Unraveling the Variability of Essential Oil Composition in Different Accessions of Bunium persicum Collected from Different Temperate Micro-Climates
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Chemical Characterization of Essential Oils from B. persicum Using GC-MS Analysis
2.2. Principal Component Analysis between 12 Different Compounds and Seven Climate Zones of North Western Himalayas
2.3. Correlation, Cluster and Network Analysis between 12 Different Compounds and Seven Climate Zones of North Western Himalayas
3. Materials and Methods
3.1. Collection of Plant Material
3.2. Essential Oil Isolation
3.3. GC-MS Analysis Conditions
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siju, E.N.; Rajalakshmi, G.R.; Hariraj, N.; Anusha, K.V.; Kuttoor, D.S.; Shirwaikar, A. Elementary analysis of Thespesia populnea fruits. Int. J. Phytopharm. 2014, 5, 139–142. [Google Scholar]
- Bozin., B.; Mimica-Dukic., N.; Simin., N.; Anackov., G. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils, Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Bansal, S.; Sharma, K.; Gautam, V.; Lone, A.A.; Malhotra, E.V.; Kumar, S.; Singh, R. A comprehensive review of Bunium persicum a valuable medicinal spice. Food Rev. Int. 2021, 1–20. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Emary, G.A.; Ali, H.F. Influence of some citrus essential oils on cell viability, glutathione-s-transferase and lipid peroxidation in Ehrlich ascites Carcinoma cells. J. Am. Sci. 2010, 6, 820–826. [Google Scholar]
- Zouari, N.; Ayadi, I.; Fakhfakh, N.; Rebai, A.; Zouari, S. Variation of chemical composition of essential oils in wild populations of Thymus algeriensis Boiss. et Reut., a North African endemic Species. Lipids Health Dis. 2012, 11, 28. [Google Scholar] [CrossRef]
- Baskin, C.; Chester, E.; Baskin, J. Complex morphological dormancy in seeds of Thaspidium pinnatifidum. Int. J. Plant Sci. 1992, 153, 565–571. [Google Scholar] [CrossRef]
- Judd, W.; Campbell, C.; Kellog, E. Plant Systematics—A Phylogenetic Approach; Sinnauer Associates Inc.: Sunderland, MA, USA, 2002; pp. 470–471. [Google Scholar]
- Panwar, K. Black caraway. In Spice Crops of India; Arya, P.S., Ed.; Kalyani Publishers: New Delhi, India, 2000; pp. 172–178. [Google Scholar]
- Panwar, K. Kala zeera, A low volume, high price crop for dry temperate hills. Ind. Farmers Digest 1992, 25, 21–23. [Google Scholar]
- Mushtaq, N.; Modi, S. Cultivation and morphological characterization of different ecotypes of Kala zeera (Bunium persicum Boiss) across Kashmir valley. Int. J. Basic Appl. Biol. 2019, 6, 72–74. [Google Scholar]
- Baser, K.H.C.; Ozek, T.; Abduganiev, B.E.; Abdullaev, U.A.; Aripov, K.N. Composition of the essential oil of Bunium persicum (Boiss.) B. Fedtsch. from Tajikistan. J. Essent. Oil Res. 1997, 9, 597–598. [Google Scholar] [CrossRef]
- Foroumadi, A.; Asadipour, F.; Arabpour, A.; Amanzadeh, Y. Composition of the essential oil of Bunium persicum (Boiss.) B. Fedtsch. from Iran. J. Essent. Oil Res. 2002, 14, 161–162. [Google Scholar] [CrossRef]
