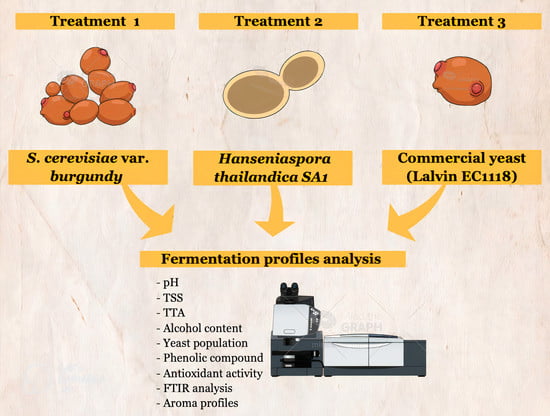
Fermentation Characteristics and Aromatic Profiles of Plum Wines Produced with Hanseniaspora thailandica Zal1 and Common Wine Yeasts
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characteristics of Plums
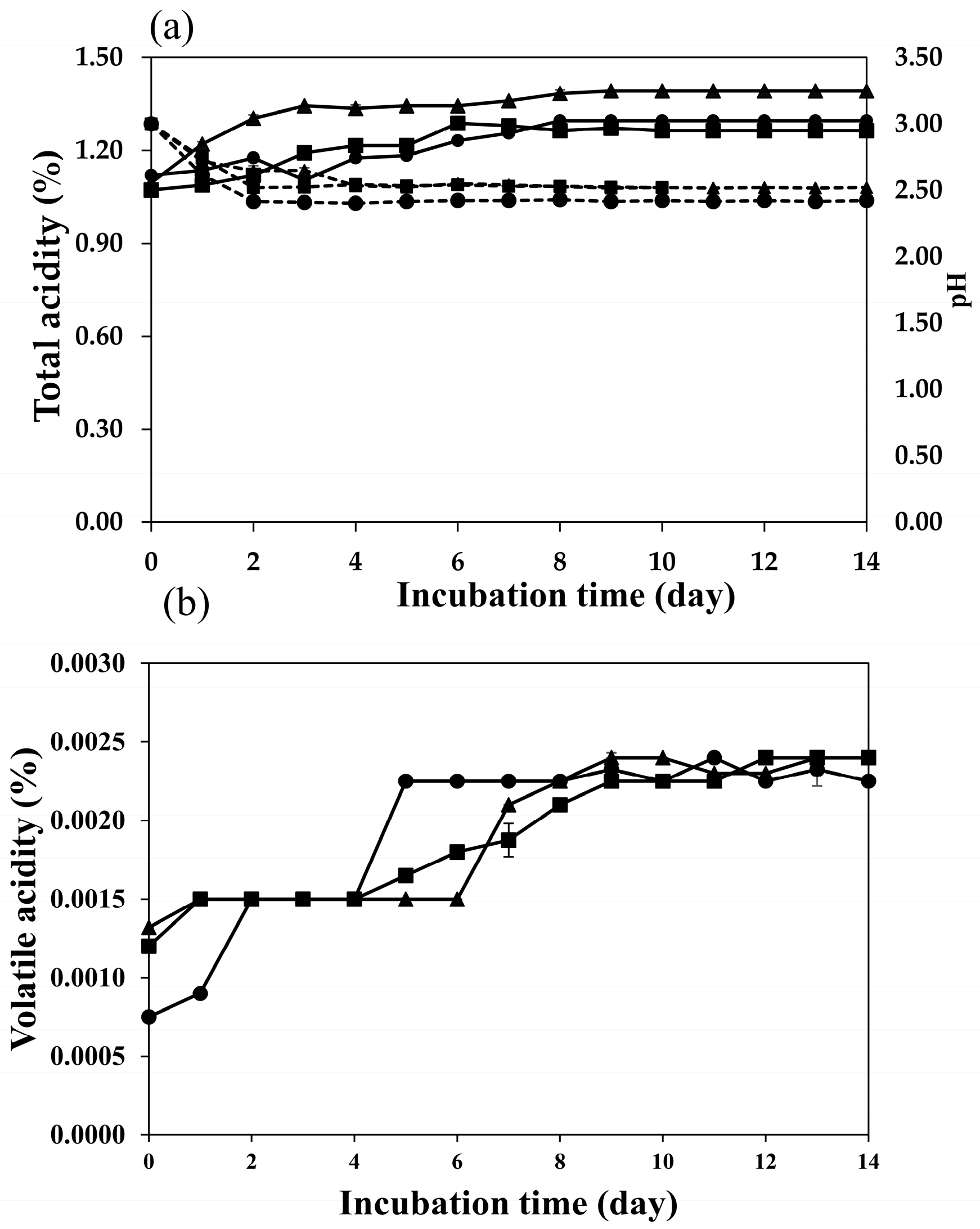
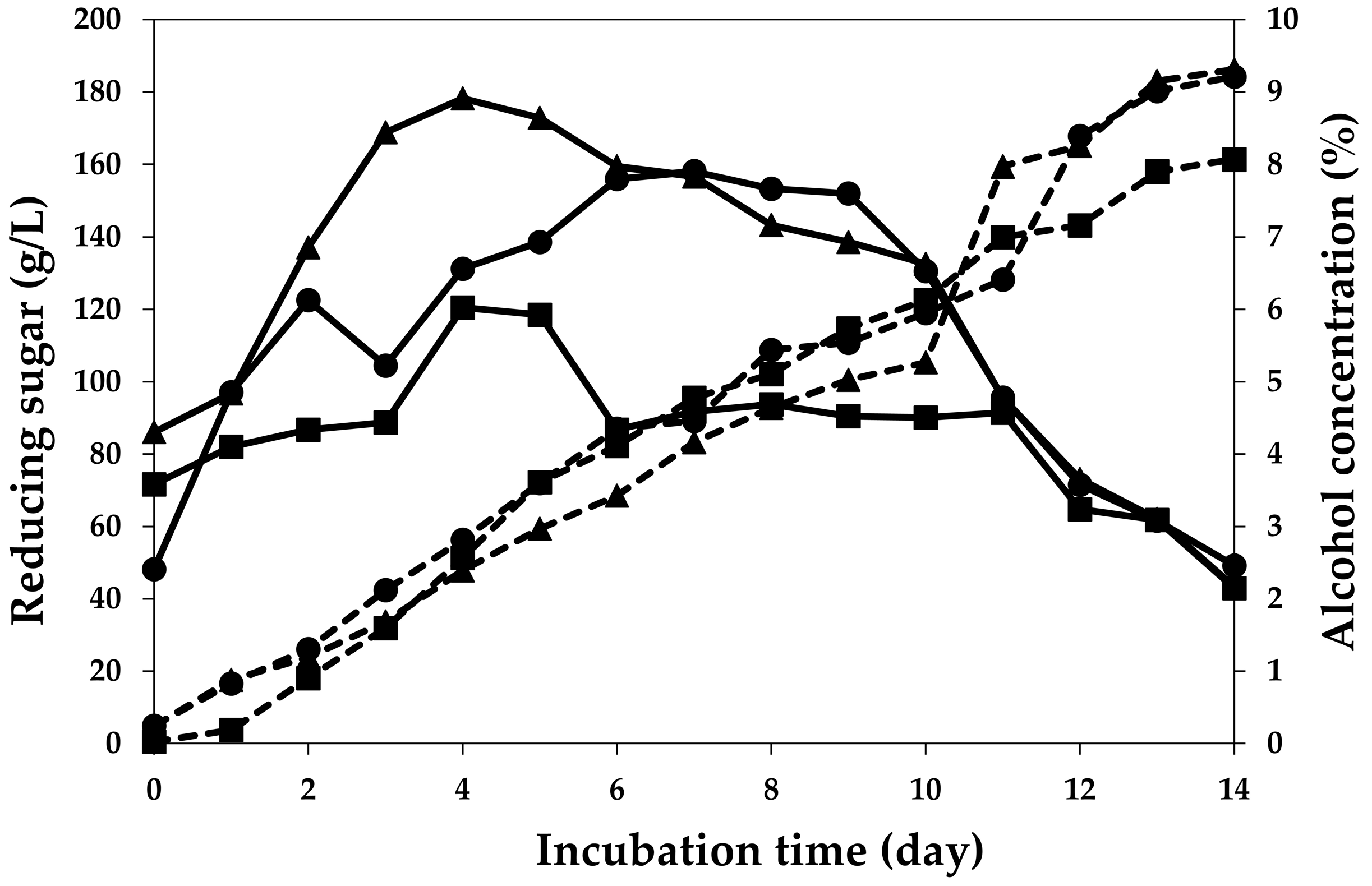
2.2. Fermentation Profiles of Plum Wine Using Different Yeasts
2.3. Total Phenolic Content and Antioxidant Activity
2.4. Wine Aroma Profiles by GC-MS
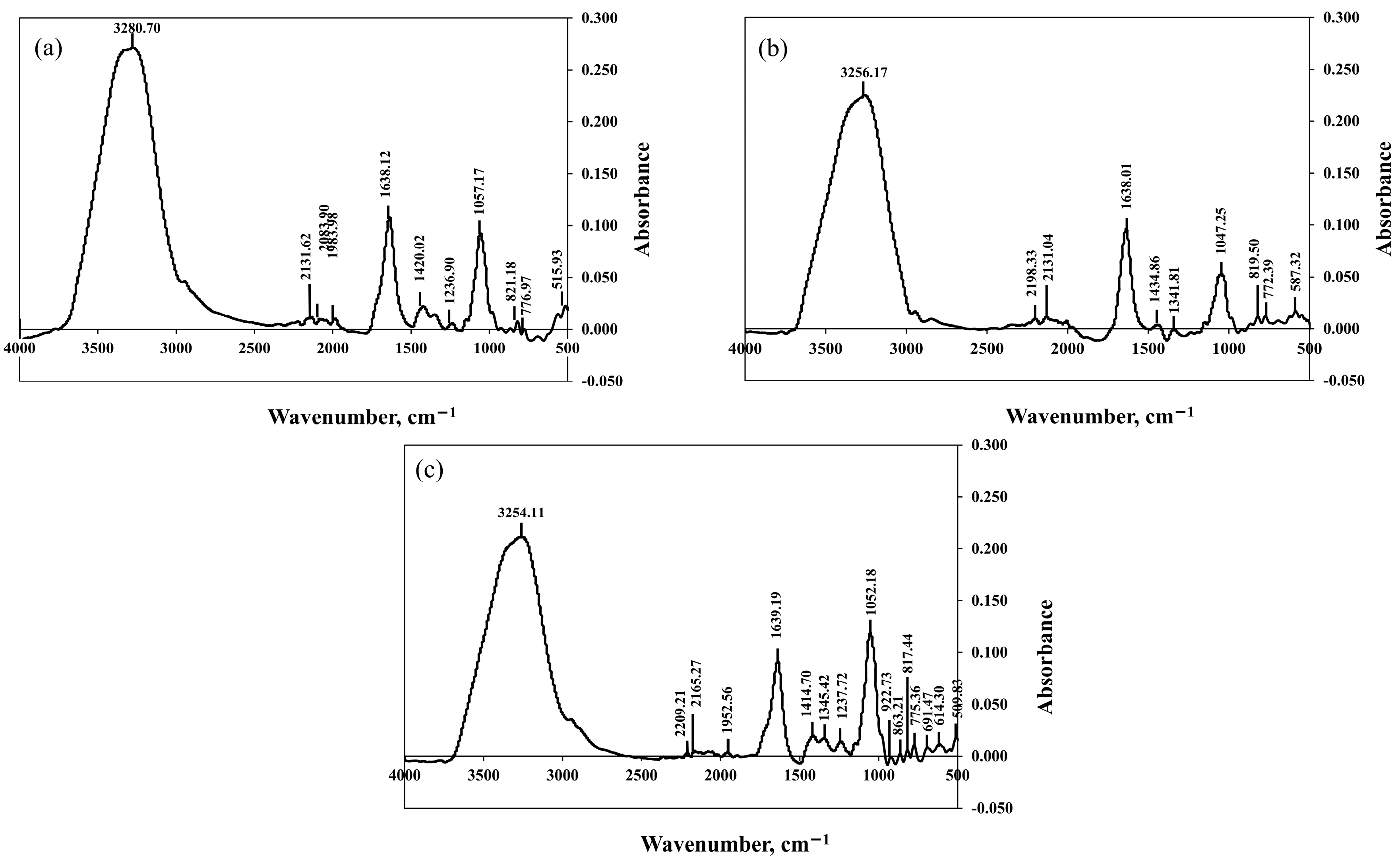
2.5. FTIR Analysis
3. Materials and Methods
3.1. Preparation of Staters
3.2. Plum Juice Preparation and Plum Wine Fermentation
3.3. Chemical Analysis
3.4. Total Phenolic (TP) Analysis
3.5. Antioxidant Activity Analysis
3.6. Fourier Transform Infrared (FTIR) Analysis
