Triterpenoids of Three Apple Cultivars—Biosynthesis, Antioxidative and Anti-Inflammatory Properties, and Fate during Processing
Abstract
1. Introduction
2. Results
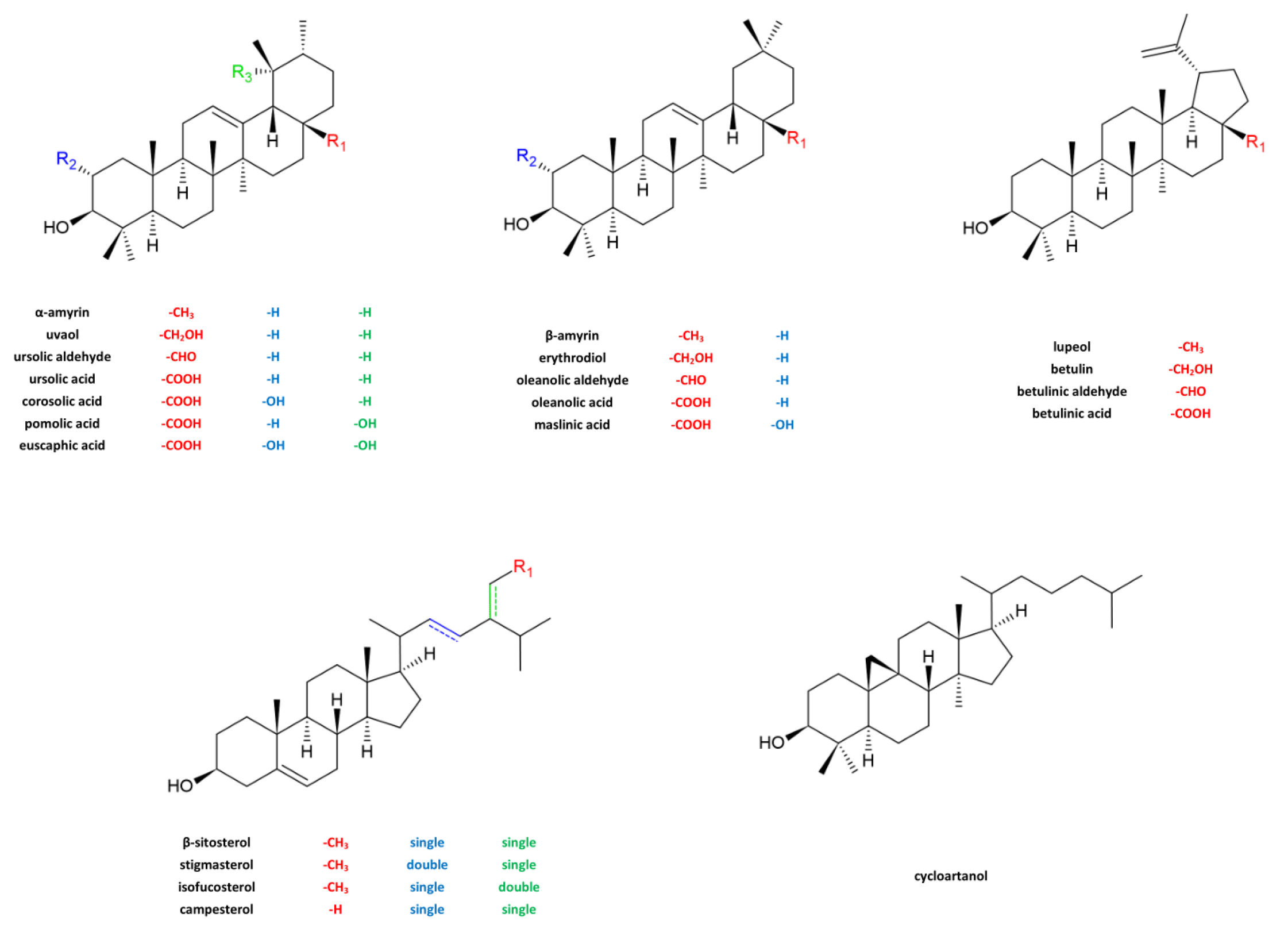
2.1. Identification of Triterpenoids
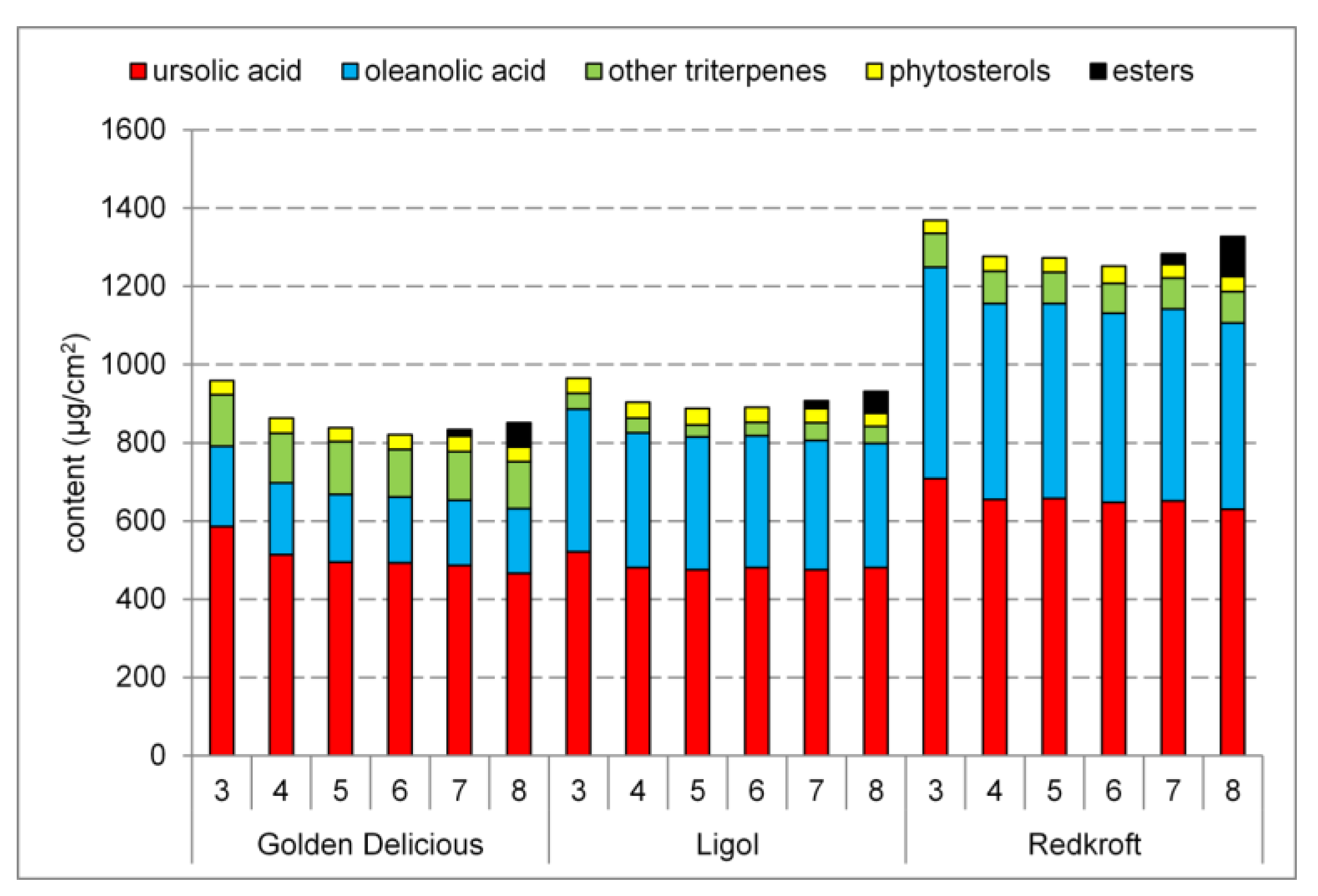
2.2. Triterpenoids in the Fruit
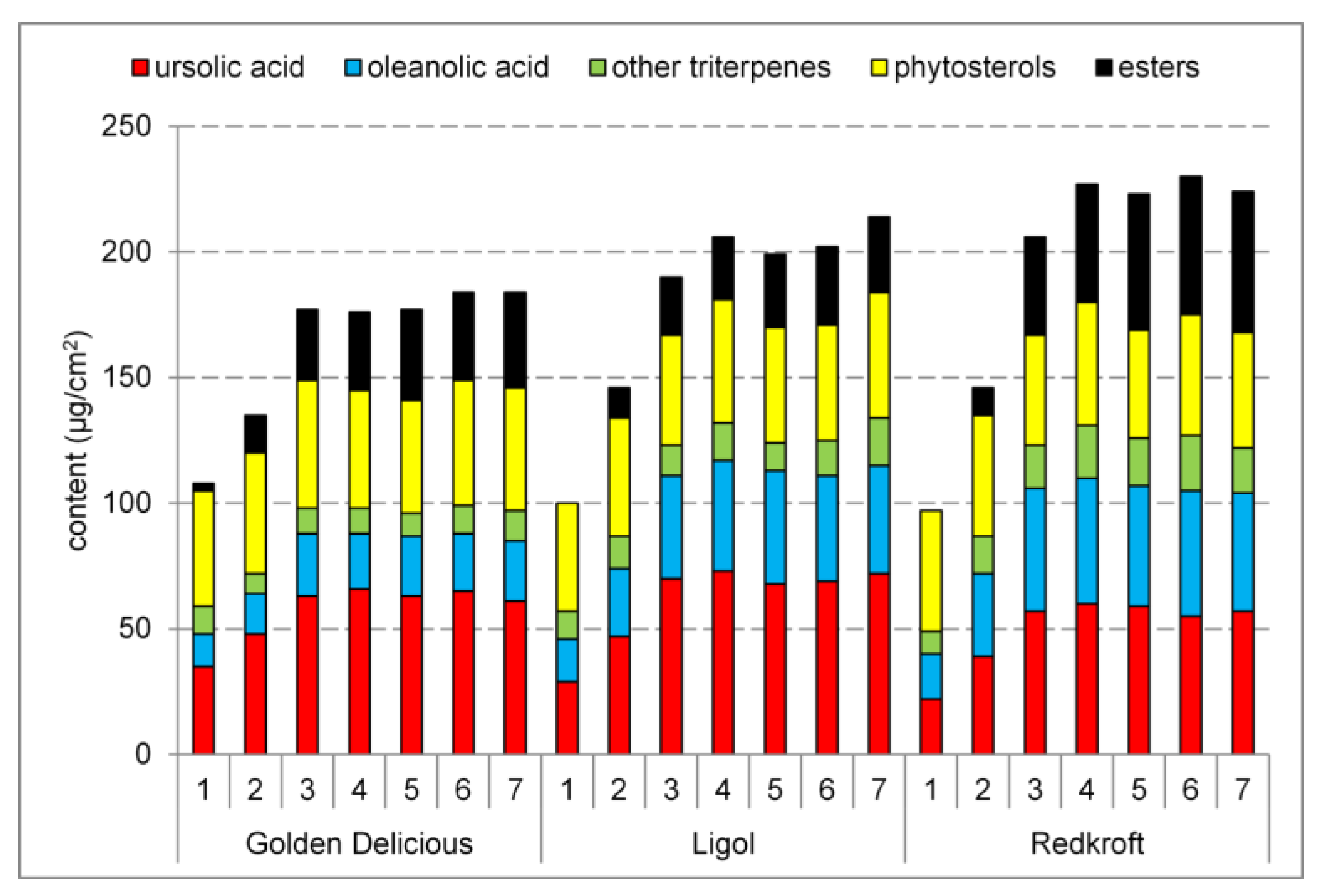
2.3. Triterpenoids in the Leaves
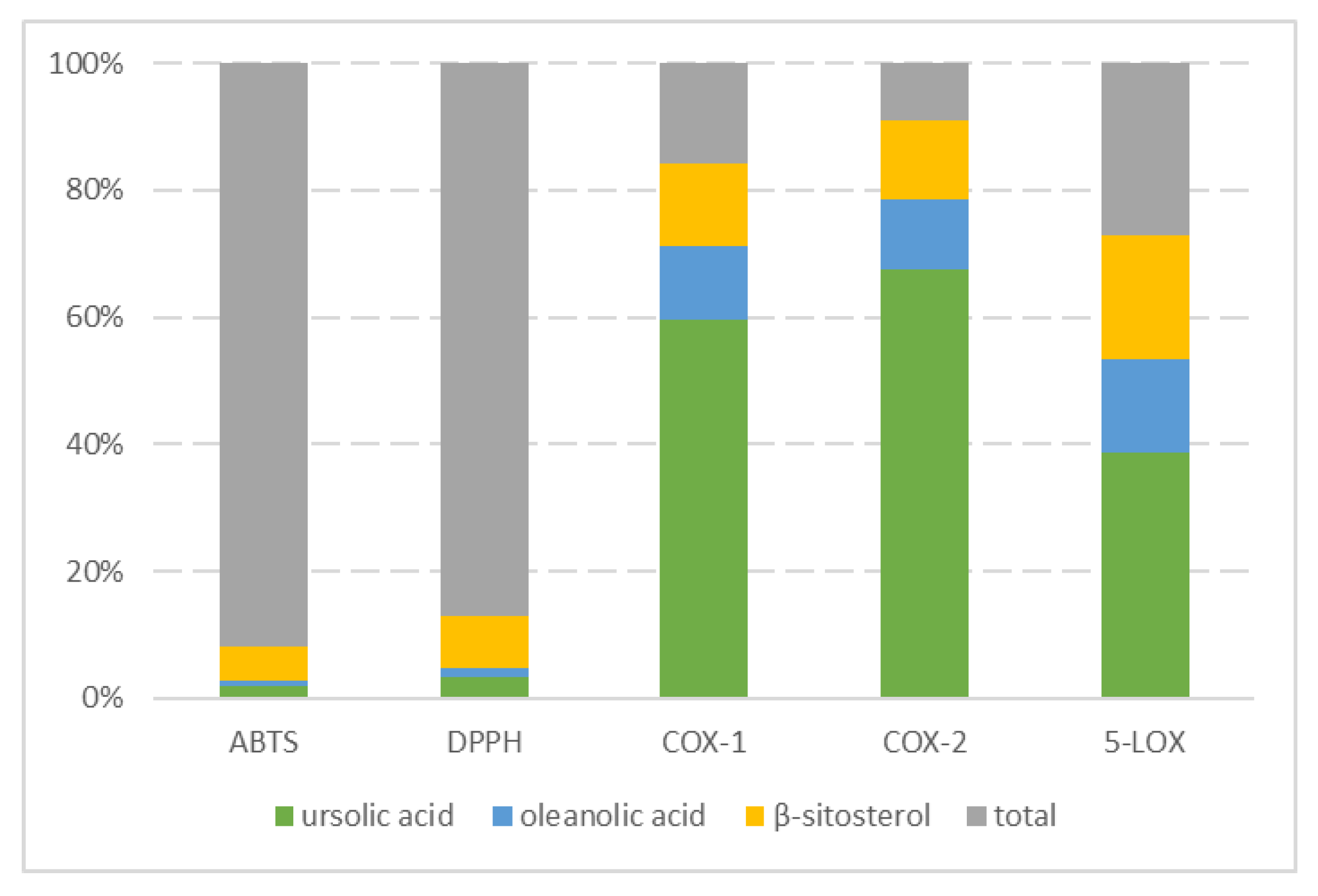
2.4. Antioxidative and Anti-Inflammatory Properties of Triterpenoids
2.5. Impact of Processing on Triterpenoid Content
3. Discussion
3.1. Triterpenoids in the Fruit
3.2. Triterpenoids in the Leaves
3.3. Antioxidative and Anti-Inflammatory Properties of Triterpenoids
3.4. Impact of Processing on Triterpenoid Content
4. Materials and Methods
4.1. Material
4.2. Chemicals and Standards
4.3. Analysis of Triterpenoid Content
4.4. Antioxidative and Anti-Inflammatory Properties
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar]
- Li, D.; Zaman, W.; Lu, J.; Niu, Q.; Zhang, X.; Ayaz, A.; Saqib, S.; Yang, B.; Zhang, J.; Zhao, H.; et al. Natural lupeol level variation among castor accessions and the upregulation of lupeol synthesis in response to light. Ind. Crops Prod. 2023, 192, 116090. [Google Scholar]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular waxes. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Bio. 2014, 65, 225–257. [Google Scholar]
- Lara, I.; Beige, B.; Goulao, L.F. A focus on the biosynthesis and composition of cuticle in fruits. J. Agric. Food Chem. 2015, 63, 4005–4019. [Google Scholar]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic acid—A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar]
- FAOSTAT. Available online: http://faostat.fao.org (accessed on 10 October 2021).
- O’Rourke, D. Economic Importance of the World Apple Industry. In The Apple Genome; Korban, S.S., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–18. [Google Scholar]
- Nezbedova, L.; McGhie, T.; Christensen, M.; Heyes, J.; Nasef, N.A.; Mehta, S. Onco-Preventive and Chemo-Protective Effects of Apple Bioactive Compounds. Nutrients 2021, 13, 4025. [Google Scholar]
