In Vitro: The Effects of the Anticoccidial Activities of Calotropis procera Leaf Extracts on Eimeria stiedae Oocysts Isolated from Rabbits
Abstract
1. Introduction
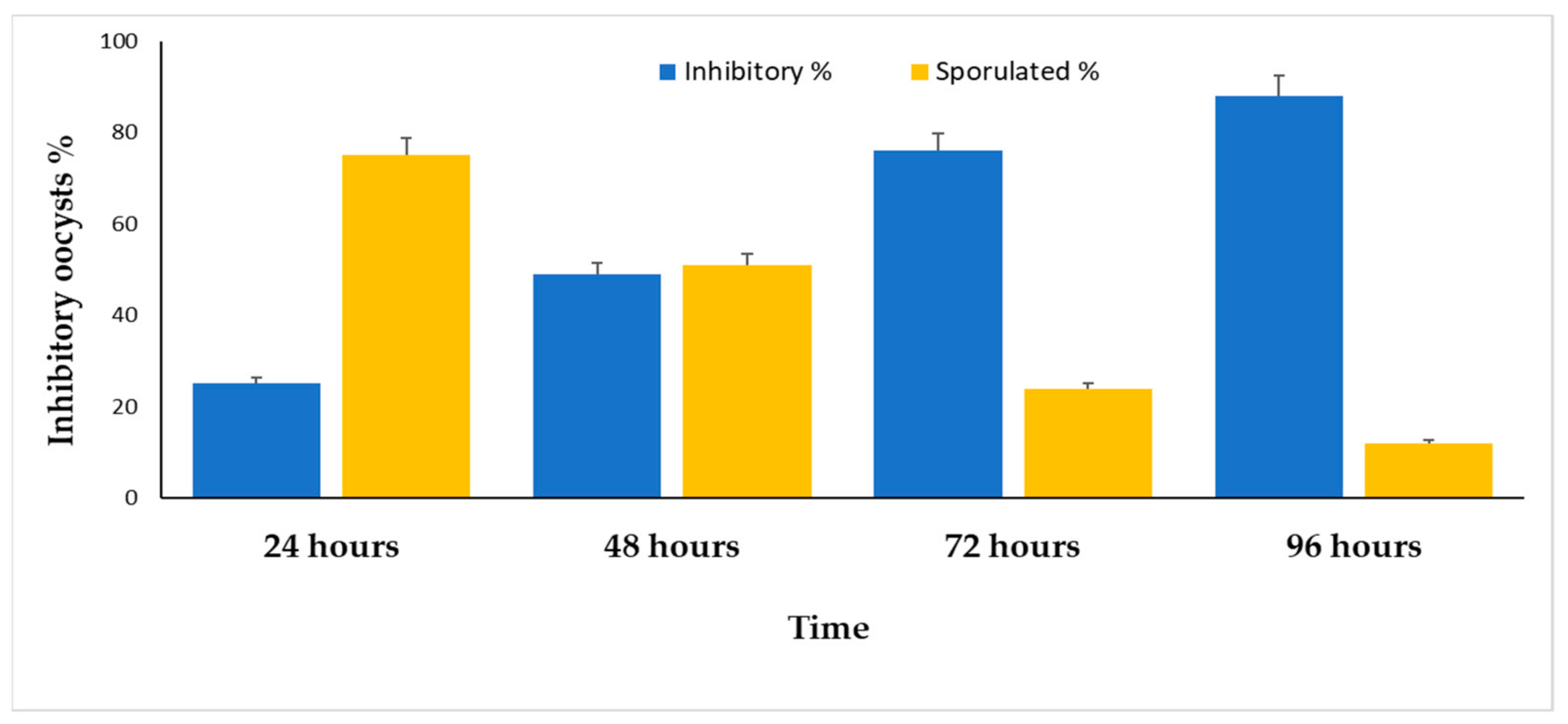
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Preparation of Extract
4.3. Analysis of Phytochemical Compounds Extracted from C. procera Leaves Using GC-MS
4.4. Oocyst Sporulation
4.5. In Vitro Effect of Extracts on Inhibition of Sporulated Oocyst
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mailafia, S.; Onakpa, M.; Owoleke, O. Problems and prospects of rabbit production in Nigeria—A review. Bayero J. Pure Appl. Sci. 2010, 3, 20–25. [Google Scholar] [CrossRef]
- Boyko, O.; Shendryk, L.; Shaban, O.; Brygadyrenko, V. Influence of essential oils on sporulation of Eimeria magna oocysts. Ann. Parasitol. 2021, 67, 11–17. [Google Scholar]
- Hofing, G.L.; Kraus, A.L. Arthropod and helminth parasites. In The Biology of the Laboratory Rabbit; Elsevier: Amsterdam, The Netherlands, 1994; pp. 231–257. [Google Scholar]
- Bangoura, B.; Daugschies, A. Eimeria. In Parasitic Protozoa of Farm Animals and Pets; Springer: Cham, Switzerland, 2018; pp. 55–101. [Google Scholar]
- Gill, B.; Ray, H. The coccidian of the domestic rabbit and the common field hare of India. Proc. Zool. Soc. 1960, 13, 128–143. [Google Scholar]
- Kvičerová, J.; Pakandl, M.; Hypša, V. Phylogenetic relationships among Eimeria spp.(Apicomplexa, Eimeriidae) infecting rabbits: Evolutionary significance of biological and morphological features. Parasitology 2008, 135, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bull, P. Incidence of coccidia (Sporozoa) in wild rabbits, Oryctolagus cuniculus (L.) in Hawke’s Bay, New Zealand. NZJ Sci. 1958, 1, 289–328. [Google Scholar]
- Wang, J.; Tsai, S. Prevalence and pathological study on rabbit hepatic coccidiosis in Taiwan. Proc. Natl. Sci. Counc. Repub. China Part B Life Sci. 1991, 15, 240–243. [Google Scholar]
- Vezzani, A.; Fujinami, R.S.; White, H.S.; Preux, P.-M.; Blümcke, I.; Sander, J.W.; Löscher, W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016, 131, 211–234. [Google Scholar] [CrossRef]
- Al-Mathal, E.M. Hepatic coccidiosis of the domestic rabbit (Oryctolagus cuniculus domesticus L.) in Saudi Arabia. World J. Zool. 2008, 3, 30–35. [Google Scholar]
- Fang, S.; Gu, X.; El-Ashram, S.; Li, X.; Yu, X.; Guo, B.; Li, H.; Liu, N.; Liu, X.; Cui, P. Immune protection provided by a precocious line trivalent vaccine against rabbit Eimeria. Vet. Parasitol. 2019, 275, 108927. [Google Scholar] [CrossRef]
