Purification and Structure Characterization of the Crude Polysaccharide from the Fruiting Bodies of Butyriboletus pseudospeciosus and Its Modulation Effects on Gut Microbiota
Abstract
1. Introduction
2. Results and Discussion
2.1. Species Identification
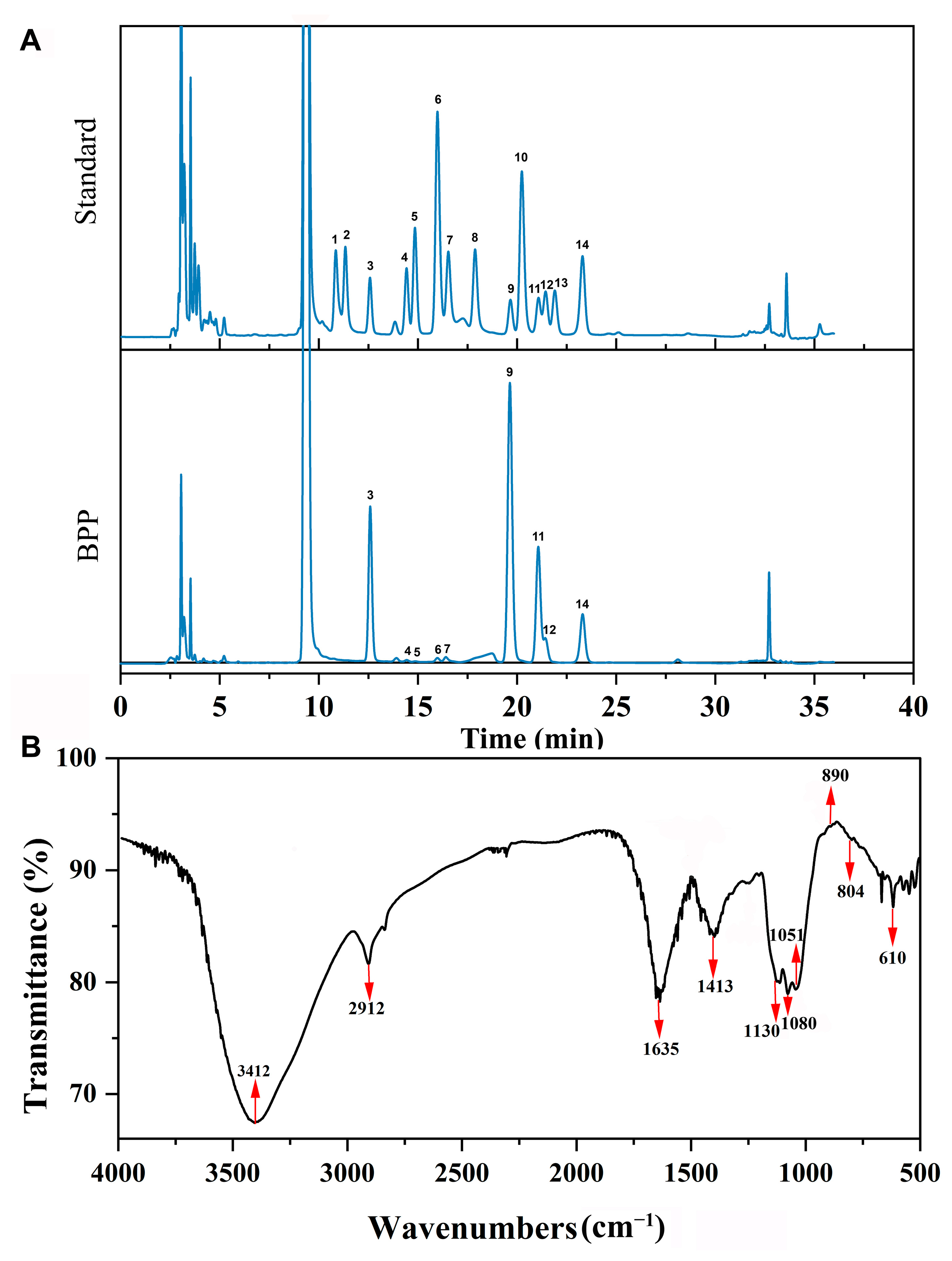
2.2. Chemical Composition of BPP
2.3. FTIR Analysis of BPP
2.4. NMR Spectroscopy Analysis
2.5. Thermal Stability of BPP
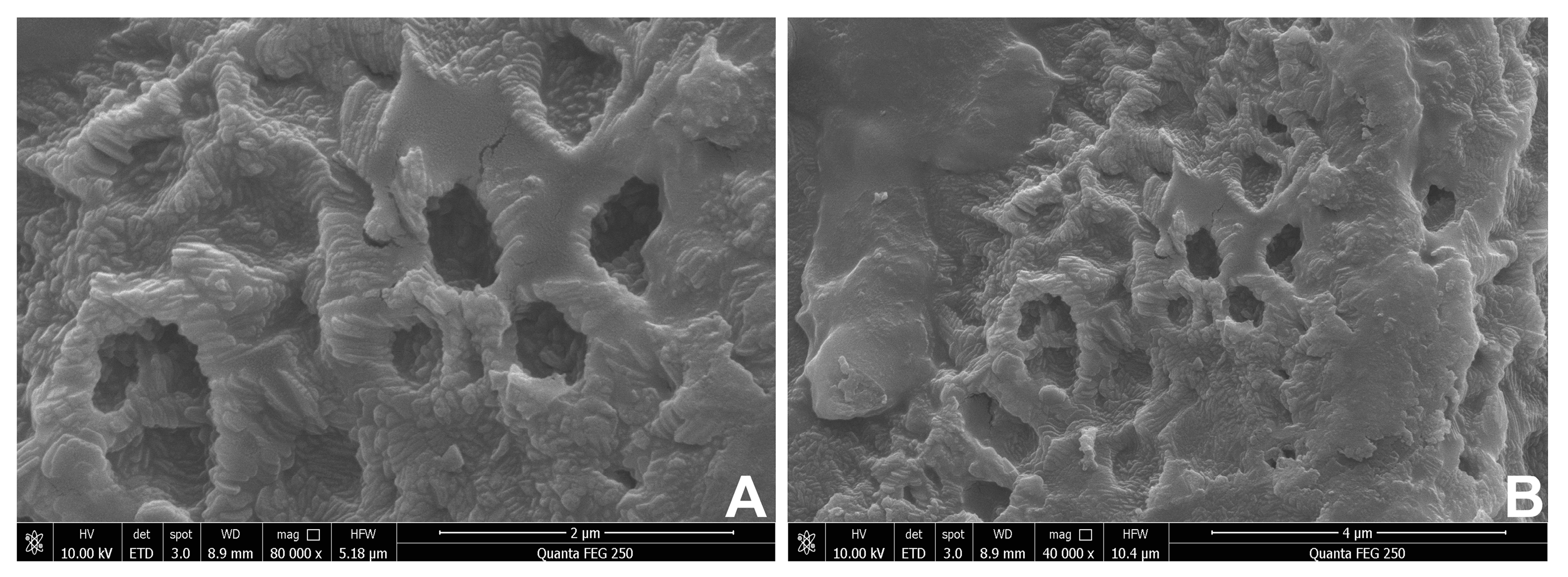
2.6. Scanning Electron Microscope (SEM) Analysis
2.7. BPP Influences Viscera Weight
2.8. Biochemical Analyses of Feces and Serum
2.9. BPP Influences Gut Microbiota
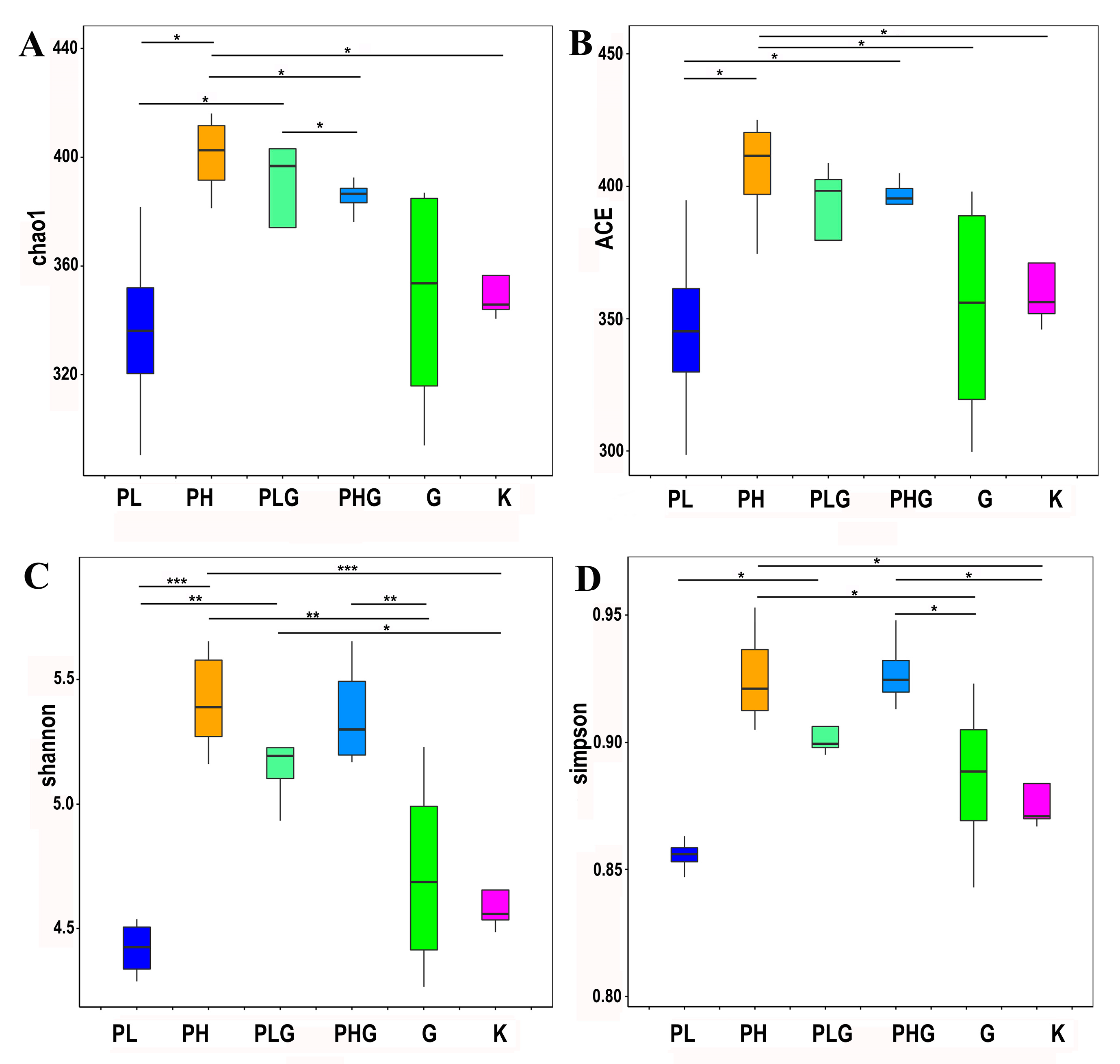
2.9.1. BPP Influences Gut Microbiota Diversity
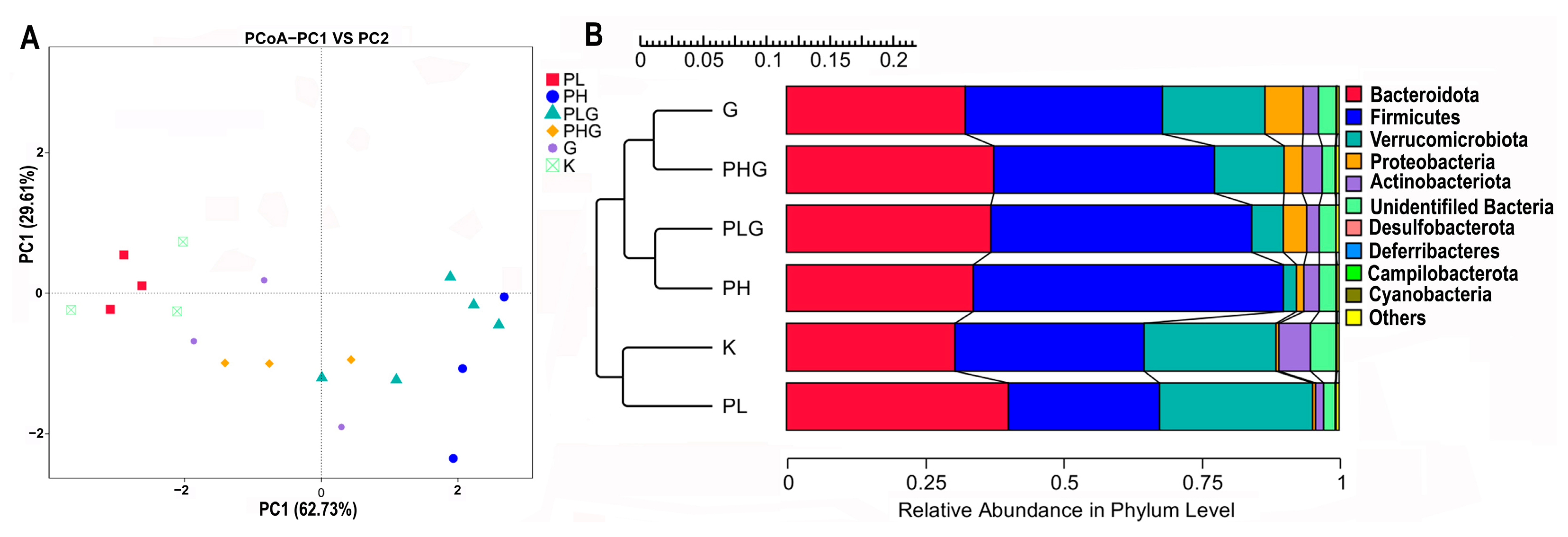
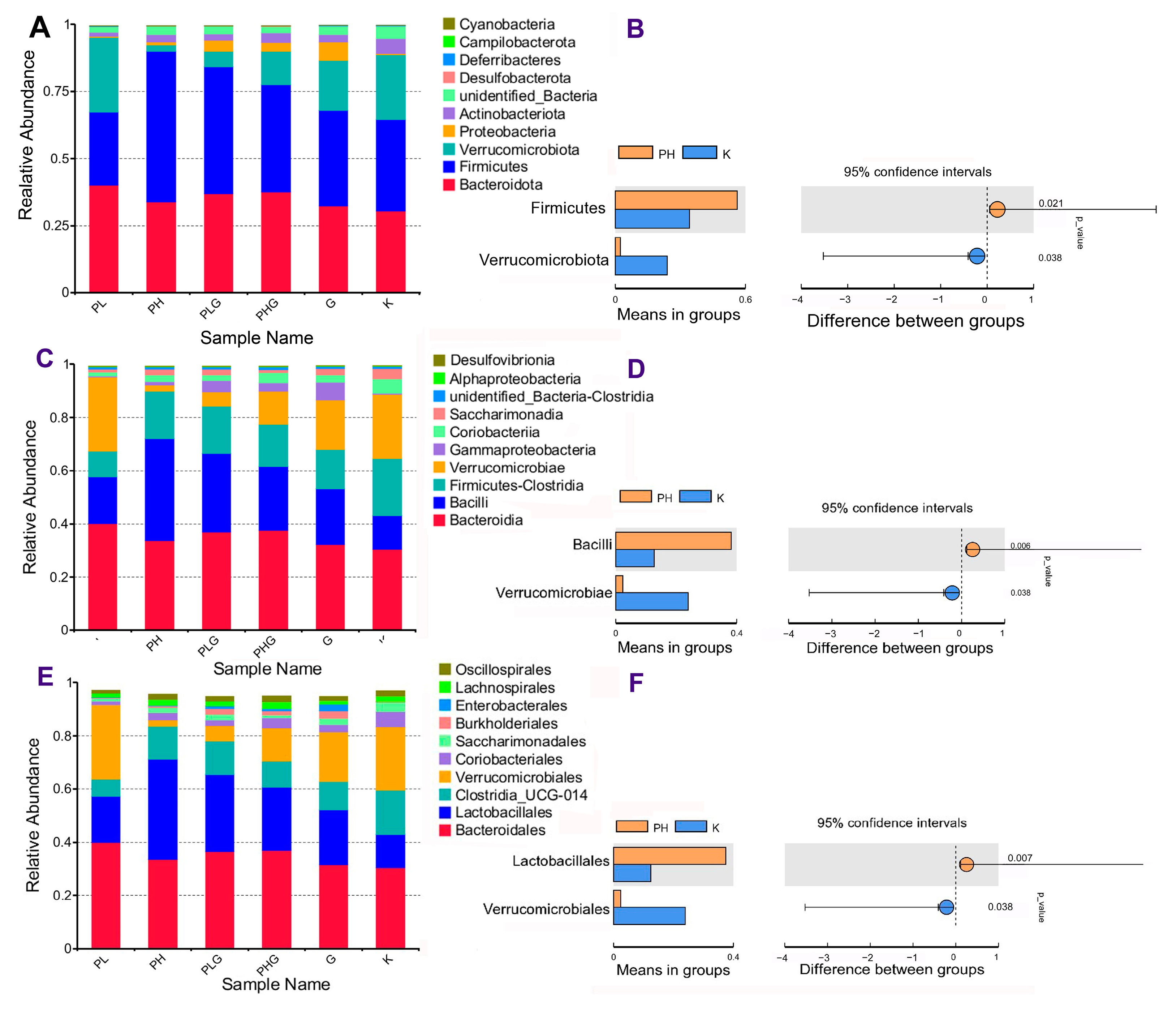
2.9.2. BPP Influences Gut Microbiota Composition
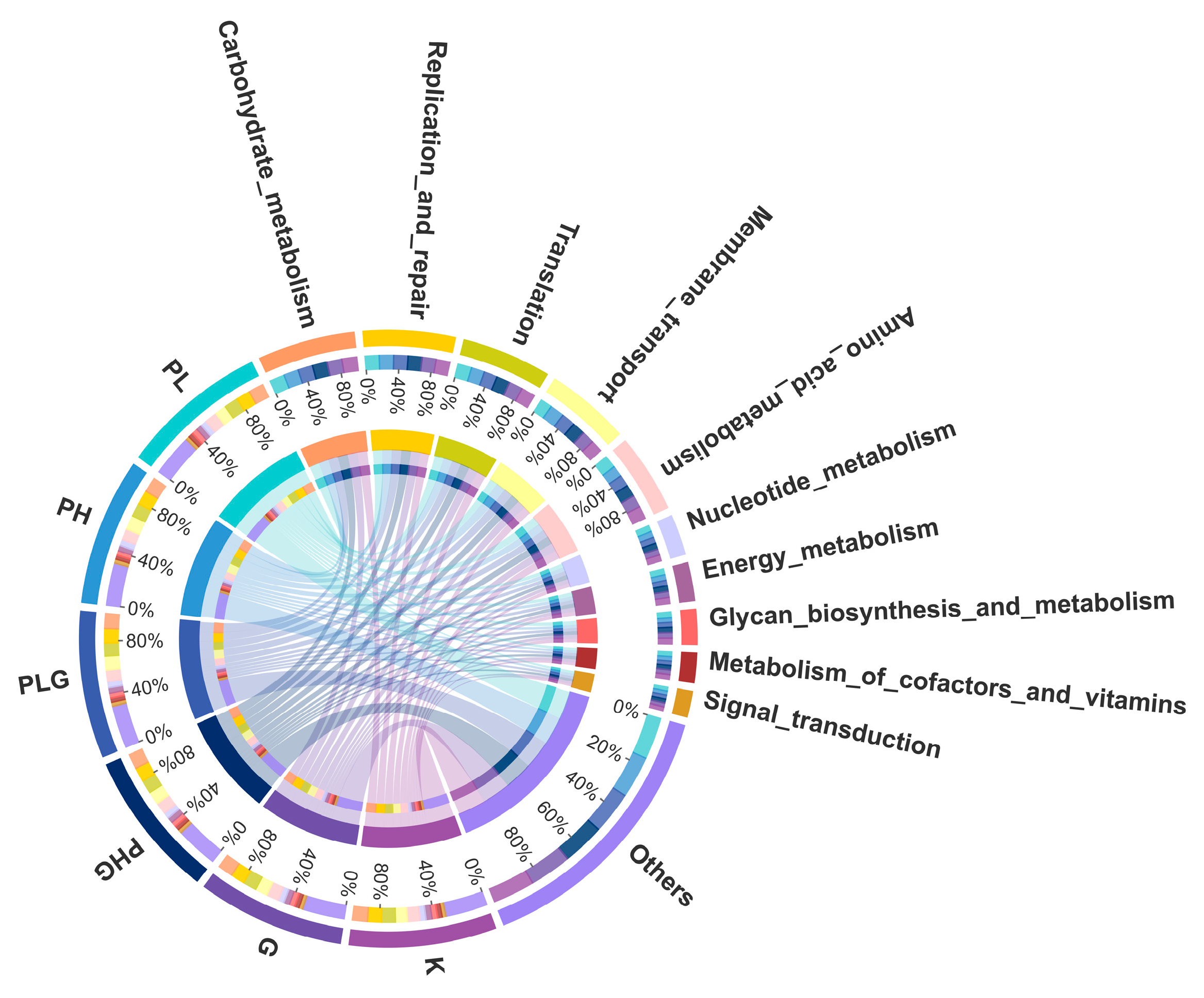
2.9.3. BPP Influences Gut Microbiota Functions
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of the Crude Polysaccharide
3.3. Monosaccharide Composition Analysis
3.4. Infrared Spectrum (IR) Analysis
3.5. Thermal Stability of BPP
3.6. NMR Spectroscopy Analysis
3.7. Scanning Electron Microscope (SEM) Analysis
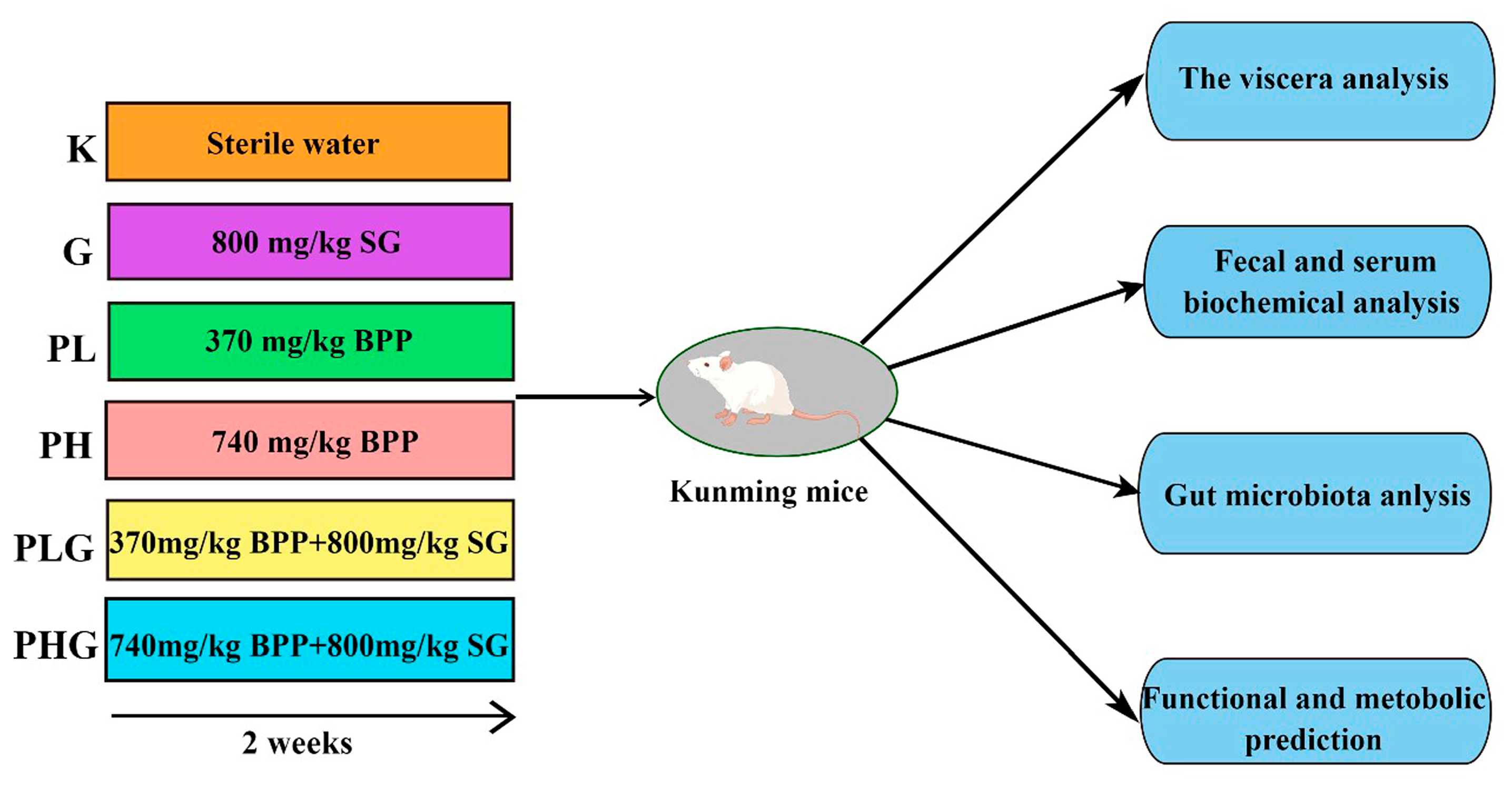
3.8. Animal Study
3.9. Serum and Feces Biochemical Analysis
3.10. Fecal Flora Genomic DNA Extraction and Amplicon Sequencing
3.11. Data Analysis and Bioinformatics
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zeng, N.K.; Tang, L.P.; Li, Y.C.; Tolgor, B.; Zhu, X.T.; Zhao, Q.; Yang, Z.L. The genus Phylloporus (Boletaceae, Boletales) from China: Morphological and multilocus DNA sequence analyses. Fungal Divers. 2013, 58, 73–101. [Google Scholar] [CrossRef]