- Oyehini, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An apple a day keeps the doctor away”: The potentials of apple bioactive constituents for chronic disease prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Farneti, B.; Masuero, D.; Costa, F.; Magnago, P.; Malnoy, M.; Costa, G.; Vrhovsek, U.; Mattivi, F. Is There Room for Improving the Nutraceutical Composition of Apple? J. Agric. Food Chem. 2015, 63, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-inflammatory procyanidins and triterepenes in 109 apple varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Paz Romero, M.; Motilva, M.J. Phytochemical profiles of new red-fleshed apple varieties compared with traditional and new white-fleshed varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Butkevičiūte, A.; Liaudanskas, M.; Kviklys, D.; Zymonė, K.; Raudonis, J.V.; Uselis, N.; Janulis, V. Detection and analysis of triterpenic compounds in apple extracts. Int. J. Food Prop. 2018, 21, 1716–1727. [Google Scholar] [CrossRef]
- Leide, J.; Xavier de Souza, A.; Papp, I.; Riederer, M. Specific characteristics of the apple fruit cuticle: Investigation of early and late season cultivars ‘Prima’ and ‘Florina’ (Malus domestica Borkh.). Sci. Hortic. 2018, 229, 137–147. [Google Scholar] [CrossRef]
- Wildner, A.C.; Ferreira, P.L.; Oliveira, S.S.; Gnoatto, S.B.; Bergold, A.M. Variation of ursolic acid and betulinic acid in five Malus domestica clones form Southern Brazil. J. Appl. Pharm. Sci. 2018, 8, 158–165. [Google Scholar]
- Andre, C.M.; Larsen, L.; Burgess, E.J.; Jensen, D.J.; Cooney, J.M.; Evers, D.; Zhang, J.; Perry, N.B.; Laing, W.A. Unusual immuno-modulatory triterpene-caffeates in the skins of russeted varieties of apples and pears. J. Agric. Food Chem. 2013, 61, 2273–2279. [Google Scholar] [CrossRef]
- He, X.; Liu, R.H. Phytochemicals in apple peels: Isolation, structure elucidation, and their antiproliferative and antioxidant activities. J. Agric. Food Chem. 2008, 56, 9905–9910. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Hudault, S.; Lunken, R.C.M.; Christeller, J.T. Apple peels, from seven cultivars, have lipase-inhibitory activity and contain numerous ursenoic acids as identified by LC-ESI-QTOF-HRMS. J. Agric. Food Chem. 2012, 60, 482–491. [Google Scholar] [CrossRef]