- Chapman, H. Milestones in avian coccidiosis research: A review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [CrossRef]
- Abbas, R.; Colwell, D.; Gilleard, J. Botanicals: An alternative approach for the control of avian coccidiosis. World’s Poult. Sci. J. 2012, 68, 203–215. [Google Scholar] [CrossRef]
- Shivaramaiah, C.; Barta, J.R.; Hernandez-Velasco, X.; Téllez, G.; Hargis, B.M. Coccidiosis: Recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet. Med. Res. Rep. 2014, 5, 23. [Google Scholar]
- Chapman, H.; Cherry, T.; Danforth, H.; Richards, G.; Shirley, M.; Williams, R. Sustainable coccidiosis control in poultry production: The role of live vaccines. Int. J. Parasitol. 2002, 32, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.R.; Silva, L.J.; Pereira, A.M.; Esteves, A.; Duarte, S.C.; Pena, A. Coccidiostats and poultry: A comprehensive review and current legislation. Foods 2022, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, F.; Al-Quraishy, S.; Steinbrenner, H.; Sies, H.; Dkhil, M.A. Towards identifying novel anti-Eimeria agents: Trace elements, vitamins, and plant-based natural products. Parasitol. Res. 2014, 113, 3547–3556. [Google Scholar] [CrossRef]
- Kumari, I.; Chaudhary, G. Calotropis procera (Arka): A tribal herb of utmost significance. Int. J. Res. Appl. Sci. Biotechnol. 2021, 8, 44–54. [Google Scholar] [CrossRef]
- Mascolo, N.; Sharma, R.; Jain, S.; Capasso, F. Ethnopharmacology of Calotropis procera flowers. J. Ethnopharmacol. 1988, 22, 211–221. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, S.; Vasudeva, N. Pharmacological profile of Calotropis gigantea in various diseases: A profound look. Int. J. Creat. Res. Thoughts 2021, 9, 2987–2996. [Google Scholar]
- Kavitha, K.; Rajendran, S. Anti-corrosive properties of an aqueous extract of Chrysanthemum indicum flower. Int. J. Corros. Scale Inhib. 2021, 10, 783–800. [Google Scholar]
- Sriranjini, S.J.; Sandhya, K.; Mamta, V.S. Ayurveda and botanical drugs for epilepsy: Current evidence and future prospects. Epilepsy Behav. 2015, 52, 290–296. [Google Scholar] [CrossRef]
- Balkrishna, A.; Misra, L. Ayurvedic plants in brain disorders: The herbal hope. J. Tradit. Med. Clin. Natur. 2017, 6, 2. [Google Scholar]
- Agharkar, S. Medicinal Plants of Bombay Presidency; Scientific Publishers: Jodhpur, India, 1991. [Google Scholar]
- Haq, F. The ethno botanical uses of medicinal plants of Allai Valley, Western Himalaya Pakistan. Int. J. Plant Res. 2012, 2, 21–34. [Google Scholar] [CrossRef]
