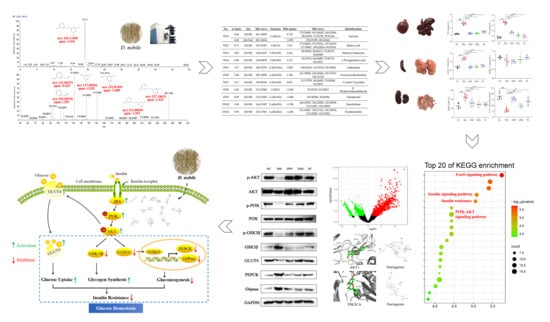
Chemical Constituents and Hypoglycemic Mechanisms of Dendrobium nobile in Treatment of Type 2 Diabetic Rats by UPLC-ESI-Q-Orbitrap, Network Pharmacology and In Vivo Experimental Verification
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Constituents of D. nobile
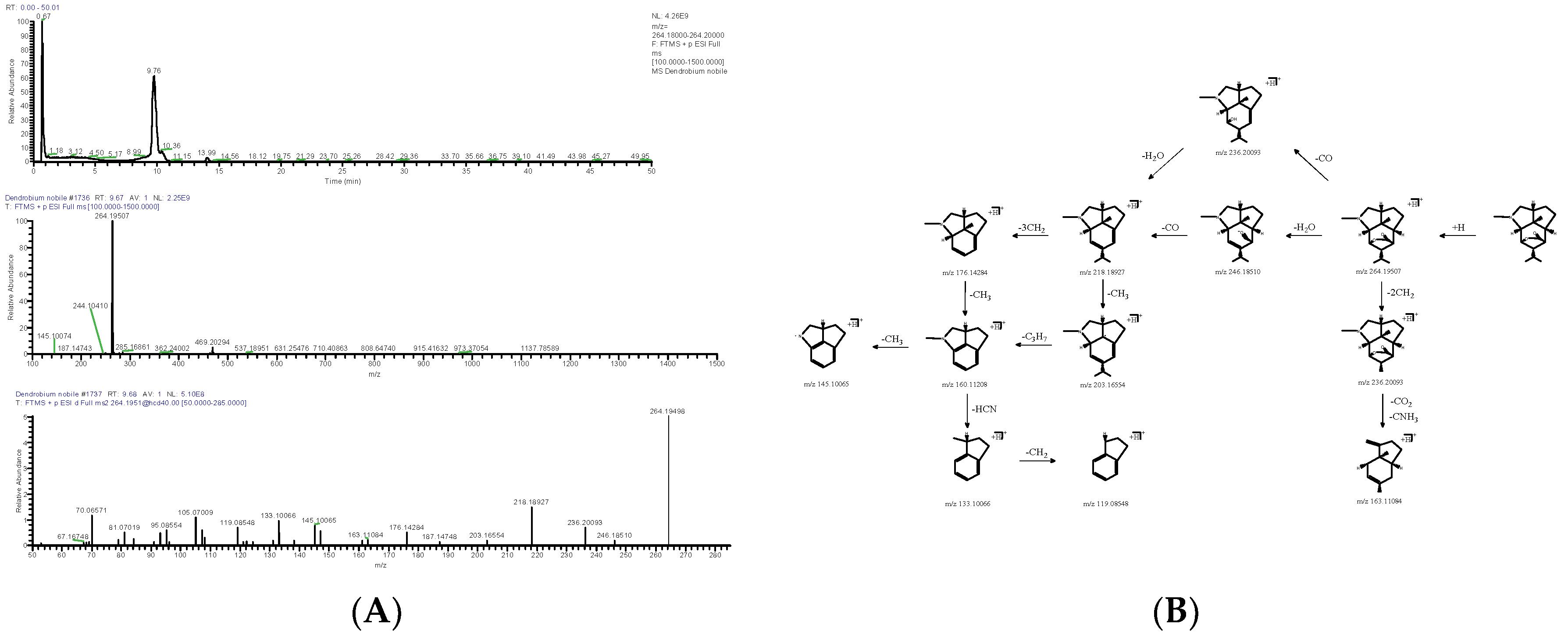
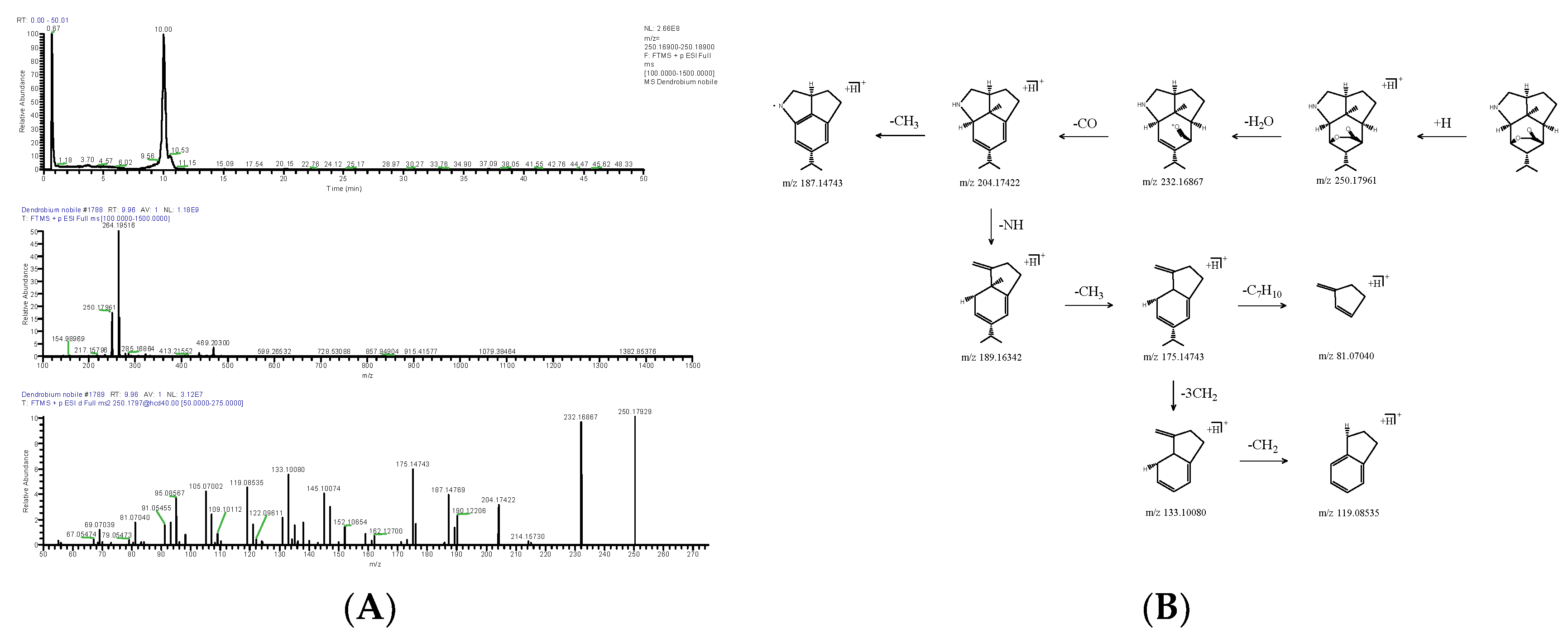
2.1.1. Alkaloids
2.1.2. Bibenzyl
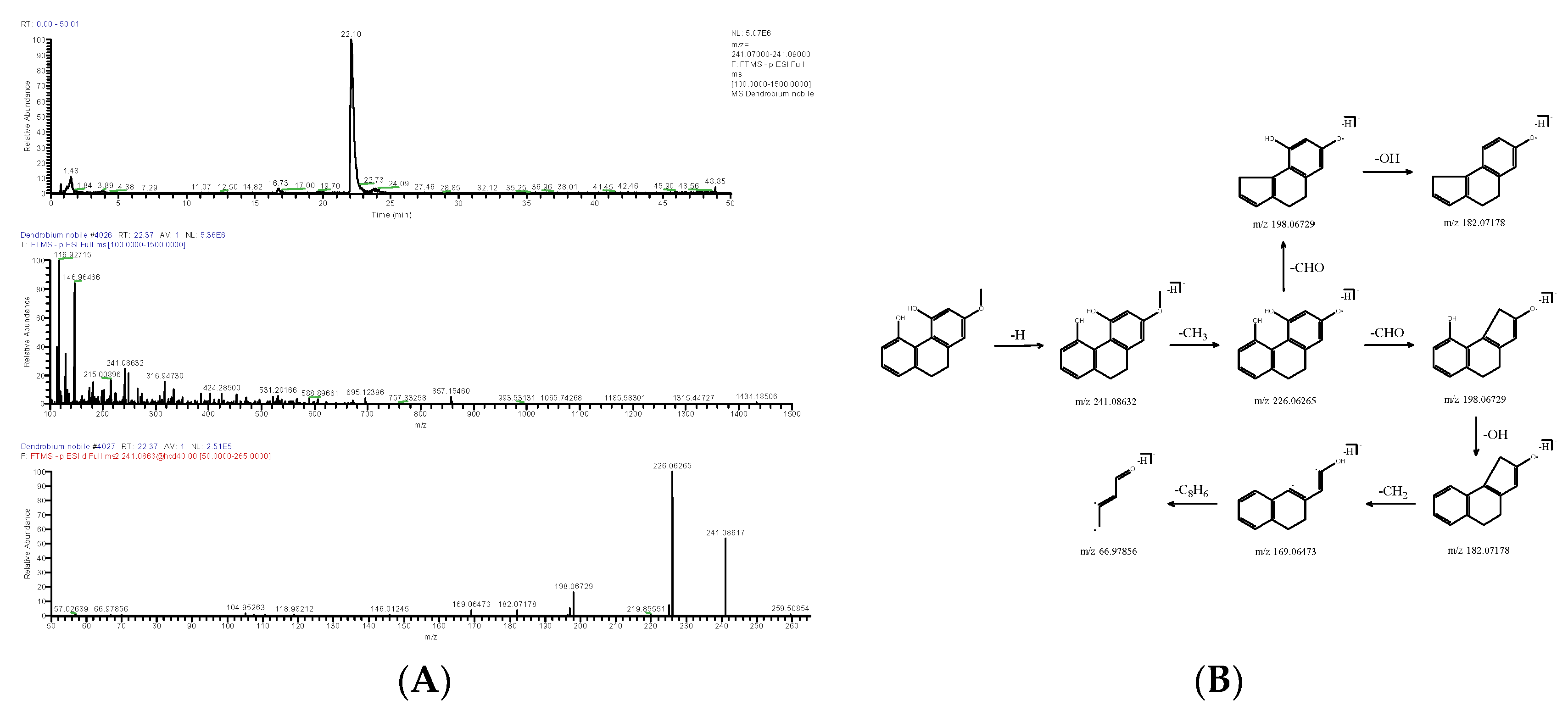
2.1.3. Phenanthrene
2.1.4. Other
2.2. Hypoglycemic Effects of D. nobile
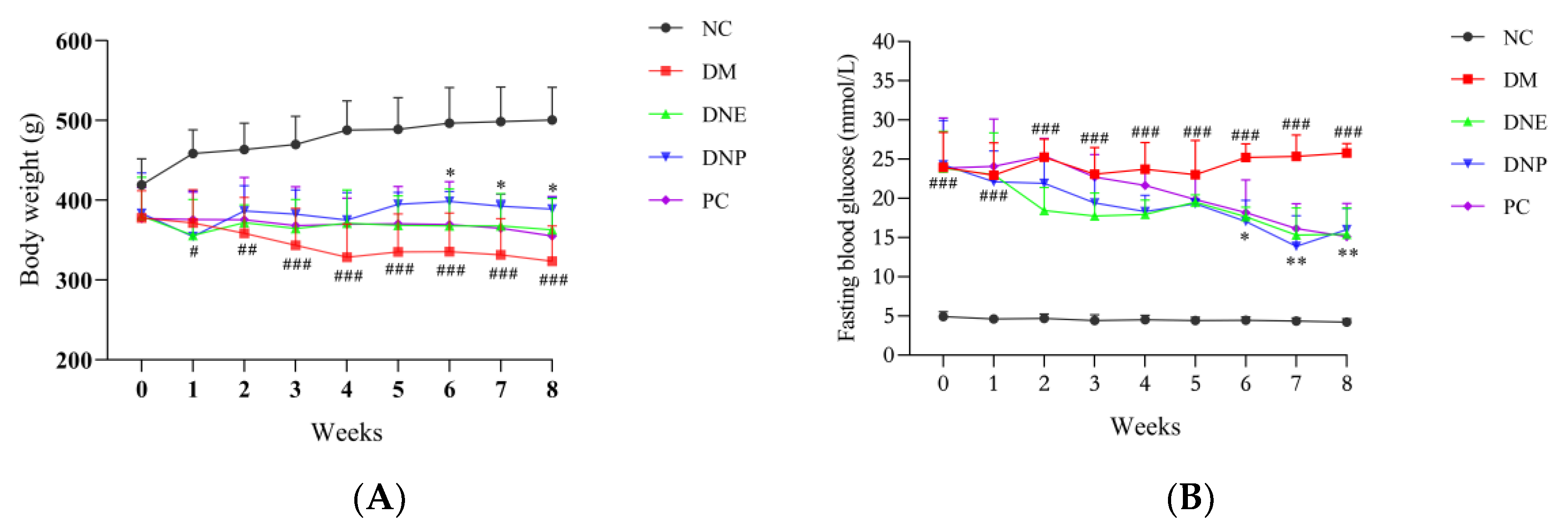
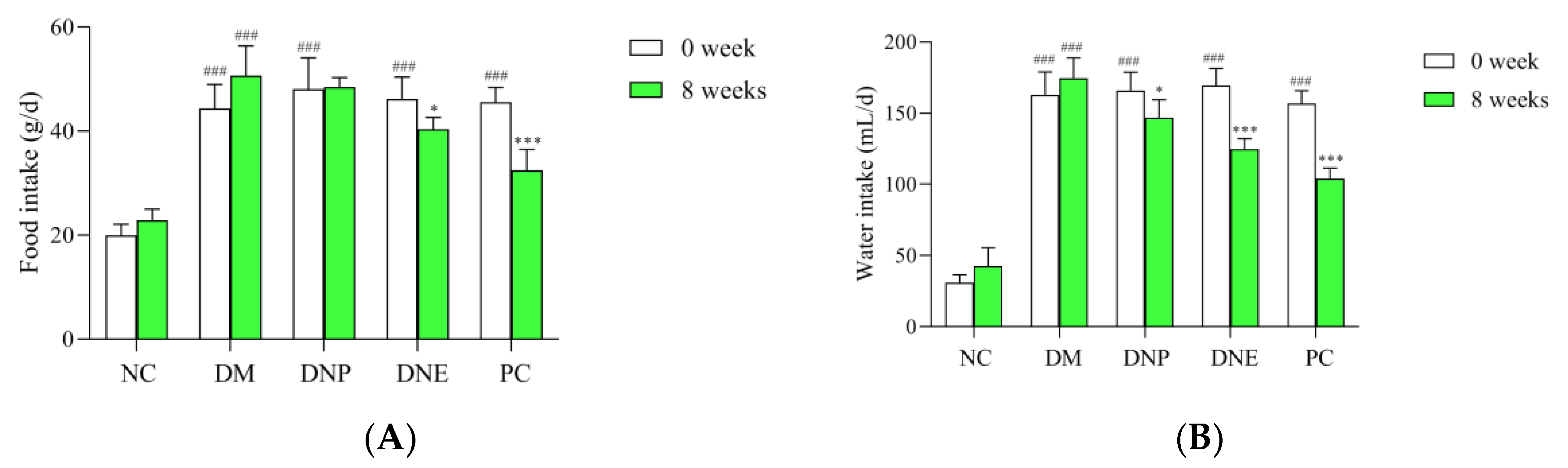
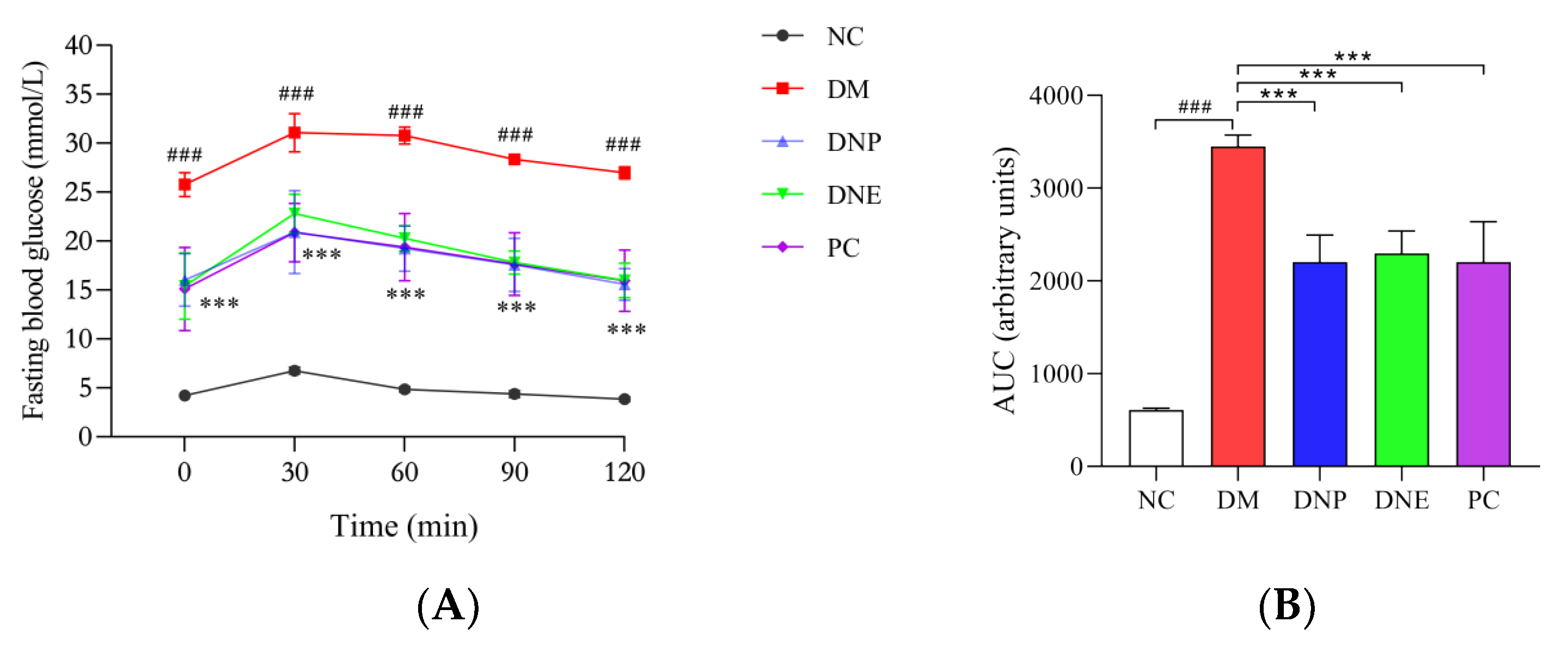
2.2.1. Effects of D. nobile on Conventional Pharmacodynamic Indexes
2.2.2. Effects of D. nobile on Biochemical Indexes
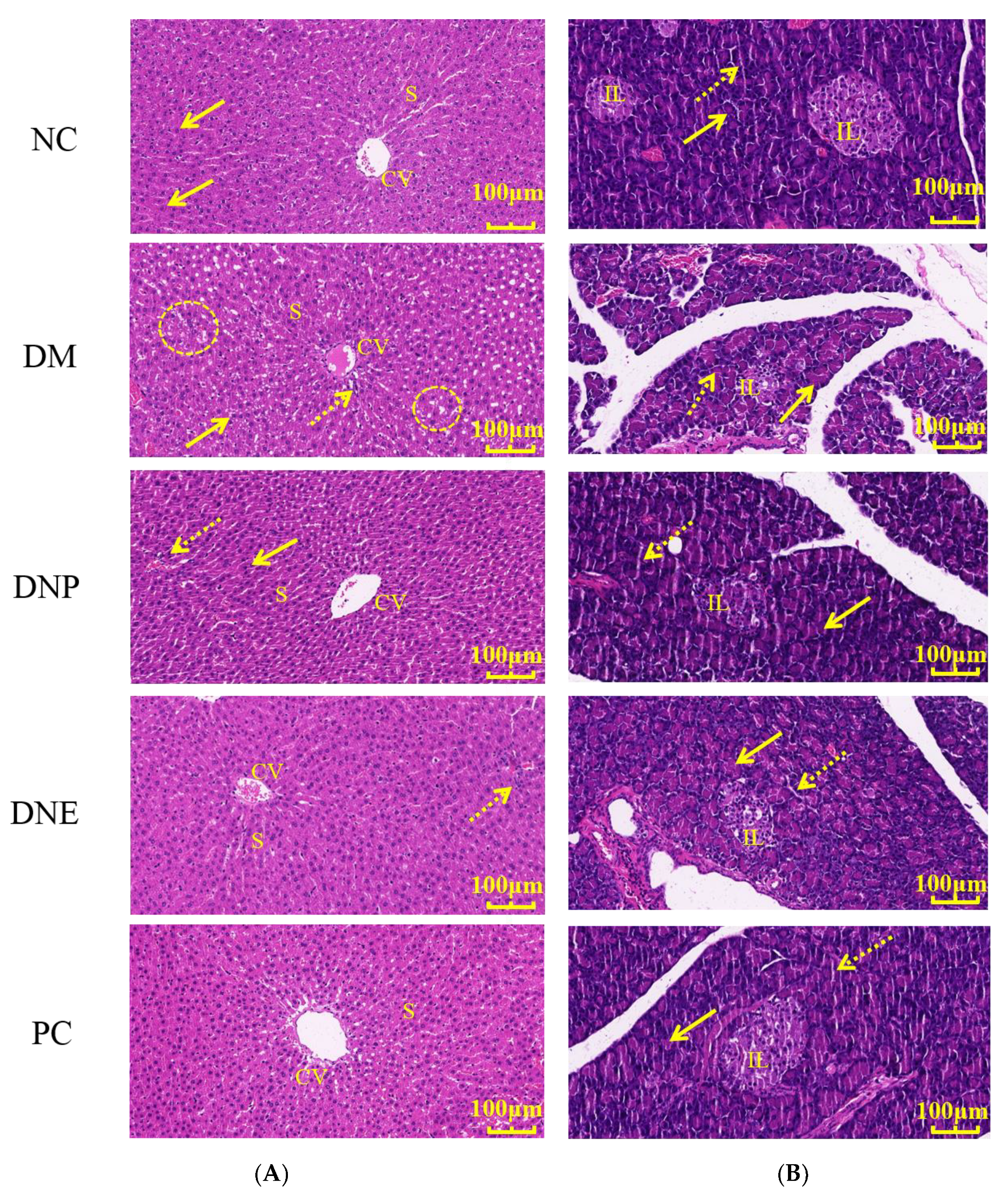
2.2.3. Effects of D. nobile on Histopathological Tissues
2.3. Hypoglycemic Mechanism of D. nobile
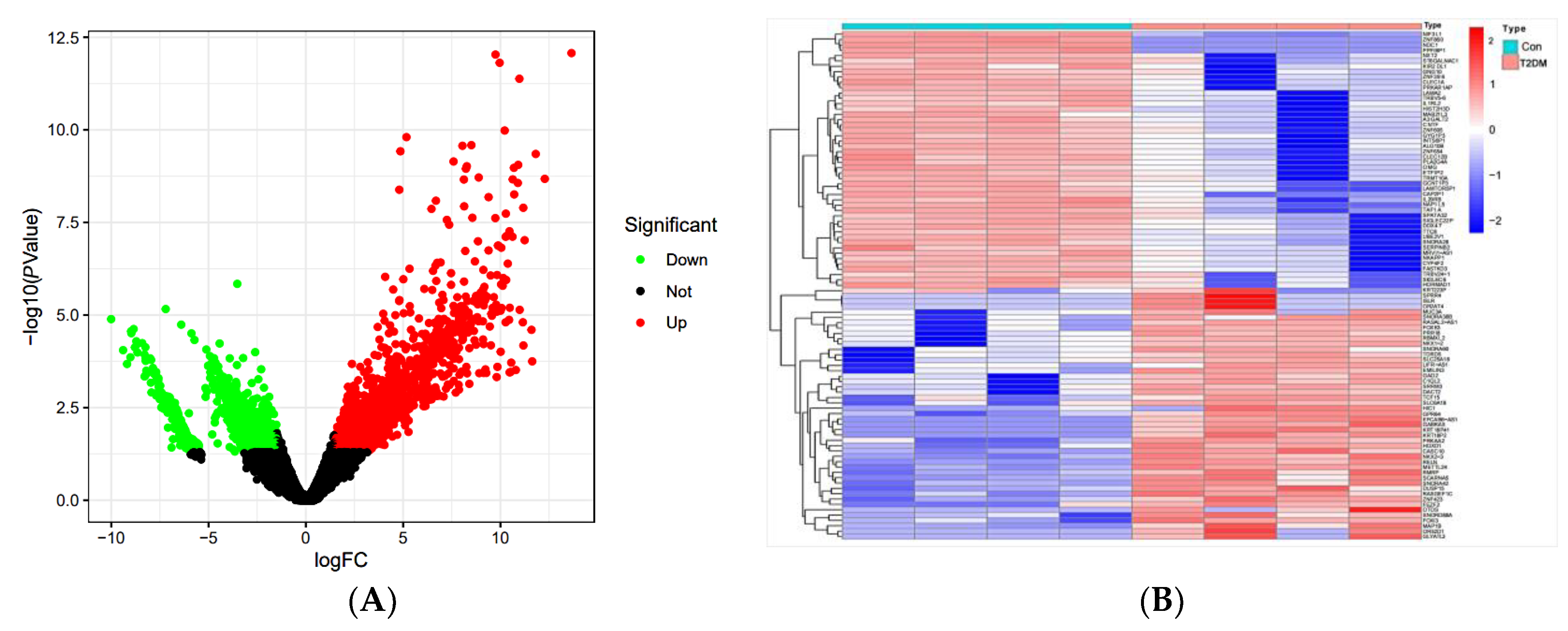
2.3.1. Network Pharmacological Analysis
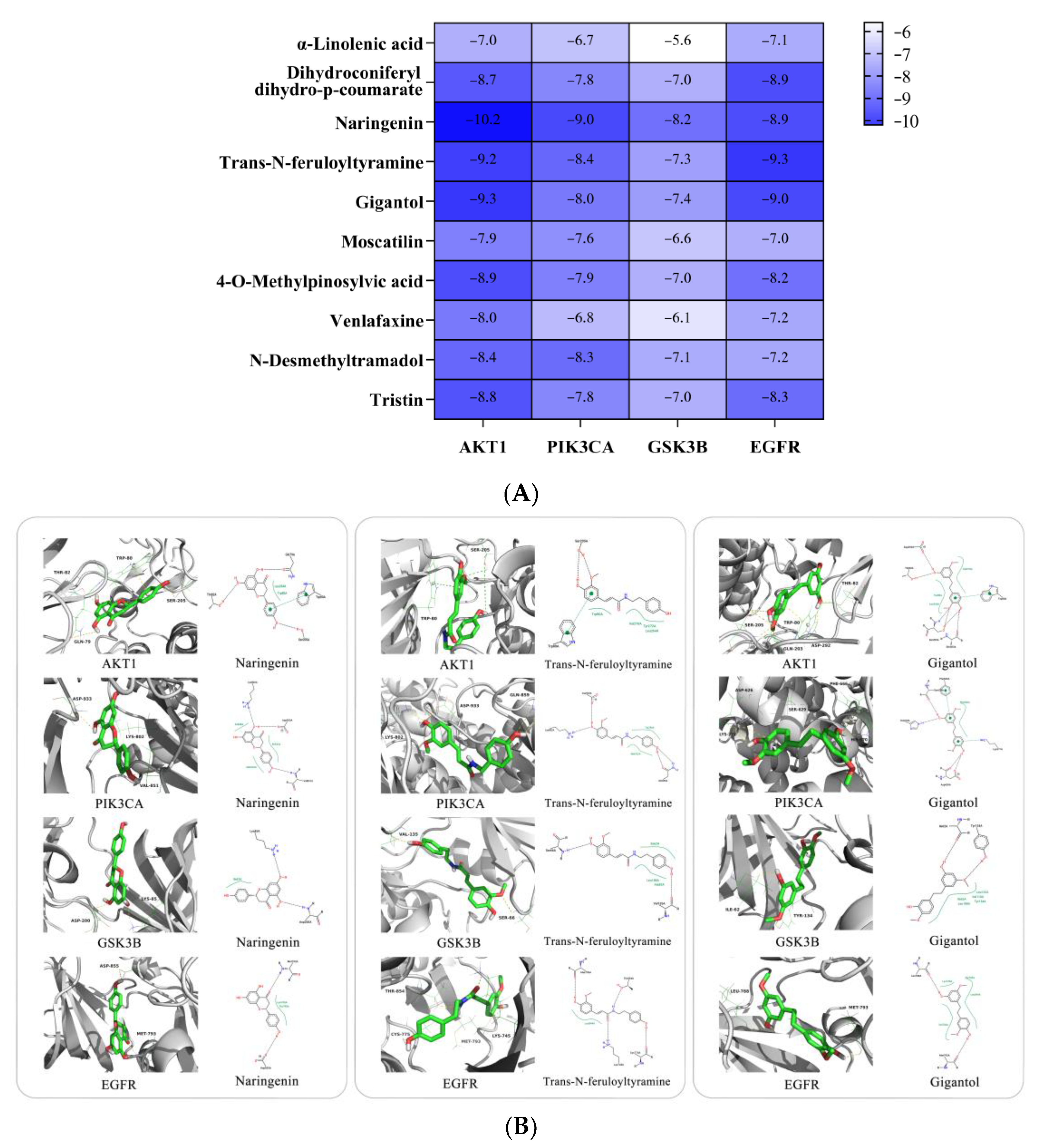
2.3.2. Molecular Docking Analysis
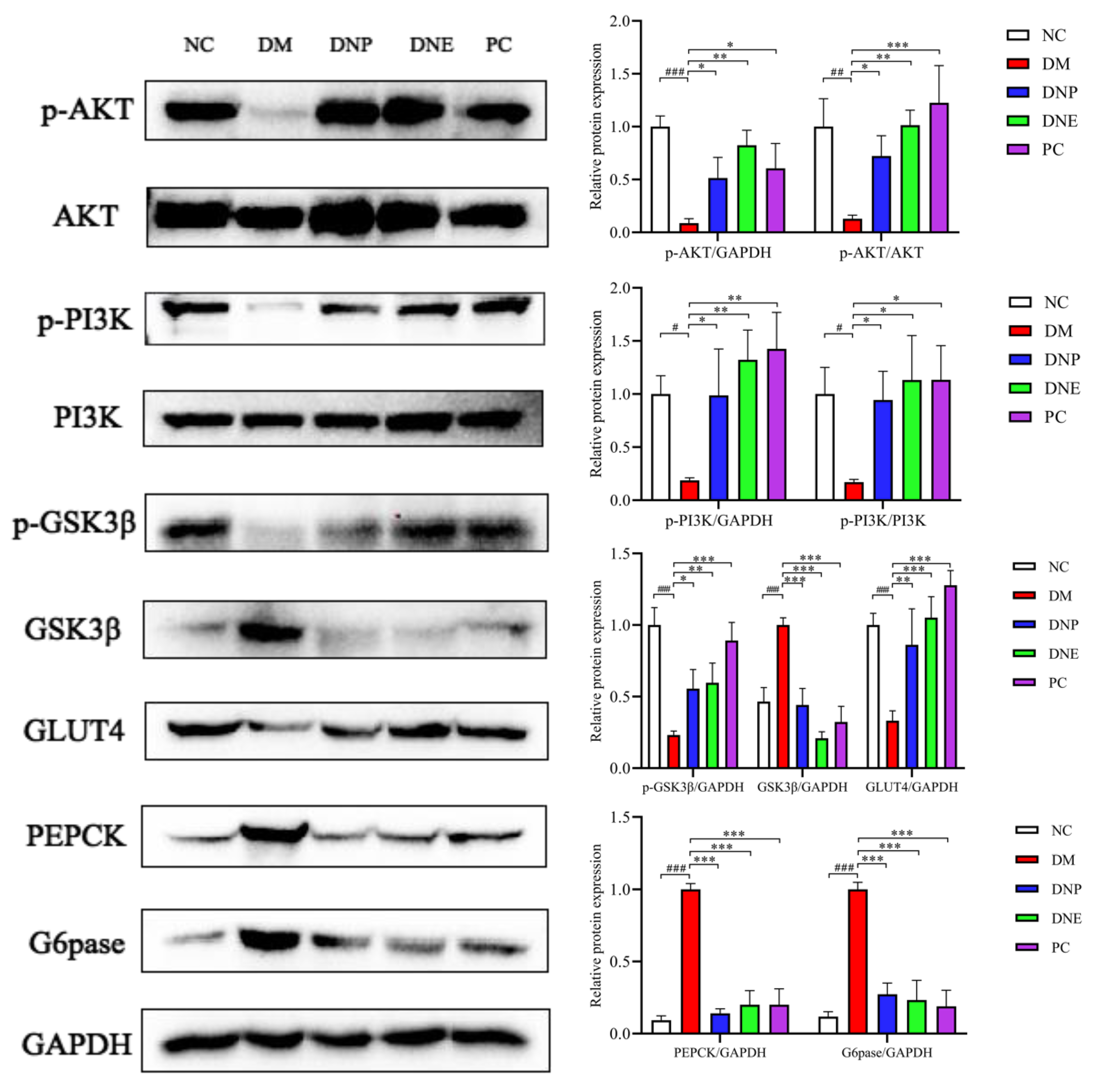
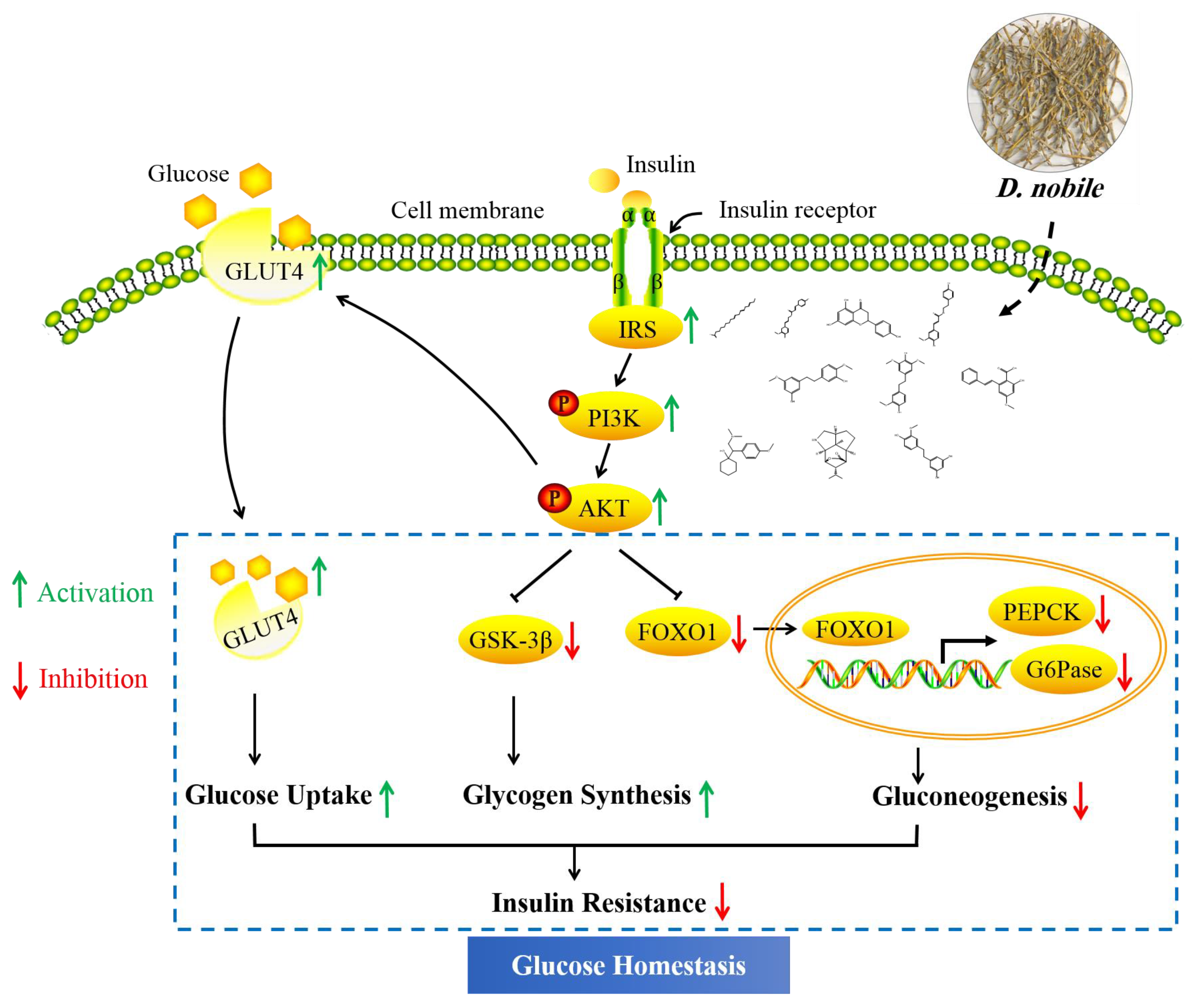
2.3.3. Insulin Signaling Pathway
3. Materials and Method
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Qualitative Analysis of D. nobile
3.4. Hypoglycemic Effect of D. nobile
3.5. Hypoglycemic Mechanism of D. nobile
3.5.1. Network Pharmacological Analysis
3.5.2. Molecular Docking Analysis
3.5.3. Western Blot
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APES: | 3-aminopropyl triethoxysilane |
| DDA: | data-dependent acquisition |
| DN: | compound from Dendrobium nobile |
| DNE: | Dendrobium nobile extract |
| DNP: | Dendrobium nobile polysaccharide |
| ESI: | electron spray ionization |
| HRMS: | high-resolution mass spectrometry |
| PPI: | protein–protein interaction |
| T2DM: | Type 2 diabetic mellitus |
| TIC: | total ions current |
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Petersmann, A.; Muller-Wieland, D.; Muller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liao, X.; Wu, Q.; Li, F.; Wang, L.Y.; Shi, J.S. Effects of Dendrobium nobile Lindl. alkaloids on blood glucose and liver fatty degeneration in diabetic rats. Chin. J. New Drugs Clin. Rem. 2013, 32, 490–493. [Google Scholar]
- Jin, H.; Tao, F.; Tang, Y.P. Effects of the aqueous extract of Dendrobium nobile lindl. on NF-κB and IL-6 expression of renal tissue in diabetic rat model. Chongqing Med. 2014, 43, 946–948. [Google Scholar]
