Uncorking Haloanisoles in Wine
Abstract
1. Introduction
2. What Are Haloanisoles in Wine?
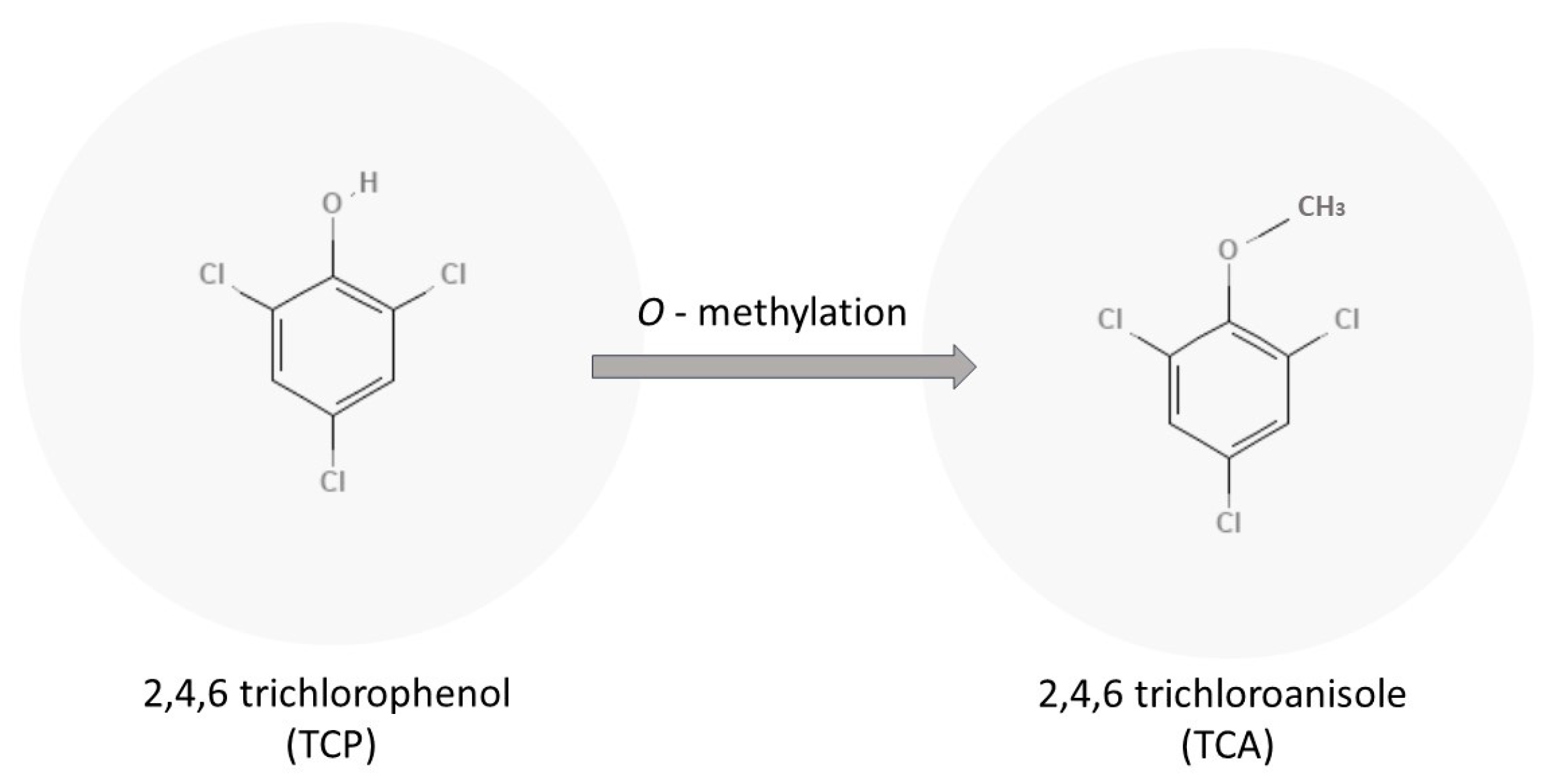
2.1. Trichloroanisole
2.2. Tribromoanisole
2.3. Tetrachloroanisole & Pentachloroanisole
3. Sensory Characteristics and Thresholds
4. Wine Closures
5. Remediation
6. Analysis Methodology
| Gas Chromatographer | Mass Spectrometer | Column Type | Sample Type | Extraction | Compounds Analyzed | Inlet Temperature | Oven Program | LOD | LOQ | Analysis Time Per Sample | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Varian 3800 | Varian Saturn 2200 Ion-trap | CP-WAX 52-CB | Wine | Dispersive liquid-liquid microextraction | TCA TBA TeCA PCA | 250 °C | 35 °C for 1 min 20 °C/min to 170 °C hold for 1 min 3 °C/min to 210 °C hold for 12 min | 3 ng/L | 10 ng/L | 32 min | [48] |
| Agilent 7890A | Agilent 7000B | DB-5 | Wine | HS-SPME 100 µm PDMS | TCA TBA TeCA PCA | 280 °C | 40 °C for 0 min 30 °C/min to 280 °C hold for 3 min | TCA, TeCA, PCA: Sub ng/L TBA: <1 ng/L | TCA, TeCA, PCA: Sub ng/L TBA: <1 ng/L | 11 min | [49] |
| (2) HP 6890 | D1: FID D2: ECD | D1: DB-XLB D2: TG-1301MS | Wine | HS-SPME 100 µm PDMS | TCA TBA TeCA | 250 °C | D1: 50 °C for 2 min 20 °C/min to 120 °C 5 °C/min to 250 °C hold for 5 min D2: 50 °C for 20 min 25 °C/min to 85 °C hold for 0.5 min 2 °C/min to 140 °C 40 °C/min to 250 °C hold for 5 min | 0.1 ng/L | TCA: 0.4 ng/L TeCA: 0.4 ng/L TBA: 20.5 ng/L | 93.75 min | [50] |
| Agilent 7890A | Agilent 5975TAD | DB-5 | Wine | HS-SPME DVB/PDMS | TCA TBA TeCA PCA | TCA: 1.5 ng/L TeCA: 0.5 ng/L PCA: 11.2 ng/L TBA: 7.5 ng/L | [51] | ||||
| Varian 3800 | uECD | VF-5ms | Wine | Vortex assisted liquid-liquid microextraction | TCA TBA TeCA PCA | 300 °C | 100 °C for 3.5 min 15 °C/min to 115 °C 1 °C/min to 150 °C 25 °C/min to 250 °C hold for 2 min | TCA: 2.1 ng/L TeCA: 2.7 ng/L PCA: 2.9 ng/L TBA: 2.1 ng/L | 44.5 min | [52] | |