- Haghirossadat, F.; Bernard, F.; Kalantar, M.; Sheikhha, M.H.; Hokmollahi, F.; Azimzadeh, M.; Hoori, M. Bunium persicum (Black caraway) of Yazd province, Chemical composition and evaluation of its antioxidant effects. JSSU J. 2010, 18, 284–291. [Google Scholar]
- Moghtader, M.; Mansori, A.I.; Salari, H.; Farahmand, A. Chemical Composition and antimicrobial activity of the essential oil of Bunium persicum (Bioss) Seed. Iran. J. Med. Aromat. Plants 2009, 25, 20–28. [Google Scholar]
- Talei, G.R.; Mosavi, Z. Chemical composition and antibacterial activity of Bunium persicum from west of Iran. Asian J. Chem. 2009, 21, 4749–4754. [Google Scholar]
- Shahsavari, N.; Barzegar, M.; Sahari, M.A.; Naghdibadi, H. Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant Foods Hum. Nutr. 2008, 63, 183–188. [Google Scholar] [CrossRef]
- Sekine, T.; Sugano, M.; Majid, A.; Fujii, Y. Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J. Chem. Ecol. 2007, 33, 2122–2123. [Google Scholar] [CrossRef]
- Kala, C.P. Medicinal Plants of Indian Trans-Himalaya: Focus on Tibetan Use of Medicinal Resources; Bishen Singh Mahendra Pal Singh: Dehradun, India, 2003. [Google Scholar]
- Thappa, R.K.; Ghosh, S.; Agarwal, S.G.; Raina, A.K.; Jamwal, P.S. Comparative studies on the major volatiles of Kala zeera (Bunium persicum seed) of wild and cultivated sources. Food Chem. 1991, 41, 129–134. [Google Scholar] [CrossRef]
- Kala, C.P. Medicinal Plants of Indian Trans-Himalaya; Bishan Singh Mahendra Pal Singh: Dehradun, India, 2003. [Google Scholar]
- Panda, H. Aromatic Plants Cultivation, Processing and Uses; Asia Pacific Business Press Inc.: New Delhi, India, 2004. [Google Scholar]
- Boskabady, M.H.; Moghaddas, A. Antihistaminic effect of Bunium persicum on guinea pig tracheal chains. Iran Biomed. J. 2004, 8, 149–155. [Google Scholar]
- Manion, C.R.; Widder, R.M. Essentials of essential oils. Am. J. Health-Syst. Pharm. 2017, 74, 153–162. [Google Scholar] [CrossRef]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils; CRC Press: London, UK, 2020; pp. 41–83. [Google Scholar]
- Cannon, J.B.; Cantrella, C.L.; Astatkieb, T.; Zheljazkovc, V.D. Modification of Yield and Composition of Essential Oils by Distillation Time. Ind. Crop Prod. 2013, 41, 214–220. [Google Scholar] [CrossRef]
- Kara, N.; Baydar, H. Essential oil characteristics of lavandins (Lavandula × intermedıa emeric ex loisel.) of Isparta province, kuyucak district, where lavender production center of Turkey. Selcuk J. Agric. Food Sci. 2011, 25, 41–45. [Google Scholar]
- Sefidkon, F.; Abbasi, K.; Khaniki, G.B. Influence of Drying and Extraction Methods on Yield and Chemical Composition of the Essential Oil of Satureja hortensis. Food Chem. 2006, 99, 19–23. [Google Scholar] [CrossRef]
- Lammerink, J.; Wallace, A.R.; Porter, N.G. Effects of harvest time and post harvest drying on oil from lavandin (Lavandula × intermedia). N. Z. J. Crop Hort. 1989, 17, 315–326. [Google Scholar] [CrossRef]
- İzmirli, A.; Yildirim, M.U. Farkli Giberellik Asit (GA3) Dozlarinin Lavantada (Lavandula angustifolia mill) Uçucu Yağ Miktarlari Ve Bileşenleri Üzerine Etkisi.Yüksek Lisans Tezi, Uşak Üniversitesi, Fen Bil.Ens.; Tarım Bilimleri ABD p (Turkish), 2018, 67.
- Brussotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological aaproach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef]
- Gani, A. Chemical Constituents and Uses Medicinal Plants of Bangladesh, 2nd ed.; Asiatic Society of Bangladesh: Ramna, Dhaka, Bangladesh, 2003. [Google Scholar]
- Fernie, A.R.; Trethewey, R.N.; Krotzky, A.J.; Willmitzer, L. Metabolite profiling, From diagnostics to systems biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 763–769. [Google Scholar] [CrossRef]
- Kell, D.B.; Brown, M.; Davey, H.M.; Dunn, W.B.; Spasic, I.; Oliver, S.G. Metabolic foot printing and systems biology, the medium is the message. Nat. Rev. Microbiol. 2005, 3, 557–565. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Sefidkon, F.; Hosseini, S.G. Supercritical carbon dioxide extraction of essential oils from Perovskia atriplicifolia Benth. J. Agric. Food Chem. 2003, 51, 5414–5419. [Google Scholar] [CrossRef]
- Azizi, M.; Davareenejad, G.; Bos, R.; Woerdenbag, H.J.; Kayser, O. Essential oil content and constituents of black zira Genet Resour Crop Evol 123 Bunium persicum Boiss Fedtsch from Iran during field cultivation (domestication). J. Essen. Oil Res. 2009, 21, 78–82. [Google Scholar] [CrossRef]
- Mazidi, S.; Rezaei, K.; Golmakani, M.T.; Sharifan, A.; Rezazadeh, S. Antioxidant activity of essential oil from Black Zira (Bunium persicum Boiss) obtained by microwave-assisted hydrodistillation. J. Agric. Sci. Tech. 2012, 14, 1013–1022. [Google Scholar]
