Anti–Zika Virus Activity and Isolation of Flavonoids from Ethanol Extracts of Curatella americana L. Leaves
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Preparation of Crude Extract and Fractionation
3.4. Isolation and Identification of Flavonoids
3.5. Spectroscopic Data for Isolated Compounds
3.6. LC–DAD–MS and LC–ESI–MS/MS Analyses
3.7. High-Resolution Mass Spectrometry Analyses
3.8. Cytotoxicity Evaluation by MTT Assay
3.9. Evaluation of Anti–Zika Virus Activity
3.9.1. Preparation of Viral Suspensions
3.9.2. Determination of Viral Infectious Titer by TCID50
3.9.3. Screening for Antiviral Activity by MTT Colorimetric Technique
3.9.4. In Vitro Cytopathic Effect Inhibition Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika virus (I). Isolations and serological specificity. Transact. Royal. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Cerbino-Neto, J.; Mesquita, E.; Souza, T.; Parreira, V.; Wittlin, B.B.; Durovni, B.; Lemos, M.C.F.; Vizzoni, A.; De Filippis, A.M.B.; Sampaio, S.A.; et al. Clinical Manifestations of Zika Virus Infection, Rio de Janeiro, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Baylis, M.; Brown, D. Zika virus and neurological disease—Approaches to the unknown. Lancet Infect. Dis. 2016, 16, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef]
- Yepez, J.B.; Murati, F.A.; Pettito, M.; Peñaranda, C.F.; de Yepez, J.; Maestre, G.; Arevalo, J.F.; Center, F.T.J.H.Z. Ophthalmic Manifestations of Congenital Zika Syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017, 135, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.O.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Thomas, D.L.; Sharp, T.M.; Torres, J.; Armstrong, P.A.; Munoz-Jordan, J.; Ryff, K.R.; Martinez-Quiñones, A.; Arias-Berríos, J.; Mayshack, M.; Garayalde, G.J.; et al. Local transmission of Zika virus—Puerto Rico, November 23, 2015–January 28, 2016. Morb. Mort Weekly Rep. 2016, 65, 154–158. [Google Scholar] [CrossRef]
- Musso, D.; Baud, D.; Gubler, D. Zika virus: What do we know? Clin. Microbiol. Infect. 2016, 22, 494–496. [Google Scholar] [CrossRef]
- Abbink, P.; LaRocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’Ang’A, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef]
- LaRocca, R.A.; Abbink, P.; Peron, J.P.S.; de A Zanotto, P.M.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’Ang’A, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef]
- Pardi, N.; Weissman, D. Nucleoside Modified mRNA Vaccines for Infectious Diseases. Methods Mol. Biol. 2017, 1499, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: www.cdc.gov/zika/ (accessed on 22 February 2023).