| Agilent 7890A | Agilent 7000B | HP-5MS HP-35MS | Atmosphere | Tenax GR | TCA TBA TeCA PCA | 280 °C | 70 °C for 2 min 25 °C/min to 150 °C 3 °C/min to 180 °C 25 °C/min to 300 °C | TCA: 0.6 pg tube-1 TeCA: 0.6 pg tube-1 PCA: 0.4 pg tube-1 TBA: 3.3 pg tube-1 | TCA: 2.1 pg tube-1 TeCA: 2.1 pg tube-1 PCA: 1.3 pg tube-1 TBA: 2.2 pg tube-1 | 25 min | [53] |
| Agilent 6890 N | Agilent 5973 N | HP-5MS | Atmosphere | Tenax TA | TCA TBA TeCA PCA | 250 °C | 55 °C for 3 min 15 °C/min to 125 °C 1.5 °C/min to 145 °C 10 °C/min to 183 °C 1.5 °C/min to 195 °C 15 °C/min to 250 °C hold for 3 min | TCA: 0.01 ng tube-1 TeCA: 0.06 ng tube-1 PCA: 0.03 ng tube-1 TBA: 0.05 ng tube-1 | TCA: 0.05 ng tube-1 TeCA: 0.1 ng tube-1 PCA: 0.1 ng tube-1 TBA: 0.1 ng tube-1 | 40 min | [54] |
| HP-17MS | Water | HS-SPME PDMS/DVB/CAB | TCA TBA | 250 °C | 45 °C for 4 min 10 °C/min to 240 °C hold for 1 min 30 °C/min to 280 °C hold for 4 min | TCA: 0.098 ng/L TBA: 0.086 ng/L | 30 min | [55] |
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, H.R.; Zanier, C.; Tanner, H. Identification of 2,4,6-Trichloroanisole as a Potent Compound Causing Cork Taint in Wine. J. Agric. Food Chem. 1982, 30, 359–362. [Google Scholar] [CrossRef]
- Alvarez-Rodríguez, M.L.; Lopez-Ocana, L.; Miguel Lopez-Coronado, J.; Rodríguez, E.; Jesus Martınez, M.; Larriba, G.; Coque, J.J.R. Cork Taint of Wines: Role of the Filamentous Fungi Isolated from Cork in the Formation of 2,4,6-Trichloroanisole by O Methylation of 2,4,6-Trichlorophenol. Appl. Environ. Microbiol. 2002, 68, 5860–5869. [Google Scholar] [CrossRef]
- Cravero, M.C. Musty and Moldy Taint in Wines: A Review. Beverages 2020, 6, 41. [Google Scholar] [CrossRef]
- Simpson, R.F.; Sefton, M.A. Origin and Fate of 2,4,6-Trichloroanisole in Cork Bark and Wine Corks. Aust. J. Grape Wine Res. 2007, 13, 106–116. [Google Scholar] [CrossRef]