- Omidbaigi, R.; Arvin, M.J. Effect of growing locations on the essential oil content and chemical compositions of Bunium persicum Boiss in wild growing in Iran. J. Essen. Oil Bear Plants 2009, 12, 34–40. [Google Scholar] [CrossRef]
- Jahansooz, F.; Sefidkon, F.; Najafi, A.; Ebrahimzadeh, H.; Najafi, M.S. Comparison of essential oils of Bunium persicum (Boiss.) populations grown in Iran, Pakistan and India. J. Essen. Oil Bear Plants 2012, 15, 761–765. [Google Scholar] [CrossRef]
- Shafie, M.S.B.; Zain Hasan, S.M.; Shah, M.S. Study of genetic variability of wormwood capillary Artemisia capillaris using inter simple sequence repeat (ISSR) in Pahang region, Malaysia. Plant Omics J. 2009, 2, 127–134. [Google Scholar]
- Purohit, S.; Vyas, S. Medicinal Plant Cultivation, A Scientific Approach Including Processing and Financial Guidelines, 1st ed.; Publishers Agrobios: Jodhpur, India, 2004; pp. 1–3. [Google Scholar]
- Ložienė, K.; Venskutonis, P.R. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005, 33, 517–525. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I.; Maccioni, S.; Baldini, R. Phytochemical typologies in some populations of Myrtus communis L. on caprione promontory (East Liguria, Italy). Food Chem. 2004, 85, 599–604. [Google Scholar] [CrossRef]
- Heywood, V.H. The conservation of genetic and chemical diversity in medicinal and aromatic plants. In Biodiversity: Biomolecular Aspects of Biodiversity and Innovative Utilization; Sener, B., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 13–22. [Google Scholar]