- Zeng, N.K.; Chai, H.; Liang, Z.Q.; Tang, L.P.; Xue, R.; Yang, Z.L. The genus Heimioporus in China. Mycologia 2018, 110, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Liang, Z.Q.; Xue, R.; Fan, Y.G.; Jiang, S.; Fu, Y.Q.; Zeng, N.K.; Su, M.S. The genus Crocinoboletus (Boletaceae, Boletales): A new species and updated information for previously described species. Phytotaxa 2019, 419, 91–99. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Z.Q.; Jiang, S.; Xu, C.; Fu, X.H.; Zeng, N.K. Baorangia duplicatopora (Boletaceae, Boletales), a new bolete from tropical China. Phytotaxa 2021, 508, 49–58. [Google Scholar] [CrossRef]
- Hou, Y.; Ding, X.; Hou, W.; Song, B.; Wang, T.; Wang, F.; Li, J.; Zeng, Y.; Zhong, J.; Xu, T.; et al. Pharmacological evaluation for anticancer and immune activities of a novel polysaccharide isolated from Boletus speciosus Frost. Mol. Med. Rep. 2014, 9, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Ke, Y.; Liu, Y.; Luo, X.; Li, C.; Zhang, Z.; Liu, A.; Shen, L.; Chen, H.; et al. Antidiabetic activity of polysaccharides from Suillellus luridus in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2018, 119, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Chai, H.; Qiu, J.Q.; Liang, Z.Q.; Xie, H.J.; Wang, Y.; Zeng, N.K. Preparation, structural characterisation, and antioxidant activities of polysaccharides from eight boletes (Boletales) in tropical China. Mycology 2022, 13, 195–206. [Google Scholar] [CrossRef]
- Tan, Y.; Zeng, N.K.; Xu, B. Chemical profiles and health-promoting effects of porcini mushroom (Boletus edulis): A narrative review. Food Chem. 2022, 390, 133199. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Yu, S.S.; Ji, H.Y.; Xu, X.M.; Liu, A.J. A novel acid polysaccharide from Boletus edulis: Extraction, characteristics and antitumor activities in vitro. Glycoconj. J. 2021, 38, 13–24. [Google Scholar] [CrossRef]
- Li, Y.; You, L.; Dong, F.; Yao, W.; Chen, J. Structural characterization, antiproliferative and immunoregulatory activities of a polysaccharide from Boletus Leccinum rugosiceps. Int. J. Biol. Macromol. 2020, 157, 106–118. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, T.; Fan, J.; Zhuang, Y.; Sun, L. Protective effects of a polysaccharide from Boletus aereus on S180 tumor-bearing mice and its structural characteristics. Int. J. Biol. Macromol. 2021, 188, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Guo, C.; Guo, D.; Wu, J.; Wang, Y.; Wang, Y.; Chen, J.; Chen, C.; Wu, K.; Na, K.; et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Hu, H.; Xiao, X.; Chen, D.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, J.; Luo, Y.; et al. Lentinan administration relieves gut barrier dysfunction induced by rotavirus in a weaned piglet model. Food Funct. 2019, 10, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wan, X.; Zeng, F.; Zhong, R.; Guo, W.; Lv, X.C.; Zhao, C.; Liu, B. Regulatory effect of Grifola frondosa extract rich in polysaccharides and organic acids on glycolipid metabolism and gut microbiota in rats. Int. J. Biol. Macromol. 2020, 155, 1030–1039. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.C.; Zhu, X.T.; Zhao, K.; Han, L.H.; Cui, Y.Y.; Li, F.; Xu, J.P.; Yang, Z.L. One hundred noteworthy boletes from China. Fungal Divers. 2016, 81, 25–188. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Anaya, N.M.; Schifman, L.A.; Oyanedel-Craver, V. Fourier transform infrared spectroscopy to assess molecular-level changes in microorganisms exposed to nanoparticles. Nanotechnol. Environ. Eng. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Hu, X.; Xu, F.; Li, J.; Li, J.; Mo, C.; Zhao, M.; Wang, L. Ultrasonic-assisted extraction of polysaccharides from coix seeds: Optimization, purification, and in vitro digestibility. Food Chem. 2022, 374, 131636. [Google Scholar] [CrossRef]
- Nicely, W.B. Infrared spectra of carbohydrates. Advances in Carbohydr. Chem. 1957, 12, 13–33. [Google Scholar]
- Jia, Y.; Wang, Y.; Li, R.; Li, S.; Zhang, M.; He, C.; Chen, H. The structural characteristic of acidic-hydrolyzed corn silk polysaccharides and its protection on the H2O2-injured intestinal epithelial cells. Food Chem. 2021, 356, 129691. [Google Scholar] [CrossRef]