- Murshed, M.; Al-Quraishy, S.; Qasem, M.A. In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite. Open Chem. 2022, 20, 1057–1064. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Delic, D.; Sies, H.; Wunderlich, F.; Abdel-Baki, A.A.S.; Dkhil, M.A.M. Differential miRNA expression in the mouse jejunum during garlic treatment of Eimeria papillata infections. Parasitol. Res. 2011, 109, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Qasem, M.A.; Dkhil, M.A.; Al-Shaebi, E.M.; Murshed, M.; Mares, M.; Al-Quraishy, S. Rumex nervosus leaf extracts enhance the regulation of goblet cells and the inflammatory response during infection of chickens with Eimeria tenella. J. King Saud Univ. Sci. 2020, 32, 1818–1823. [Google Scholar] [CrossRef]
- Murshed, M.; Al-Quraishy, S.; Alghamdi, J.; Aljawdah, H.; Mares, M.M. The Anticoccidial Effect of Alcoholic Vitis vinifera Leaf Extracts on Eimeria papillate Oocysts Isolated in Mice In Vitro and In Vivo. Vet. Sci. 2023, 10, 97. [Google Scholar] [CrossRef]
- Abdullah, H. Pakistan Wa Hindustan Ki Jari Bootian. In Idar-e-Matbout-C-Sulemani Urdu Bazar Lahore; Adara Matabat e Sulaman: Lahore, Pakistan, 1975; pp. 29–67. [Google Scholar]
- Kumar, P.S.; Suresh, E.; Kalavathy, S. Review on a potential herb Calotropis gigantea (L.) R. Br. Sch. Acad. J. Pharm. 2013, 2, 135–143. [Google Scholar]
- Ramadan, A.M.; Azeiz, A.A.; Baabad, S.; Hassanein, S.; Gadalla, N.O.; Hassan, S.; Algandaby, M.; Bakr, S.; Khan, T.; Abouseadaa, H.H. Control of β-sitosterol biosynthesis under light and watering in desert plant Calotropis procera. Steroids 2019, 141, 1–8. [Google Scholar] [CrossRef]
- Mezzetti, T.; Orzalesi, G.; Bellavita, V. Chemistry of ursolic acid. Planta Med. 1971, 20, 244–252. [Google Scholar] [CrossRef]
- Singh, B.; Rastogi, R. Structure of asclepin and some observations on the NMR spectra of Calotropis glycosides. Phytochemistry 1972, 11, 757–762. [Google Scholar] [CrossRef]
- Choedon, T.; Mathan, G.; Arya, S.; Kumar, V.L.; Kumar, V. Anticancer and cytotoxic properties of the latex of Calotropis procera in a transgenic mouse model of hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 2517. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, H.I.; Ferreira, P.M.; Moura, E.S.; Torres, M.R.; Alves, A.P.; Pessoa, O.D.; Costa-Lotufo, L.V.; Moraes, M.O.; Pessoa, C. In vitro and in vivo antiproliferative activity of Calotropis procera stem extracts. An. Acad. Bras. Ciências 2010, 82, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Smit, H.; Woerdenbag, H.; Singh, R.; Meulenbeld, G.; Labadie, R.; Zwaving, J. Āyurvedic herbal drugs with possible cytostatic activity. J. Ethnopharmacol. 