- Basalma, D.; Gürbüz, B.; Sarıhan, E.O.; İpek, A.; Arslan, N.; Duran, A.; Kendir, H. Essential oil composition of Salvia heldreichiana (Boiss) Ex Bentham Described Endemic Species from Turkey. Asian J. Chem. 2007, 19, 2130–2134. [Google Scholar]
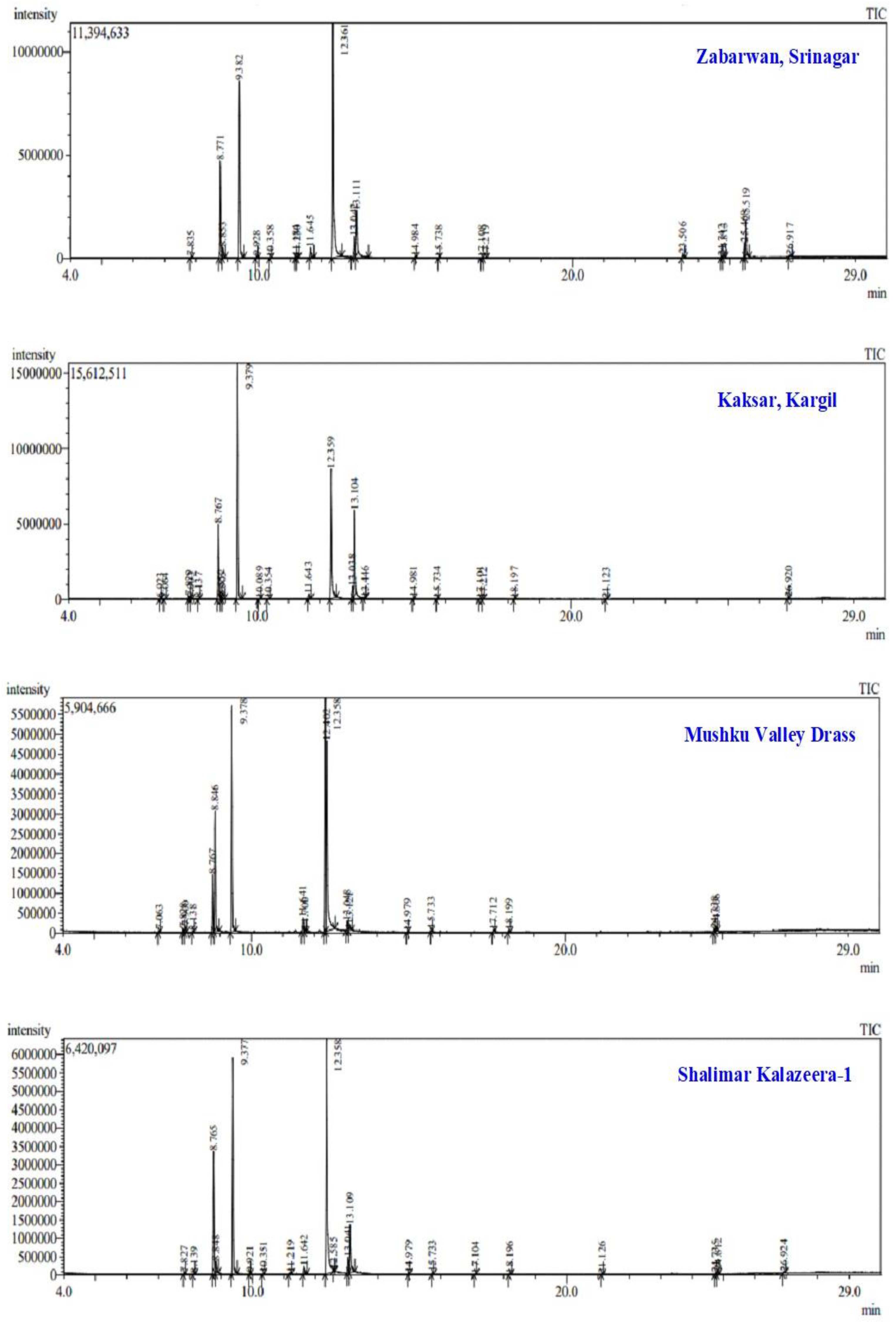

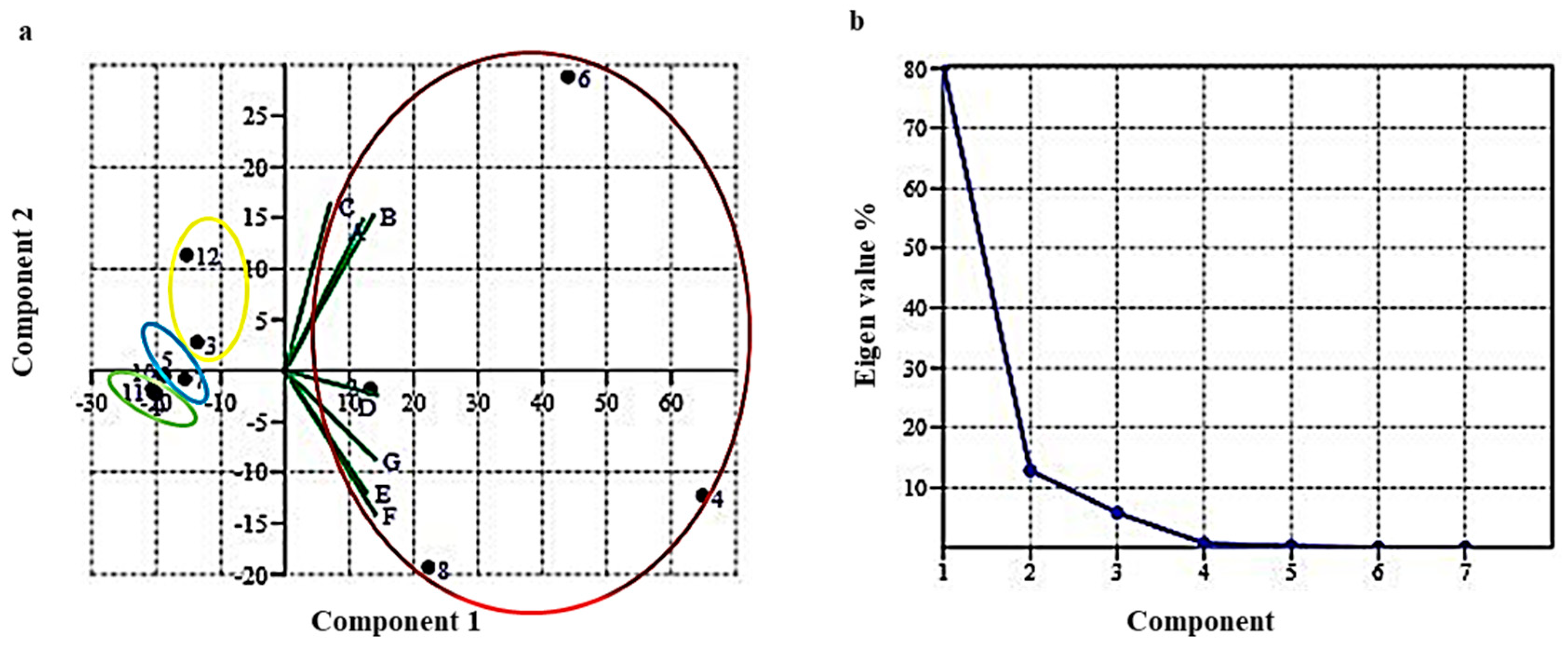

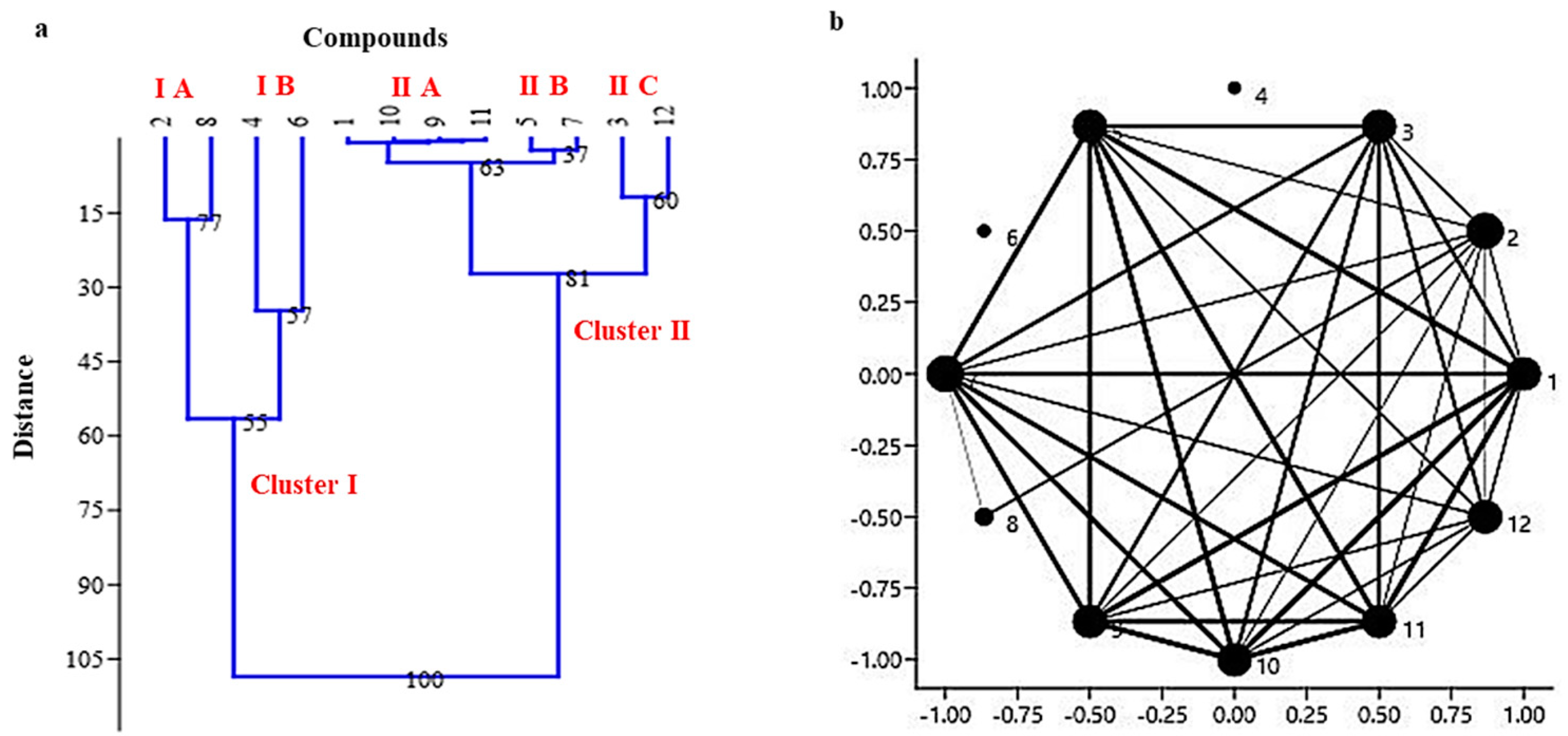
| Compound | Area (%) | Mean (µ) | SD (σ) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Bicyclo[3.1.0]hexane, 4-methylene-1-(1-methylethyl)- | 0.17 | 0.24 | 0.29 | 0.40 | 0.53 | 0.71 | 0.50 | 0.41 | 0.19 |
| p-Cymene | 12.11 | 15.18 | 5.98 | 12.44 | 14.27 | 9.88 | 18.76 | 12.66 | 4.06 |
| D-Limonene | 1.64 | 1.95 | 12.59 | 0.71 | 6.78 | 0.71 | 0.66 | 3.58 | 4.52 |
| Gamma-Terpinene | 21.92 | 26.51 | 22.45 | 36.36 | 36.42 | 40.66 | 40.22 | 32.08 | 8.21 |
| 3-p-Menthen-7-al | 1.59 | 1.13 | 1.64 | 0.94 | 0.33 | 0.56 | 0.36 | 0.94 | 0.55 |
| Cumic aldehyde | 39.55 | 41.54 | 24.10 | 25.64 | 13.44 | 14.53 | 16.72 | 25.07 | 11.53 |
| 4-Isopropylcyclohexa-1,3-dienecarbaldehyde | 3.25 | 2.32 | 1.33 | 2.33 | 1.38 | 1.96 | 1.83 | 2.06 | 0.66 |
| 1,4-p-Menthadien-7-al | 9.57 | 8.56 | 1.12 | 19.47 | 24.15 | 27.51 | 17.78 | 15.45 | 9.40 |
| Caryophyllene | 0.18 | 0.17 | 0.09 | 0.22 | 0.04 | 0.61 | 0.07 | 0.20 | 0.19 |
| Cycloheptasiloxane, tetradecamethyl- | 0.09 | 0.19 | 0.28 | 0.14 | 0.04 | - | 0.09 | 0.14 | 0.09 |