- Poirier, B.C.; Buchanan, D.A.; Rudell, D.R.; Mattheis, J.P. Differential partitioning of triterpenes and triterpene esters in apple peel. J. Agric. Food Chem. 2018, 66, 1800–1806. [Google Scholar] [CrossRef]
- Andre, C.M.; Legay, S.; Deleruelle, A.; Nieuwenhuizen, N.; Punter, M.; Brendolise, C.; Cooney, J.M.; Lateur, M.; Hausman, J.F.; Larondelle, Y.; et al. Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids. New Phytol. 2016, 211, 1279–1294. [Google Scholar] [CrossRef]
- Dashbaldan, S.; Pączkowski, C.; Szakiel, A. Variations in triterpenoid deposition in cuticular waxes during development and maturation of selected fruits from Rosaceae family. Int. J. Mol. Sci. 2020, 21, 9762. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Szakiel, A.; Pączkowski, C.; Marszałek, K.; Skąpska, S.; Kowalska, H.; Jędrzejczak, R. Extraction of triterpenic acids and phytosterols from apple pomace with supercritical carbon dioxide: Impact of process parameters, modelling of kinetics, and scaling-up study. Molecules 2018, 23, 2790. [Google Scholar] [CrossRef]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpene acids and phenolics from ancient apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS study and in vitro activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef]
- Lv, Y.; Tahir, I.; Olsson, M. Changes in triterpene content during storage of three apple cultivars. Acta Hortic. 2015, 1071, 365–368. [Google Scholar] [CrossRef]
- Lv, Y.; Tahir, I.; Olsson, M. Factors affecting the content of the ursolic acid and oleanolic acid in apple peel: Influence of cultivars, sun exposure, storage conditions, bruising and Penicillium expanum infections. J. Sci. Food Agric. 2015, 96, 2161–2169. [Google Scholar] [CrossRef]
- Lv, Y.; Tahir, I.; Olsson, M. Influence of rootstock, harvest time, and storage conditions on triterpene content of apple peel. Acta Hortic. 2016, 1120, 405–408. [Google Scholar] [CrossRef]
- Ju, Z.; Bramlage, W.J. Developmental changes of cuticular constituents and their association with ethylene during fruit ripening in ‘Delicious’ apples. Postharvest Biol. Technol. 2001, 21, 257–263. [Google Scholar] [CrossRef]