- Saurina, J.; Sentellas, S. Liquid chromatography coupled to mass spectrometry for metabolite profiling in the field of drug discovery. Expert Opin. Drug Dis. 2019, 14, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Heidari, H.; Junghani, M.S.; Absari, R.; Khoogar, M.; Ghaedi, E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin-induced type II diabetic male mice. Res. Pharm. Sci. 2017, 12, 416–424. [Google Scholar]
- He, Y.Q.; Lu, Y.L.; Li, L.S.; Nie, J. Analysis of Alkaloids from Dendrobium nobile Stem by UPLC-ESI-Orbitrap-MS. Chin. J. Experiment. Tradit. Med. Formulae 2017, 23, 30–35. [Google Scholar]
- Lu, A.J.; Cao, L.G.; Tan, D.P.; Qin, L.; Lu, Y.L.; Zhao, Y.X.; Qian, Y.; Bai, C.J.; Yang, J.Y.; Ling, H.; et al. UPLC-Q/TOF-MS coupled with multivariate analysis for comparative analysis of metabolomic in Dendrobium nobile from different growth altitudes. Arab. J. Chem. 2022, 15, 104208. [Google Scholar] [CrossRef]
- Orrego-Lagaron, N.; Vallverdu-Queralt, A.; Martinez-Huelamo, M.; Lamuela-Raventos, R.M.; Escribano-Ferrer, E. Metabolic profile of naringenin in the stomach and colon using liquid chromatography/electrospray ionization linear ion trap quadrupole-Orbitrap-mass spectrometry (LC-ESI-LTQ-Orbitrap-MS) and LC-ESI-MS/MS. J. Pharm. Biomed. Anal. 2016, 120, 38–45. [Google Scholar] [CrossRef]
- Gromiha, M.M.; Yugandhar, K.; Jemimah, S. Protein-protein interactions: Scoring schemes and binding affinity. Curr. Opin. Struc. Biol. 2017, 44, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Zhang, Y.L.; Ma, Y.H.; Yu, H.Y.; Zhao, X.Z.; Zhang, L.H.; Ge, S.Q.; Zhang, G.W.; Qin, X.D. A network pharmacology approach to investigate the mechanism of Shuxuening injection in the treatment of ischemic stroke. J. Ethnopharmacol. 2020, 257, 112891. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, F.; Wu, Q.; Shi, J.S. Effects of Dendrobium nobile Lindl. Alkaloids on alloxan-induced hyperglycemia in rats. J. Zunyi Med. Univ. 2009, 32, 451–453. [Google Scholar]
- De Abreu, A.M.; Copetti, C.L.K.; Hauschild, D.B.; Pietro, P.F.D.; Wazlawik, E. Effects of supplementation with vegetable sources of alpha-linolenic acid (ALA) on inflammatory markers and lipid profile in individuals with chronic kidney disease: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.H.; Ma, Q.; Shen, S.R.; Xu, G.T.; Das, U.N. Effect of α-linolenic acid on streptozotocin-induced diabetic retinopathy indices in vivo. Arch. Med. Res. 2013, 44, 514–520. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.W.; Wu, F.H.; Wang, S.S.; Pan, C.; Wang, L.Y.; Zeng, B.; Ma, S.P.; Liang, J.Y. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.D.; Wang, C.; Chen, Z.Q.; Chen, Y.; Santhanam, R.K.; Xue, Z.H.; Ma, Q.Q.; Guo, Q.W.; Liu, W.; Zhang, M.; et al. Effects of N-trans-feruloyltyramine isolated from laba garlic on antioxidant, cytotoxic activities and H2O2-induced oxidative damage in HepG2 and L02 cells. Food Chem. Toxicol. 