1995, 47, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, P.M.; Eswara, R.; Krishnapriya, M.; Nagalakshimi, V.; Silpa, P.; Anjali, M. An overview of phytochemical and pharmacological activities of Calotropis procera. FS J. Pharm. Res. 2012, 1, 18–25. [Google Scholar]
- Bhagat, M.; Arora, J.S.; Saxena, A.K. In vitro cytotoxicity of extracts and fractions of Calotropis procera (Ait.) roots against human cancer cell lines. Int. J. Green Pharm. 2010, 4. [Google Scholar]
- Jain, P.; Kumar, N.; Verma, R. Clinical trials of Arka Mula Tuvaka, bark of Calotropios procera Ait.(R. Br.) on atisar and Pravihika-A preliminary study. J. Res. Aurveda Siddha 1985, 6, 89–91. [Google Scholar]
- Perumal, G.M.; Prabhu, K.; Janaki, R.M.; Kalaivannan, J.; Kavimani, M. The GC MS Analysis of Ethyl Acetate Extract Of ‘Flueggea Leucopyrus. NVEO J. 2021, 8, 4035–4040. [Google Scholar]
- Sakthivel, K.; Palani, S. Santhosh kalash R, Devi K, Senthil kumar B. Phytoconstituents analysis by GC-MS cardioprotective and anti oxidant activity of Buchania axillaris against doxorubicin induced cardiotoxicity in albino rats. Int. J. Pharm. Stud. Res. 2010, 1, 34–48. [Google Scholar]
- Bassaganya-Riera, J.; Hontecillas, R.; Beitz, D. Colonic anti-inflammatory mechanisms of conjugated linoleic acid. Clin. Nutr. 2002, 21, 451–459. [Google Scholar] [CrossRef]
- Giulitti, F.; Petrungaro, S.; Mandatori, S.; Tomaipitinca, L.; De Franchis, V.; D’Amore, A.; Filippini, A.; Gaudio, E.; Ziparo, E.; Giampietri, C. Anti-tumor effect of oleic acid in hepatocellular carcinoma cell lines via autophagy reduction. Front. Cell Dev. Biol. 2021, 9, 629182. [Google Scholar] [CrossRef]
- Pegoraro, N.S.; Camponogara, C.; Cruz, L.; Oliveira, S.M. Oleic acid exhibits an expressive anti-inflammatory effect in croton oil-induced irritant contact dermatitis without the occurrence of toxicological effects in mice. J. Ethnopharmacol. 2021, 267, 113486. [Google Scholar] [CrossRef]
- Kalaivani, C.; Sathish, S.S.; Janakiraman, N.; Johnson, M. GC-MS studies on Andrographis paniculata (Burm. f.) Wall. Ex Nees—A medicinally important plant. Int. J. Med. Arom. Plants 2012, 2, 69–74. [Google Scholar]
- Williams, R. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int. J. Parasitol. 1999, 29, 1209–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, C.; Liu, L.; Xu, J.; Jiang, H.; Li, D.; Lan, J.; Li, J.; Yang, J.; Tu, Q. ZnO-based multifunctional nanocomposites to inhibit progression and metastasis of melanoma by eliciting antitumor immunity via immunogenic cell death. Theranostics 2020, 10, 11197. [Google Scholar] [CrossRef]