- Wan, X.; Jin, X.; Wu, X.; Yang, X.; Lin, D.; Li, C.; Fu, Y.; Liu, Y.; Liu, X.; Lv, J.; et al. Structural characterization and antitumor activity against non-small cell lung cancer of polysaccharides from Sanghuangporus vaninii. Carbohydr. Polym. 2022, 276, 118798. [Google Scholar] [CrossRef]
- Yu, S.; Ji, H.; Dong, X.; Liu, A.J.; Yu, J. FAS/FAS-L-mediated apoptosis and autophagy of SPC-A-1 cells induced by water-soluble polysaccharide from Polygala tenuifolia. Int. J. Biol. Macromol. 2020, 150, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N.; Neiss, T.G. Solution NMR spectroscopy of food polysaccharides. Poly. Rev. 2012, 52, 81–114. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, B.; Li, C.; Huang, Q.; Fu, X.; Liu, R.H. Structure and in vitro hypoglycemic activity of a homogenous polysaccharide purified from Sargassum pallidum. Food Funct. 2019, 10, 2828–2838. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.R.; Chen, L.H.; Zeng, Y.J. Structure, antioxidant activity and in vitro hypoglycemic activity of a polysaccharide purified from Tricholoma matsutake. Foods 2021, 10, 2184. [Google Scholar] [CrossRef]
- Qu, H.; Gao, X.; Zhao, H.T.; Wang, Z.Y.; Yi, J.J. Structural characterization and in vitro hepatoprotective activity of polysaccharide from pine nut (Pinus koraiensis Sieb. et Zucc.). Carbohydr. Polym. 2020, 1223, 115056. [Google Scholar] [CrossRef]
- Kuang, M.T.; Xu, J.Y.; Li, J.Y.; Yang, L.; Hou, B.; Zhao, Q.; Hu, J.M. Purification, structural characterization and immunomodulatory activities of a polysaccharide from the fruiting body of Morchella sextelata. Int. J. Biol. Macromol. 2022, 213, 394–403. [Google Scholar] [CrossRef]
- Yu, S.; Ma, R.; Dong, X.; Ji, H.; Liu, A. A novel polysaccharide from Boletus edulis: Extraction, purification, characterization and immunologic activity. Ind. Crops Prod. 2022, 186, 115206. [Google Scholar] [CrossRef]
