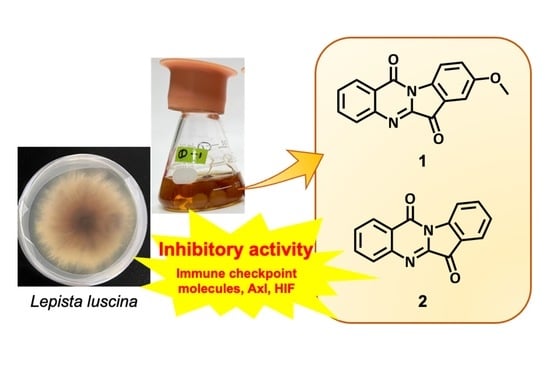
Axl, Immune Checkpoint Molecules and HIF Inhibitors from the Culture Broth of Lepista luscina
Abstract
1. Introduction
2. Results and Discussion
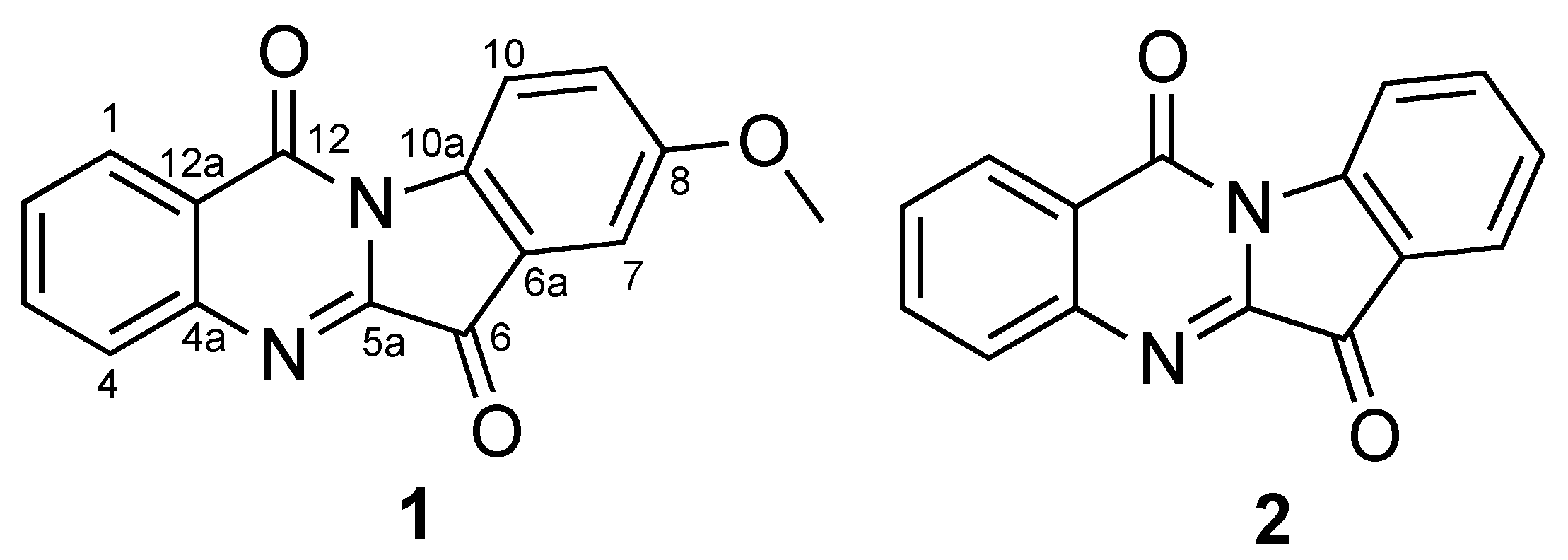
2.1. Identification of Compounds
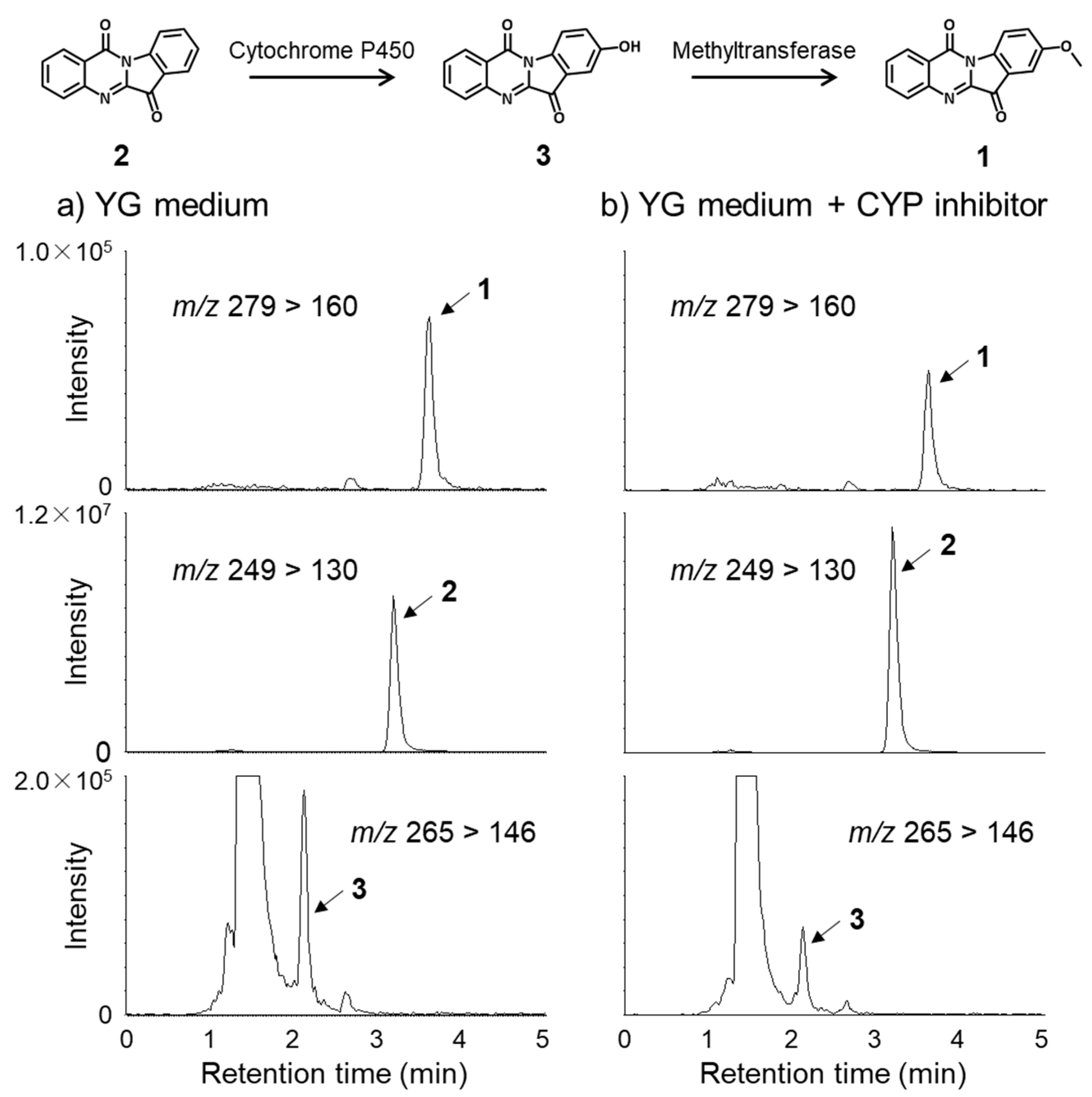
2.2. Metabolic Pathway of Tryptanthrin (2) and Its Analogues (1 and 3)
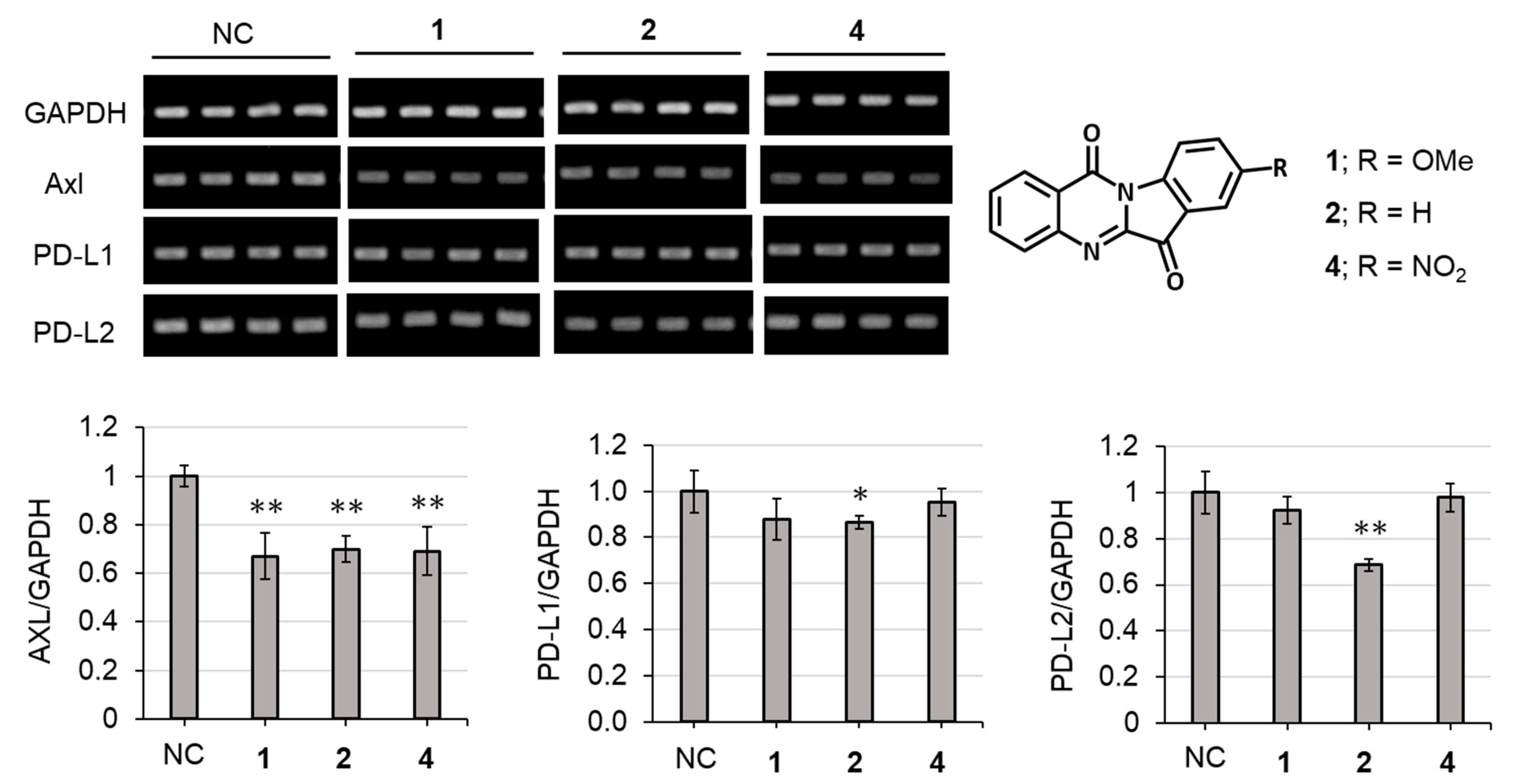
2.3. Biological Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. X-ray Crystallography Analysis
3.5. Feeding Studies Using CYP-Inhibitor 1-Aminobenzotriazole
3.6. LC-MS Spectrometry
3.7. Axl and Immune Checkpoint Molecules Assay
3.8. HIF Luciferase Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Azuma, K.; Ota, K.; Kawahara, A.; Hattori, S.; Iwama, E.; Harada, T.; Matsumoto, K.; Takayama, K.; Takamori, S.; Kage, M.; et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann. Oncol. 2014, 25, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.; Giaccia, A. The Receptor Tyrosine Kinase AXL in Cancer Progression. Cancers 2016, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wong, T.Y. Current Epidemiology of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diabetes Rep. 2012, 12, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Del Re, M.; Rofi, E.; Posarelli, C.; Figus, M.; Danesi, R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye 2018, 32, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 5049. [Google Scholar] [CrossRef]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [CrossRef]
- Shoda, C.; Miwa, Y.; Nimura, K.; Okamoto, K.; Yamagami, S.; Tsubota, K.; Kurihara, T. Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy. Nutrients 2020, 12, 1055. [Google Scholar] [CrossRef]
- Ibuki, M.; Lee, D.; Shinojima, A.; Miwa, Y.; Tsubota, K.; Kurihara, T. Rice Bran and Vitamin B6 Suppress Pathological Neovascularization in a Murine Model of Age-Related Macular Degeneration as Novel HIF Inhibitors. Int. J. Mol. Sci. 2020, 21, 8940. [Google Scholar] [CrossRef]