- Wong, S.S.-Y.; Poon, R.W.-S.; Wong, S.C.-Y. Zika virus infection—The next wave after dengue? J. Formos. Med. Assoc. 2016, 115, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.; Khalil, A.; Aarons, E.; Russell, K.; O’Brien, P. Management of Zika virus in pregnancy: A review. Br. Med. Bull. 2017, 124, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Naik, T.N. Antivirals of Ethnomedicinal Origin: Structure-activity Relationship and Scope. Mini Rev. Med. Chem. 2007, 7, 275–301. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Field, H.J. Antiviral prodrugs-the development of successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol. 2006, 147, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.C.; Silva, B.M.; de Moura, H.M.M.; Pereira, G.R.; Brandão, G.C. Anti-Zika virus activity and chemical characterization by ultra-high performance liquid chromatography (UPLC-DAD-UV-MS) of ethanol extracts in Tecoma species. BMC Complement. Med. Ther. 2020, 20, 246. [Google Scholar] [CrossRef]
- Reis, A.C.C.; de Moura, H.M.M.; Silva, B.M.; de Oliveira, A.B.; Brandão, G.C. Antiviral activity and chemical characterization of Cissus erosa (Vitaceae) ethanol extracts. Rodriguésia 2020, 71, 1–9. [Google Scholar] [CrossRef]
- Ferreira, G.M.; Silva, B.D.M.; de Souza, G.H.B.; de Oliveira, A.B.; Brandão, G.C. Anti-Zika Activity of Ouratea semiserrata and Dereplication of Its Constituents. Rev. Bras. Farm. 2021, 31, 121–125. [Google Scholar] [CrossRef]
- Reis, A.C.C.; Valente, G.M.; Silva, B.D.M.; Magalhães, C.L.D.B.; Kohlhoff, M.; Brandão, G.C. Anti-arboviral activity and chemical characterization of hispidulin and ethanolic extracts from Millingtonia hortensis L.f. and Oroxylum indicum (L.) Kurz (Bignoniaceae). Nat. Prod. Res. 2022, 37, 613–617. [Google Scholar] [CrossRef]
- Dhole, A.E.; Yarasu, R.B.; Lata, D.B.; Baraskar, S.S.; Shaw, D. Mathematical modeling for the performance and emission parameters of dual-fuel diesel engine using producer gas as secondary fuel. Biomass Convers. Bioref. 2015, 5, 257–270. [Google Scholar] [CrossRef]
- Doss, V.A.; Thangavel, K.P. Antioxidant and antimicrobial activity using different extracts of Anacardium occidentale L. Int. J. Appl. Bio. Pharma Tech. 2011, 2, 436–443. [Google Scholar]
- Aquino, F.G.; Pereira, C.S.; Passos, F.B.; de Oliveira, M.C. Composição florística e estrutural de um cerrado sentido restrito na área de proteção de Manancial Mestre D’Armas, Distrito Federal. Biosci. J. 2014, 30, 564–575. [Google Scholar]