- Alzorqi, I.; Sudheer, S.; Lu, T.J.; Manickam, S. Ultrasonically extracted β-d-glucan from artificially cultivated mushroom, characteristic properties and antioxidant activity. Ultrason. Sonochem. 2017, 35, 531–540. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Isolation, purification and physicochemical properties of polysaccharide from fruiting body of Hericium erinaceus and its effect on colonic health of mice. Int. J. Biol. Macromol. 2018, 107, 1310–1319. [Google Scholar] [CrossRef]
- Nataraj, A.; Govindan, S.; Ramani, P.; Subbaiah, K.A.; Sathianarayanan, S.; Venkidasamy, B.; Thiruvengadam, M.; Rebezov, M.; Shariati, M.A.; Lorenzo, J.M.; et al. Antioxidant, anti-tumour, and anticoagulant activities of polysaccharide from Calocybe indica (apk2). Antioxidants 2022, 11, 1694. [Google Scholar] [CrossRef]
- Hu, J.; Nie, S.; Li, C.; Xie, M. In vitro fermentation of polysaccharide from the seeds of Plantago asiatica L. by human fecal microbiota. Food Hydrocoll. 2013, 33, 384–392. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Honzawa, Y.; Nakase, H.; Matsuura, M.; Chiba, T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: Importance of evaluation of small intestinal permeability. Inflamm. Bowel Dis. 2011, 17, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Llorente, C.; Hartmann, P.; Yang, A.M.; Chen, P.; Schnabl, B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods. 2015, 421, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Ye, J.; Wang, X.; Wang, K.; Deng, Y.; Yang, Y.; Ali, R.; Chen, F.; Wu, Z.; Liao, W.; Mao, L. A novel polysaccharide isolated from Flammulina velutipes, characterization, macrophage immunomodulatory activities and its impact on gut microbiota in rats. J. Anim. Physio. Anim. Nutr. 2020, 104, 735–748. [Google Scholar] [CrossRef]
- Li, K.; Zhuo, C.; Teng, C.; Yu, S.; Wang, X.; Hu, Y.; Ren, G.; Yu, M.; Qu, J. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int. J. Biol. Macromol. 2016, 93, 904–912. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Li, Z.; Jia, H.; Liang, Y.; Hao, G. Effects of Baohe pills on blood lipid in SD rats with a high-fat diet on the basis of intestinal Microbiota. World, J. Chin. Med. 2018, 13, 2107–2116. [Google Scholar]
