Comparative Analysis of Traditional Oriental Herbal Fruits as Potential Sources of Polyphenols and Minerals for Nutritional Supplements
Abstract
1. Introduction
2. Results and Discussion
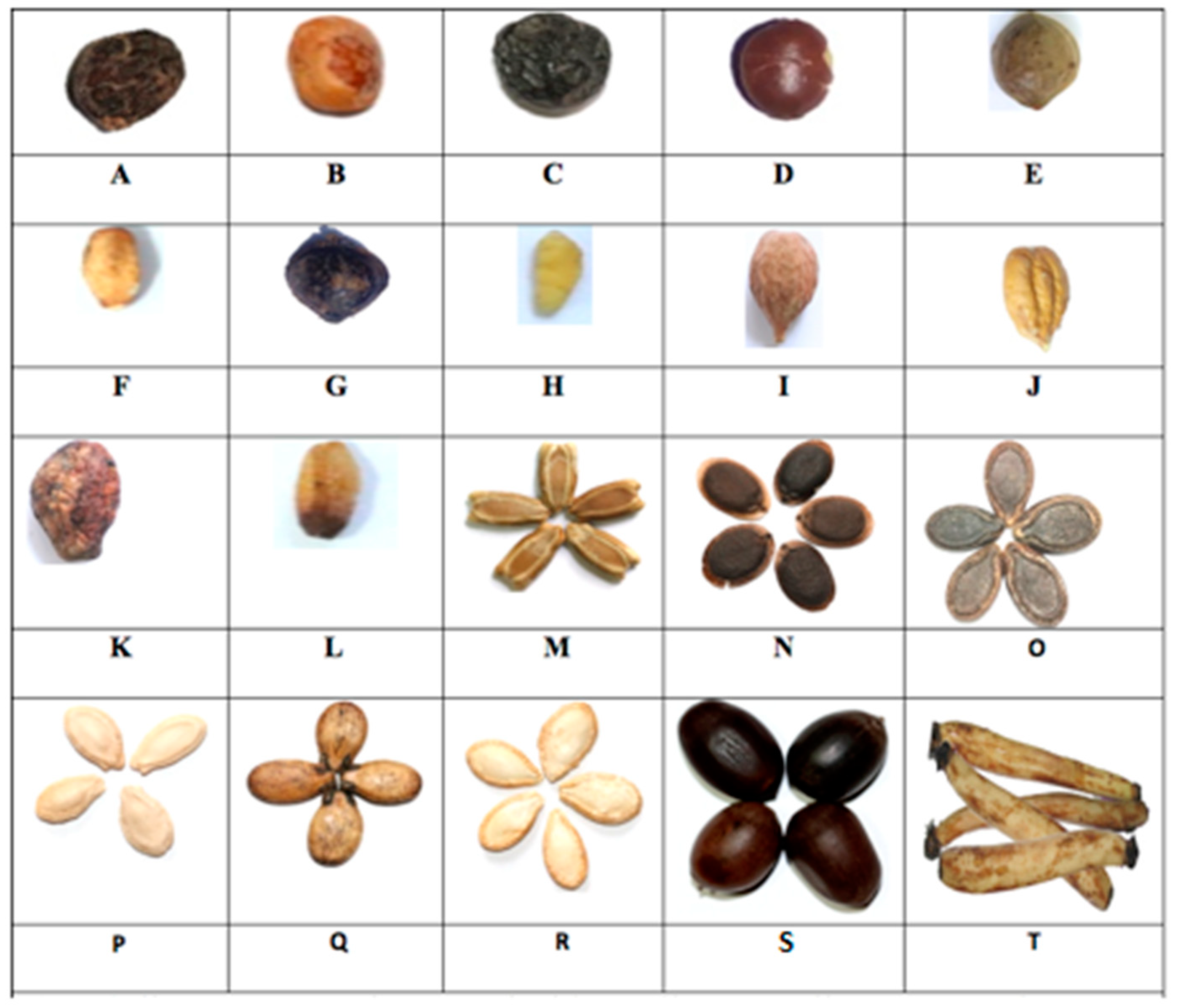
2.1. Physical Characteristics
2.2. Total Polyphenol (TPh) and Mineral Contents
2.3. Detailed Description of the Minerals
2.4. Viability of Herbal Fruits as a Source of Polyphenols and Minerals for Nutritional Supplements
3. Materials and Methods
3.1. Sampling of Herbal Fruits
3.2. Sample Preparation
3.3. Analysis of Polyphenols
3.4. Analysis of Minerals
3.5. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ros, E.; Hu, F.B. Consumption of plant seeds and cardiovascular health: Epidemiologic and clinical trial evidence. Circulation 2013, 128, 553–565. [Google Scholar] [CrossRef]
- Welch, R.M. The impact of mineral nutrients in food crops on global human health. Plant Soil. 2002, 247, 83–90. [Google Scholar] [CrossRef]
- Heaney, R.P. Sodium, Potassium, Phosphorus, and Magnesium. In Nutrition and Bone Health; Nutrition and Health Series; Holick, M.F., Dawson-Hughes, B., Eds.; Humana Press Inc.: New York, NY, USA, 2004; pp. 327–344. [Google Scholar]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Senhaji, S.; Lamchouri, F.; Boulfia, M.; Lachkar, N.; Bouabid, K.; Toufik, H. Mineral Composition, Content of Phenolic Compounds and in Vitro Antioxidant and Antibacterial Activities of Aqueous and Organic Extracts of the Seeds of Peganum harmala L. S. Afr. J. Bot. 2022, 147, 697–712. [Google Scholar] [CrossRef]
- Salami, M.; Heidari, B.; Tan, H. Comparative Profiling of Polyphenols and Antioxidants and Analysis of Antiglycation Activities in Rapeseed (Brassica napus L.) under Different Moisture Regimes. Food Chem. 2023, 399, 133946. [Google Scholar] [CrossRef]
- Ginocchio, R.; Muñoz-Carvajal, E.; Velásquez, P.; Giordano, A.; Montenegro, G.; Colque-Perez, G.; Sáez-Navarrete, C. Mayten Tree Seed Oil: Nutritional Value Evaluation According to Antioxidant Capacity and Bioactive Properties. Foods 2021, 10, 729. [Google Scholar] [CrossRef]
- Palejkar, C.J.; Palejkar, J.H.; Patel, A.J.; Patel, M.A. A plant review on Ziziphus mauritiana. Int. J. Univ. Pharm. Life Sci. 2012, 2, 203–211. [Google Scholar]
- Lee, S.M.; Park, J.G.; Lee, Y.H.; Lee, C.G.; Min, B.S.; Kim, J.H.; Lee, H.K. Anti-complementary activity of triterpenoides from fruits of Zizyphus jujube. Biol. Pharm. Bull. 2004, 27, 1883–1886. [Google Scholar] [CrossRef]