- Alvarado, P.; Moreno, G.; Vizzini, A.; Consiglio, G.; Manjón, J.L.; Setti, L. Atractosporocybe, Leucocybe and Rhizocybe: Three new clitocyboid genera in the Tricholomatoid clade (Agaricales) with notes on Clitocybe and Lepista. Mycologia 2015, 107, 123–136. [Google Scholar] [CrossRef]
- Couch, H.B. Diseases of Turfgrasses, 3rd ed.; Krieger: Malabar, FL, USA, 1995. [Google Scholar]
- Choi, J.-H.; Abe, N.; Tanaka, H.; Fushimi, K.; Nishina, Y.; Morita, A.; Kiriiwa, Y.; Motohashi, R.; Hashizume, D.; Koshino, H.; et al. Plant-Growth Regulator, Imidazole-4-Carboxamide, Produced by the Fairy Ring Forming Fungus Lepista sordida. J. Agric. Food Chem. 2010, 58, 9956–9959. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Fushimi, K.; Abe, N.; Tanaka, H.; Maeda, S.; Morita, A.; Hara, M.; Motohashi, R.; Matsunaga, J.; Eguchi, Y.; et al. Disclosure of the “Fairy” of Fairy-Ring-Forming Fungus Lepista sordida. Chembiochem 2010, 11, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Ohnishi, T.; Yamakawa, Y.; Takeda, S.; Sekiguchi, S.; Maruyama, W.; Yamashita, K.; Suzuki, T.; Morita, A.; Ikka, T.; et al. The Source of “Fairy Rings”: 2-Azahypoxanthine and Its Metabolite Found in a Novel Purine Metabolic Pathway in Plants. Angew. Chem. Int. Ed. 2014, 53, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Mitchinson, A. Fairy chemicals. Nature 2014, 505, 298. [Google Scholar] [CrossRef]
- Aoshima, H.; Matsumoto, T.; Ibuki, R.; Kawagishi, H. Safety evaluation of 2-aza-8-oxohypoxanthine by in vitro skin sensitization and human tests. Fundam. Toxicol. Sci. 2021, 8, 123–133. [Google Scholar] [CrossRef]
- Aoshima, H.; Ibuki, R.; Ito, M.; Kawagishi, H. Clinical Evaluation of Topical Lotion Containing 2-Aza-8-Oxohypoxanthine on Skin Barrier Function against Water Loss. Cosmetics 2021, 8, 83. [Google Scholar] [CrossRef]