2019, 130, 130–141. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, H.K.; Xu, M.M.; Huang, K.D.; Zhang, T.; Duan, J.N. Immobilized lipase catalytic synthesis of phenolamides and their potential against α-glucosidase. J. Biotechnol. 2021, 334, 51–57. [Google Scholar] [CrossRef]
- Kadiroglu, A.K.; Sit, D.; Kayabasi, H.; Tuzcu, A.K.; Tasdemir, N.; Yilmaz, M.E. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2008, 22, 241–245. [Google Scholar] [CrossRef]
- Chen, M.F.; Liou, S.S.; Hong, T.Y.; Kao, S.T.; Liu, I.M. Gigantol has Protective Effects against High Glucose-Evoked Nephrotoxicity in Mouse Glomerulus Mesangial Cells by Suppressing ROS/MAPK/NF-κB Signaling Pathways. Molecules 2018, 24, 80. [Google Scholar] [CrossRef]
- Lai, M.C.; Liu, W.Y.; Liou, S.S.; Liu, I.M. A Bibenzyl Component Moscatilin Mitigates Glycation-Mediated Damages in an SH-SY5Y Cell Model of Neurodegenerative Diseases through AMPK Activation and RAGE/NF-κB Pathway Suppression. Molecules 2020, 25, 4574. [Google Scholar] [CrossRef]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Braz. J. Pharmacogn. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Lee, H.; Lim, Y. Tocotrienol-rich fraction supplementation reduces hyperglycemia-induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice. J. Nutr. Biochem. 2018, 57, 77–85. [Google Scholar] [CrossRef]
- Mokashi, P.; Bhatt, L.K.; Khanna, A.; Pandita, N. Swertisin rich fraction from Enicostema littorale ameliorates hyperglycemia and hyperlipidemia in high-fat fed diet and low dose streptozotacin induced type 2 diabetes mellitus in rats. Biomed. Pharmacother. 2017, 96, 1427–1437. [Google Scholar] [CrossRef]
- Huang, D.W.; Chang, W.C.; Wu, J.S.B.; Shih, R.W.; Shen, S.C. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr. Res. 2016, 36, 150–160. [Google Scholar] [CrossRef]
- Han, H.F.; Hu, C.X.; Wang, L.; Zhang, G.Y.; Liu, S.Z.; Li, F.; Sun, D.; Hu, S.Y. Duodenal-jejunal bypass surgery suppresses hepatic de novo lipogenesis and alleviates liver fat accumulation in a diabetic rat model. Obes. Surg. 2014, 24, 2152–2160. [Google Scholar] [CrossRef]
- Song, Z.L.; Wu, L.L.; Qin, L.L.; Sun, W.; Qin, T.Y.; Pan, Y.J.; Liu, T.H. Research Progress of PI3K/AKT Signaling Pathway and Diabetes Mellitus. World Sci. Technol. Modern. Tradit. Chin. Med. 2019, 21, 1264–1269. [Google Scholar]