- Frighetto, R.T.S.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N.; Siani, A.C. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar] [CrossRef]
- Bartley, I.M. Lipid metabolism of ripening apples. Phytochemistry 1985, 24, 2857–2859. [Google Scholar] [CrossRef]
- Han, J.H.; Yang, Y.X.; Feng, M.Y. Content of phytosterols in vegetables and fruits commonly consumed in China. Biomed. Environ. Sci. 2008, 21, 449–453. [Google Scholar] [CrossRef]
- Piironen, V.; Toivo, J.; Puupponen-Pimiä, R.; Lampi, A.M. Plant sterols in vegetables, fruits, and berries. J. Sci. Food Agric. 2003, 83, 330–337. [Google Scholar] [CrossRef]
- Normén, L.; Johnsson, M.; Andersson, H.; van Gameren, Y.; Dutta, P. Plant sterols in vegetables and fruits commonly consumed in Sweden. Eur. J. Nutr. 1999, 38, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Dashbaldan, S.; Becker, R.; Pączkowski, C.; Szakiel, A. Various patterns of composition and accumulation of steroids and triterpenoids in cuticular waxes from selected Ericaceae and Caprifoliaceae berries during fruit development. Molecules 2019, 24, 3826. [Google Scholar] [CrossRef]
- Salvador, Â.C.; Rocha, S.M.; Silvestre, A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): Influence of ripening, cultivar and season. Ind. Crops Prod. 2015, 71, 15–23. [Google Scholar] [CrossRef]
- Bringe, K.; Schumacher, C.F.A.; Schmitz-Eiberger, M.; Steriner, U.; Oerke, E.C. Ontogenic variation in chemical and physical characteristics of adaxial apple leaf surfaces. Phytochemistry 2006, 67, 161–170. [Google Scholar] [CrossRef]
- Jetter, R.; Schäffer, S.; Riederer, M. Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: Evidence from Prunus laurocerasus L. Plant Cell Environ. 2000, 23, 619–628. [Google Scholar] [CrossRef]
- Pensec, F.; Pączkowski, C.; Grabarczyk, M.; Woźniak, A.; Bénard-Gellon, M.; Bertsch, C.; Chong, J.; Szakiel, A. Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the Upper Rhine Valley. J. Agric. Food Chem. 2014, 62, 7998–8007. [Google Scholar] [CrossRef]