- Khatoon, N.; Gupta, R.K.; Tyagi, Y.K. Nutraceutical potential and phytochemical screening of Buchanania lanzan, an underutilized exotic Indian nut and its use as a source of functional food. J. Pharmacogn. Phytochem. 2015, 4, 87–94. [Google Scholar]
- Paudel, K.R.; Panth, N. Phytochemical profile and biological activity of Nelumbo nucifera. Evid. Based Complement. Altern. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef]
- Cock, I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, K.; Gupta, A.; Sharma, D.K.; Gill, N.S.; Goyal, A. A Review on the Medicinally Important Plants of the Family Cucurbitaceae. Asian J. Clin. Nutr. 2012, 4, 16–26. [Google Scholar] [CrossRef]
- Ajuru, M.; Nmom, F. A Review on the economic uses of species of Cucurbitaceae and their sustainability in Nigeria. Am. J. Plant Biol. 2017, 2, 17–24. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Lamien, E.C.; Compaoré, M.M.Y.; Meda, R.N.T.; Kiendrebeogo, M.; Zeba, B.; Millogo, J.F.; Nacoulma, O.G. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 2008, 13, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Tabiri, B.; Agbenorhevi, J.K.; Wireko-Manu, F.D.; Ompouma, E.I. Watermelon seeds as food: Nutrient composition, phytochemicals and antioxidant activity. Int. J. Nutri. Food Sci. 2016, 5, 139–144. [Google Scholar] [CrossRef]
- Seymen, M.; Uslu, N.; Türkmen, Ö.; Juhaimi, F.A.; Özcan, M.M. Chemical compositions and mineral contents of some hull-less pumpkin seed and oils. J. Am. Oil Chem. Soc. 2016, 93, 1095–1099. [Google Scholar] [CrossRef]
- Valenzuela, G.M.; Soro, A.S.; Tauguinas, A.L.; Gruszycki, M.R.; Cravzov, A.L.; Giménez, M.C.; Wirth, A. Evaluation of polyphenol content and antioxidant activity in extracts of Cucurbita spp. OALib 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Chakradhari, S.; Rajhans, K.; Patel, K.; Towett, E.; Martín-Gil, J.; Martín-Ramos, P. Nutritional and spectral characteristics of terminalia plants. Eur. J. Med. Plants 2019, 27, 1–13. [Google Scholar] [CrossRef]
- Elinge, C.M.; Muhammad, A.; Atiku, F.A.; Itodo, A.U.; Peni, I.J.; Sanni, O.M.; Mbongo, A.N. Proximate, mineral and anti-nutrient composition of pumpkin (Cucurbitapepo L.) seeds extract. Int. J. Plant Res. 2012, 2, 146–150. [Google Scholar] [CrossRef]