- Rathinaswamy, M.K.; Burke, J.E. Class I phosphoinositide 3-kinase (PI3K) regulatory subunits and their roles in signaling and disease. Adv. Biol. Regul. 2020, 75, 100657. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.D.; Zhong, P.; Hu, J.; Lin, F.; Qian, Y.Y.; Xu, Z.; Wang, J.Y.; Zeng, C.L.; Li, X.K.; Liang, G. EGFR inhibition protects cardiac damage and remodeling through attenuating oxidative stress in STZ-induced diabetic mouse model. J. Mol. Cell. Cardiol. 2015, 82, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.P.; Wang, H.X.; Liu, Y.G.; Shui, W.Z.; Wang, J.F.; Cao, P.; Wang, H.J.; You, R.X.; Zhang, Y. Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. J. Funct. Foods 2018, 40, 261–271. [Google Scholar] [CrossRef]
- Bao, S.Y.; Wu, Y.L.; Wang, X.Z.; Han, S.Y.; Cho, S.B.; Ao, W.L.J.; Nan, J.X. Agriophyllum oligosaccharides ameliorate hepatic injury in type 2 diabetic db/db mice targeting INS-R/IRS-2/PI3K/AKT/PPAR-γ/Glut4 signal pathway. J. Ethnopharmacol. 2020, 257, 112863. [Google Scholar] [CrossRef]
- Fang, G.; Cheng, C.J.; Zhang, M.Q.; Ma, X.Y.; Yang, S.N.; Hou, X.T.; Deng, J.G.; Hou, Y.Y.; Bai, G. The glucuronide metabolites of kaempferol and quercetin, targeting to the AKT PH domain, activate AKT/GSK3β signaling pathway and improve glucose metabolism. J. Funct. Foods 2021, 82, 104501. [Google Scholar] [CrossRef]
- Govers, R. Molecular mechanisms of GLUT4 regulation in adipocytes. Diabetes Metab. 2014, 40, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Qiu, Y.; Chen, Q.G.; Han, X.; Cai, M.J.; Hao, L. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG(2) cells. Biomed. Pharmacother. 2021, 137, 111325. [Google Scholar] [CrossRef] [PubMed]
- He, L.L.; Jiang, H.; Lan, T.H.; Qiu, Y.; Yang, K.F.; Chen, K.J.; Yao, X.S.; Yao, Z.H.; Lu, W.H. Chemical profile and potential mechanisms of Huo-Tan-Chu-Shi decoction in the treatment of coronary heart disease by UHPLC-Q/TOF-MS in combination with network pharmacology analysis and experimental verification. J. Chromatogr. B 2021, 1175, 122729. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
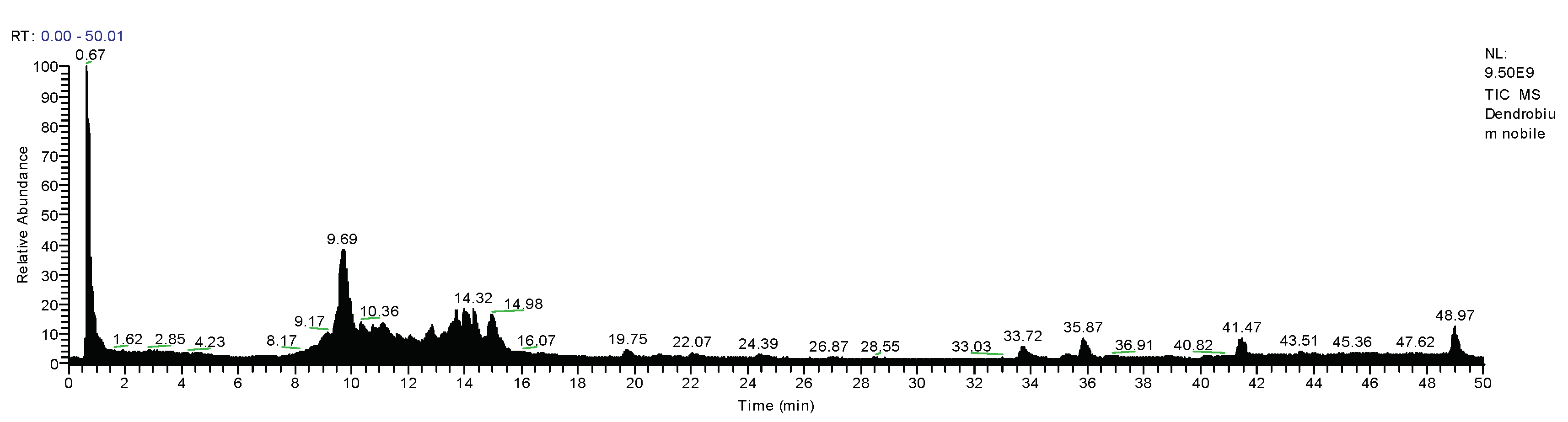

| No. | tR (min) | Ion | MS1 (m/z) | Formula | Diff (ppm) | MS2 (m/z) | Identification | Category | CAS | InChIKey |
|---|---|---|---|---|---|---|---|---|---|---|
| DN1 | 0.66 | [M-H]− | 341.10809 | C12H22O11 | 0.739 | 179.05489, 161.04433, 143.03384, 89.02295, 71.01239, 59.01244 | Sucrose | Glycoside | 57-50-1 | CZMRCDWAGMRECN-UGDNZRGBSA-N |
| 0.68 | [M+Na]+ | 365.10434 | −2.992 | 203.05199, 185.04161 | ||||||
| DN2 | 0.71 | [M-H]− | 191.05507 | C7H12O6 | 0.29 | 173.04453, 155.03311, 137.02310, 127.03867, 109.02816, 93.03314 | Quinic acid | Other | 77-95-2 | AAWZDTNXLSGCEK-LNVDRNJUSA-N |
| DN3 | 0.85 | [M+H]+ | 144.10167 | C7H13NO2 | 1.632 | 98.09663, 84.08115, 70.06570, 58.06583 | Homocycloleucine | Alkaloids | 2756-85-6 | WOXWUZCRWJWTRT-UHFFFAOYSA-N |