- Pensec, F.; Szakiel, A.; Pączkowski, C.; Woźniak, A.; Grabarczyk, M.; Bertsch, C.; Fischer, M.J.C.; Chong, J. Characterization of triterpenoid profiles and triterpene synthase expression in the leaves of eight Vitis vinifera cultivars grown in the Upper Rhine Valley. J. Plant Res. 2016, 129, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant structure-activity relationship analysis of five dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Balanehru, S.; Nagarajan, B. Protective effect of oleanolic and ursolic acid against lipid peroxidation. Biochem. Int. 1991, 24, 981–990. [Google Scholar]
- Ramachandran, S.; Rajendra Prasad, N.; Pugalendi, K.V.; Menon, V.P. Modulation of UVB-induced oxidative stress by ursolic acid in human blood lymphocytes. Asian J. Biochem. 2008, 3, 11–18. [Google Scholar] [CrossRef]
- Ramos, A.A.; Pereira-Wilson, C.; Collins, A.R. Protective effects of ursolic acid and luteolin against oxidative DNA damage include enhancement of DNA repair in Caco-2 cells. Mutat. Res. 2010, 692, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Rajendra Prasad, N. Effect of ursolic acid, a triterpenoid antioxidant, on ultraviolet-B radiation-induced cytotoxicity, lipid peroxidation and DNA damage in human lymphocytes. Chem. Biol. Interact. 2008, 176, 99–107. [Google Scholar] [CrossRef]
- Ringbom, T.; Segura, L.; Noreen, Y.; Perera, P.; Bohlin, L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J. Nat. Prod. 1998, 61, 1212–1215. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Mulabagal, V.; Liu, Y.; Nair, M.G. Ursolic acid analogues: Non-phenolic functional food components in Jamaican raspberry fruits. Food Chem. 2009, 116, 633–637. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslovac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase inhibition by plant extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef]
- Brooks, P.; Emery, P.; Evans, J.F.; Fenner, H.; Hawkey, C.J.; Patrono, C.; Smolen, J.; Breedveld, F.; Day, R.; Dougados, M.; et al. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumathology 1999, 38, 779–788. [Google Scholar] [CrossRef]