- Wang, Y.; Zhang, N.; Kan, J.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Liu, J.; Qian, C.; Jin, C. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt fruit attenuates hyperglycemia and hyperlipidemia and regulates colon microbiota in diabetic db/db mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Ji, X.; Zhou, Z.; Liu, Y.; Sun, T.; Zhang, L. Effects of fucoidan on gut flora and tumor prevention in 1,2-dimethylhydrazine-induced colorectal carcinogenesis. J. Nutr. Biochem. 2020, 82, 108396. [Google Scholar] [CrossRef] [PubMed]
- Bellaguarda, E.; Chang, E.B. IBD and the gut microbiota–From bench to personalized medicine. Curr. Gastroenterol. Rep. 2015, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Satish Kumar, C.S.; Kondal Reddy, K.; Reddy, A.G.; Vinoth, A.; Ch, S.R.; Boobalan, G.; Rao, G.S. Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. Int. Immunopharmacol. 2015, 25, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Perelmuter, K.; Fraga, M.; Zunino, P. In vitro activity of potential probiotic Lactobacillus murinus isolated from the dog. J. Appl. Microbiol. 2008, 104, 1718–1725. [Google Scholar] [CrossRef]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S Rrna data. Bioinf. 2015, 31, 2882–2884. [Google Scholar] [CrossRef]
- Su, L.; Li, D.; Su, J.; Zhang, E.; Chen, S.; Zheng, C.; Luo, T.; Li, M.; Chen, X.; Huang, G.; et al. Polysaccharides of sporoderm-broken spore of Ganoderma lucidum modulate adaptive immune function via gut microbiota regulation. Evid. Based Complement Alternat. Med. 2021, 2021, 8842062. [Google Scholar] [CrossRef]