| beta.-Myrcene | 0.16 | 0.11 | 0.15 | 0.14 | 0.38 | 0.39 | 0.31 | 0.23 | 0.12 |
| D-Carvone | - | - | 27.60 | - | - | - | - | 27.60 | - |
| Compound | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 |
|---|---|---|---|---|---|---|---|
| Bicyclo[3.1.0]hexane, 4-methylene-1-(1-methylethyl)- | −19.877 | −2.3037 | −3.0839 | −0.1287 | −0.7254 | −0.2367 | 0.08317 |
| p-Cymene | 13.266 | −1.7894 | −4.4934 | −6.6893 | 1.5689 | 0.38245 | 0.10253 |
| D-Limonene | −13.574 | 2.7936 | 7.0385 | 0.40952 | 4.4759 | −0.3107 | −0.1015 |
| Gamma-Terpinene | 64.874 | −12.262 | 8.0665 | −1.6293 | −1.5443 | −0.3239 | −0.0913 |
| 3-p-Menthen-7-al | −18.691 | −0.4942 | −2.8329 | 0.20041 | −0.7894 | 0.05656 | −0.1665 |
| Cumic aldehyde | 43.963 | 28.866 | −8.4323 | 2.0817 | 0.0748 | −0.0287 | 0.02121 |
| 4-Isopropylcyclohexa- 1,3-dienecarbaldehyde | −15.503 | −0.8088 | −3.8088 | 0.22301 | −0.9262 | 0.48871 | −0.219 |
| 1,4-p-Menthadien-7-al | 22.24 | −19.324 | −2.84 | 5.2228 | 1.3014 | 0.34354 | 0.10481 |
| Caryophyllene | −20.422 | −2.0897 | −3.3217 | 0.1183 | −0.9407 | −0.2357 | 0.1578 |
| Cycloheptasiloxane, tetradecamethyl- | −20.686 | −1.7734 | −3.194 | −0.2515 | −0.7236 | −0.3012 | −0.0644 |
| beta.-Myrcene | −20.34 | −2.1809 | −3.2041 | −0.1842 | −0.6225 | −0.1488 | 0.07826 |
| D-Carvone | −15.252 | 11.367 | 20.106 | 0.6271 | −1.149 | 0.31443 | 0.0949 |
| PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 | |
|---|---|---|---|---|---|---|---|