- Jacob, A.G.; Etong, D.I.; Tijjani, A. Proximate, mineral and anti-nutritional compositions of melon (Citrullus lanatus) seeds. Br. J. Res. 2015, 2, 142–151. [Google Scholar]
- Kwiri, R.; Winini, C.; Musengi, A.; Mudyiwa, M.; Nyambi, C.; Muredzi, P.; Malunga, A. Proximate composition of pumpkin gourd (Cucurbita pepo) seeds from Zimbabwe. Int. J. Nutri. Food Sci. 2014, 3, 279–283. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Gil-Hernández, Á.; Artacho Martín-Lagos, R.; Ruiz-López, M.D. (Eds.) Tratado de Nutrición. Tomo 3. Composición y Calidad Nutritiva de Los Alimentos, 3rd ed.; Médica Panamericana: Madrid, Spain, 2017; Volume 3, ISBN 9788491101925. [Google Scholar]
- Madrid Vicente, A. Bromatología: Ciencia de Los Alimentos: Con Ejercicios Prácticos Resueltos/Antonio Madrid; Madrid Vicente: Madrid, Spain, 2021; ISBN 9788412309300. [Google Scholar]
- Obadi, M.; Sun, J.; Xu, B. Highland Barley: Chemical Composition, Bioactive Compounds, Health Effects, and Applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Costello, R.B.; Rosanoff, A.; Dai, Q.; Saldanha, L.G.; Potischman, N.A. Perspective: Characterization of Dietary Supplements Containing Calcium and Magnesium and Their Respective Ratio—Is a Rising Ratio a Cause for Concern? Adv. Nutr. Int. Rev. J. 2021, 12, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Mata, A.; Sahu, P.K.; Chakradhari, S.; Sahu, Y.K.; Patel, K.S.; Singh, S.; Towett, E.K.; Martín-Ramos, P.; Quesada-Granados, J.J.; Rufián-Henares, J.A. Plant Seeds as Source of Nutrients and Phytochemicals for the Indian Population. Int. J. Food Sci. Technol. 2022, 57, 525–532. [Google Scholar] [CrossRef]
- Rao, N.D.; Min, J.; DeFries, R.; Ghosh-Jerath, S.; Valin, H.; Fanzo, J. Healthy, Affordable and Climate-Friendly Diets in India. Glob. Environ. Chang. 2018, 49, 154–165. [Google Scholar] [CrossRef]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Dekant, W.; Engel, K.H.; Gontard, N.; Gott, D.; Grilli, S.; et al. Safety of aluminium from dietary intake. EFSA J. 2008, 6, 754. [Google Scholar] [CrossRef]
- FAO/WHO. ALINORM 01/12A; Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commission 2001, Food Additives and Contaminants. FAO/WHO: Rome, Italy, 2001; pp. 1–286.
- ISO 874:1980(EN); Fresh Fruits and Vegetables—Sampling. International Organization for Standardization: Geneva, Switzerland, 1980.