- Alexandre-Moreira, M.S.; Piuvezam, M.R.; Araújo, C.C.; Thomas, G. Studies on the anti-inflammatory and analgesic activity of Curatella americana L. J. Ethnopharmacol. 1999, 67, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Felfili, J.M.; Nogueira, P.E.; Silva Júnior, M.C.D.; Marimon, B.S.; Delitti, W.B.C. Composição florística e fitossociologia do cerrado sentido restrito no município de Água Boa-MT. Acta Bot. Bras. 2002, 16, 103–112. [Google Scholar] [CrossRef]
- Kissmann, K.G.; Groth, D. Plantas infestantes e nocivas. Tomo 3: Plantas dicotiledôneas por orden de familias: Geraniaceae a Verbenaceae; Basf: São Paulo, Brasil, 1995. [Google Scholar]
- Guerrero, M.F.; Puebla, P.; Carrón, R.; Martın, M.L.; Arteaga, L.; San Román, L. Assessment of the antihypertensive and vasodi-lator effects of ethanolic extracts of some Colombian medicinal plants. J. Ethnopharmacol. 2002, 80, 37–42. [Google Scholar] [CrossRef]
- Costa, E.S.; Hiruma-Lima, C.A.; Lima, E.O.; Sucupira, G.C.; Bertolin, A.O.; Lolis, S.F.; Andrade, F.D.P.; Vilegas, W.; Souza-Brito, A.R.M. Antimicrobial activity of some medicinal plants of the Cerrado, Brazil. Phytot. Res. Int. J. Pharmacol. Toxicol. Eval. Nat. Prod. Derivat. 2008, 22, 705–707. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Rodrigues, C.M.; Kushima, H.; Moraes, T.M.; de Fatima Lolis, S.; Feitosa, S.B.; Magri, L.P.; Soares, F.R.; Cola, M.M.; Andrade, F.D.P.; et al. The anti-ulcerogenic effects of Curatella americana L. J. Ethnopharmacol. 2009, 121, 425–432. [Google Scholar] [CrossRef]
- De Toledo, C.E.M.; Britta, E.A.; Ceole, L.F.; Silva, E.R.; de Mello, J.C.; Dias Filho, B.P.; Nakamura, C.V.; Ueda-Nakamura, T. An-timicrobial and cytotoxic activities of medicinal plants of the Brazilian cerrado, using Brazilian cachaça as extractor liquid. J. Ethnopharmacol. 2011, 133, 420–425. [Google Scholar] [CrossRef]
- De Toledo, C.E.M.; Santos, P.R.; de Mello, J.C.P.; Dias Filho, B.P.; Nakamura, C.V.; Ueda-Nakamura, T. Antifungal properties of crude extracts, fractions, and purified compounds from bark of Curatella americana L. (Dilleniaceae) against Candida species. Evi.-Bas. Comp. Alt. Med. 2015, 2015, 673962. [Google Scholar]
- Lopes, R.H.O.; Macorini, L.F.B.; Antunes, K.Á.; Espindola, P.P.D.T.; Alfredo, T.M.; Rocha, P.D.S.D.; Pereira, Z.V.; dos Santos, E.L. Antioxidant and hypolipidemic activity of the hydroethanolic extract of Curatella americana L. leaves. Oxi. Med. Cell. Long. 2016, 2016, 9681425. [Google Scholar]