| Zabarwan, Srinagar | 0.36103 | 0.43949 | −0.36015 | 0.21627 | 0.10026 | 0.69623 | −0.072439 |
| Shalimar, Kalazeera-1 | 0.40537 | 0.45059 | −0.33667 | −0.15151 | 0.071625 | −0.5932 | 0.37326 |
| Mushku Valley, Drass | 0.20698 | 0.48385 | 0.84939 | 0.030137 | −0.015923 | 0.019998 | 0.0043217 |
| Kaksar, Kargil | 0.42256 | −0.070291 | −0.063804 | 0.16946 | −0.30868 | −0.29656 | −0.77491 |
| Padder Valley, Kishtwar | 0.37377 | −0.35323 | 0.12638 | 0.029115 | 0.84215 | −0.044791 | −0.086501 |
| Atholi, Kishtwar | 0.41537 | −0.41703 | 0.10769 | 0.53065 | −0.34112 | 0.039087 | 0.49243 |
| Dawr, Gurez | 0.41437 | −0.25708 | 0.061978 | −0.78626 | −0.2524 | 0.26738 | 0.070434 |
| PC | Eigen Value | % Variance |
|---|---|---|
| Zabarwan, Srinagar | 861.114 | 80.393 |
| Shalimar, Kalazeera-1 | 137.792 | 12.864 |
| Mushku Valley, Drass | 61.9786 | 5.7863 |
| Kaksar, Kargil | 7.25369 | 0.6772 |
| Padder Valley, Kishtwar | 2.8821 | 0.26907 |
| Atholi, Kishtwar | 0.0938095 | 0.008758 |
| Dawr, Gurez | 0.0152141 | 0.0014204 |
| S No | Ecotypes | Altitude (mts) | Coordinates (Degree) |
|---|---|---|---|
| 1 | Zabarwan Srinagar | 1587 | 34.14 N, 74.96 E |
| 2 | Shalimar Kalazeera-1 | 1587 | 34.08 N 74.83 E |
| 3 | Mushku Valley Drass | 3722 | 34.19 N, 75.33 E |
| 4 | Kaksar Kargil | 2773 | 35.61 N, 75.81 E |
| 5 | Padder Valley Kishtwar | 1640 | 33.15 N, 76.09 E |
| 6 | Atholi Kishtwar | 1640 | 33.15 N, 76.09 E |
