separation of oil/water emulsions. ACS Nano 2015, 9, 3791–3799. [Google Scholar] [CrossRef]
- Loh, J.; Goh, X.Y.; Nguyen, P.T.; Thai, Q.B.; Ong, Z.; Duong, H.M. Advanced aerogels from wool waste fibers for oil spill cleaning applications. J. Polym. Environ. 2022, 30, 681–694. [Google Scholar] [CrossRef]
- Chen, N.; Pan, Q. Versatile fabrication of ultralight magnetic foams and application for oil—Water separation. ACS Nano 2013, 7, 6875–6883. [Google Scholar] [CrossRef] [PubMed]
- Thai, Q.B.; Le, D.K.; Do, N.H.; Le, P.K.; Phan-Thien, N.; Wee, C.Y.; Duong, H.M. Advanced aerogels from waste tire fibers for oil spill-cleaning applications. J. Environ. Chem. Eng. 2020, 8, 104016. [Google Scholar] [CrossRef]
- Do, N.H.; Nguyen, T.H.; Pham, B.T.; Nguyen, P.T.; Nguyen, S.T.; Duong, H.M.; Le, P.K. Green fabrication of flexible aerogels from polypropylene fibers for heat insulation and oil/water separation. J. Porous Mater. 2021, 28, 617–627. [Google Scholar] [CrossRef]
- Shi, M.; Tang, C.; Yang, X.; Zhou, J.; Jia, F.; Han, Y.; Li, Z. Superhydrophobic silica aerogels reinforced with polyacrylonitrile fibers for adsorbing oil from water and oil mixtures. RSC Adv. 2017, 7, 4039–4045. [Google Scholar] [CrossRef]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Do, N.H.; Truong, B.Y.; Nguyen, P.T.; Le, K.A.; Duong, H.M.; Le, P.K. Composite aerogels of TEMPO-oxidized pineapple leaf pulp and chitosan for dyes removal. Sep. Purif. Technol. 2022, 283, 120200. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Senturk, E.; Ozkan, T. Adsorption kinetics for the removal of chromium (VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. Water SA 2004, 30, 533–539. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, Y.; Fan, B.; Yao, Q.; Wang, H.; Jin, C.; Sun, Q. Cellulose as an adhesion agent for the synthesis of lignin aerogel with strong mechanical performance, Sound-absorption and thermal Insulation. Sci. Rep. 2016, 6, 32383. [Google Scholar] [CrossRef]
- Le, D.K.; Ng, G.N.; Koh, H.W.; Zhang, X.; Thai, Q.B.; Phan-Thien, N.; Duong, H.M. Methyltrimethoxysilane-coated recycled polyethylene terephthalate aerogels for oil spill cleaning applications. Mater. Chem. Phys. 2020, 239, 122064. [Google Scholar] [CrossRef]


| Aluminum | 4 wt.% (A1) | 6 wt.% (A2) | 8 wt.% (A3) | |
|---|---|---|---|---|
| Oil Absorption Capacity, Qmax (g/g) | 9.73 | 6.82 | 6.13 | |
| Pseudo-first-order | R2 | 0.8891 | 0.9889 | 0.9789 |
| k1 | 0.0150 | 0.0141 | 0.0145 | |
| Pseudo-second-order | R2 | 0.9986 | 0.9975 | 0.9973 |
| k2 | 0.0954 | 0.1265 | 0.1364 | |
| Sample | Al Content (wt.%) | CMC Content (wt.%) | PAA Content (wt.%) | Young’s Modulus (kPa) |
|---|---|---|---|---|
| A1 | 4.00 | 1.50 | 1.50 | 130.5 |
| A2 | 6.00 | 225.2 | ||
| A3 | 8.00 | 170.9 | ||
| A6 | 6.00 | 1.00 | 1.00 | 70.6 |
| A7 | 2.00 | 2.00 | 333.2 |
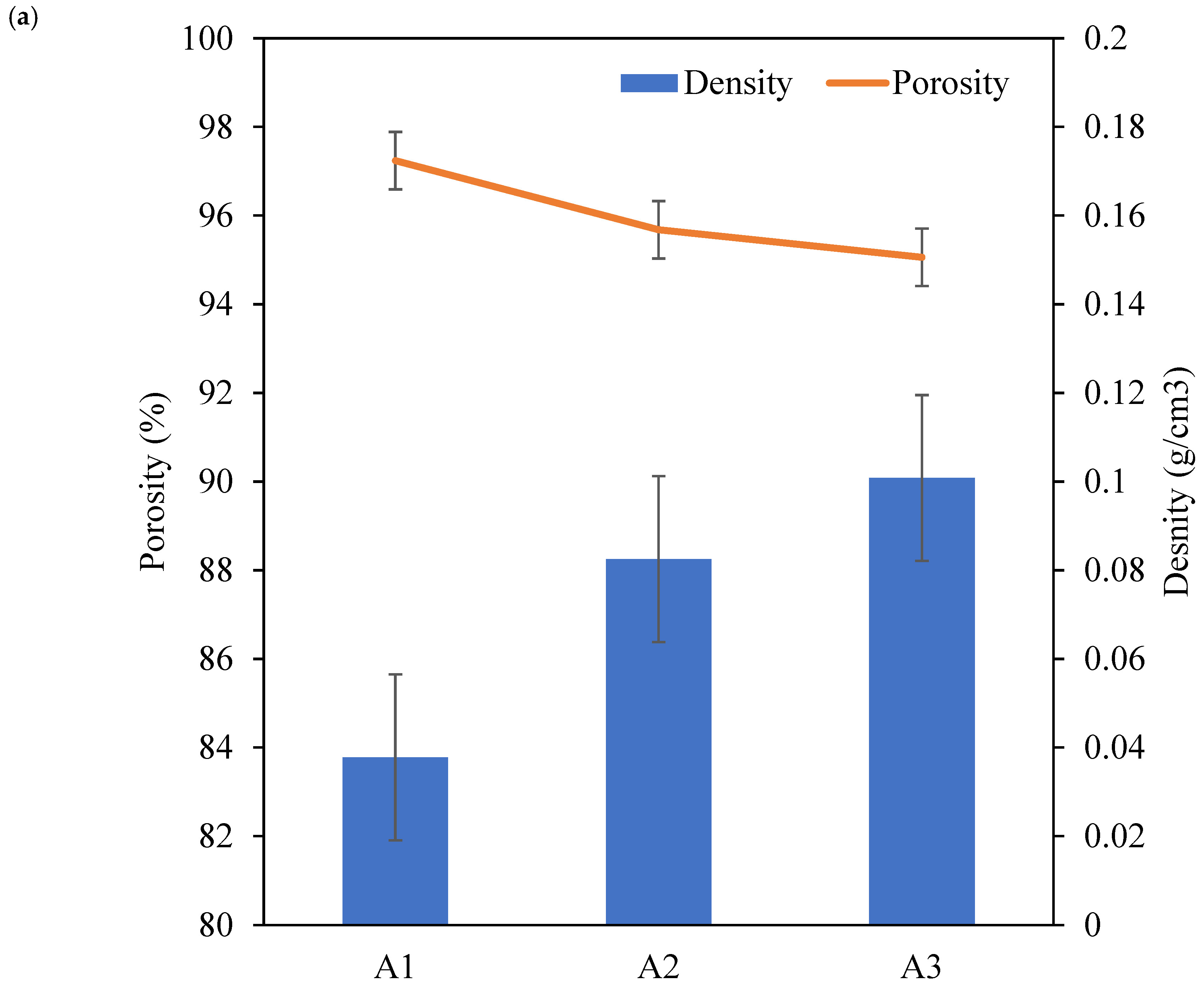
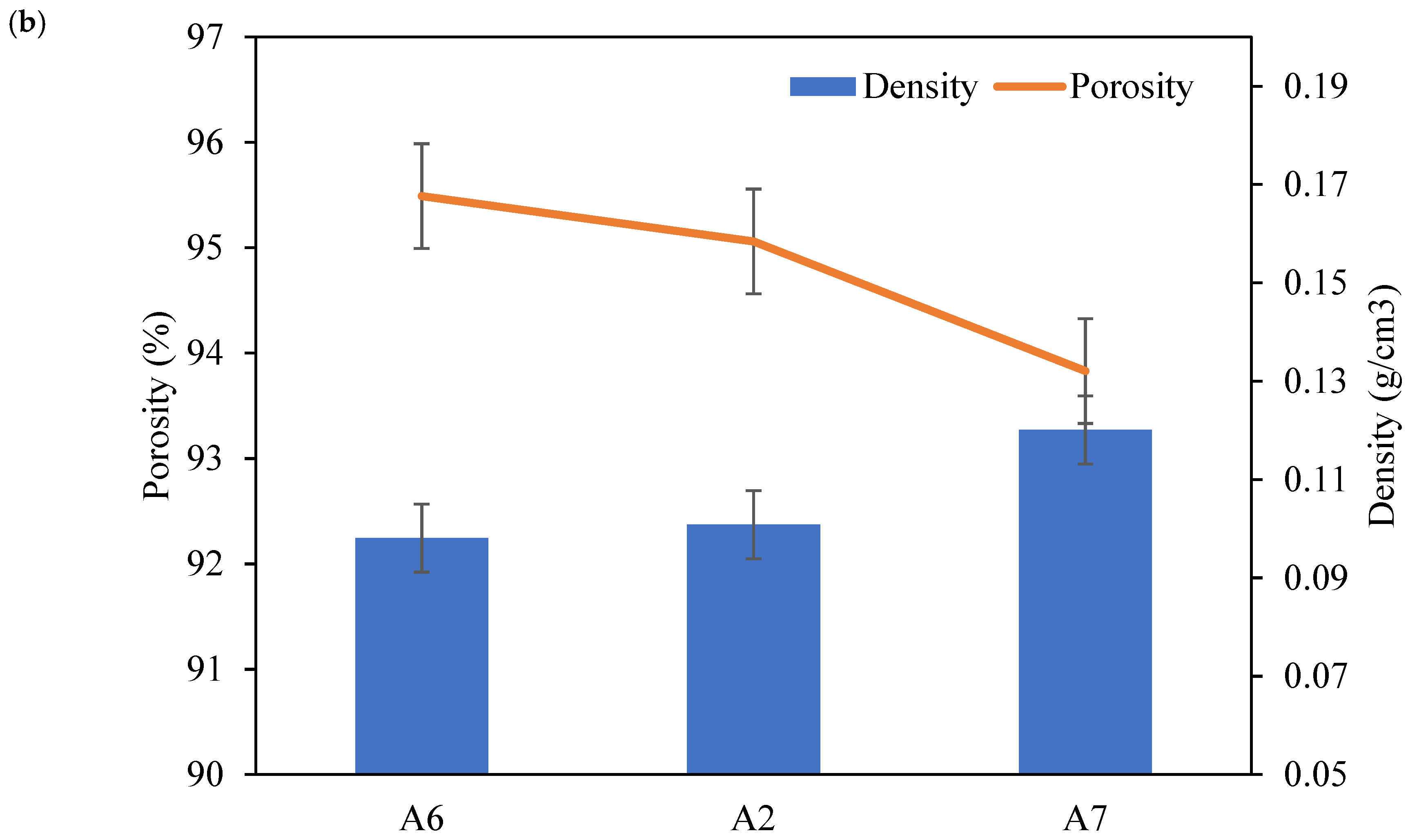
| Sample | Al (wt.%) | CMC (wt.%) | PAA (wt.%) | Density (g/cm3) | Porosity (%) | Thermal Conductivity (W/m·K) |
|---|---|---|---|---|---|---|
| A1 | 4.00 | 1.50 | 1.50 | 0.08 ± 0.02 | 95.68 ± 0.06 | 0.038 ± 0.001 |
| A2 | 6.00 | 0.10 ± 0.03 | 95.06 ± 0.02 | 0.039 ± 0.001 | ||
| A3 | 8.00 | 0.12 ± 0.03 | 94.25 ± 0.04 | 0.041 ± 0.001 | ||