| DN4 | 0.96 | [M+H]+ | 130.04958 | C5H7NO3 | 2.227 | 102.0553, 84.04489, 70.06570, 56.05017 | L-Pyroglutamic acid | Alkaloids | 98-79-3 | ODHCTXKNWHHXJC-VKHMYHEASA-N |
| DN5 | 1.07 | [M+H]+ | 268.10291 | C10H13N5O4 | 2.836 | 136.06163, 119.03513, 85.02884 | Adenosine | Glycoside | 58-61-7 | OIRDTQYFTABQOQ-KQYNXXCUSA-N |
| DN6 | 3.08 | [M+H]+ | 280.18994 | C16H25NO3 | 2.784 | 262.18994, 234.18460, 220.13251, 166.12218 | 6-hydroxydendrobine | Alkaloids | 7668-75-9 | QSNCUGULHPBRGR-FYPZSMAZSA-N |
| DN7 | 3.57 | [M+H]+ | 158.08104 | C7H11NO3 | 0.821 | 112.07591, 84.04493, 70.06568, 56.05017 | N-acetyl-D-proline | Alkaloids | 59785-68-1 | GNMSLDIYJOSUSW-ZCFIWIBFSA-N |
| DN8 | 6.06 | [M-H]− | 121.02806 | C7H6O2 | −2.445 | 93.03320, 65.03816 | p-Hydroxybenzaldehyde | Other | 123-08-0 | RGHHSNMVTDWUBI-UHFFFAOYSA-N |
| DN9 | 8.99 | [M+H]+ | 278.21063 | C17H27NO2 | −2.968 | 105.06984, 58.06584 | Venlafaxine | Alkaloids | 93413-69-5 | PNVNVHUZROJLTJ-UHFFFAOYSA-N |
| DN10 | 9.68 | [M+H]+ | 264.19507 | C16H25NO2 | −2.784 | 246.18503, 236.20000, 218.18958, 176.14381, 145.10034 | Dendrobine | Alkaloids | 2115-91-5 | RYAHJFGVOCZDEI-UFFNCVEVSA-N |
| DN11 | 9.96 | [M+H]+ | 250.17961 | C15H23NO2 | −2.181 | 232.16867, 204.17422, 145.10074, 133.10080, 105.07002 | Nordendrobin | Alkaloids | 73806-55-0 | VUMQHLSPUAFKKK-UHFFFAOYSA-N |
| DN12 | 10.32 | [M+H]+ | 285.16846 | C15H24O5 | −4.174 | 267.15814, 249.14789, 145.10077, 133.10095, 119.08533, 105.06994 | Dendronobilin B | Other | - | ROURONGLEFVLGL-RQJMXFNBSA-N |
| 10.73 | [M+Na]+ | 307.15051 | −3.533 | 289.13968, 157.06676 | ||||||
| DN13 | 10.37 | [M-H]− | 593.14978 | C27H30O15 | −0.534 | 503.11746, 473.10797, 383.07654, 353.06586, 325.07150, 297.07630, 93.03333 | Vicenin II | Glycoside | 23666-13-9 | FIAAVMJLAGNUKW-VQVVXJKKSA-N |
| DN14 | 10.91 | [M+H]+ | 267.15805 | C15H22O4 | −3.877 | 249.14771, 231.13695, 203.14226, 155.06976, 96.08576 | Verrucarol | Other | 2198-92-7 | ZSRVBNXAPSQDFY-OJVARPOJSA-N |
| DN15 | 11.17 | [M+H]+ | 235.16855 | C15H22O2 | −3.004 | 217.15810, 205.15822, 189.16325, 175.11128, 161.09561, 147.11646, 133.10085 | Curcumenol | Other | 19431-84-6 | ISFMXVMWEWLJGJ-NZBPQXDJSA-N |
| DN16 | 11.24 | [M+H]+ | 306.20541 | C18H27NO3 | −3.136 | 249.14839, 175.14801, 121.10115 | Unknown 1 | - | - | - |
| DN17 | 13 | [M+H]+ | 183.07768 | C6H15O4P | −2.141 | 155.04639, 127.01531, 98.98456 | Triethyl phosphate | Other | 78-40-0 | DQWPFSLDHJDLRL-UHFFFAOYSA-N |
| DN18 | 13.83 | [M-H]− | 461.23892 | C22H38O10 | 1.726 | 179.05470, 161.04404, 119.03348 | 2-(4-Methyl-3-cyclohexen-1-yl)-2-propanyl-6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside | Glycoside | - | - |