- El-Azizi, M.M.; Ateya, A.M.; Svoboda, G.H.; Schiff, P.L., Jr.; Slatkin, D.J.; Knapp, J.E. Chemical constituents of Curatella americana (Dilleniaceae). J. Pharml. Sci. 1980, 69, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.A.T.; Silva, N.D.S.R.D.; Ramos, R.D.S.; Ferreira, E.F.B.; dos Santos, K.L.B.; Silva, C.H.T.D.P.D.; da Silva, J.O.; Rosa, J.M.C.; dos Santos, C.B.R. An Antioxidant Potential, Quantum-Chemical and Molecular Docking Study of the Major Chemical Constituents Present in the Leaves of Curatella americana Linn. Pharmaceuticals 2018, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.A.T.; Sá, D.M.C.; Lima, C.M.D.S.; Bittencourt, J.A.H.M.; Pereira, W.L.A.; Muribeca, A.D.J.B.; e Silva, C.Y.Y.; da Silva, M.N.; de Sousa, F.F.O.; dos Santos, C.B.R.; et al. Chemical profiling of Curatella americana Linn leaves by UPLC-HRMS and its wound healing activity in mice. PLoS ONE 2020, 15, e0225514. [Google Scholar] [CrossRef] [PubMed]
- Bézivin, C.; Tomasi, S.; Lohézic-Le Dévéhat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Yasumura, Y.; Kawakita, Y. Studies on SV40 in tissue culture-preliminary step for cancer research in vitro. Nihon. Rinsho. 1963, 21, 1201–1215. [Google Scholar]
- Kim, W.Y.; Sharpless, N.E. The regulation of INK4/ARF in cancer and aging. Cell 2006, 127, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Osada, N.; Kohara, A.; Yamaji, T.; Hirayama, N.; Kasai, F.; Sekizuka, T.; Kuroda, M.; Hanada, K. The Genome Landscape of the African Green Monkey Kidney-Derived Vero Cell Line. DNA Res. 2014, 21, 673–683. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a human diploid cell designated MRC-5. Nature 1970, 227, 168–170. [Google Scholar] [CrossRef]
- MacKenzie, K.L.; Franco, S.; May, C.; Sadelain, M.; Moore, M.A. Mass cultured human fibroblasts overexpressing hTERT en-counter a growth crisis following an extended period of proliferation. Exp. Cell Res. 2000, 259, 336–350. [Google Scholar] [CrossRef]
- Brandão, G.C.; Kroon, E.G.; Filho, J.D.S.; Oliveira, A.B. Antiviral Activity of Fridericia formosa (Bureau) L. G. Lohmann (Bignoniaceae) Extracts and Constituents. J. Trop. Med. 2017, 2017, 6106959. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.G. Antiviral effect of methylated flavonol isorhamnetin against Influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef] [PubMed]
- Calland, N.; Sahuc, M.E.; Belouzard, S.; Pène, V.; Bonnafous, P.; Mesalam, A.A.; Deloison, G.; Descamps, V.; Sahpaz, S.; Wy-chowski, C.; et al. Polyphenols inhibit Hepatitis C virus entry by a new mechanism of action. J. Virol. 2015, 89, 10053–10063. [Google Scholar] [CrossRef] [PubMed]