- Ekberg, O. Literature Reveiw of Fungi in Buildings and Their Ability to Methylate Chlorophenols into Malodorous Chloroanisoles. J. Phys. Conf. Ser. 2021, 2069, 012207. [Google Scholar] [CrossRef]
- Callejón, R.M.; Ubeda, C.; Ríos-Reina, R.; Morales, M.L.; Troncoso, A.M. Recent Developments in the Analysis of Musty Odour Compounds in Water and Wine: A Review | Elsevier Enhanced Reader. J. Chromatogr. A 2015, 1428, 72–85. [Google Scholar] [CrossRef]
- BSEF. Electronics and Electrical Equipment—Flame Retardants—Preventing Firesand Protecting People. Available online: http://flameretardantsguide.com/flame-retardants-applications/electronics-and-electrical-equipment/ (accessed on 20 December 2022).
- US EPA. Methyl Bromide. Available online: https://www.epa.gov/ods-phaseout/methyl-bromide (accessed on 20 December 2022).
- Hinz, R.; Mannetje, A.; Glass, B.; McLean, D.; Douwes, J. Airborne Fumigants and Residual Chemicals in Shipping Containers Arriving in New Zealand. Ann. Work Expo. Health 2022, 66, 481–494. [Google Scholar] [CrossRef]
- Sela, S.; Schroeder, T.; Mamoru, M.; Ormsby, M. Explanatory document for International Standards for Phytosanitary Measures 15 (Regulation of Wood Packaging Material in International Trade); International Plant Protection Convention: Rome, Italy, 2017. [Google Scholar]
- Chatonnet, P.; Fleury, A.; Boutou, S. Identification of a New Source of Contamination of Quercus Sp. Oak Wood by 2,4,6-Trichloroanisole and Its Impact on the Contamination of Barrel-Aged Wines. J. Agric. Food Chem. 2010, 58, 10528–10538. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Bonnet, S.; Boutou, S.; Labadie, M.-D. Identification and Responsibility of 2,4,6-Tribromoanisole in Musty, Corked Odors in Wine. J. Agric. Food Chem. 2004, 52, 1255–1262. [Google Scholar] [CrossRef]
- PubChem 2,3,4,6-Tetrachlorophenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6028 (accessed on 19 December 2022).