- Pieri, F.; Silva, V.; Vargas, F.; Veiga Junior, V.; Moreira, M. Antimicrobial activity of Copaifera langsdorffii oil and evaluation of its most bioactive fraction against bacteria of dog’s dental plaque. Pak. Vet. J. 2014, 34, 165–169. [Google Scholar]
- Xiao, C.; Ji, Q.; Rajput, Z.; Wei, Q.; Liu, Y.; Bao, G. Antifungal Efficacy of Phellodendron amurense Ethanol Extract against Trichophyton mentagrophytes in Rabbits. Pak. Vet. J. 2014, 34, 219–223. [Google Scholar]
- Cedric, Y.; Payne, V.; Nadia, N.; Kodjio, N.; Kollins, E.; Megwi, L.; Kuiate, J. In vitro antioxidant and anticoccidial studies of Pentaclethra macrophylla extracts against Eimeria magna, Eimeria flavescens, Eimeria intestinalis Eimeria stiedae oocysts and sporozoites of rabbits. J. Adv. Parasitol. 2018, 5, 38–48. [Google Scholar]
- Molan, A.L.; Liu, Z.; De, S. Effect of pine bark (Pious radiata) extracts on sporulation of coccidian oocysts. Folia Parasitol. 2009, 56, 1. [Google Scholar] [CrossRef]
- Wang, L.; Gong, T.; Chen, R.Y. Two new prenylflavonoids from Morus nigra L. Chin. Chem. Lett. 2009, 20, 1469–1471. [Google Scholar] [CrossRef]
- Fornazier, R.F.; Ferreira, R.R.; Vitoria, A.; Molina, S.; Lea, P.; Azevedo, R. Effects of cadmium on antioxidant enzyme activities in sugar cane. Biol. Plant. 2002, 45, 91–97. [Google Scholar] [CrossRef]
- Narsih, K.S.; Wignyanto, W.S. Identification of aloin and saponin and chemical composition of volatile constituents from Aloe vera (L.) Peel. J. Agric. Food Tech. 2012, 2, 79–84. [Google Scholar]
- Abbas, R.; Abbas, A.; Iqbal, Z.; Raza, M.; Hussain, K.; Ahmed, T.; Shafi, M. In vitro anticoccidial activity of Vitis vinifera extract on oocysts of different Eimeria species of broiler chicken. J. Hell. Vet. Med. Soc. 2020, 71, 2267–2272. [Google Scholar] [CrossRef]
- Olaku, O.O.; Ojukwu, M.O.; Zia, F.Z.; White, J.D. The role of grape seed extract in the treatment of chemo/radiotherapy induced toxicity: A systematic review of preclinical studies. Nutr. Cancer 2015, 67, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Al-Quraishy, S.; Abdel Moneim, A.E.; Delic, D. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol. Res. 2013, 112, 101–106. [Google Scholar] [CrossRef]
- Amer, O.S.; Dkhil, M.A.; Hikal, W.M.; Al-Quraishy, S. Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. BioMed Res. Int. 2015, 2015, 219670. [Google Scholar] [CrossRef]