- Bertaud, F.; Tapin-Lingua, S.; Pizzi, A.; Navarrete, P.; Petit-Conil, M. Characterisation of Industrial Barks for Their Tannin Contents for Further Green-Wood Based Adhesives Applications. In Tech Fibres COST FP0901-Hamburg; InTechFibres: Gières, France, 2010; Available online: http://web.abo.fi/fak/tkf/spk/costfp0901/Hamburg_2010/01_Hamburg_presentation_Bertaud.pdf (accessed on 17 January 2023).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Sahu, P.K.; Cervera-Mata, A.; Chakradhari, S.; Singh Patel, K.; Towett, E.K.; Quesada-Granados, J.J.; Martín-Ramos, P.; Rufián-Henares, J.A. Seeds as Potential Sources of Phenolic Compounds and Minerals for the Indian Population. Molecules 2022, 27, 3184. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Zurita-Ortega, A.; Cervera-Mata, A.; Delgado, G.; Zurita-Ortega, F.; Rufián-Henares, J.A.; Pastoriza, S. Mineral profile of weight loss related foods marketed in Spain. Food Chem. 2020, 313, 126156. [Google Scholar] [CrossRef] [PubMed]


| Species | Family | Part | Seed Mass (mg) | Kernel Mass (%) | Moisture (%) |
|---|---|---|---|---|---|
| Buchanania lanzan | Anacardiaceae | PC + SC | 310.0 | 24.0 | 5.2 |
| Buchanania lanzan | Anacardiaceae | KE | - | - | 1.9 |
| Ziziphus mauritiana | Rhamnaceae | PC + SC | 978.0 | 6.1 | 6.1 |
| Ziziphus mauritiana | Rhamnaceae | KE | - | - | 1.2 |
| Nilumbonucifera -I | Nelumbonaceae | Rh | - | 10.5 | 10.5 |
| Nilumbo nucifera -II | Nelumbonaceae | Rh | - | 10.4 | 10.4 |
| Nilumbo nucifera -I | Nelumbonaceae | SE | 1213.0 | 5.4 | 7.2 |
| Nilumbo nucifera -II | Nelumbonaceae | SE | 1180.0 | 5.3 | 6.9 |
| Terminaliacatappa -I | Combretaceae | PC + SC | 4763.0 | 8.3 | 4.8 |
| Terminalia catappa -II | Combretaceae | PC + SC | 5770.0 | 8.4 | 4.3 |
| Terminalia catappa -I | Combretaceae | KE | - | - | 2.8 |
| Terminalia catappa -II | Combretaceae | KE | - | - | 2.2 |
| Terminalia arjuna | Combretaceae | PC + SC | 3885.0 | 3.1 | 4.2 |
| Terminalia arjuna | Combretaceae | KE | - | - | 3.8 |
| Terminalia bellirica | Combretaceae | PC + SC | 4373.0 | 11.0 | 5.2 |
| Terminalia bellirica | Combretaceae | KE | - | - | 3.5 |
| Terminalia chebula | Combretaceae | PC + SC | 5426.0 | 3.1 | 4.9 |
| Terminalia chebula | Combretaceae | KE | - | - | 3.0 |
| Lagenaria siceraria -I | Cucurbitaceae | CA | - | - | 12.6 |
| Lagenaria siceraria -II | Cucurbitaceae | CA | - | - | 13.5 |
| Lagenaria siceraria -I | Cucurbitaceae | SC | 240.0 | 47.0 | 4.5 |
| Lagenaria siceraria -II | Cucurbitaceae | SC | 216.0 | 58.0 | 5.0 |