- Inoue, C.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Toda, M.; Fridman D’Alessandro, V.; Inoue, R.; Fujimoto, H.; Kobori, H.; Tharavecharak, S.; Takeshita, A.; et al. The Fairy Chemical Imidazole-4-carboxamide Inhibits the Expression of Axl, PD-L1, and PD-L2 and Improves Response to Cisplatin in Melanoma. Cells 2022, 11, 374. [Google Scholar] [CrossRef]
- Noël-Suberville, C.; Cruz, C.; Guinberteau, J.; Montury, M. Correlation between Fatty Acid Content and Aromatic Compound Release in Fresh Blewit (Lepista nuda). J. Agric. Food Chem. 1996, 44, 1180–1183. [Google Scholar] [CrossRef]
- Mercan, N.; Duru, M.E.; Turkoglu, A.; Gezer, K.; Kivrak, I.; Turkoglu, H. Antioxidant and antimicrobial properties of ethanolic extract from Lepista nuda (Bull.) Cooke. Ann. Microbiol. 2006, 56, 339–344. [Google Scholar] [CrossRef]
- Vaz, J.A.; Heleno, S.A.; Martins, A.; Almeida, G.M.; Vasconcelos, M.H.; Ferreira, I.C. Wild mushrooms Clitocybe alexandri and Lepista inversa: In vitro antioxidant activity and growth inhibition of human tumour cell lines. Food Chem. Toxicol. 2010, 48, 2881–2884. [Google Scholar] [CrossRef]
- Fortin, H.; Tomasi, S.; Delcros, J.-G.; Bansard, J.-Y.; Boustie, J. In vivo antitumor activity of clitocine, an exocyclic amino nucleoside isolated from Lepista inversa. ChemMedChem Chem. Enabling Drug Discov. 2006, 1, 189–196. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, J.; Wang, Z.; Liu, Y.; Li, Y.; Ma, D.; Wang, Q. Discovery of Tryptanthrins as Novel Antiviral and Anti-Phytopathogenic-Fungus Agents. J. Agric. Food Chem. 2020, 68, 5586–5595. [Google Scholar] [CrossRef] [PubMed]
- Honda, G.; Tabata, M. Isolation of Antifungal Principle Tryptanthrin, from Strobilanthes Cusia O. Kuntze. Planta Medica 1979, 36, 85–86. [Google Scholar] [CrossRef]