- Alzahrani, F.; Al-Shaebi, E.M.; Dkhil, M.A.; Al-Quraishy, S. In vivo anti-eimeria and in vitro anthelmintic activity of Ziziphus spina-christi leaf extracts. Pak. J. Zool. 2016, 48, 409–413. [Google Scholar]
- Thagfan, F.A.; Al-Megrin, W.A.; Al-Quraishy, S.; Dkhil, M.A.M. Mulberry extract as an ecofriendly anticoccidial agent: In vitro and in vivo application. Rev. Bras. Parasitol. Veterinária 2020, 29, e009820. [Google Scholar] [CrossRef]
- Alcicek, A.; Bozkurt, M.; Çabuk, M. The effect of a mixture of herbal essential oils, an organic acid or a probiotic on broiler performance. S. Afr. J. Anim. Sci. 2004, 34, 217–222. [Google Scholar]
- Gracia, M.; Millán, C.; Sánchez, J.; Guyard-Nicodeme, M.; Mayot, J.; Carre, Y.; Csorbai, A.; Chemaly, M.; Medel, P. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: Part B. Poult. Sci. 2016, 95, 886–892. [Google Scholar] [CrossRef]
- Garcia, V.; Catala-Gregori, P.; Hernandez, F.; Megias, M.; Madrid, J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poult. Res. 2007, 16, 555–562. [Google Scholar] [CrossRef]
- Waghorn, T.; Molan, A.; Deighton, M.; Alexander, R.; Leathwick, D.; McNabb, W.; Meagher, L. In vivo anthelmintic activity of Dorycnium rectum and grape seed extract against Ostertagia (Teladorsagia) circumcincta and Trichostrongylus colubriformis in sheep. N. Z. Vet. J. 2006, 54, 21–27. [Google Scholar] [CrossRef]
- Marizvikuru, M.; Patrick, J.M. Point prevalence study of gastro-intestinal parasites in village chickens of Centane district, South Africa. Afr. J. Agric. Res. 2011, 6, 2033–2038. [Google Scholar]
- Onan, K.S.; Toure, A.; Ouattara, K.; Djaman, A.J.; N’Guessan, J.D. In vitro anticoccidial activity of Thonningia sanguinea extract on Eimeria tenella and Eimeria necatrix sporozoites cell. Afr. J. Microbiol. Res. 2012, 6, 6247–6251. [Google Scholar]
- Aboelhadid, S.M.; El-Ashram, S.; Hassan, K.M.; Arafa, W.M.; Darwish, A.B. Hepato-protective effect of curcumin and silymarin against Eimeria stiedae in experimentally infected rabbits. Livest. Sci. 2019, 221, 33–38. [Google Scholar] [CrossRef]
- Manikandan, P.; Letchoumy, P.V.; Gopalakrishnan, M.; Nagini, S. Evaluation of Azadirachta indica leaf fractions for in vitro antioxidant potential and in vivo modulation of biomarkers of chemoprevention in the hamster buccal pouch carcinogenesis model. Food Chem. Toxicol. 2008, 46, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Kanthal, L.K.; Dey, A.; Satyavathi, K.; Bhojaraju, P. GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. Pharmacogn. Res. 2014, 6, 58. [Google Scholar] [CrossRef]