| DN19 | 15.05 | [M+H]+ | 219.17361 | C15H22O | −3.339 | 163.11115, 145.10080, 131.08527, 105.07000 | α-Cyperone or Germacrone | Other | 4674-50-4 | WTOYNNBCKUYIKC-JMSVASOKSA-N |
| DN20 | 15.22 | [M-H]− | 312.12369 | C18H19NO4 | 2.1 | 297.10040, 190.04984, 178.04990, 148.05173, 135.04387 | Trans-N-feruloyltyramine | Alkaloids | 66648-43-9 | NPNNKDMSXVRADT-WEVVVXLNSA-N |
| DN21 | 16.28 | [M+H]+ | 219.17357 | C15H22O | −3.522 | 159.11636, 145.10075, 107.08556, 105.06995 | Germacrone or α-Cyperone | Other | 6902-91-6 | CAULGCQHVOVVRN-SWZPTJTJSA-N |
| DN22 | 16.42 | [M-H]− | 259.09708 | C15H16O4 | 2.295 | 244.07341, 137.02312, 121.02820 | Tristin | Bibenzyl | 139101-67-0 | KPFFMALTIRFAHW-UHFFFAOYSA-N |
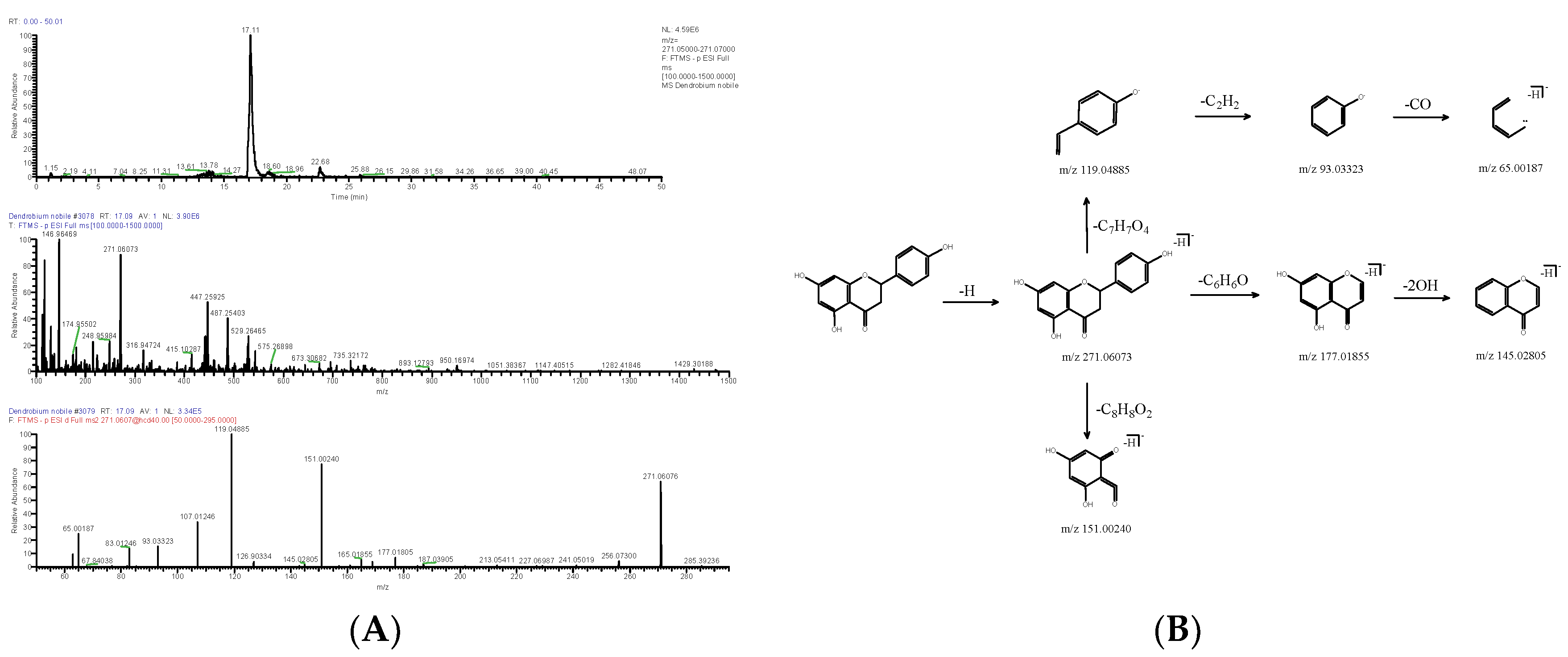
| DN23 | 17.09 | [M-H]− | 271.06073 | C15H12O5 | 2.324 | 119.04885, 151.00240, 107.01246 | Naringenin | Flavonoids | 67604-48-2 | FTVWIRXFELQLPI-UHFFFAOYSA-N |
| DN24 | 18.69 | [M+H]+ | 305.1369 | C17H20O5 | 4.753 | 181.08534, 166.06190, 151.07495, 137.05937, 119.04906, 91.05453 | Moscatilin | Bibenzyl | 108853-14-1 | YTRAYUIKLRABOQ-UHFFFAOYSA-N |
| DN25 | 18.99 | [M-H]− | 329.23279 | C18H34O5 | 1.638 | 229.14378, 211.13306, 171.10156, 139.11140 | (15Z)-9,12,13-Trihydroxy-15-octadecenoic acid | Other | - | DNWUYCUUEGGVPR-CLTKARDFSA-N |
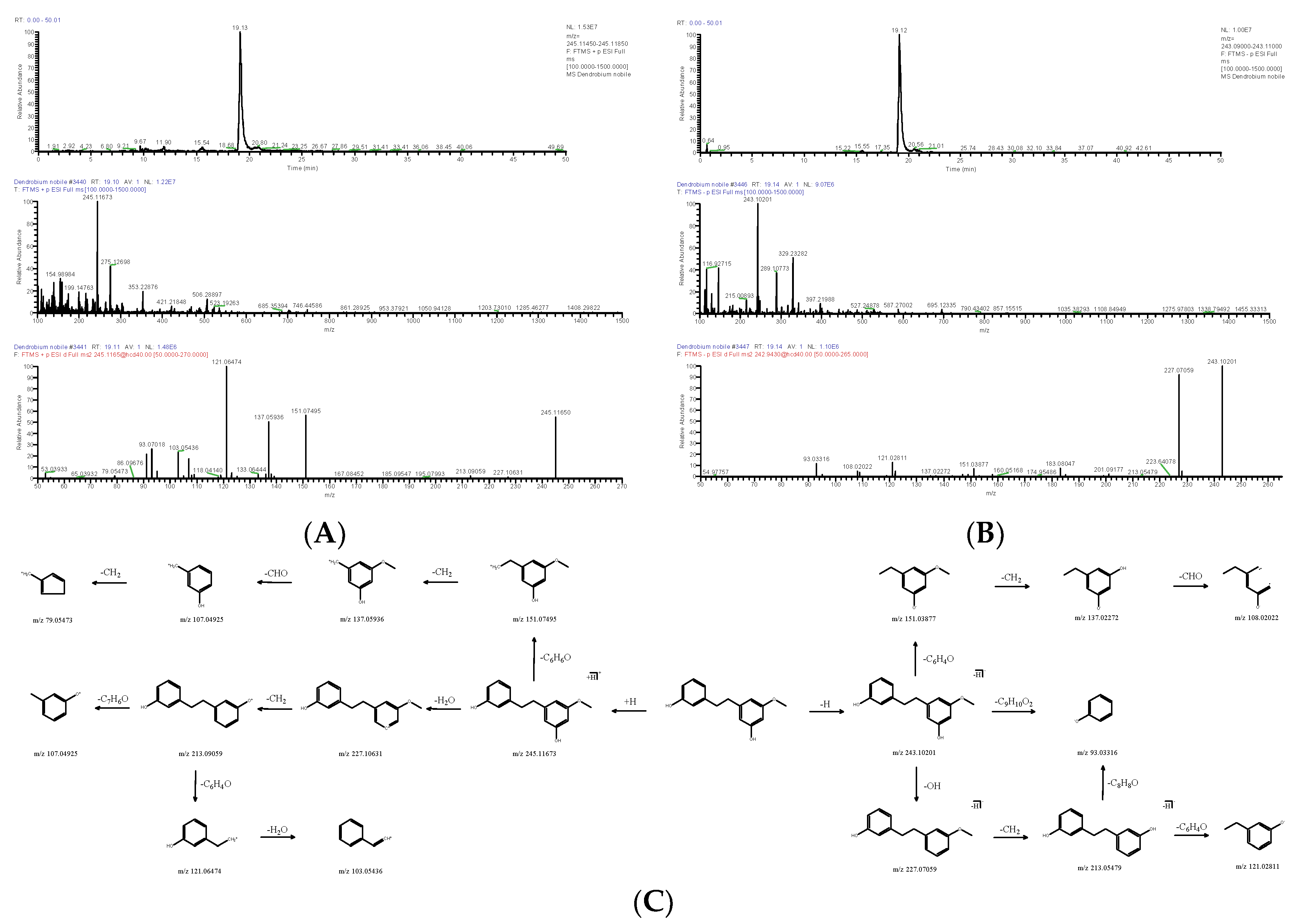
| DN26 | 19.11 | [M+H]+ | 245.11673 | C15H16O3 | −2.003 | 227.10631, 213.09059, 151.07495, 137.05936, 121.06474, 103.05436, 93.07018 | Batatasin III | Bibenzyl | 56684-87-8 | VYQXIUVIYICVCM-UHFFFAOYSA-N |
| 19.14 | [M-H]− | 243.10201 | 1.806 | 227.07059, 213.05479, 151.03877, 93.03316 | ||||||
| DN27 | 20.71 | [M+H]+ | 228.26805 | C15H33N | −2.307 | 85.10143, 71.08615, 60.08149 | Dodecyltrimethylammonium | Other | 10182-91-9 | VICYBMUVWHJEFT-UHFFFAOYSA-N |
| DN28 | 20.77 | [M-H]− | 329.1387 | C19H22O5 | 1.063 | 208.07326, 181.08633, 179.07018, 149.05962, 121.02794 | Dihydroconiferyl dihydro-p-coumarate | Other | - | ZXQHQUXJMNGVJP-UHFFFAOYSA-N |
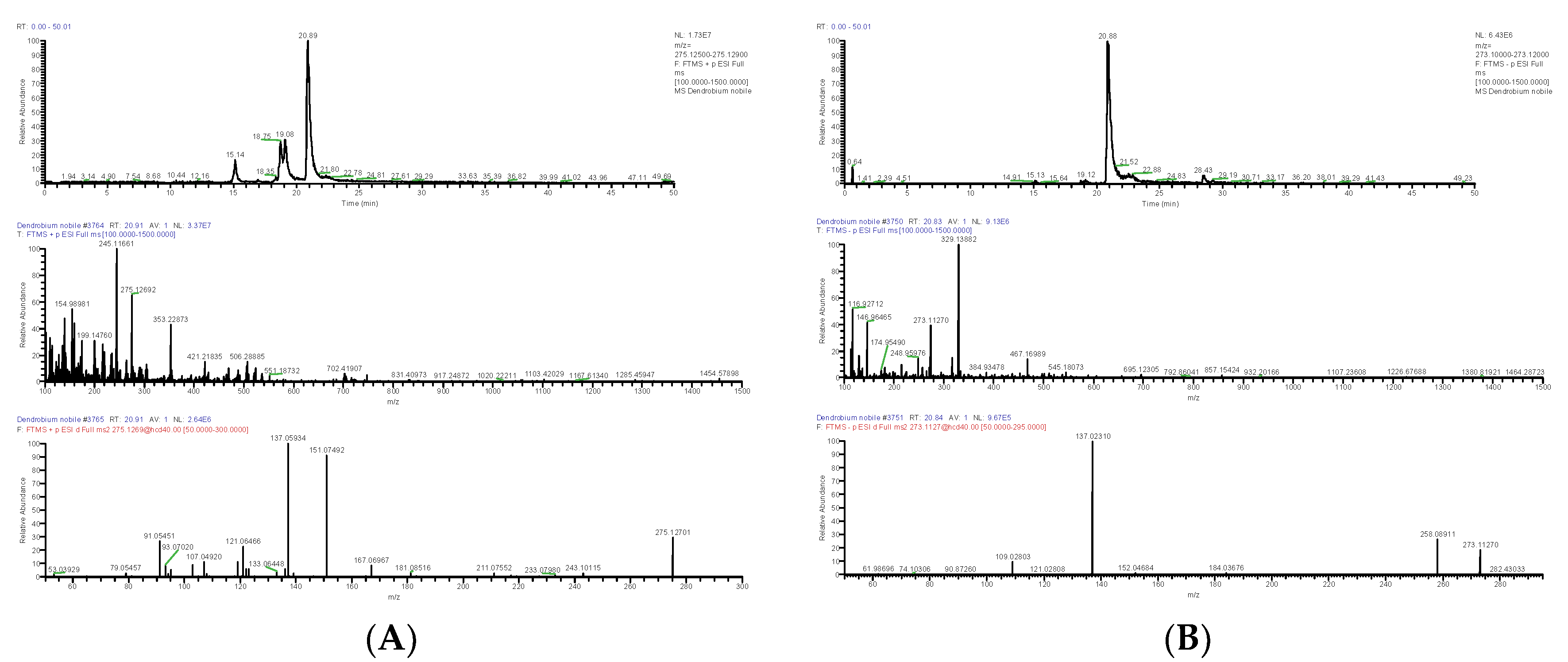
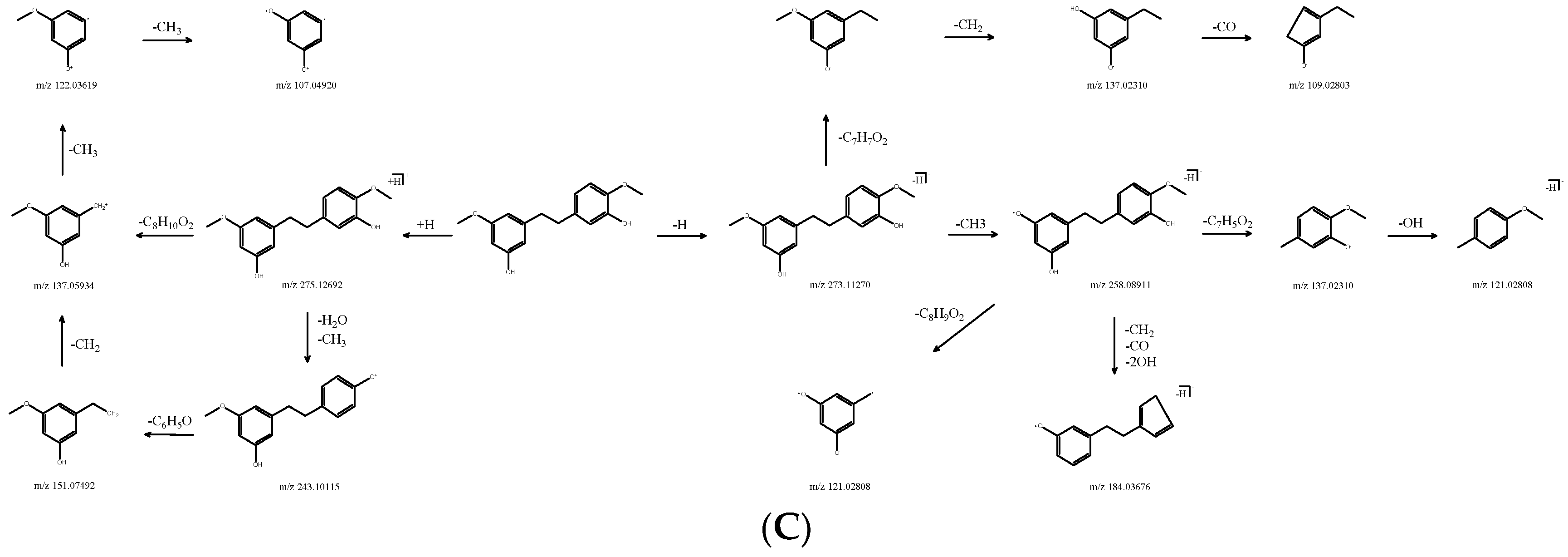
| DN29 | 20.91 | [M+H]+ | 275.12692 | C16H18O4 | −3.146 | 243.10115, 151.07492, 137.05934, 121.06466, 107.04920 | Gigantol | Bibenzyl | 67884-30-4 | SDXKZPQOVUDXIY-UHFFFAOYSA-N |
| 20.84 | [M-H]− | 273.1127 | −2.819 | 258.08911, 137.02310, 121.02808, 109.02803 | ||||||
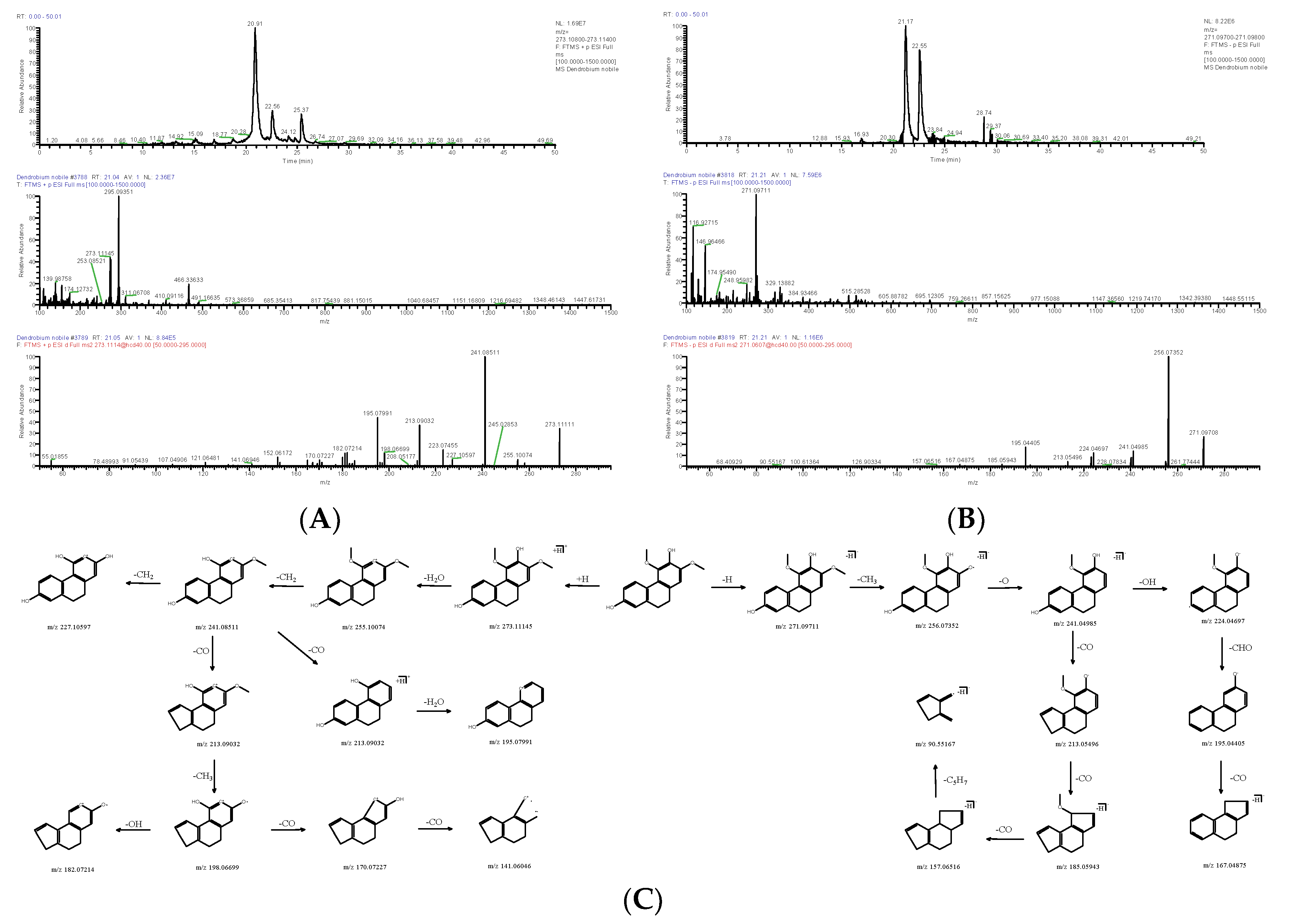
| DN30 | 21.05 | [M+H]+ | 273.11145 | C16H16O4 | −2.510 | 255.10074, 241.08511, 213.09032, 195.07991, 182.07214, 170.07227 | Ephemeranthol B | Phenanthrene | 130827-44-0 | SOHTUOALNFQEMJ-UHFFFAOYSA-N |