- Honda, G.; Tosirisuk, V.; Tabata, M. Isolation of an Antidermatophytic, Tryptanthrin, from Indigo Plants, Polygonum tinctorium and Isatis tinctoria. Planta Medica 1980, 38, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Egestad, B.; Lindstrom, J.O. The structure and properties of some indolic constituents in Couroupita guianensis aubl. Tetrahedron 1985, 41, 2879–2881. [Google Scholar] [CrossRef]
- Chavda, V. “Cannonball tree”: The alchemist plant. Innorig. Int. J. Sci. 2015, 2, 6–9. [Google Scholar]
- Brufani, M.; Fedeli, W.; Mazza, F.; Gerhard, A.; Keller-Schierlein, W. The structure of tryptanthrin. Experientia 1971, 27, 1249–1250. [Google Scholar] [CrossRef]
- Pergola, C.; Jazzar, B.; Rossi, A.; Northoff, H.; Hamburger, M.; Sautebin, L.; Werz, O. On the inhibition of 5-lipoxygenase product formation by tryptanthrin: Mechanistic studies and efficacy in vivo. J. Cereb. Blood Flow Metab. 2012, 165, 765–776. [Google Scholar] [CrossRef]
- Krivogorsky, B.; Nelson, A.C.; Douglas, K.A.; Grundt, P. Tryptanthrin derivatives as Toxoplasma gondii inhibitors—Structure–activity-relationship of the 6-position. Bioorg. Med. Chem. Lett. 2013, 23, 1032–1035. [Google Scholar] [CrossRef]
- Han, N.-R.; Moon, P.-D.; Kim, H.-M.; Jeong, H.-J. Tryptanthrin ameliorates atopic dermatitis through down-regulation of TSLP. Arch. Biochem. Biophys. 2014, 542, 14–20. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X.; Wang, C.-L.; Wang, L.; Sun, C.; Zhang, D.-B.; Liu, J.-L.; Liang, Y.-N.; Tang, D.-X.; Tang, Z.-S. Tryptanthrin Protects Mice against Dextran Sulfate Sodium-Induced Colitis through Inhibition of TNF-α/NF-κB and IL-6/STAT3 Pathways. Molecules 2018, 23, 1062. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, A.; Seepersaud, M.; Maxwell, A.; Jayaraman, J.; Ramsubhag, A. Isolation and Antibacterial Activity of Indole Alkaloids from Pseudomonas aeruginosa UWI-1. Molecules 2020, 25, 3744. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Lee, C.-L.; Yen, H.-R.; Chang, Y.-S.; Lin, Y.-P.; Huang, S.-H.; Lin, C.-W. Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63. Biomolecules 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.-Y.; Park, S.-E.; Liang, J.L.; Jahng, Y.; Kwon, Y. Benzo[b]tryptanthrin Inhibits MDR1, Topoisomerase Activity, and Reverses Adriamycin Resistance in Breast Cancer Cells. Chemmedchem 2015, 10, 827–835. [Google Scholar] [CrossRef]
- Kim, W.; Youn, H.; Kwon, T.; Kang, J.; Kim, E.; Son, B.; Yang, H.J.; Jung, Y.; Youn, B. PIM1 kinase inhibitors induce radiosensitization in non-small cell lung cancer cells. Pharmacol. Res. 2013, 70, 90–101. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Ma, G.; Yan, J.; Wang, H.; Yang, Q. Transport Characteristics of Tryptanthrin and its Inhibitory Effect on P-gp and MRP2 in Caco-2 Cells. J. Pharm. Pharm. Sci. 2011, 14, 325–335. [Google Scholar] [CrossRef]
- Gao, J.-Y.; Chang, C.-S.; Lien, J.-C.; Chen, T.-W.; Hu, J.-L.; Weng, J.-R. Synthetic Tryptanthrin Derivatives Induce Cell Cycle Arrest and Apoptosis via Akt and MAPKs in Human Hepatocellular Carcinoma Cells. Biomedicines 2021, 9, 1527. [Google Scholar] [CrossRef]
- Latypova, D.K.; Shmakov, S.V.; Pechkovskaya, S.A.; Filatov, A.S.; Stepakov, A.V.; Knyazev, N.A.; Boitsov, V.M. Identification of Spiro-Fused Pyrrolo[3,4-a]pyrrolizines and Tryptanthrines as Potential Antitumor Agents: Synthesis and In Vitro Evaluation. Int. J. Mol. Sci. 2021, 22, 11997. [Google Scholar] [CrossRef]
- Miao, S.; Shi, X.; Zhang, H.; Wang, S.; Sun, J.; Hua, W.; Miao, Q.; Zhao, Y.; Zhang, C. Proliferation-Attenuating and Apoptosis-Inducing Effects of Tryptanthrin on Human Chronic Myeloid Leukemia K562 Cell Line in Vitro. Int. J. Mol. Sci. 2011, 12, 3831–3845. [Google Scholar] [CrossRef]