- Heelan, J.S.; Ingersoll, F.W.; Ingersoll, F.W. Essentials of Human Parasitology; Delmar Publishers: Huntington Beach, CA, USA, 2002. [Google Scholar]
- You, M.-J. Suppression of Eimeria tenella sporulation by disinfectants. Korean J. Parasitol. 2014, 52, 435. [Google Scholar] [CrossRef] [PubMed]



| Part of the Plant | Chemical Class | Chemical Ingredient | Reference |
|---|---|---|---|
| Leaves | Flavonoid | α-Amyrin | [30] |
| α-Amyrin acetate | [31] | ||
| β-sitosterol | [32] | ||
| Ursolic acid | [33] | ||
| Calotropin | [34] | ||
| Calotropagenin | [34] | ||
| Procesterol (A new steroidal hydroxyl ketone) | [35] | ||
| Other constituents | Calotropenylacetate | [36] | |
| Multiflorenol | [37] | ||
| 16-α-Hydroxy calotropagenin | [38] | ||
| O-Pyrocatechuic acid | [39] | ||
| Quercetin-3-rutinoside | [40] | ||
| α- and β-calotropeols | [40] | ||
| 3-epimoretenol | [40] | ||
| Procerain | [40] | ||
| Histamine | [40] |
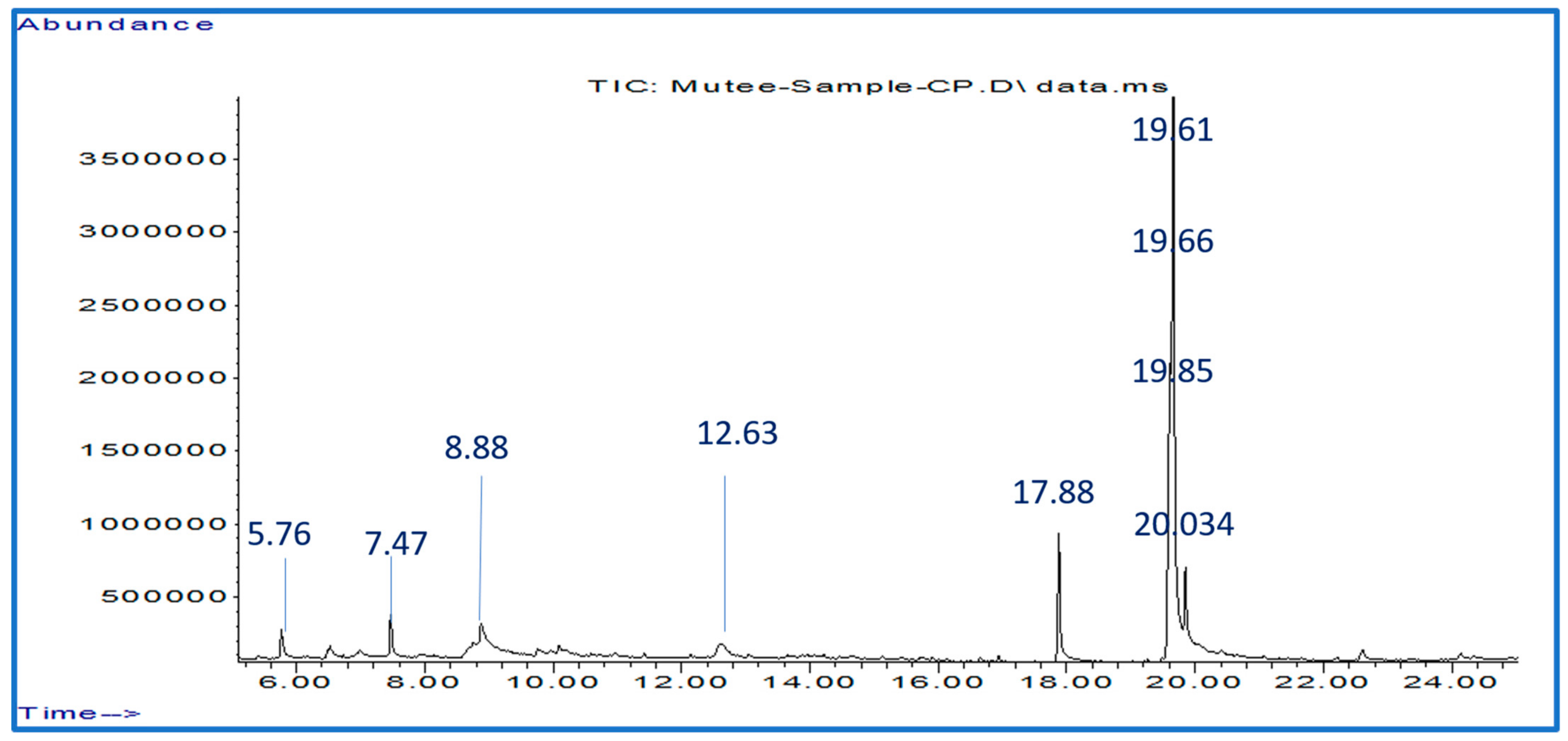
| NO | RT (min) | Bioactive Phytochemicals Compounds | Molecular Formula | [M − H] (m/z) | Peak Area % |
|---|---|---|---|---|---|
| 1 | 5.76 | 1-Amino-2,6-dimethylpiperidine | C7H16N2 | 128 | 2.09 |
| 2 | 7.47 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 2.82 |
| 3 | 8.88 | 7-Ethyl-4-decen-6-one | C12H22O | 182 | 10.7 |
| 4 | 12.63 | β-D-Glucopyranose, 1,6-anhydro- | C6H10O5 | 162 | 4.5 |
| 5 | 17.88 | n-Hexadecanoic acid | C16H32O2 | 256 | 9.39 |
| 6 | 19.61 | Linoleic acid | C18H32O2 | 280 | 18.44 |
| 7 | 19.66 | Oleic Acid | C18H34O2 | 282 | 47.39 |
| 8 | 19.85 | Octadecanoic acid | C18H36O2 | 284 | 3.43 |
| 9 | 22.61 | 9,12-Octadecadienoyl chloride, (Z, Z)- | C18H31ClO | 298 | 1.24 |
| Compound Name | Compound Nature | Function | Reference |
|---|---|---|---|
| 1-Amino-2,6-dimethylpiperidine | Nature | Cardiotoxicity Antioxidants Anti-inflammatory arthritis, and muscle and joint pain Antimicrobial | [41] |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | Alkaloid | Used for gastrointestinal disorders, gonorrhea, menorrhagia. Antifungal, anti-cancer, sclerosis, and warts | [41] |
| 7-Ethyl-4-decen-6-one | Flavonoid | Antimalarial activity Antifungal Antibacterial Antidiarrhoeal activity | [38] |
| β-D-Glucopyranose, 1,6-anhydro | Sugar moiety | Antiviral, antifungal Anthelmintic activity Antidiarrhoeal activity Anticonvulsant activity | [38] |
| n-Hexadecanoic acid | Palmitic acid | Antibacterial, antifungal Antibiofilms Antioxidant and anti-cancer | [42] |