- Cotin, S.; Calliste, C.A.; Mazeron, M.C.; Hantz, S.; Duroux, J.L.; Rawlinson, W.D.; Ploy, M.; Alain, S. Eight flavonoids and their potential as inhibitors of human Cytomegalovirus replication. Antiviral. Res. 2012, 96, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Duarte dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs Dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; He, S.; Siragam, V.; Bi, Y.; Mbikay, M.; Chretien, M.; Qiu, X. Antiviral activity of quercetin-3-β-OD-glucoside against Zika virus infection. Virol. Sin. 2017, 32, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and Chikungunya virus infection by inhibiting cell binding. Antiv. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Desprès, P.; El-Kalamouni, C. The flavonoid isoquercitrin precludes initiation of Zika virus infection in human cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef]
- Sharma, N.; Murali, A.; Singh, S.K.; Giri, R. Epigallocatechin gallate, an active green tea compound inhibits the Zika virus entry into host cells via binding the envelope protein. Int. J. Bio. Macromol. 2017, 104, 1046–1054. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2021, 36, 266–278. [Google Scholar] [CrossRef]
- Roy, A.; Lim, L.; Srivastava, S.; Lu, Y.; Song, J. Solution conformations of Zika NS2B-NS3pro and its inhibition by natural products from edible plants. PLoS ONE 2017, 12, e0180632. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Nguyen, T.T.H.; Kim, N.M.; Park, J.S.; Jang, T.S.; Kim, D. Inhibitory effect of flavonoids against NS2B-NS3 protease of Zika virus and their structure activity relationship. Biotechnol. Lett. 2017, 39, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Liu, H.; Li, J.; Yao, X.; Chen, Y.; Ke, C.; Liu, S. Structure-activity relationship of flavonoid bifunctional inhibitors against Zika virus infection. Biochem. Pharmacol. 2020, 177, 113962. [Google Scholar] [CrossRef] [PubMed]
- Kadota, S.; Takamori, Y.; Nyein, K.N.; Kikuchi, T.; Tanaka, K.; Ekimoto, H. Constituents of the leaves of Woodfordia fruticosa Kurz. I: Isolation, structure, and proton and carbon-13 nuclear magnetic resonance signal assignments of Woodfruticosin (Woodfordin C), an inhibitor of deoxyribonucleic acid topoisomerase II. Chem. Pharm. Bull. 1990, 38, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Son, Y.K.; Han, Y.N. Tissue factor inhibitory flavonoids from the fruits of Chaenomeles sinensis. Arch. Phar. Res. 2002, 25, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hou, J.; Fang, Q.; Sun, H.; Liu, X.; Zhang, L.; Wang, P.G. Synthesis of flavonol 3-O-glycoside by UGT78D1. Glycoconj. J. 2012, 29, 425–432. [Google Scholar] [CrossRef]