- Su, J.; Li, D.; Chen, Q.; Li, M.; Su, L.; Luo, T.; Liang, D.; Lai, G.; Shuai, O.; Jiao, C.; et al. Anti-breast cancer enhancement of a polysaccharide from spore of Ganoderma lucidum with paclitaxel: Suppression on tumor metabolism with gut microbiota reshaping. Front Microbiol. 2018, 9, 3099. [Google Scholar] [CrossRef]
- Chai, H.; Liang, Z.Q.; Xue, R.; Jiang, S.; Luo, S.H.; Wang, Y.; Wu, L.L.; Tang, L.P.; Chen, Y.; Hong, D.; et al. New and noteworthy boletes from subtropical and tropical China. MycoKeys 2019, 18, 55–96. [Google Scholar] [CrossRef]
- Yang, B.; Luo, Y.; Sang, Y.; Kan, J. Isolation, purification, structural characterization, and hypoglycemic activity assessment of polysaccharides from Hovenia dulcis (Guai Zao). Int. J. Biol. Macromol. 2022, 208, 1106–1115. [Google Scholar] [CrossRef]
- Joseph, J.; Sajeesh, A.K.; Nagashri, K.; Edinsha Gladis, E.H.; Shamina, J.; Dhanaraj, C. Determination of ammonia content in various drinking water sources in Malappuram District, Kerala and its removal by adsorption using agricultural waste materials. Mater. Today Proc. 2020, 4, 121–127. [Google Scholar] [CrossRef]
- Jin, R.; Shan, Q.; Yuan, C. Structural characterization of camellia seed meal polysaccharide and its effect on intestinal microflora. Farm Prod. Process. 2022, 1, 7–13. [Google Scholar]


| Chemical Composition (w/w, %) | |
|---|---|
| Carbohydrate | 64.00 |
| Protein | 1.95 |
| Uronic acid | 1.19 |
| Monosaccharide Component (%) | |
| Mannose (Man) | 17.12 |
| Ribose (Rib) | 0.16 |
| Rhamnose (Rham) | 0.018 |
| Glucosamine(GlcN) | 0.11 |
| Glucuronic acid(GlcUA) | 0.38 |
| Glucose (Glc) | 54.87 |
| Galactose (Gal) | 20.58 |
| Xylose (Xyl) | 3.02 |
| Fucose (Fuc) | 3.82 |
| Groups | Liver (%) | Spleen (%) | Thymus (%) |
|---|---|---|---|
| K | 4.42 ± 0.33 | 0.19 ± 0.01 | 0.86 ± 0.10 |
| G | 4.85 ± 0.62 | 0.26 ± 0.03 * | 1.11 ± 0.22 * |
| PL | 4.47 ± 0.62 | 0.24 ± 0.05 | 1.11 ± 0.08 * |
| PH | 4.50 ± 0.46 | 0.26 ± 0.03 * | 1.12 ± 0.17 * |
| PLG | 4.45 ± 0.49 | 0.25 ± 0.04 | 1.12 ± 0.24 * |
| PHG | 5.16 ± 0.44 * | 0.27 ± 0.04 * | 1.12 ± 0.19 * |
| Groups | pH Value | Ammonia Content (μg/g) |