| Lagenaria siceraria -I | Cucurbitaceae | KE | - | - | 4.6 |
| Lagenaria siceraria -II | Cucurbitaceae | KE | - | - | 4.4 |
| Luffa aegyptiaca | Cucurbitaceae | KE | 105.0 | 43.0 | 5.5 |
| Praecitrullus fistulosus | Cucurbitaceae | KE | 90.0 | 46.0 | 9.6 |
| Benincasa hispida | Cucurbitaceae | KE | 64.0 | 47.0 | 8.3 |
| Citrullus lanatus var. lanatus | Cucurbitaceae | KE | 38.0 | 49.0 | 6.5 |
| Cucurbita maxima | Cucurbitaceae | KE | 132.0 | 18.0 | 7.8 |
| Species | Part | TPh mg Rutin Hydrate/100 g | Fla mg Quercetin/100 g |
|---|---|---|---|
| Buchanania lanzan | PC + SC | 1525.0 ± 10.44 | 805.0 ± 8.72 |
| Buchanania lanzan | KE | 2553.0 ± 13.53 | 406.0 ± 5.57 |
| Ziziphus mauritiana | PC + SC | 1094.0 ± 15.10 | 820.0 ± 6.24 |
| Ziziphus mauritiana | KE | 1210.0 ± 6.24 | 1065.0 ± 6.08 |
| Nilumbo nucifera -I | Rh | 778.0 ± 12.53 | 20.0 ± 2.65 |
| Nilumbo nucifera -II | Rh | 857.0 ± 11.27 | 28.0 ± 3.61 |
| Nilumbo nucifera -I | SE | 883.0 ± 7.55 | 41.0 ± 2.65 |
| Nilumbo nucifera -II | SE | 1030.0 ± 7.81 | 44.0 ± 3.61 |
| Terminalia catappa -I | PC + SC | 2219.0 ± 7.94 | 1053.0 ± 7.55 |
| Terminalia catappa -II | PC + SC | 1978.0 ± 11.36 | 932.0 ± 5.57 |
| Terminalia catappa -I | KE | 576.0 ± 6.56 | 300.0 ± 6.93 |
| Terminalia catappa -II | KE | 544.0 ± 6.24 | 265.0 ± 4.58 |
| Terminalia arjuna | PC + SC | 3144.0 ± 8.19 | 3070.0 ± 13.08 |
| Terminalia arjuna | KE | 1943.0 ± 9.85 | 830.0 ± 6.56 |
| Terminalia bellirica | PC + SC | 2505.0 ± 8.66 | 525.0 ± 5.29 |
| Terminalia bellirica | KE | 584.0 ± 4.58 | 360.0 ± 4.58 |
| Terminalia chebula | PC + SC | 3085.0 ± 13.89 | 2942.0 ± 11.27 |
| Terminalia chebula | KE | 2554.0 ± 9.54 | 1970.0 ± 9.64 |
| Lagenaria siceraria -I | CA | 831.0 ± 7.94 | 214.0 ± 3.46 |
| Lagenaria siceraria -II | CA | 778.0 ± 7.55 | 208.0 ± 3.61 |
| Lagenaria siceraria -I | SC | 1480.0 ± 9.64 | 939.0 ± 9.85 |
| Lagenaria siceraria -II | SC | 1440.0 ± 8.72 | 890.0 ± 8.54 |
| Lagenaria siceraria -I | KE | 1340.0 ± 7.21 | 1205.0 ± 7.94 |
| Lagenaria siceraria -II | KE | 1256.0 ± 10.58 | 1140.0 ± 6.56 |
| Luffa aegyptiaca | KE | 777.0 ± 6.56 | 252.0 ± 3.46 |
| Praecitrullus fistulosus | KE | 1136.0 ± 9.64 | 466.0 ± 4.58 |
| Benincasa hispida | KE | 407.0 ± 5.57 | 228.0 ± 4.36 |
| Citrullus lanatus var. lanatus | KE | 828.0 ± 8.19 | 728.0 ± 6.24 |
| Cucurbita maxima | KE | 1230.0 ± 8.89 | 1100.0 ± 9.54 |
| Species | Part | Al | Ca | Cr | Cu | Fe (**) | K | Mg (**) | Mn |
|---|---|---|---|---|---|---|---|---|---|
| BL | PC + SC | 5.17 ± 0.08 | 3662.57 ± 3.01 | 1.41 ± 0.19 | 8.44 ± 0.37 | 22.57 ± 0.19 | 701.81 ± 3.87 ϕ | 602.93 ± 6.28 | 6.72 ± 0.08 |