- Li, Y.-L.; Li, J.; Wang, N.-L.; Yao, X.-S. Flavonoids and a New Polyacetylene from Bidens parviflora Willd. Molecules 2008, 13, 1931–1941. [Google Scholar] [CrossRef]
- Da Fonseca, J.M.; Reis, A.C.C.; Pereira, G.R.; de Moura, H.M.M.; Filho, J.D.S.; Silva, B.D.M.; Brandão, G.C. Chromatographic profile of xanthones and flavonoids in the anti-dengue extracts of Fridericia samydoides (Cham.) L.G. Lohmann (Bignoniaceae). Braz. J. Pharm. Sci. 2022, 58, 1–15. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Kenichi, T.; Satoshi, T.; Ken, A.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass. Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Allard, P.M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immun. Met. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Twentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemo-sensitivity. Br. J. Cancer 1987, 56, 279. [Google Scholar] [CrossRef] [PubMed]
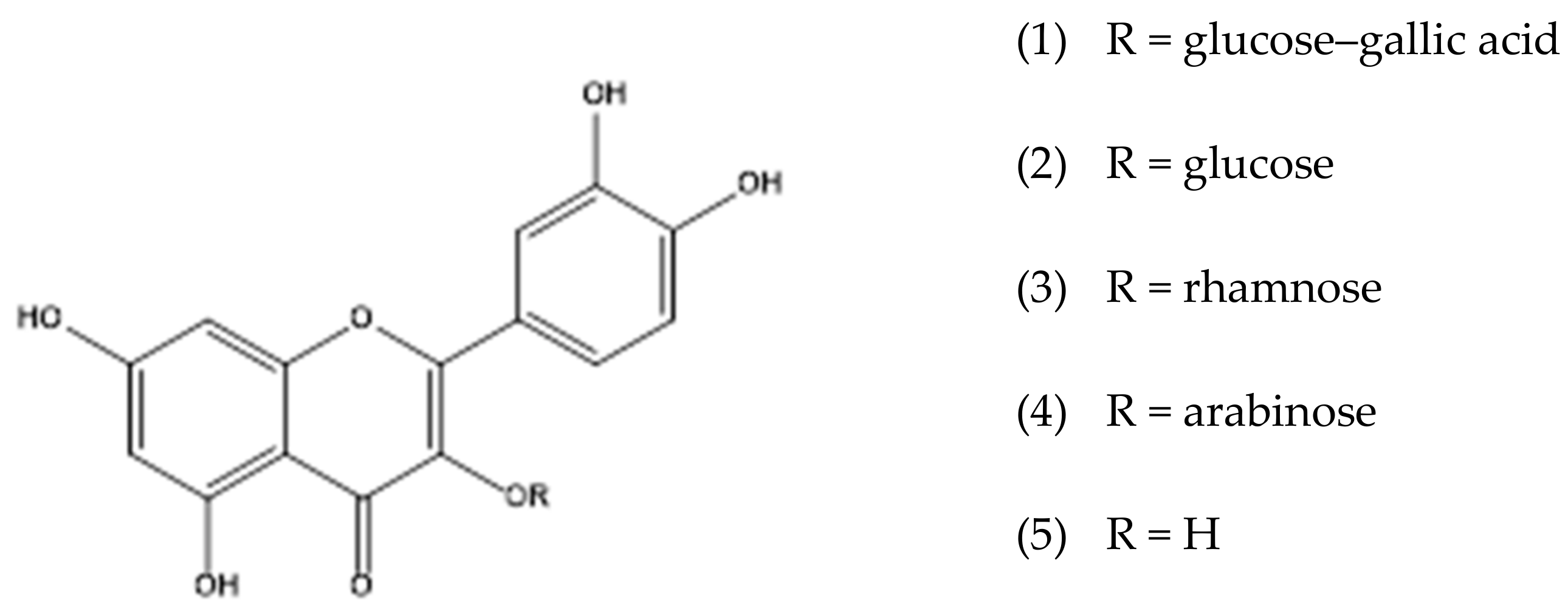

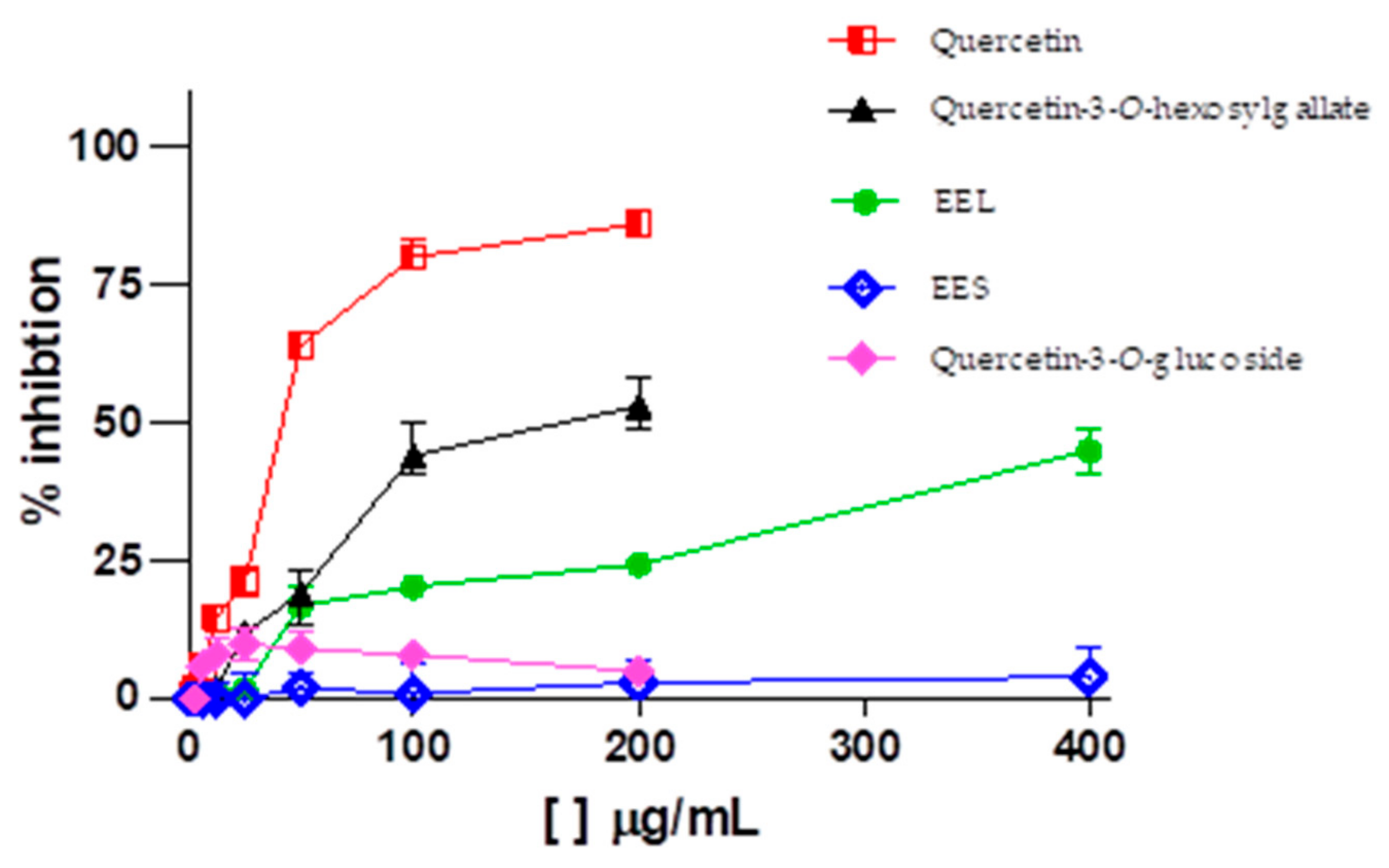
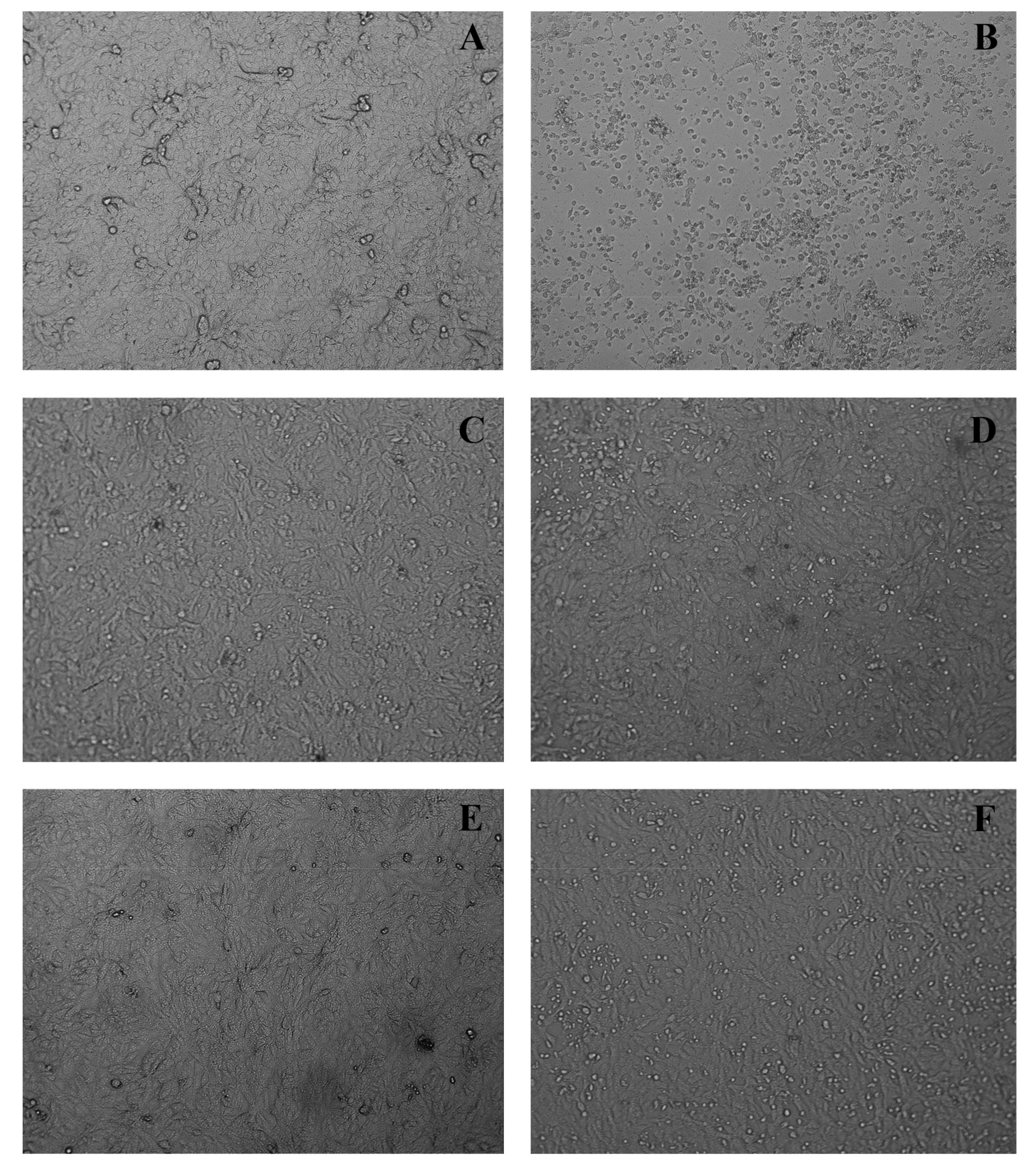
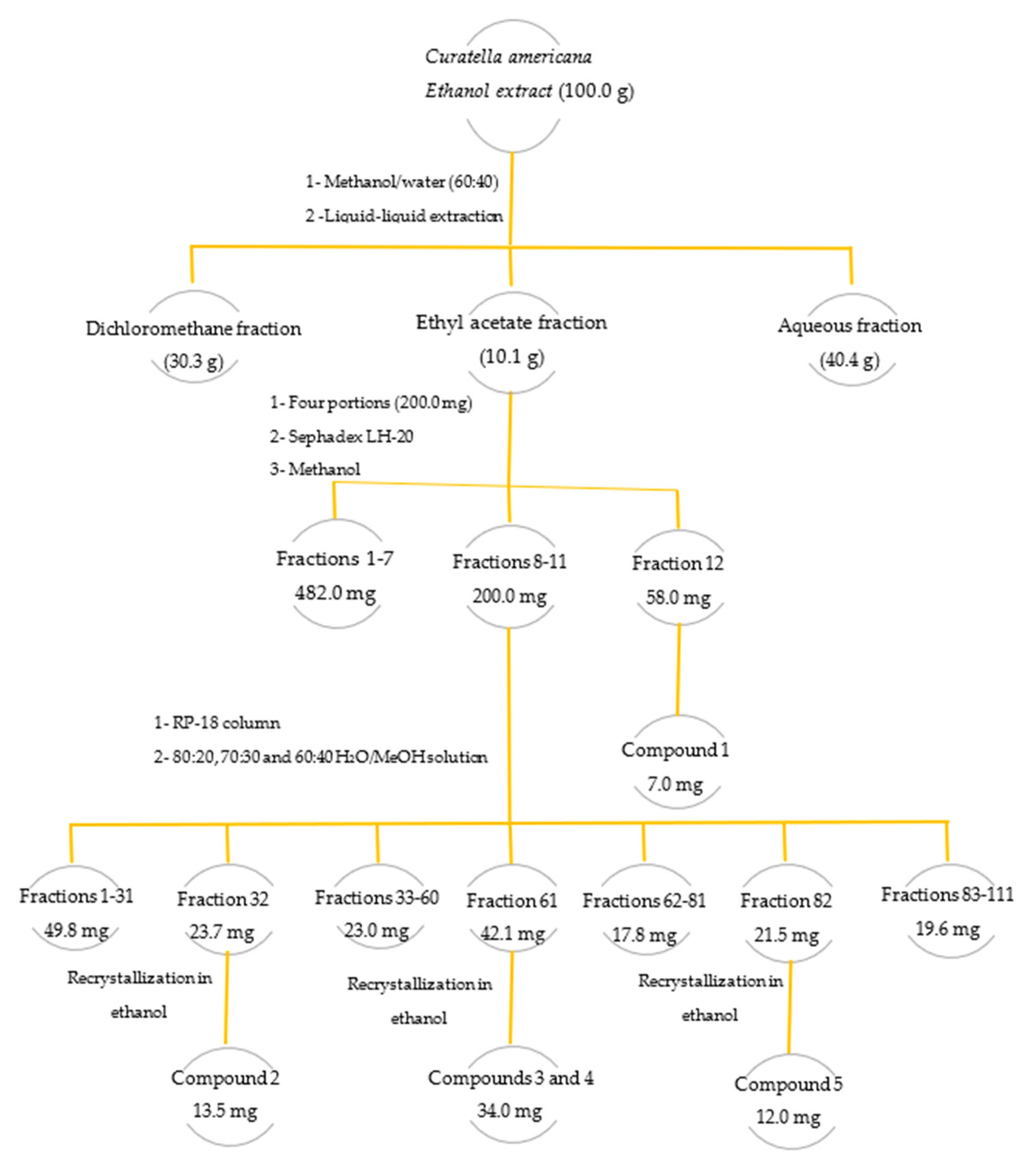
| Compounds | Molecular Formula | RT * (min) | UV (nm) | [M-H]− (m/z) | Characteristic m/z of Ions in Positive Ion Mode (%) | HRMS [M + H]+ (m/z) | Error (ppm) | |
|---|---|---|---|---|---|---|---|---|
| 1 | (Epi)catechin | C15H14O6 | 13.1 | 278 | 288.95 | 291.0860 (30.02), 139.0387 (100.0), 123.0437 (45.4) | 291.0858 | 3.4 |
| 2 | (Epi)catechin | C15H14O6 | 14.3 | 279 | 289.14 | 291.0859 (34.8), 147.0439 (24.3), 139.0386 (100.0) | 291.0859 | 3.1 |
| 3 | B-type dimer of (epi)catechin-(epi)catechin-O-gallate | C37H30O16 | 14.4 | 277 | 729.27 | 731.1636 (16.4), 579.1163 (13.4), 291.0860 (12.8), 153.0180 (100.0) | 731.1607 | 0.7 |
| 4 | Homoorientin | C21H20O11 | 15.3 | 265, 350 | 447.17 | 449.1087 (73.1), 329.0650 (71.7), 299.0546 (100.0) | 449.1074 | 2.0 |
| 5 | Orientin | C21H20O11 | 15.5 | 265, 350 | 447.26 | 449.1084 (100.0), 329.0651 (67.0), 299.0546 (38.7) | 449.1077 | 1.3 |
| 7 | Quercetin-3-O-hexosyl-arabinoside | C26H28O16 | 15.5 | 269, 350 | 595.33 | 597.1469 (1.8), 465.1037 (7.7), 303.0500 (100.0) | 597.1449 | 1.8 |
| 8 | Quercetin-3-O-hexosylgallate | C28H24O16 | 16.0 | 264, 354 | 615.20 | 617.1156 (18.8), 303.0500 (97.3), 153.0182 (100.0) | 617.1137 | 1.0 |
| 9 | Isovitexin | C21H20O10 | 16.1 | 267, 350 | 431.09 | 433.1138 (100.0), 313.0708 (59.2), 283.0600 (42.9) | 433.1129 | 1.1 |
| 10 | Vitexin | C21H20O10 | 16.2 | 267, 351 | 431.23 | 433.1137 (100.0), 313.0709 (57.3), 283.0601 (42.2) | 433.1130 | 0.9 |