| 21.21 | [M+H]− | 271.09711 | 2.304 | 256.07352, 241.04985, 224.04697, 195.04405, 185.05943, 167.04875 | ||||||
| DN31 | 21.4 | [M+H]+ | 271.09546 | C16H14O4 | 3.783 | 253.08495, 238.06938, 225.09015, 210.06674, 182.07204, 165.06932 | Nudol or 4-O-Methylpinosylvic acid | Phenanthrene or Bibenzyl | 86630-46-8 | JZIYNZGPIKGKQC-UHFFFAOYSA-N |
| DN32 | 22.37 | [M-H]− | 241.08632 | C15H14O3 | 1.656 | 241.08617, 226.06265, 198.06729, 182.07178, 169.06473 | 2-Methoxy-9,10-dihydrophenanthrene-4,5-diol | Phenanthrene | - | KQMGXHNRKZYDEK-UHFFFAOYSA-N |
| DN33 | 28.17 | [M+H]+ | 279.23123 | C18H30O2 | 2.244 | 261.22159, 209.15300, 195.13731, 123.11677, 109.10130, 95.08582 | α-Linolenic acid | Other | 463-40-1 | DTOSIQBPPRVQHS-PDBXOOCHSA-N |
| DN34 | 28.51 | [M+H]+ | 271.09512 | C16H14O4 | −4.869 | 253.08514, 238.06178, 225.09023, 210.06693, 182.07220, 165.06952 | 4-O-Methylpinosylvic acid or Nudol | Bibenzyl or Phenanthrene | 149697-30-3 | PICDNGANOHNCPT-BQYQJAHWSA-N |
| DN35 | 30.15 | [M-H]− | 303.15955 | C18H24O4 | 1.532 | 259.17096, 205.04944, 161.05963 | Unknown 2 | - | - | - |
| DN36 | 34.17 | [M+H]+ | 256.26239 | C16H33NO | −4.297 | 144.13774, 130.12199, 116.10680, 102.09145, 88.07596, 74.06048 | Hexadecanamide | Other | 629-54-9 | HSEMFIZWXHQJAE-UHFFFAOYSA-N |
| DN37 | 35.68 | [M-H]− | 339.23215 | C23H32O2 | 0.865 | 163.1116, 147.08009, 107.04906 | 2,2′-Methylenebis(4-methyl-6-tert-butylphenol) | Other | 119-47-1 | KGRVJHAUYBGFFP-UHFFFAOYSA-N |
| DN38 | 36.07 | [M-H]− | 279.23236 | C18H32O2 | 1.802 | 175.20976 | Unknown 3 | - | - | - |
| DN39 | 37.89 | [M+H]+ | 284.29401 | C18H37NO | −2.748 | 186.18478, 144.13776, 130.12242, 116.10702, 102.09155, 88.07607 | Stearamide | Other | 124-26-5 | LYRFLYHAGKPMFH-UHFFFAOYSA-N |
| DN40 | 40.18 | [M+H]+ | 165.0907 | C10H12O2 | 1.855 | 147.08064, 137.09578, 119.08543, 109.10125, 91.05452 | 4-Phenylbutyric acid | Other | 1821-12-1 | OBKXEAXTFZPCHS-UHFFFAOYSA-N |
| DN41 | 41.29 | [M+H]+ | 338.34058 | C22H43NO | −3.433 | 321.31415, 226.21616, 149.13222, 135.11647 | Erucamide | Other | 112-84-5 | UAUDZVJPLUQNMU-KTKRTIGZSA-N |
| DN42 | 48.82 | [M+H]+ | 141.11308 | C16H12N4 | −2.005 | 112.08706, 98.07157, 85.07648, 71.06096 | Hexamethylenetetramine | Alkaloids | 100-97-0 | VKYKSIONXSXAKP-UHFFFAOYSA-N |
| Items | NC | DM | DNP | DNE | PC |
|---|---|---|---|---|---|
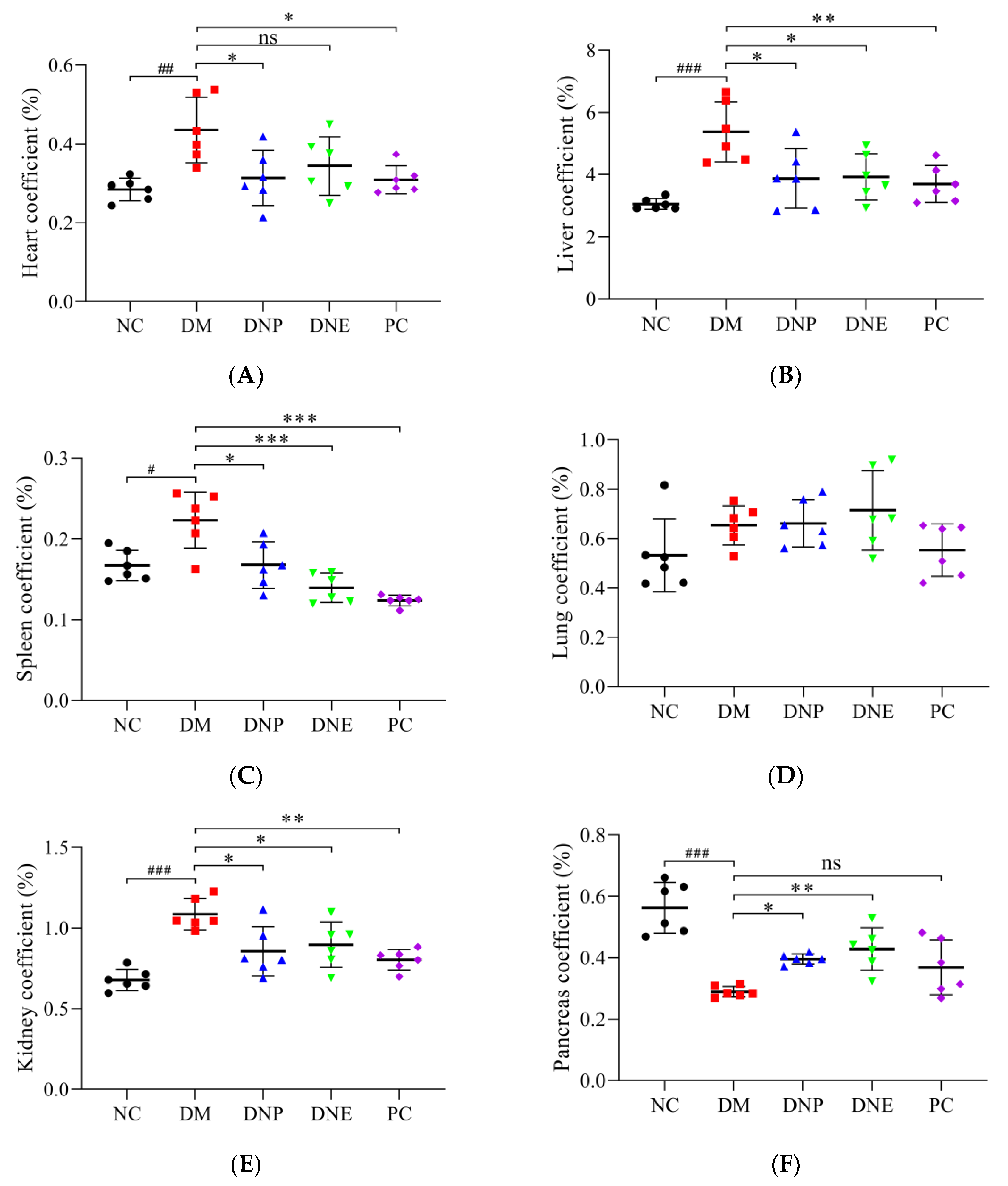
| Heart coefficient (%) | 0.28 ± 0.03 | 0.44 ± 0.082 ## | 0.31 ± 0.07 * | 0.34 ± 0.07 | 0.31 ± 0.04 * |
| Liver coefficient (%) | 3.05 ± 0.17 | 5.38 ± 0.96 ### | 3.87 ± 0.96 * | 3.93 ± 0.75 * | 3.69 ± 0.59 ** |
| Spleen coefficient (%) | 0.17 ± 0.02 | 0.22 ± 0.04 # | 0.17 ± 0.03 * | 0.14 ± 0.02 *** | 0.12 ± 0.01 *** |