- Zhang, F.; Daimaru, E.; Ohnishi, M.; Kinoshita, M.; Tokuji, Y. Oleanolic acid and ursolic acid in commercial dried fruits. Food Sci. Technol. Res. 2013, 19, 113–116. [Google Scholar] [CrossRef]
- Zhang, X.; Julien-David, D.; Miesch, M.; Geoffroy, P.; Raul, F.; Roussi, S.; Aoudé-Werner, D.; Marchioni, E. Identification and quantitative analysis of β-sitosterol oxides in vegetable oils by capillary gas chromatography–mass spectrometry. Steroids 2005, 70, 896–906. [Google Scholar] [CrossRef]
- Fuliaş, A.; Ledeţi, I.; Vlase, G.; Vlase, T.; Şoica, C.; Dehelean, C.; Oprean, C.; Bojin, F.; Şuta, L.M.; Bercean, V.; et al. Thermal degradation, kinetic analysis, and apoptosis induction in human melanoma for oleanolic and ursolic acids. J. Therm. Anal. Calorim. 2016, 125, 759–768. [Google Scholar] [CrossRef]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.R.; et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef]
- Marcotte, B.V.; Verheyde, M.; Pomerleau, S.; Doyen, A.; Couillard, C. Health Benefits of Apple Juice Consumption: A Review of Interventional Trials on Humans. Nutrients 2022, 14, 821. [Google Scholar] [CrossRef]
- Clayton, M.; Amos, N.D.; Banks, N.H.; Morton, R.H. Estimation of apple fruit surface area. New Zeal. J. Crop Hort. Sci. 1995, 23, 345–349. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R. Novel method for HPLC analysis of triterpenic acids using 9-anthryldiazomethane derivatization and fluorescence detection. Chromatographia 2017, 80, 1527–1533. [Google Scholar] [CrossRef]
- Janicsák, G.; Veres, K.; Kállai, M.; Máthé, I. Gas chromatographic method for routine determination of oleanolic and ursolic acids in medicinal plants. Chromatographia 2003, 58, 295–299. [Google Scholar]
- Sánchez Ávila, N.; Priego Capote, F.; Luque de Castro, M.D. Ultrasound-assisted extraction and silylation prior to gas chromatography-mass spectrometry for the characterization of the triterpenic fraction in olive leaves. J. Chromatogr. A 2007, 1665, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
| Content (μg cm−2) | ‘Golden Delicious’ | ‘Ligol’ | ‘Redkroft’ |
|---|---|---|---|
| Ursolic acid | 466.2 ± 24.0 c | 581.0 ± 14.2 b | 630.4 ± 11.0 a |
| 13.1 ± 6.4 b | 12.1 ± 2.8 b | 31.5 ± 3.1 a | |