| BL | KE | 6.03 ± 0.24 | 122.25 ± 0.27 | 0 | 0.75 ± 0.06 | 8.59 ± 0.38 | 932.57 ± 2.90 ϕ | 517.21 ± 3.30 | 2.25 ± 0.10 |
| ZM | PC + SC | 1.91 ± 0.17 | 220.58 ± 0.14 | 0.25 ± 0.07 | 0 | 6.52 ± 0.19 | 1656.54 ± 11.04 | 142.46 ± 2.34 | 1.16 ± 0.12 √ |
| ZM | KE | 0.73 ± 0.15 | 145.96 ± 0.14 | 0.31 ± 0.05 | 0.27 ± 0.04 | 9.13 ± 0.12 | 991.93 ± 2.69 | 421.96 ± 3.59 | 2.94 ± 0.11 √ |
| NN | Rh | 57.81 ± 2.30 | 168.74 ± 0.14 | 0.11 ± 0.03 | 0 | 64.64 ± 0.13 | 2983.08 ± 7.54 | 101.92 ± 3.40 | 8.69 ± 0.17 ϕ®£¥∞©√ |
| NN | SE | 8.67 ± 0.34 | 184.03 ± 0.09 | 0 | 0.66 ± 0.08 | 11.12 ± 0.93 | 1626.71 ± 6.12 | 172.75 ± 6.91 | 6.54 ± 0.26 ϕ®£¥∞©√ |
| TC | PC + SC | 8.36 ± 0.33 ϕ | 183.87 ± 0.06 | 0 | 18.96 ± 0.80 | 13.39 ± 0.35 | 3231.41 ± 12.53 | 61.31 ± 3.05 | 0.85 ± 0.16 ∞ |
| TC | KE | 388.17 ± 5.46 ϕ | 473.05 ± 0.20 | 0.39 ± 0.03 | 1.98 ± 0.13 | 12.86 ± 0.75 | 1484.08 ± 15.52 | 664.31 ± 3.23 | 2.27 ± 0.11 ∞ |
| TA | PC + SC | 30.42 ± 1.41 | 582.98 ± 0.20 | 0.13 ± 0.04 | 181.64 ± 0.95 ϕ | 35.84 ± 0.97 | 3427.72 ± 25.25 ϕ | 185.58 ± 4.39 | 4.12 ± 0.10 |
| TA | KE | 1.08 ± 0.07 | 1874.19 ± 1.23 | 0.10 ± 0.02 | 3.22 ± 0.10 ϕ | 8.01 ± 0.39 | 2583.91 ± 35.78 ϕ | 1455.81 ± 9.44 | 5.47 ± 0.13 |
| TB | PC + SC | 4.35 ± 0.17 | 982.94 ± 1.16 | 0 | 0 | 11.45 ± 0.29 | 2969.25 ± 31.13 | 174.46 ± 2.15 | 2.79 ± 0.12 ¥ |
| TB | KE | 1.02 ± 0.06 | 1195.66 ± 1.25 | 0.12 ± 0.02 | 1.79 ± 0.20 | 4.86 ± 0.27 | 1391.55 ± 35.95 | 532.91 ± 4.51 | 1.41 ± 0.08 ¥ |
| TCH | PC + SC | 1.75 ± 0.13 | 79.77 ± 0.32 | 0.10 ± 0.03 | < | 9.00 ± 0.29 | 1797.79 ± 11.84 | 57.92 ± 5.13 | 0.49 ± 0.13 © |
| TCH | KE | 1.75 ± 0.15 | 533.23 ± 0.20 | 0.14 ± 0.04 | 2.39 ± 0.08 | 5.74 ± 0.27 | 1379.31 ± 10.01 | 970.77 ± 9.58 | 3.83 ± 0.15 © |
| LS | CA | 4.98 ± 0.15 ϕ | 424.48 ± 0.16 | 0.11 ± 0.04 | 0 ϕ | 9.55 ± 0.41 | 2549.35 ± 10.71 | 140.05 ± 4.31 | 2.06 ± 0.12 ϕ |
| LS | SC | 3.65 ± 0.11 ϕ | 58.03 ± 0.12 | 0.02 ± 0.02 | 1.04 ± 0.08 ϕ | 5.96 ± 0.32 | 1175.24 ± 6.01 | 52.89 ± 2.01 | 1.72 ± 0.10 ϕ |
| LS | KE | 1.03 ± 0.03 ϕ | 50.38 ± 0.15 | 0 | 4.35 ± 0.09 ϕ | 9.35 ± 0.13 | 942.15 ± 5.33 | 798.03 ± 8.22 | 3.24 ± 0.12 ϕ |
| LA | KE | 0.45 ± 0.02 | 33.81 ± 0.11 | 0.15 ± 0.06 | 0.51 ± 0.06 | 4.77 ± 0.57 | 1312.13 ± 9.91 | 709.21 ± 7.31 | 2.04 ± 0.11 ® |
| PF | KE | 2.29 ± 0.07 | 172.19 ± 0.10 | 0 | 0.68 ± 0.05 | 11.47 ± 0.27 | 1048.62 ± 14.42 | 853.17 ± 6.82 | 2.23 ± 0.08 £ |
| BH | KE | 4.83 ± 0.15 | 81.24 ± 0.24 | 0 | 0.76 ± 0.39 | 7.84 ± 0.71 | 954.82 ± 11.19 | 786.11 ± 9.98 | 5.85 ± 0.23 |