| Linoleic acid | Linoleic acid | Antibacterial Anti-tumor, anti-virus, and anti-inflammatory | [43] |
| Oleic Acid | Palmitic acid | Antibacterial, antifungal Antibiofilms Antioxidant and anti-cancer | [44] |
| Octadecanoic acid | Linoleic acid | Antibacterial, antifungal Antibiofilms Antioxidant and anticancer | [45] |
| 9,12-Octadecadienoyl chloride, (Z, Z)- | solely chloride | Antisecretory, antispermigenic, antitonsilitic, antitubercular, choleretic, contraceptive | [46] |
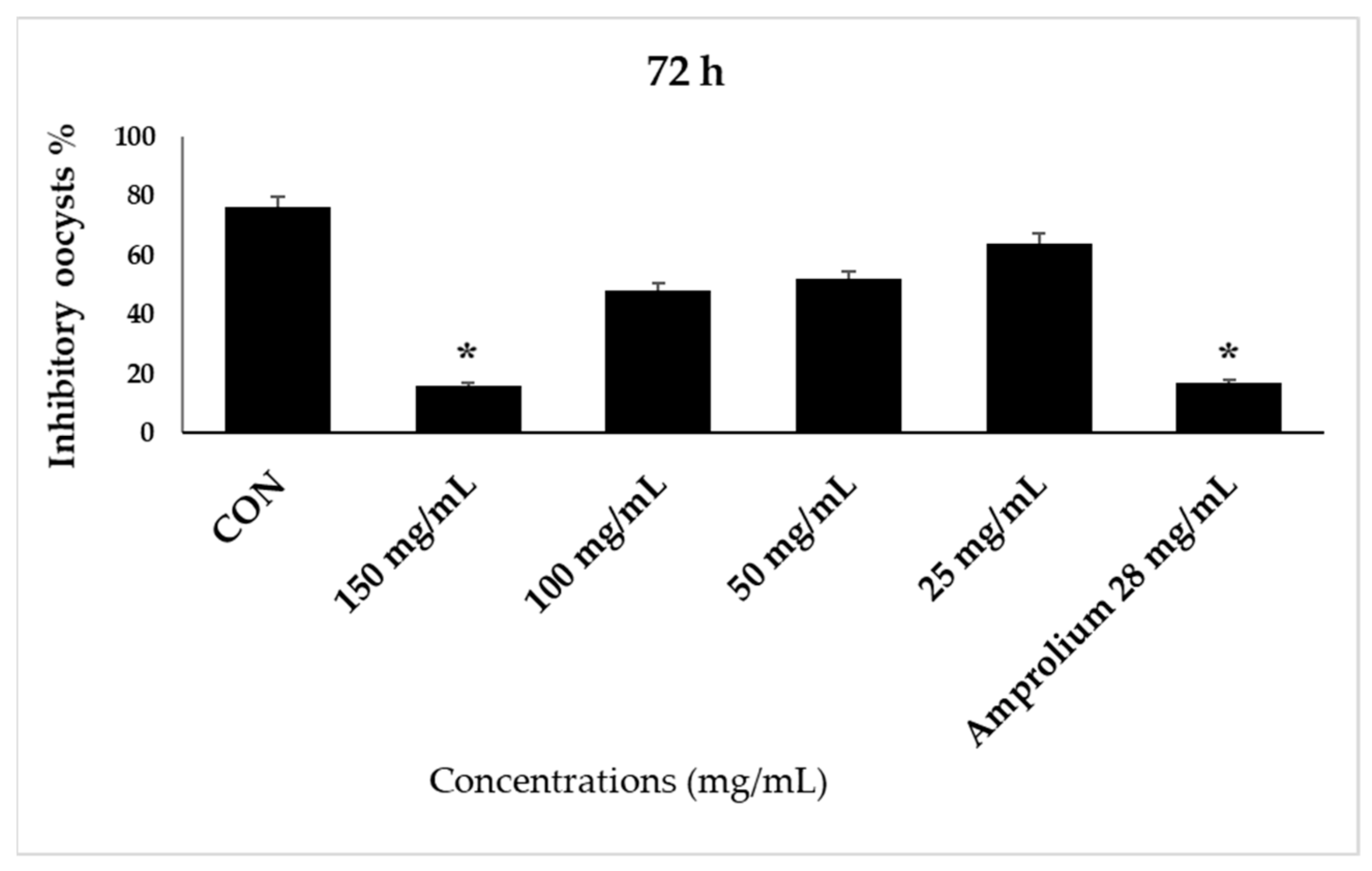
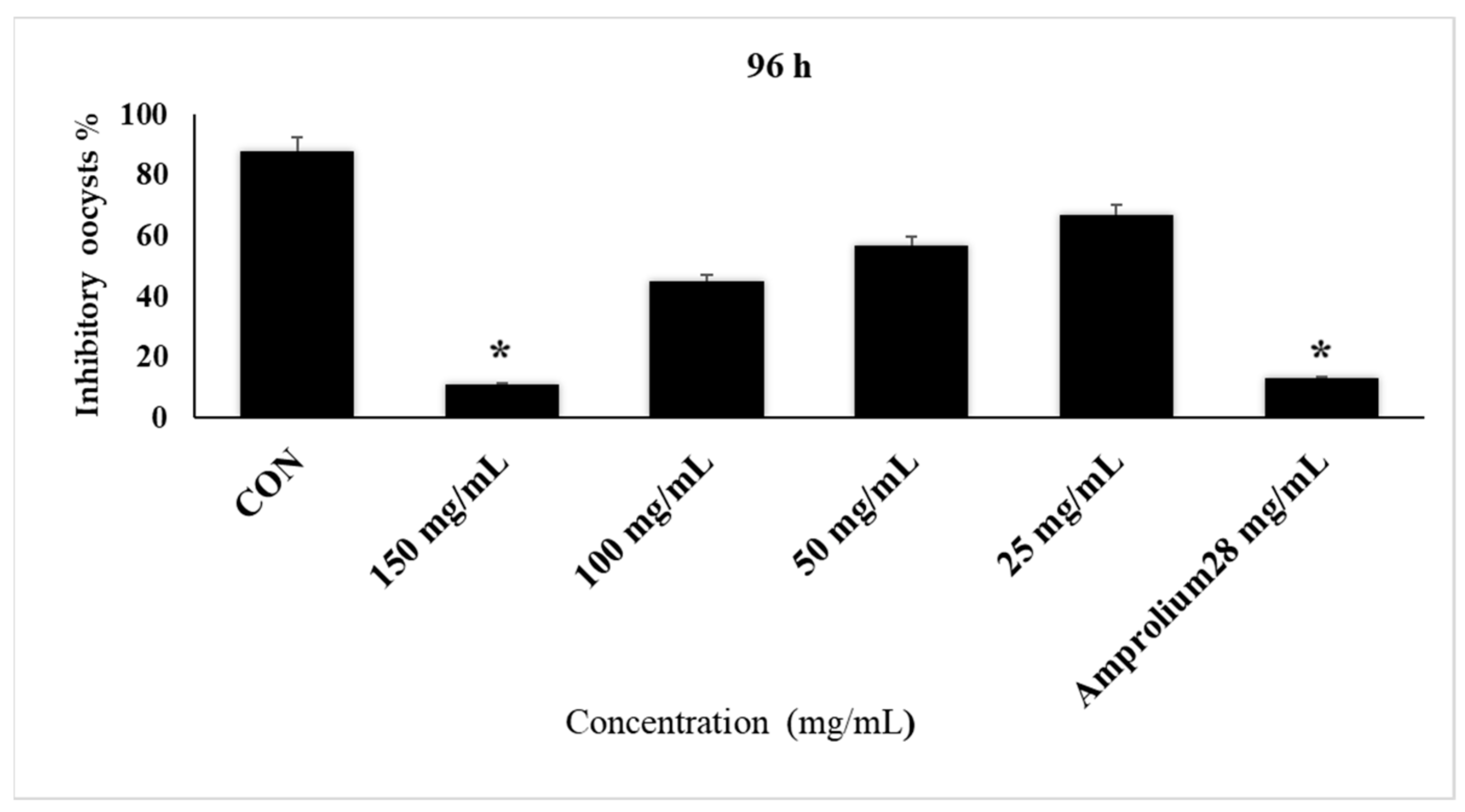
| 24 h | 48 h | 72 h | 96 h | |||||
|---|---|---|---|---|---|---|---|---|
| Inhibition | Sporulation | Inhibition | Sporulation | Inhibition | Sporulation | Inhibition | Sporulation | |
| CON | 46 | 54 | 32 | 68 | 24 | 76 | 7 | 93 |
| 150 mg/mL | 97 | 3 | 92 | 8 | 88 | 12 | 96 | 4 |
| 100 mg/mL | 78 | 22 | 78 | 22 | 52 | 48 | 55 | 45 |
| 50 mg/mL | 69 | 31 | 61 | 39 | 48 | 52 | 43 | 57 |
| 25 mg/mL | 61 | 39 | 53 | 47 | 26 | 64 | 33 | 67 |
| Amprolium 28 mg/mL | 92 | 8 | 96 | 4 | 97 | 3 | 97 | 3 |
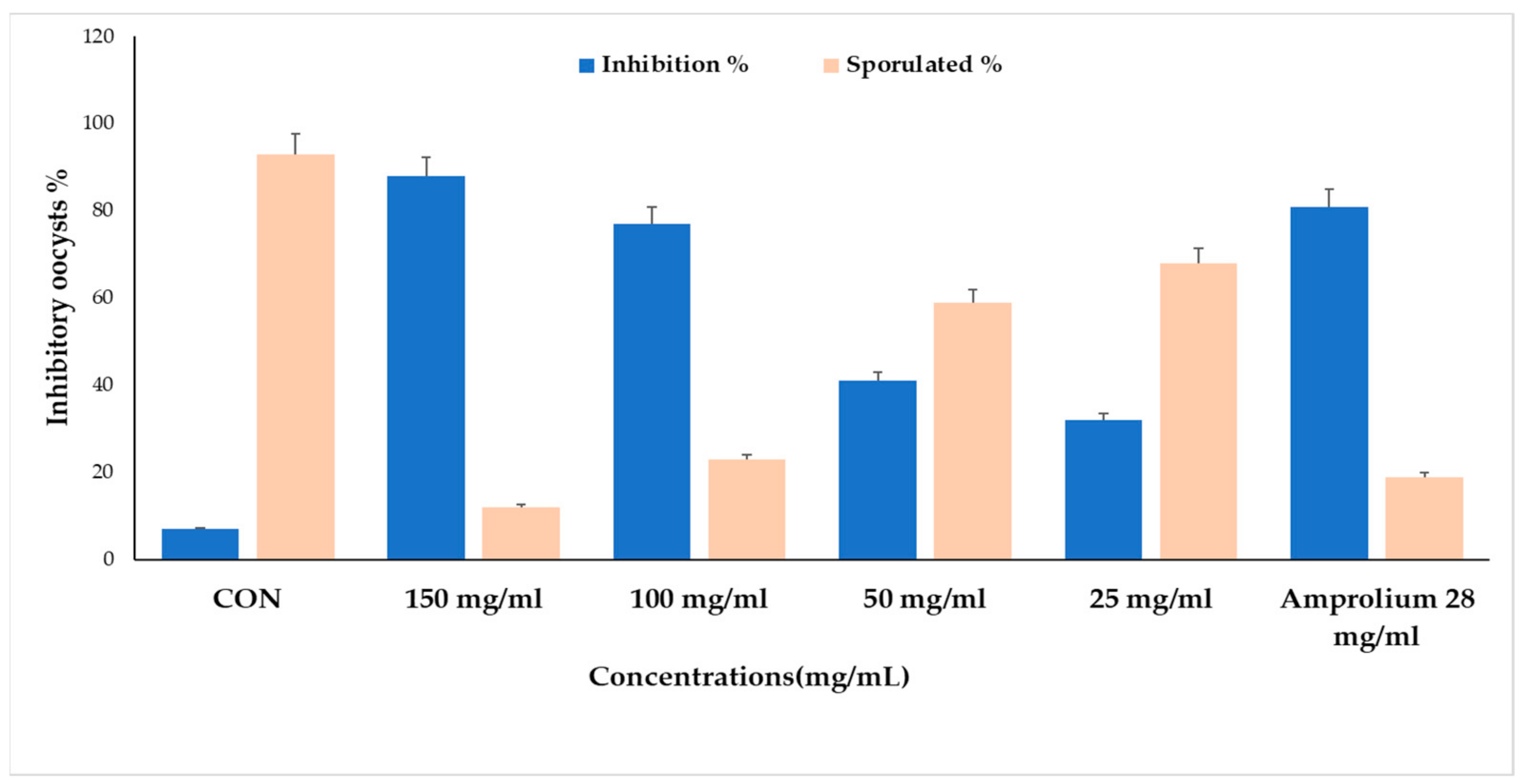
| CON | 150 mg/mL | 100 mg/mL | 50 mg/mL | 25 mg/mL | Amprolium 28 mg/mL | |
|---|---|---|---|---|---|---|
| Inhibition% | 11 | 93 | 77 | 41 | 32 | 83 |
| Sporulation% | 89 | 7 | 23 | 59 | 68 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murshed, M.; Aljawdah, H.M.A.; Mares, M.; Al-Quraishy, S. In Vitro: The Effects of the Anticoccidial Activities of Calotropis procera Leaf Extracts on Eimeria stiedae Oocysts Isolated from Rabbits. Molecules 2023, 28, 3352. https://doi.org/10.3390/molecules28083352
Murshed M, Aljawdah HMA, Mares M, Al-Quraishy S. In Vitro: The Effects of the Anticoccidial Activities of Calotropis procera Leaf Extracts on Eimeria stiedae Oocysts Isolated from Rabbits. Molecules. 2023; 28(8):3352. https://doi.org/10.3390/molecules28083352
Chicago/Turabian StyleMurshed, Mutee, Hossam M. A. Aljawdah, Mohammed Mares, and Saleh Al-Quraishy. 2023. "In Vitro: The Effects of the Anticoccidial Activities of Calotropis procera Leaf Extracts on Eimeria stiedae Oocysts Isolated from Rabbits" Molecules 28, no. 8: 3352. https://doi.org/10.3390/molecules28083352
APA StyleMurshed, M., Aljawdah, H. M. A., Mares, M., & Al-Quraishy, S. (2023). In Vitro: The Effects of the Anticoccidial Activities of Calotropis procera Leaf Extracts on Eimeria stiedae Oocysts Isolated from Rabbits. Molecules, 28(8), 3352. https://doi.org/10.3390/molecules28083352