- Danz, H.; Stoyanova, S.; Thomet, O.A.; Simon, H.-U.; Dannhardt, G.; Ulbrich, H.; Hamburger, M. Inhibitory Activity of Tryptanthrin on Prostaglandin and Leukotriene Synthesis. Planta Medica 2002, 68, 875–880. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, G.H.; Kim, D.H.; Kim, D.H.; Jahng, Y.; Jeong, T.C. Identification of a Tryptanthrin Metabolite in Rat Liver Microsomes by Liquid Chromatography/Electrospray Ionization-Tandem Mass Spectrometry. Biol. Pharm. Bull. 2007, 30, 1991–1995. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Li, Y.; Yang, D.; Hu, N.; Guo, Z.; Kuang, C.; Yang, Q. Establishment of a human indoleamine 2,3-dioxygenase 2 (hIDO2) bioassay system and discovery of tryptanthrin derivatives as potent hIDO2 inhibitors. Eur. J. Med. Chem. 2016, 123, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-M.; Oh, T.; Kaneko, T.; Upton, A.M.; Franzblau, S.G.; Ma, Z.; Cho, S.-N.; Kim, P. Design, Synthesis, and Structure–Activity Relationship Studies of Tryptanthrins as Antitubercular Agents. J. Nat. Prod. 2012, 76, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Scovill, J.; Blank, E.; Konnick, M.; Nenortas, E.; Shapiro, T. Antitrypanosomal Activities of Tryptanthrins. Antimicrob. Agents Chemother. 2002, 46, 882–883. [Google Scholar] [CrossRef]
- Malya, I.Y.; Wu, J.; Harada, E.; Toda, M.; D’Alessandro-Gabazza, C.N.; Yasuma, T.; Gabazza, E.C.; Choi, J.-H.; Hirai, H.; Kawagishi, H. Plant growth regulators and Axl and immune checkpoint inhibitors from the edible mushroom Leucopaxillus giganteus. Biosci. Biotechnol. Biochem. 2020, 84, 1332–1338. [Google Scholar] [CrossRef]
- Ridwan, A.Y.; Wu, J.; Harada, E.; D’alessandro-Gabazza, C.N.; Toda, M.; Yasuma, T.; Gabazza, E.C.; Choi, J.-H.; Hirai, H.; Kawagishi, H. Axl and immune checkpoints inhibitors from fruiting bodies of Pleurocybella porrigens. J. Antibiot. 2020, 73, 733–736. [Google Scholar] [CrossRef]