| 11 | Quercetin-3-O-glucoside | C21H20O12 | 16.3 | 256, 354 | 463.17 | 465.1035 (2.0), 303.0501 (100.0) | 465.1030 | 0.6 |
| 12 | Kaempferol-3-O-hexosyl-arabinoside | C26H28O15 | 16.4 | 256, 354 | 579.20 | 449.1083 (11.1), 287.0552 (100.0) | 581.1499 | 1.2 |
| 13 | Kaempferol-3-O-hexosylgallate | C28H24O15 | 16.5 | 269,353 | 599.06 | 601.1212 (7.4), 287.0549 (100.0), 153.0181 (75.5) | 601.1185 | 1.3 |
| 14 | Quercetin-3-O-arabinoside | C20H18O11 | 16.8 | 256, 354 | 433.32 | 435.0927 (1.2), 303.0500 (100.0), 153.0182 (2.7) | 435.0922 | 1.1 |
| 15 | Methylquercetin-3-O-glucuronylgallate | C29H26O16 | 16.8 | 270, 365 | 629.24 | 631.1308 (9.5), 329.0868 (19.1), 303.0500 (59.7), 167.0341 (100.0) | 631.1291 | 1.3 |
| 16 | Kaempferol-3-O-hexosylgallate | C28H24O15 | 17.1 | 257, 354 | 599.25 | 601.1209 (19.7), 315.0711 (18.8), 287.0551 (99.3), 153.0183 (100.0) | 601.1189 | 0.7 |
| 17 | Kaempferol-3-O-glucoside | C21H20O11 | 17.4 | 256, 349 | 447.30 | 287.0551 (100.0), 153.0182 (4.4) | 449.1079 | 0.9 |
| 18 | Quercetin-3-O-rhamnoside | C21H20O11 | 17.5 | 256, 348 | 447.17 | 303.0503 (100.0), 153.0182 (11.7) | 449.1080 | 0.7 |
| 19 | Quercetin-3-O-hexosyl-O-acetyl | C23H22O13 | 17.6 | 256, 354 | 505.18 | 507.1143 (8.4), 303.0501 (100.0), 187.0599 (6.3) | 507.1133 | 1.0 |
| 20 | Kaempferol-3-O-arabinoside | C20H18O10 | 17.7 | 256, 354 | 417.31 | 419.0967 (2.1), 287.0551 (100.0), 153.0184 (3.7) | 419.0973 | 1.2 |
| 21 | Quercetin-3-O-hexosyl-O-acetyl | C23H22O13 | 18.0 | 256, 354 | 505.21 | 507.1145 (3.6), 303.0501 (100.0), 205.0707 (3.3) | 507.1135 | 0.6 |
| 22 | Kaempferol-3-O-rhamnoside | C21H20O10 | 18.5 | 270, 355 | 431.29 | 287.0552 (100.0), 153.0182 (2.6) | 433.1130 | 0.9 |
| 23 | Quercetin-3-O-arabinosyl-O-acetyl | C22H20O12 | 18.7 | 256, 354 | 475.13 | 303.0501 (100.0), 175.0602 (12.2) | 477.1028 | 1.0 |
| 24 | Kaempferol-3-O-hexosyl-O-acetyl | C23H22O12 | 18.8 | 256, 354 | 489.17 | 287.0550 (100.0), 187.0603 (5.9) | 491.1184 | 1.0 |
| 25 | Quercetin-3-O-(6”-O-E-p-coumaroyl)-glucopyranoside | C30H26O14 | 19.2 | 277, 354 | 609.25 | 611.1407 (4.9), 303.0500 (25.8), 147.0441 (100.0) | 611.1394 | 2.3 |
| 26 | Quercetin | C15H10O7 | 20.1 | 275, 371 | 301.29 | 303.0500 (100.0), 229.0496 (7.4), 153.0182 (10.5), 137.0232 (4.5) | 303.0498 | 2.0 |
| 27 | Kaempferol-3-O-(6”-O-E-p-coumaroyl)-glucopyranoside | C30H26O13 | 20.2 | 277, 350 | 593.18 | 595.1456 (4.2), 309.0968 (15.3), 287.0549 (29.4), 147.0441 (100.0) | 595.1446 | 0.8 |
| 28 | Kaempferol-3-O-(2”-O-E-p-coumaroyl)-glucopyranoside | C30H26O13 | 20.6 | 254, 369 | 593.31 | 595.1459 (7.6), 309.0965 (7.3) 287.0549 (65.4), 147.0440 (100.0) | 595.1444 | 1.2 |