| Oleanolic acid | 166.1 ± 13.2 c | 317.8 ± 19.8 b | 476.1 ± 7.7 a |
| 39.4 ± 2.0 b | 38.1 ± 5.4 b | 63.1 ± 3.4 a | |
| Betulinic acid | 30.2 ± 4.1 b | nd | 52.1 ± 7.1 a |
| 1.4 ± 0.7 a | nd | 4.3 ± 2.0 a | |
| Pomolic acid | 49.4 ± 5.7 a | 10.2 ± 2.5 b | nd |
| 4.1 ± 2.4 | nd | nd | |
| Corosolic acid | 10.2 ± 1.5 | nd | nd |
| 1.1 ± 0.4 | nd | nd | |
| α-amyrin | 9.0 ± 0.7 a | 8.2 ± 0.5 ab | 6.3 ± 0.5 b |
| nd | nd | nd | |
| Uvaol | 4.1 ± 0.7 a | 5.1 ± 0.8 a | 5.3 ± 0.6 a |
| nd | nd | nd | |
| β-amyrin | 7.1 ± 0.9 a | 5.0 ± 1.1 ab | 3.3 ± 0.2 b |
| nd | nd | nd | |
| Erythrodiol | 3.2 ± 0.5 a | 2.1 ± 0.3 a | 2.9 ± 0.2 a |
| nd | nd | nd | |
| β-sitosterol | 35.2 ± 1.6 a | 33.3 ± 1.0 a | 37.4 ± 1.9 a |
| 1.4 ± 0.3 a | 1.7 ± 0.4 a | 1.4 ± 0.4 a | |
| Campesterol | 1.4 ± 0.2 a | 1.0 ± 0.1 a | 1.2 ± 0.2 a |
| nd | nd | nd |
| Content (μg cm−2) | ‘Golden Delicious’ | ‘Ligol’ | ‘Redkroft’ |
|---|---|---|---|
| Ursolic acid | 61.2 ± 4.1 a | 71.9 ± 5.0 a | 57.3 ± 3.2 a |
| 12.1 ± 1.4 b | 13.9 ± 1.2 ab | 18.3 ± 1.9 a | |
| Oleanolic acid | 24.0 ± 2.0 b | 43.4 ± 1.6 a | 46.5 ± 2.4 a |
| 18.4 ± 1.2 b | 12.1 ± 0.9 c | 31.5 ± 0.7 a | |
| Betulinic acid | 1.6 ± 0.2 b | nd | 8.2 ± 1.4 a |
| nd | nd | 0.9 ± 0.4 | |
| Pomolic acid | 1.4 ± 0.2 a | 0.7 ± 0.0 b | nd |
| 0.5 ± 0.2 | nd | nd | |
| Corosolic acid | nd | nd | nd |
| nd | nd | nd | |
| α-amyrin | 4.1 ± 0.6 ab | 5.2 ± 0.3 a | 3.7 ± 0.5 b |
| 0.6 ± 0.2 a | 0.8 ± 0.0 a | 0.5 ± 0.2 a | |
| Uvaol | 2.1 ± 0.4 a | 1.7 ± 0.3 a | 1.9 ± 0.3 a |
| nd | nd | nd | |
| β-amyrin | 2.0 ± 0.2 b | 4.9 ± 0.7 a | 2.6 ± 0.4 b |
| nd | 0.6 ± 0.1 | nd | |
| Erythrodiol | 1.1 ± 0.3 ab | 1.4 ± 0.2 a | 0.8 ± 0.0 b |
| nd | nd | nd | |
| β-sitosterol | 45.8 ± 1.6 a | 48.3 ± 2.1 a | 44.7 ± 1.3 a |
| 2.6 ± 0.4 a | 3.4 ± 0.5 a | 2.5 ± 0.2 a | |
| Campesterol | 1.7 ± 0.4 a | 1.2 ± 0.2 a | 1.0 ± 0.3 a |
| nd | nd | nd |
| Test | Ursolic Acid | Oleanolic Acid | β-Sitosterol | Chlorogenic Acid | Phloridzin | Apple Extract |
|---|---|---|---|---|---|---|
| ABTS•+ | 163 ± 8 | 155 ± 7 | 130 ± 4 | 23 ± 2 | 34 ± 4 | 140 ± 8 |
| DPPH• | 94 ± 3 | 96 ± 5 | 88 ± 5 | 21 ± 3 | 23 ± 3 | 82 ± 9 |
| COX-1 | 52 ± 4 | 104 ± 6 | 542 ± 6 | 1047 ± 73 | 960 ± 61 | 205 ± 12 |