|---|---|---|
| K | 8.32 ± 0.07 | 44.72 ± 4.13 |
| G | 8.22 ± 0.09 * | 40.38 ± 0.66 ** |
| PL | 8.23 ± 0.05 * | 41.75 ± 0.66 * |
| PH | 8.18 ± 0.01 ** | 41.43 ± 0.27 * |
| PLG | 8.04 ± 0.05 ** | 42.89 ± 0.47 |
| PHG | 8.03 ± 0.09 ** | 40.89 ± 0.11 * |
| Groups | DAO (pg/mL) | ET (EU/mL) |
|---|---|---|
| K | 430.56 ± 34.69 | 29.43 ± 1.34 |
| G | 234.41 ± 32.52 ** | 30.70 ± 1.40 |
| PL | 322.75 ± 24.97 ** | 26.69 ± 3.75 * |
| PH | 339.30 ± 26.34 ** | 23.13 ± 1.74 ** |
| PLG | 333.08 ± 29.43 ** | 37.70 ± 2.04 ** |
| PHG | 319.35 ± 37.83 ** | 37.11 ± 1.06 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, R.; Wu, L.-L.; Li, H.-F.; Liang, Z.-Q.; Li, P.-H.; Wang, Y.; Zeng, N.-K. Purification and Structure Characterization of the Crude Polysaccharide from the Fruiting Bodies of Butyriboletus pseudospeciosus and Its Modulation Effects on Gut Microbiota. Molecules 2023, 28, 2679. https://doi.org/10.3390/molecules28062679
Tian R, Wu L-L, Li H-F, Liang Z-Q, Li P-H, Wang Y, Zeng N-K. Purification and Structure Characterization of the Crude Polysaccharide from the Fruiting Bodies of Butyriboletus pseudospeciosus and Its Modulation Effects on Gut Microbiota. Molecules. 2023; 28(6):2679. https://doi.org/10.3390/molecules28062679
Chicago/Turabian StyleTian, Run, Lu-Ling Wu, Hong-Fu Li, Zhi-Qun Liang, Pei-Hu Li, Yong Wang, and Nian-Kai Zeng. 2023. "Purification and Structure Characterization of the Crude Polysaccharide from the Fruiting Bodies of Butyriboletus pseudospeciosus and Its Modulation Effects on Gut Microbiota" Molecules 28, no. 6: 2679. https://doi.org/10.3390/molecules28062679
APA StyleTian, R., Wu, L.-L., Li, H.-F., Liang, Z.-Q., Li, P.-H., Wang, Y., & Zeng, N.-K. (2023). Purification and Structure Characterization of the Crude Polysaccharide from the Fruiting Bodies of Butyriboletus pseudospeciosus and Its Modulation Effects on Gut Microbiota. Molecules, 28(6), 2679. https://doi.org/10.3390/molecules28062679