- Yasuma, T.; Toda, M.; Kobori, H.; Tada, N.; D’Alessandro-Gabazza, C.N.; Gabazza, E.C. Subcritical Water Extracts from Agaricus blazei Murrill’s Mycelium Inhibit the Expression of Immune Checkpoint Molecules and Axl Receptor. J. Fungi 2021, 7, 590. [Google Scholar] [CrossRef]
- Robinson, A.; Keely, S.; Karhausen, J.; Gerich, M.E.; Furuta, G.T.; Colgan, S.P. Mucosal Protection by Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibition. Gastroenterology 2008, 134, 145–155. [Google Scholar] [CrossRef]
- Malek, G.; Busik, J.; Grant, M.B.; Choudhary, M. Models of retinal diseases and their applicability in drug discovery. Expert Opin. Drug Discov. 2018, 13, 359–377. [Google Scholar] [CrossRef]
- Hellinen, L.; Hagström, M.; Knuutila, H.; Ruponen, M.; Urtti, A.; Reinisalo, M. Characterization of artificially re-pigmented ARPE-19 retinal pigment epithelial cell model. Sci. Rep. 2019, 9, 13761. [Google Scholar] [CrossRef]
- Sayyad, Z.; Sirohi, K.; Radha, V.; Swarup, G. 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 2017, 7, 16855. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, W.; Mazza, F.J. Crystal structure of tryptanthrin(indolo[2,1-b]quinazoline-6,12-dione). J. Chem. Soc. Perkin Trans. 2 1974, 13, 1621–1623. [Google Scholar] [CrossRef]
- Kunimi, H.; Miwa, Y.; Inoue, H.; Tsubota, K.; Kurihara, T. A Novel HIF Inhibitor Halofuginone Prevents Neurodegeneration in a Murine Model of Retinal Ischemia-Reperfusion. Int. J. Mol. Sci. 2019, 20, 3171. [Google Scholar] [CrossRef]
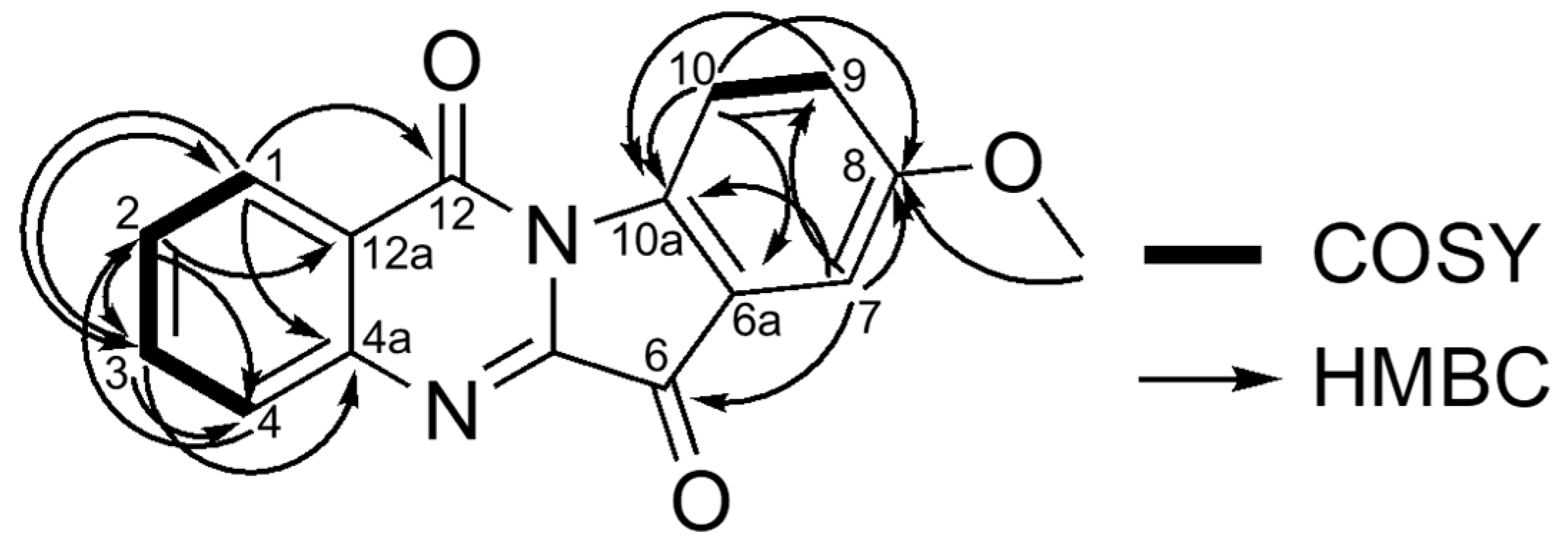


| Position | δH (J in Hz) | δc |
|---|---|---|
| 1 | 8.43 (dd, 8.0, 1.7) | 127.4 |
| 2 | 7.67 (ddd, 8.0, 8.2, 1.1) | 130.2 |
| 3 | 7.84 (ddd, 8.2, 8.1, 1.7) | 134.9 |
| 4 | 8.02 (br d, 8.1) | 130.7 |
| 4a | - | 146.6 |
| 5a | - | 144.8 |
| 6 | - | 182.7 |
| 6a | - | 123.0 |
| 7 | 7.38 (d, 2.2) | 108.4 |
| 8 | - | 158.8 |
| 9 | 7.31 (dd, 9.2, 2.9) | 125.1 |
| 10 | 8.52 (d, 9.2) | 119.2 |