| CL | KE | 4.95 ± 0.15 | 68.74 ± 0.26 | 0 | 0.93 ± 0.07 | 7.77 ± 0.19 | 1054.87 ± 13.15 | 680.03 ± 6.26 | 3.21 ± 0.12 |
| CM | KE | 0.70 ± 0.11 | 32.34 ± 0.09 | 0.02 ± 0.01 | 0.76 ± 0.08 | 9.04 ± 0.64 | 992.10 ± 18.35 | 841.09 ± 7.87 | 4.89 ± 0.20 |
| Species | Part | Mo | Na | Ni | P (**) | Pb (*) | S (**) | Zn | As (*) |
| BL | PC + SC | 0 | 14.90 ± 0.32 | 0.23 ± 0.03 | 130.91 ± 0.35 | 0 | 113.00 ± 0.28 | 0 | 0 |
| BL | KE | 0 | 7.94 ± 0.10 | 0.27 ± 0.04 | 936.12 ± 0.81 | 0 | 285.89 ± 3.94 | 2.19 ± 0.07 | 0 |
| ZM | PC + SC | 0 | 4.23 ± 0.07 ¥ | 0 ® | 111.64 ± 1.41 | 0 | 63.47 ± 0.46 | 0 | 0 |
| ZM | KE | 0 | 4.75 ± 0.05 ¥ | 0 ® | 870.25 ± 2.22 | 0.53 ± 0.07 | 337.09 ± 2.80 | 4.82 ± 0.13 | 0 |
| NN | Rh | 0 | 51.63 ± 0.25 | 0.17 ± 0.04 | 404.67 ± 1.52 | 0.22 ± 0.03 | 196.78 ± 0.31 | 0 | 0 |
| NN | SE | 0 | 9.66 ± 0.10 | 0.50 ± 0.05 | 743.22 ± 2.27 | 0 | 237.51 ± 1.36 | 0 | 0 |
| TC | PC + SC | 0 | 60.01 ± 0.96 ϕ®£¥ | 0.01 ± 0.01 | 78.03 ± 0.76 | 2.94 ± 0.08 | 72.40 ± 0.65 | 0 | 0 |
| TC | KE | 0 | 1549.87 ± 12.94 ϕ®£¥ | 0.82 ± 0.03 | 1193.62 ± 4.24 | 2.49 ± 0.09 | 251.42 ± 0.57 | 10.76 ± 0.10 | 0 |
| TA | PC + SC | 0 | 22.39 ± 0.18 | 0.52 ± 0.09 | 64.15 ± 1.09 | 0 | 86.50 ± 0.55 | 0 | 0 |
| TA | KE | 0 | 7.78 ± 0.12 | 0.86 ± 0.05 | 1617.28 ± 3.25 | 1.76 ± 0.06 | 366.76 ± 0.56 | 1.76 ± 0.09 | 0 |
| TB | PC + SC | 0.05 ± 0.01 | 7.01 ± 0.13 ® | 0.28 ± 0.02 | 59.74 ± 1.75 | 1.30 ± 0.04 | 74.07 ± 1.47 | 0 | 0 |
| TB | KE | 0 | 11.83 ± 0.16 ® | 0.76 ± 0.04 | 771.51 ± 1.31 | 0 | 233.10 ± 1.23 | 0 | 0 |
| TCH | PC + SC | < | 2.31 ± 0.09 £ | < | 25.95 ± 0.87 | < | 32.28 ± 0.77 | < | < |
| TCH | KE | < | 7.12 ± 0.11 £ | 0.78 ± 0.06 | 897.02 ± 5.59 | < | 339.92 ± 0.88 | 3.47 ± 0.30 | < |
| LS | CA | 0 | 7.36 ± 0.08 ϕ | 0.29 ± 0.05 ϕ | 161.56 ± 0.39 | 0 | 80.81 ± 0.41 | 0 | 1.27 ± 0.04 |
| LS | SC | 0 | 6.64 ± 0.10 ϕ | 0 ϕ | 86.04 ± 0.60 | 0 | 41.36 ± 1.13 | 0 | 0 |
| LS | KE | 0 | 8.00 ± 0.11 ϕ | 0 ϕ | 1150.64 ± 1.52 | 0.29 ± 0.03 | 294.11 ± 0.48 | 1.71 ± 0.17 | 0 |
| LA | KE | 0 | 4.93 ± 0.07 | 0 | 1044.37 ± 1.19 | 0.33 ± 0.03 | 250.00 ± 0.64 | 0.57 ± 0.06 | 4.75 ± 0.05 |
| PF | KE | 0 | 18.59 ± 0.21 | 0.35 ± 0.10 | 1127.10 ± 2.01 | 0 | 307.79 ± 0.83 | 1.82 ± 0.06 | 0 |
| BH | KE | 0 | 8.82 ± 0.14 | 0.18 ± 0.05 | 1191.30 ± 5.48 | 0 | 292.87 ± 0.90 | 2.38 ± 0.08 | 1.86 ± 0.04 |
| CL | KE | 0 | 8.05 ± 0.11 | 0.95 ± 0.05 ϕ | 1091.43 ± 7.07 | 0.41 ± 0.04 | 367.84 ± 0.88 | 1.44 ± 0.10 | 0 |
| CM | KE | 0 | 18.81 ± 0.08 | 0 | 1218.13 ± 3.01 | 0 | 287.02 ± 0.91 | 2.19 ± 0.07 | 0 |
| Species | Part | Lead | Arsenic | Viability |
|---|---|---|---|---|