| Lung coefficient (%) | 0.53 ± 0.15 | 0.65 ± 0.08 | 0.66 ± 0.10 | 0.71 ± 0.16 | 0.55 ± 0.11 |
| Kidney coefficient (%) | 0.68 ± 0.07 | 1.09 ± 0.10 ### | 0.86 ± 0.15 * | 0.90 ± 0.14 * | 0.80 ± 0.06 ** |
| Pancreas coefficient (%) | 0.56 ± 0.08 | 0.29 ± 0.02 ### | 0.40 ± 0.02 * | 0.43 ± 0.07 ** | 0.37 ± 0.09 |
| Final body weight (g) | 500.60 ± 41.02 | 323.30 ± 39.66 ### | 362.20 ± 31.10 * | 388.30 ± 11.18 | 355.00 ± 44.41 |
| Items | NC | DM | DNP | DNE | PC |
|---|---|---|---|---|---|
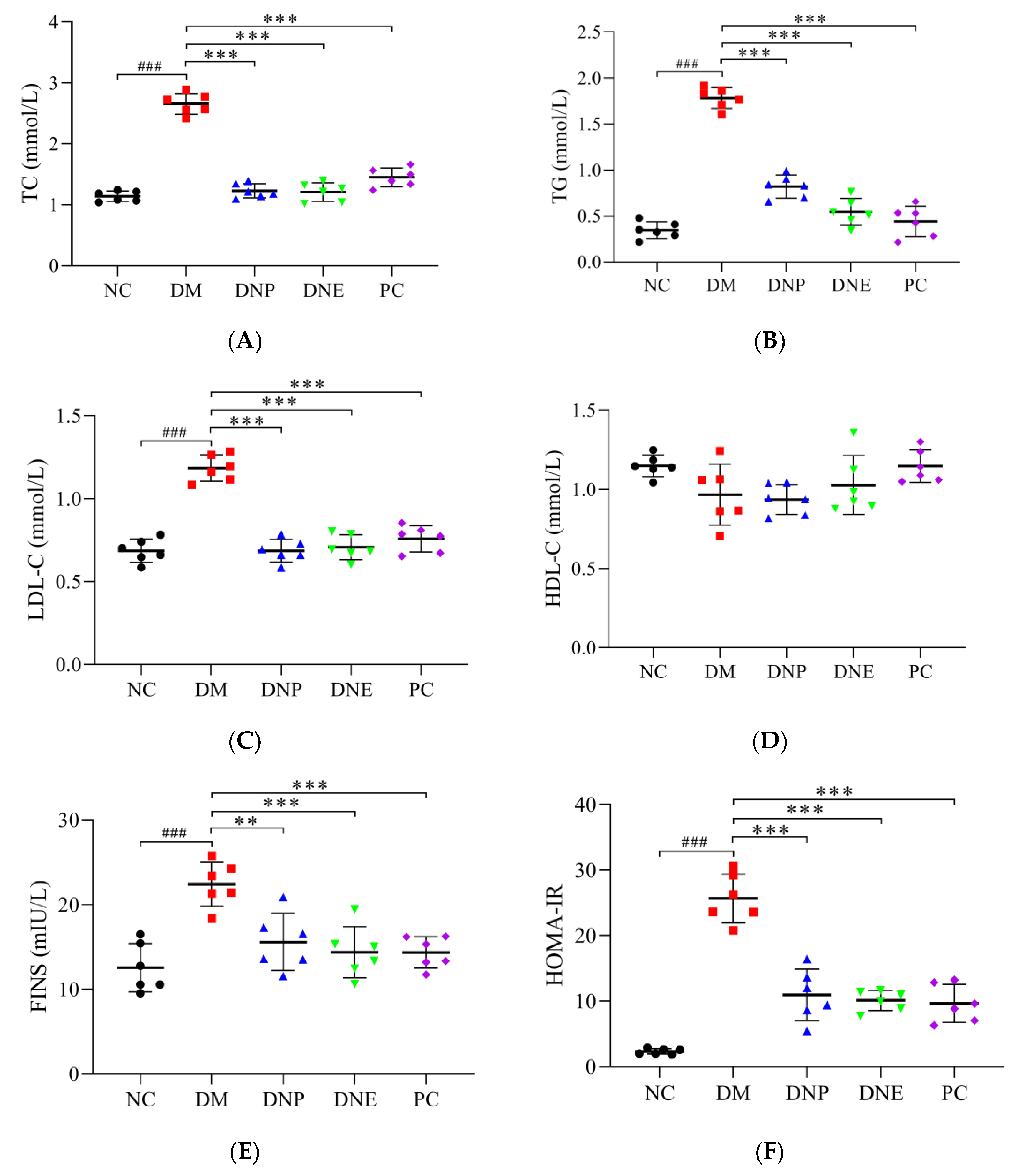
| TC (mmol/L) | 1.14 ± 0.08 | 2.65 ± 0.17 ### | 1.22 ± 0.11 *** | 1.21 ± 0.15 *** | 1.45 ± 0.15 *** |
| TG (mmol/L) | 0.34 ± 0.09 | 1.77 ± 0.11 ### | 0.82 ± 0.12 *** | 0.54 ± 0.14 *** | 0.44 ± 0.16 *** |
| LDL-C (mmol/L) | 0.68 ± 0.07 | 1.18 ± 0.08 ### | 0.68 ± 0.06 *** | 0.71 ± 0.07 *** | 0.75 ± 0.07 *** |
| HDL-C (mmol/L) | 1.15 ± 0.06 | 0.97 ± 0.19 | 0.93 ± 0.09 | 1.03 ± 0.18 | 1.15 ± 0.10 |
| FINS (mIU/L) | 12.55 ± 2.86 | 22.40 ± 2.61 ### | 15.56 ± 3.36 ** | 14.36 ± 3.03 *** | 14.33 ± 1.85 *** |
| HOMA-IR | 2.31 ± 0.42 | 25.67 ± 3.73 ### | 10.93 ± 3.92 *** | 10.11 ± 1.54 *** | 9.63 ± 2.89 *** |
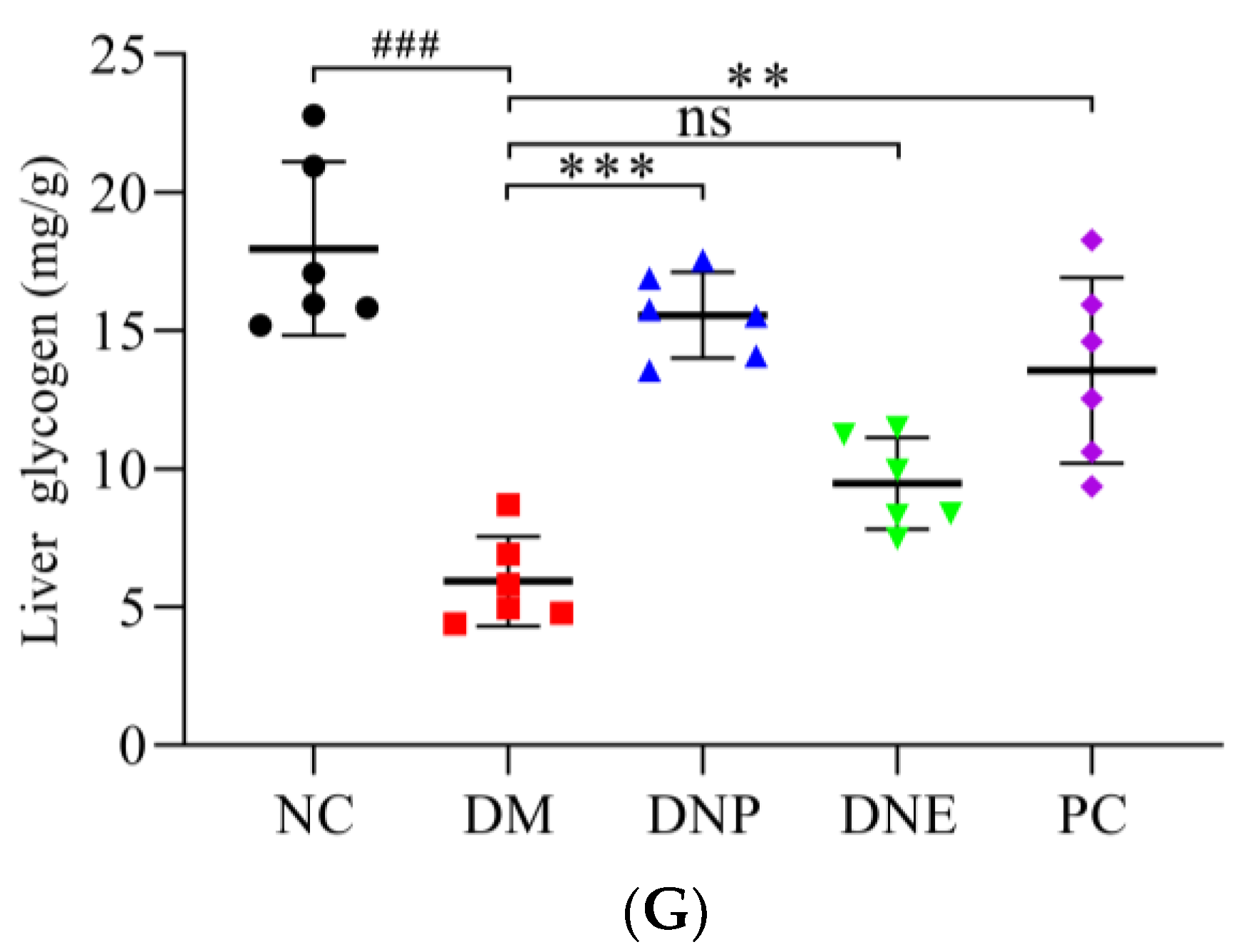
| Liver glycogen (mg/g) | 17.96 ± 3.14 | 5.92 ± 1.63 ### | 15.56 ± 1.55 *** | 9.47 ± 1.66 | 13.55 ± 3.35 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zeng, M.; Geng, K.; Lai, D.; Xu, Z.; Zhou, W. Chemical Constituents and Hypoglycemic Mechanisms of Dendrobium nobile in Treatment of Type 2 Diabetic Rats by UPLC-ESI-Q-Orbitrap, Network Pharmacology and In Vivo Experimental Verification. Molecules 2023, 28, 2683. https://doi.org/10.3390/molecules28062683
Li Z, Zeng M, Geng K, Lai D, Xu Z, Zhou W. Chemical Constituents and Hypoglycemic Mechanisms of Dendrobium nobile in Treatment of Type 2 Diabetic Rats by UPLC-ESI-Q-Orbitrap, Network Pharmacology and In Vivo Experimental Verification. Molecules. 2023; 28(6):2683. https://doi.org/10.3390/molecules28062683
Chicago/Turabian StyleLi, Zhaoyang, Meiling Zeng, Keyong Geng, Donna Lai, Zhi Xu, and Wei Zhou. 2023. "Chemical Constituents and Hypoglycemic Mechanisms of Dendrobium nobile in Treatment of Type 2 Diabetic Rats by UPLC-ESI-Q-Orbitrap, Network Pharmacology and In Vivo Experimental Verification" Molecules 28, no. 6: 2683. https://doi.org/10.3390/molecules28062683
APA StyleLi, Z., Zeng, M., Geng, K., Lai, D., Xu, Z., & Zhou, W. (2023). Chemical Constituents and Hypoglycemic Mechanisms of Dendrobium nobile in Treatment of Type 2 Diabetic Rats by UPLC-ESI-Q-Orbitrap, Network Pharmacology and In Vivo Experimental Verification. Molecules, 28(6), 2683. https://doi.org/10.3390/molecules28062683