| A4 | 6.00 | 3.00 | 0.00 | 0.10 ± 0.03 | 95.34 ± 0.03 | 0.045 ± 0.001 |
| A5 | 0.00 | 3.00 | 0.10 ± 0.02 | 94.54 ± 0.05 | 0.041 ± 0.001 | |
| A6 | 1.00 | 1.00 | 0.10 ± 0.03 | 95.49 ± 0.02 | 0.043 ± 0.001 | |
| A7 | 2.00 | 2.00 | 0.12 ± 0.02 | 93.83 ± 0.04 | 0.041 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, X.Y.; Ong, R.H.; Nguyen, P.T.T.; Bai, T.; Aw, D.; Li, T.; Nguyen, L.T.; Duong, H.M. Advanced Fabrication and Multi-Properties of Aluminum-Based Aerogels from Aluminum Waste for Thermal Insulation and Oil Absorption Applications. Molecules 2023, 28, 2727. https://doi.org/10.3390/molecules28062727
Goh XY, Ong RH, Nguyen PTT, Bai T, Aw D, Li T, Nguyen LT, Duong HM. Advanced Fabrication and Multi-Properties of Aluminum-Based Aerogels from Aluminum Waste for Thermal Insulation and Oil Absorption Applications. Molecules. 2023; 28(6):2727. https://doi.org/10.3390/molecules28062727
Chicago/Turabian StyleGoh, Xue Yang, Ren Hong Ong, Phuc T. T. Nguyen, Tianliang Bai, Dave Aw, Tian Li, Luon Tan Nguyen, and Hai M. Duong. 2023. "Advanced Fabrication and Multi-Properties of Aluminum-Based Aerogels from Aluminum Waste for Thermal Insulation and Oil Absorption Applications" Molecules 28, no. 6: 2727. https://doi.org/10.3390/molecules28062727
APA StyleGoh, X. Y., Ong, R. H., Nguyen, P. T. T., Bai, T., Aw, D., Li, T., Nguyen, L. T., & Duong, H. M. (2023). Advanced Fabrication and Multi-Properties of Aluminum-Based Aerogels from Aluminum Waste for Thermal Insulation and Oil Absorption Applications. Molecules, 28(6), 2727. https://doi.org/10.3390/molecules28062727