| Buchanania lanzan | PC + SC | yes | ||
| Buchanania lanzan | KE | yes | ||
| Ziziphus mauritiana | PC + SC | yes | ||
| Ziziphus mauritiana | KE | + | not | |
| Nilumbo nucifera -I | Rh | + | not | |
| Nilumbo nucifera -II | Rh | + | not | |
| Nilumbo nucifera -I | SE | yes | ||
| Nilumbo nucifera -II | SE | yes | ||
| Terminalia catappa -I | PC + SC | + | not | |
| Terminalia catappa -II | PC + SC | + | not | |
| Terminalia catappa -I | KE | + | not | |
| Terminalia catappa -II | KE | + | not | |
| Terminalia arjuna | PC + SC | yes | ||
| Terminalia arjuna | KE | + | not | |
| Terminalia bellirica | PC + SC | + | not | |
| Terminalia bellirica | KE | yes | ||
| Terminalia chebula | PC + SC | yes | ||
| Terminalia chebula | KE | yes | ||
| Lagenaria siceraria -I | CA | + | not | |
| Lagenaria siceraria -II | CA | + | not | |
| Lagenaria siceraria -I | SC | yes | ||
| Lagenaria siceraria -II | SC | yes | ||
| Lagenaria siceraria -I | KE | + | not | |
| Lagenaria siceraria -II | KE | + | not | |
| Luffa aegyptiaca | KE | + | + | not |
| Praecitrullus fistulosus | KE | yes | ||
| Benincasa hispida | KE | + | not | |
| Citrullus lanatus var. lanatus | KE | + | not | |
| Cucurbita maxima | KE | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quesada-Granados, J.J.; Rufián-Henares, J.Á.; Chakradhari, S.; Sahu, P.K.; Sahu, Y.K.; Patel, K.S. Comparative Analysis of Traditional Oriental Herbal Fruits as Potential Sources of Polyphenols and Minerals for Nutritional Supplements. Molecules 2023, 28, 2682. https://doi.org/10.3390/molecules28062682
Quesada-Granados JJ, Rufián-Henares JÁ, Chakradhari S, Sahu PK, Sahu YK, Patel KS. Comparative Analysis of Traditional Oriental Herbal Fruits as Potential Sources of Polyphenols and Minerals for Nutritional Supplements. Molecules. 2023; 28(6):2682. https://doi.org/10.3390/molecules28062682
Chicago/Turabian StyleQuesada-Granados, José Javier, José Ángel Rufián-Henares, Suryakant Chakradhari, Pravin Kumar Sahu, Yaman Kumar Sahu, and Khageshwar Singh Patel. 2023. "Comparative Analysis of Traditional Oriental Herbal Fruits as Potential Sources of Polyphenols and Minerals for Nutritional Supplements" Molecules 28, no. 6: 2682. https://doi.org/10.3390/molecules28062682
APA StyleQuesada-Granados, J. J., Rufián-Henares, J. Á., Chakradhari, S., Sahu, P. K., Sahu, Y. K., & Patel, K. S. (2023). Comparative Analysis of Traditional Oriental Herbal Fruits as Potential Sources of Polyphenols and Minerals for Nutritional Supplements. Molecules, 28(6), 2682. https://doi.org/10.3390/molecules28062682