| 7 | Dawr Gurez | 2580 | 34.63 N, 74.83 E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.H.; Dar, N.A.; Alie, B.A.; Dar, S.A.; Lone, A.A.; Mir, G.H.; Fayaz, U.; Ali, S.; Tyagi, A.; El-Sheikh, M.A.; et al. Unraveling the Variability of Essential Oil Composition in Different Accessions of Bunium persicum Collected from Different Temperate Micro-Climates. Molecules 2023, 28, 2404. https://doi.org/10.3390/molecules28052404
Khan MH, Dar NA, Alie BA, Dar SA, Lone AA, Mir GH, Fayaz U, Ali S, Tyagi A, El-Sheikh MA, et al. Unraveling the Variability of Essential Oil Composition in Different Accessions of Bunium persicum Collected from Different Temperate Micro-Climates. Molecules. 2023; 28(5):2404. https://doi.org/10.3390/molecules28052404
Chicago/Turabian StyleKhan, Mudasir Hafiz, Niyaz Ahmad Dar, Bashir Ahmad Alie, Sher Ahmad Dar, Ajaz Ahmad Lone, Ghulam Hassan Mir, Uzma Fayaz, Sajad Ali, Anshika Tyagi, Mohamed A. El-Sheikh, and et al. 2023. "Unraveling the Variability of Essential Oil Composition in Different Accessions of Bunium persicum Collected from Different Temperate Micro-Climates" Molecules 28, no. 5: 2404. https://doi.org/10.3390/molecules28052404
APA StyleKhan, M. H., Dar, N. A., Alie, B. A., Dar, S. A., Lone, A. A., Mir, G. H., Fayaz, U., Ali, S., Tyagi, A., El-Sheikh, M. A., & Alansi, S. (2023). Unraveling the Variability of Essential Oil Composition in Different Accessions of Bunium persicum Collected from Different Temperate Micro-Climates. Molecules, 28(5), 2404. https://doi.org/10.3390/molecules28052404