| COX-2 | 31 ± 3 | 73 ± 7 | 382 ± 4 | 612 ± 31 | 644 ± 38 | 144 ± 10 |
| 5-LOX | 717 ± 43 | 641 ± 22 | 1740 ± 52 | >5000 | >5000 | 2084 ± 301 |
| Content (mg L−1) or (mg kg−1) | Ursolic Acid | Oleanolic Acid | β-Sitosterol |
|---|---|---|---|
| Apple | 56.1 ± 3.1 a | 24.1 ± 1.3 a | 80.5 ± 3.1 a |
| Purée (laboratory) | 19.6 ± 2.1 b | 8.0 ± 1.1 c | 53.6 ± 4.2 b |
| Cloudy juice (laboratory) | 6.2 ± 0.9 c | 2.8 ± 0.4 d | 17.0 ± 2.9 d |
| Clear juice (laboratory) | <0.1 d | <0.1 e | <0.1 e |
| Purée (commercial) | 22.3 ± 3.9 b | 15.2 ± 4.0 b | 34.1 ± 11.1 c |
| Cloudy juice (commercial) | 4.1 ± 2.0 c | 2.3 ± 0.5 d | 14.1 ± 5. d |
| Clear juice (commercial) | <0.1 d | <0.1 e | <0.1 e |
| Term | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Date | 21 April | 12 May | 2 June | 27 June | 19 July | 14 August | 8 September | 29 September |
| Fruits | - | - | + | + | + | + | + | + |
| Leaves | + | + | + | + | + | + | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, Ł.; Szakiel, A.; Głowacka, A.; Rozpara, E.; Marszałek, K.; Skąpska, S. Triterpenoids of Three Apple Cultivars—Biosynthesis, Antioxidative and Anti-Inflammatory Properties, and Fate during Processing. Molecules 2023, 28, 2584. https://doi.org/10.3390/molecules28062584
Woźniak Ł, Szakiel A, Głowacka A, Rozpara E, Marszałek K, Skąpska S. Triterpenoids of Three Apple Cultivars—Biosynthesis, Antioxidative and Anti-Inflammatory Properties, and Fate during Processing. Molecules. 2023; 28(6):2584. https://doi.org/10.3390/molecules28062584
Chicago/Turabian StyleWoźniak, Łukasz, Anna Szakiel, Agnieszka Głowacka, Elżbieta Rozpara, Krystian Marszałek, and Sylwia Skąpska. 2023. "Triterpenoids of Three Apple Cultivars—Biosynthesis, Antioxidative and Anti-Inflammatory Properties, and Fate during Processing" Molecules 28, no. 6: 2584. https://doi.org/10.3390/molecules28062584
APA StyleWoźniak, Ł., Szakiel, A., Głowacka, A., Rozpara, E., Marszałek, K., & Skąpska, S. (2023). Triterpenoids of Three Apple Cultivars—Biosynthesis, Antioxidative and Anti-Inflammatory Properties, and Fate during Processing. Molecules, 28(6), 2584. https://doi.org/10.3390/molecules28062584