| 29 | Kaempferol | C15H10O6 | 22.0 | 269, 350 | 285.09 | 287.0550 (100.0), 153.0180 (14.5), 137.0232 (2.2) | 287.0548 | 2.4 |
| Extracts | Used Part | CC50 Vero µg/mL | CC50 MRC-5 µg/mL | EC50 µg/mL | SI Vero | SI MRC-5 |
|---|---|---|---|---|---|---|
| Curatella americana | Stems | 54.6 ± 1.4 | >400 | Inactive | - | - |
| Curatella americana | Leaves | 161.5 ± 2.0 | >400 | 85.2 ± 1.6 | 1.9 | >2.5 |
| Fractions | ||||||
| Fraction DCM | Leaves | 58.9 ± 1.3 | 115.2 ± 1.8 | Inactive | - | - |
| Fraction AcOEt | Leaves | 56.2 ± 1.2 | >400 | 40.7 ± 2.3 | 1.4 | 9.8 |
| Fraction H2O/MeOH | Leaves | 101.0 ± 1.2 | >400 | Inactive | - | - |
| Isolated Compounds | ||||||
| Quercetin-3-O-hexosylgallate | Leaves | 305.6 ± 1.4 (496.1 ± 1.4 µM) | >100 (>162.3 µM) | 152.8 ± 2.0 (248.1 ± 2.0 µM) | 2.0 | >0.7 |
| Quercetin-3-O-glucoside | Leaves | >100 (>215.5 µM) | >100 (>215.5 µM) | Inactive | - | - |
| Quercetin | Leaves | 71.3 ± 1.3 (235.8 ±1.3 µM) | NT | 18.61 ± 2.8 (61.6 ± 2.8 µM) | 3.8 | - |
| Ribavirin (positive control) | - | 370.4 ± 1.2 (1516.7 ± 1.2 µM) | NT | 94.47 ± 2.7 (386.8 ± 2.7 µM) | 3.9 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.D.; Reis, A.C.C.; Sousa, J.A.C.; Valente, G.M.; de Mello Silva, B.; Magalhães, C.L.d.B.; Kohlhoff, M.; Teixeira, L.F.d.M.; Brandão, G.C. Anti–Zika Virus Activity and Isolation of Flavonoids from Ethanol Extracts of Curatella americana L. Leaves. Molecules 2023, 28, 2546. https://doi.org/10.3390/molecules28062546
Lima LD, Reis ACC, Sousa JAC, Valente GM, de Mello Silva B, Magalhães CLdB, Kohlhoff M, Teixeira LFdM, Brandão GC. Anti–Zika Virus Activity and Isolation of Flavonoids from Ethanol Extracts of Curatella americana L. Leaves. Molecules. 2023; 28(6):2546. https://doi.org/10.3390/molecules28062546
Chicago/Turabian StyleLima, Lienne D’Auria, Adriana Cotta Cardoso Reis, Jordano Augusto Carvalho Sousa, Gabriel Mendonça Valente, Breno de Mello Silva, Cíntia Lopes de Brito Magalhães, Markus Kohlhoff, Luiz Fernando de Medeiros Teixeira, and Geraldo Célio Brandão. 2023. "Anti–Zika Virus Activity and Isolation of Flavonoids from Ethanol Extracts of Curatella americana L. Leaves" Molecules 28, no. 6: 2546. https://doi.org/10.3390/molecules28062546
APA StyleLima, L. D., Reis, A. C. C., Sousa, J. A. C., Valente, G. M., de Mello Silva, B., Magalhães, C. L. d. B., Kohlhoff, M., Teixeira, L. F. d. M., & Brandão, G. C. (2023). Anti–Zika Virus Activity and Isolation of Flavonoids from Ethanol Extracts of Curatella americana L. Leaves. Molecules, 28(6), 2546. https://doi.org/10.3390/molecules28062546