| 10a | - | 140.5 |
| 12 | - | 157.8 |
| 12a | - | 123.9 |
| -OCH3 | 3.90 (s) | 56.0 |
| Position | Compound 2 | Thyptanthrin [33] |
|---|---|---|
| δc | δc | |
| 1 | 127.5 | 127.6 |
| 2 | 130.2 | 130.3 |
| 3 | 135.1 | 135.2 |
| 4 | 130.7 | 130.8 |
| 4a | 146.6 | 146.7 |
| 5a | 144.3 | 144.4 |
| 6 | 182.5 | 182.5 |
| 6a | 121.9 | 122.0 |
| 7 | 125.4 | 125.4 |
| 8 | 127.2 | 127.2 |
| 9 | 138.3 | 138.3 |
| 10 | 118.0 | 118.0 |
| 10a | 146.3 | 146.4 |
| 12 | 158.1 | 158.1 |
| 12a | 123.7 | 123.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotajima, M.; Choi, J.-H.; Kondo, M.; D’Alessandro-Gabazza, C.N.; Toda, M.; Yasuma, T.; Gabazza, E.C.; Miwa, Y.; Shoda, C.; Lee, D.; et al. Axl, Immune Checkpoint Molecules and HIF Inhibitors from the Culture Broth of Lepista luscina. Molecules 2022, 27, 8925. https://doi.org/10.3390/molecules27248925
Kotajima M, Choi J-H, Kondo M, D’Alessandro-Gabazza CN, Toda M, Yasuma T, Gabazza EC, Miwa Y, Shoda C, Lee D, et al. Axl, Immune Checkpoint Molecules and HIF Inhibitors from the Culture Broth of Lepista luscina. Molecules. 2022; 27(24):8925. https://doi.org/10.3390/molecules27248925
Chicago/Turabian StyleKotajima, Mihaya, Jae-Hoon Choi, Mitsuru Kondo, Corina N. D’Alessandro-Gabazza, Masaaki Toda, Taro Yasuma, Esteban C. Gabazza, Yukihiro Miwa, Chiho Shoda, Deokho Lee, and et al. 2022. "Axl, Immune Checkpoint Molecules and HIF Inhibitors from the Culture Broth of Lepista luscina" Molecules 27, no. 24: 8925. https://doi.org/10.3390/molecules27248925
APA StyleKotajima, M., Choi, J.-H., Kondo, M., D’Alessandro-Gabazza, C. N., Toda, M., Yasuma, T., Gabazza, E. C., Miwa, Y., Shoda, C., Lee, D., Nakai, A., Kurihara, T., Wu, J., Hirai, H., & Kawagishi, H. (2022). Axl, Immune Checkpoint Molecules and HIF Inhibitors from the Culture Broth of Lepista luscina. Molecules, 27(24), 8925. https://doi.org/10.3390/molecules27248925