- Ruckdeschel, G.; Renner, G. Effects of Pentachlorophenol and Some of Its Known and Possible Metabolites on Fungi. Appl. Environ. Microbiol. 1986, 51, 1370–1372. [Google Scholar] [CrossRef]
- Prescott, J.; Norris, L.; Kunst, M.; Kim, S. Estimating a “Consumer Rejection Threshold” for Cork Taint in White Wine. Elsevier 2004, 16, 345–349. [Google Scholar] [CrossRef]
- McKay, M.; Bauer, F.F.; Panzeri, V.; Buica, A. Testing the Sensitivity of Potential Panelists for Wine Taint Compounds Using a Simplified Sensory Strategy. Foods 2018, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, V.; Maggi, L. Effect of Wine Style on the Perception of 2,4,6-Trichloroanisole, a Compound Related to Cork Taint in Wine. Elsevier 2006, 40, 694–699. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kato, H.; Kurahashi, T. 2,4,6-Trichloroanisole Is a Potent Suppressor of Olfactory Signal Transduction. Proc. Natl. Acad. Sci. USA 2013, 110, 16235–16240. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.R. Analytical Methods for Determination of Cork-Taint Compounds in Wine | Elsevier Enhanced Reader. Trends Anal. Chem. 2012, 37, 135–147. [Google Scholar] [CrossRef]
- Tarasov, A.; Rauhut, D.; Jung, R. “Cork Taint” Responsible Compounds. Determination of Haloanisoles and Halophenols in Cork Matrix: A Review. Elsevier 2017, 175, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Godden, P. Summary of AWRI Closure Trials and Other Investigations into Closure Performance. AWRI 2012, No. 248, 6–12. [Google Scholar]
- Atkin, T.; Dove, D. Rodney Strong Winery: The Great Cork Debate. J. Int. Acad. Case Stud. 2006, 12, 77–89. [Google Scholar]
- Marin, A.B.; Jorgensen, E.M.; Kennedy, J.A.; Ferrier, J. Effects of Bottle Closure Type on Consumer Perceptions of Wine Quality. Am. J. Enol. Vitic. 2007, 58, 182–191. [Google Scholar] [CrossRef]
- Lopes, P.; Marques, J.; Lopes, T.; Lino, J.; Coelho, J.; Alves, C.; Roseira, I.; Mendes, A.; Cabral, M. Permeation of D5-2,4,6-Trichloroanisole via Vapor Phase through Different Closures into Wine Bottles. Am. J. Enol. Vitic. 2011, 62, 245–249. [Google Scholar] [CrossRef]
- Pereira, B.; Lopes, P.; Marques, J.; Pimenta, M.; Alves, C.; Roseira, I.; Mendes, A.; Cabral, M. Sealing Effectiveness of Different Types of Closures towards Volatile Phenols and Haloanisoles. OENO One 2013, 47, 145. [Google Scholar] [CrossRef]
- Cork Quality Council Incoming Natural Corks—Average TCA Score. Available online: https://www.corkqc.com/products/incoming-natural-corks-average-tca-score (accessed on 20 December 2022).
- Taylor, M.K.; Thomas, M.Y.; Butzke, C.E.; Ebeler, S.E. Supercritical Fluid Extraction of 2,4,6-Trichloroanisole from Cork Stoppers. J. Agric. Food. Chem. 2000, 48, 2208–2211. Available online: https://pubs.acs.org/doi/pdf/10.1021/jf991045q (accessed on 15 January 2023). [CrossRef]
- Diam. Available online: https://www.diam-closures.com/cork-closures-manufacturing-process-wine-and-champagne (accessed on 20 December 2022).
- Cunningham, J. Highly Selective Molecular Confinement for the Prevention and Removal of Taint in Foods and Beverages. U.S. Patent 7629009B2, 28 November 2007. [Google Scholar]
- Garde-Cerdan, T. Molecularly Imprinted Polymer-Assisted Simple Clean-up of 2,4,6-Trichloroanisole and Ethylphenols from Aged Red Wines. Am. J. Enol. Vitic. 2008, 59, 5. [Google Scholar] [CrossRef]
- Jung, R.; Schaefer, V.; Christmann, M.; Hey, M.; Fischer, S.; Rauhut, D. Removal of 2,4,6-Trichloroanisole (TCA) and 2,4,6-Tribromoanisole (TBA) from Wine. Mitt. Klosterneubg. 2008, 10, 58–67. [Google Scholar]
- Swan, J. Process For Removing Off-Flavors and Odors From Foods and Beverages. US6610342B2, 4 May 2001. [Google Scholar]
- Valdés, O.; Marican, A.; Avila-Salas, F.; Castro, R.; John, A.; Laurie, F.; Santos, L. Polyaniline Based Materials as Response to Eliminate Haloanisoles in Spirits Beverages. Ind. Eng. Chem. Res. 2018, 57, 8308–8318. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Tempère, S.; Teissedre, P.-L.; Chira, K. Use of Alimentary Film for Selective Sorption of Haloanisoles from Contaminated Red Wine. Food Chem. 2021, 350, 128364. [Google Scholar] [CrossRef]
- Vuchot, P.; Puech, C.; Fernandez, O.; Fauveau, C.; Pellerin, P.; Vidal, S. Elimination des goûts de bouchon/moisi et de l’OTA à l’aide d’écorces de levures hautement adsorbantes. Rev. Vitic. Oenol. 2007, 2, 62–72. [Google Scholar]
- OIV. Part III—Good Practices Guide for Bulk Wine Transportation, in International Code of Oenological Practices; OIV: Paris, France, 2022.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6884, 2,4,6-Trichloroanisole. 2D-Structure. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_4_6-Trichloroanisole (accessed on 20 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6914, 2,4,6-Trichlorophenol. 2D-Structure. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_4_6-Trichlorophenol (accessed on 20 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11839, 2,4,6-Tribromoanisole. 2D-Structure. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_4_6-Tribromoanisole (accessed on 20 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1483, 2,4,6-Tribromophenol. 2D-Structure. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_4_6-Tribromophenol (accessed on 20 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 13647, 2,3,4,6-Tetrachloroanisole. 2D-Structure. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_3_4_6-Tetrachloroanisole (accessed on 20 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6028, 2,3,4,6-Tetrachlorophenol. 2D-Structure. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_3_4_6-Tetrachlorophenol (accessed on 20 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 15767, Pentachloroanisole. 2D-Structure. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pentachloroanisole (accessed on 20 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 992, Pentachlorophenol. 2D-Structure. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pentachlorophenol (accessed on 20 December 2022).
- Garde-Cedan, T.; Lorenzo, C.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Using near Infrared Spectroscopy to Determine Haloanisoles and Halophenols in Barrel Aged Red Wines. LWT- Food Sci. Technol. 2012, 46, 401–405. [Google Scholar] [CrossRef]
- Varelas, V.; Sanvicens, N.; M-Pilar-Marco; Kintzios, S. Development of a Cellular Biosensor for the Detection of 2,4,6-Trichloroanisole (TCA). Talanta 2011, 84, 936–940. [Google Scholar] [CrossRef]
- Cappellin, L.; Lopez-Hilfiker, F.D.; Pospisilova, V.; Ciotti, L.; Pastore, P.; Gonin, M.; Hutterli, M.A. Thermal Desorption–Vocus Enables Online Nondestructive Quantification of 2,4,6-Trichloroanisole in Cork Stoppers below the Perception Threshold. Anal. Chem. 2020, 92, 9823–9829. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Sáenz-González, C.; Pérez-del-Notario, N.; González-Sáiz, J.M. Development of a Dispersive Liquid–Liquid Microextraction Method for the Simultaneous Determination of the Main Compounds Causing Cork Taint and Brett Character in Wines Using Gas Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1576–1584. [Google Scholar] [CrossRef]
- Hjelmeland, A.K.; Collins, T.S.; Mitchell, A.E.; Ebeler, S.E. Using HS-SPME and GC/Triple Quadrupole MS for High Throughput Analysis of Haloanisoles in Wines at Sub-Ng/L Levels. Am. J. Enol. Vitic. 2012, 63, 495–499. [Google Scholar] [CrossRef]
- Slabizki, P.; Schmarr, H.-G. Analysis of Corky Off-Flavour Compounds at Ultra Trace Level with Multidimensional Gas Chromatography-Electron Capture Detection | Elsevier Enhanced Reader. J. Chromatogr. A 2013, 1271, 181–184. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Dziadas, M.; Majcher, M. Different Headspace Solid Phase Microextraction—Gas Chromatography/Mass Spectrometry Approaches to Haloanisoles Analysis in Wine. J. Chromatogr. A 2013, 1313, 185–193. [Google Scholar] [CrossRef]
- Pizarro, C.; Perez-del-Norario, N.; Saenz-Mateo, A.; Gonzales-Saiz, J.M. A Simple and Sensitive Vortex Assisted Liquid–Liquid Microextraction Method for the Simultaneous Determination of Haloanisoles and Halophenols in Wines | Elsevier Enhanced Reader. Talanta 2014, 128, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Camino-Sanchez, F.J.; Ruiz-Garcia, J.; Zafra-Gomez, A. Development of a Thermal Desorption Gas Chromatography–Mass Spectrometry Method for Quantitative Determination of Haloanisoles and Halophenols in Wineries’ Ambient Air | Elsevier Enhanced Reader. J. Chromatogr. A 2013, 1305, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Jove, P.; Vives-Mestres, M.; De Nadal, R.; Verdum, M. Development, Optimization and Validation of a Sustainable and Quantifiable Methodology for the Determination of 2,4,6-Trichloroanisole, 2,3,4,6 Tetrachloroanisole, 2,4,6-Tribromoanisole, Pentachloroanisole, 2-Methylisoborneole and Geosmin in Air. Processes 2021, 9, 1571. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, B.; Qi, F.; Kumirska, J. The Occurrence of Haloanisoles as an Emerging Odorant in Municipal Tap Water of Typical Cities in China. Water Res. 2016, 98, 242–249. [Google Scholar] [CrossRef] [PubMed]
| Name | Abbreviation | Chemical Structure | Sources * | Thresholds | Remediation |
| 2,4,6-Trichloroanisole | TCA |  [37] | 3–10 ng/L [15,16] | ||
| 2,4,6-Trichlorophenol | TCP | 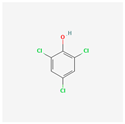 [38] |
| - | - |
| 2,4,6-Tribromoanisole | TBA | 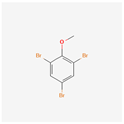 [39] |
| 2–7.9 ng/L [6] | |
| 2,4,6-Tribromophenol | TBP | 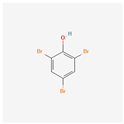 [40] |
| - | - |
| 2,3,4,6-Tetrachloroanisole | TeCA | 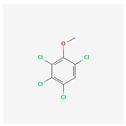 [41] |
| 5–15 ng/L [19] |
|
| 2,3,4,6-Tetrachlorophenol | TeCP |  [42] |
| - | - |
| Pentachloroanisole | PCA |  [43] |
| 10,000 ng/L [19] |
|
| Pentachlorophenol | PCP | 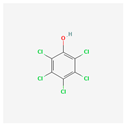 [44] |
| - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keng, A.; Botezatu, A. Uncorking Haloanisoles in Wine. Molecules 2023, 28, 2532. https://doi.org/10.3390/molecules28062532
Keng A, Botezatu A. Uncorking Haloanisoles in Wine. Molecules. 2023; 28(6):2532. https://doi.org/10.3390/molecules28062532
Chicago/Turabian StyleKeng, Abigail, and Andreea Botezatu. 2023. "Uncorking Haloanisoles in Wine" Molecules 28, no. 6: 2532. https://doi.org/10.3390/molecules28062532
APA StyleKeng, A., & Botezatu, A. (2023). Uncorking Haloanisoles in Wine. Molecules, 28(6), 2532. https://doi.org/10.3390/molecules28062532