3.7. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, J.; Twito, T.; Zhang, Z.; Chao, C.T. Genetic Relationships among Fruiting-Mei (Prunus mume Sieb et Zucc.) Cultivars Evaluated with AFLP and SNP Markers. Genome 2006, 49, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Kono, R.; Okuno, Y.; Inada, K.; Tokuda, A.; Hashizume, H.; Yoshida, M.; Nakamura, M.; Utsunomuya, H. A Prunus mume extract stimulated the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biosci. Biotechnol. Biochem. 2011, 75, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Mitani, T.; Horinishi, A.; Kishida, K.; Kawabata, T.; Yano, F.; Mimura, H.; Inaba, N.; Yamanishi, H.; Oe, T.; Negoro, K.; et al. Phenolics profile of mume, Japanese apricot (Prunus mume Sieb. et Zucc.) fruit. Biosci. Biotechnol. Biochem. 2013, 77, 1623–1627. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Takekoshi, S.; Gato, N.; Utatsu, H.; Motley, E.D.; Eguchi, K.; Fitzgerald, T.G.; Mifune, M.; Frank, G.D.; Eguchi, S. Fruit-juice concentrate of Asian plum inhibits growth signals of vascular smooth muscle cells induced by angiotensin II. Life sciences. 2002, 72, 659–667. [Google Scholar] [CrossRef]
- Toolapong, P. Fruit Trees in the Cold Zone; Department of Horticulture, Faculty of Agricultural Production, Maejo University Press: Chiang Mai, Thailand, 1996; p. 177. [Google Scholar]
- Ministry of Agriculture and Cooperatives. Japanese Apricot. Available online: http://www.moac.go.th/builder/bhad/Apricot.php (accessed on 20 October 2022).
- Fine Wine Master. Plum Wine. Available online: https://finewinemaster.com/a-simple-guide-to-plum-wine/ (accessed on 20 October 2022).
- Jindamorakot, S.; Ninomiya, S.; Limtong, S.; Yongmanitchai, W.; Tuntirungkij, M.; Potacharoen, W.; Tanaka, K.; Kawasaki, H.; Nakase, T. Three new species of bipolar budding yeasts of the genus Hanseniaspora and its anamorph Kloeckera isolated in Thailand. FEMS Yeast Res. 2009, 9, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, P.; Mahendran, S.; Muthuselvi, T.; Chaiyasut, C.; Sivamaruthi, B. Preparation and validation of fermented fruit juices (wines) using Hanseniaspora thailandica MM7 as starter culture. Asian J. Microbiol. Biotechnol. Environ. Sci. 2019, 21, 269–275. [Google Scholar]
- Quast, E.; Vieira, I.; Nogueira, A.; Schmidt, F.L. Chemical and physical characterization of mume fruit collected from different locations and at different maturity stages in São Paulo State. Food Sci. Biotechnol. 2013, 33, 441–445. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.-S.; Liu, X.-M.; Cheng, J.-R.; Yang, C.-Y. Chemical composition and antioxidant activities of five samples of Prunus mume Umezu from different factories in south and east China. J. Food Qual. 2017, 2017, 4878926. [Google Scholar] [CrossRef]
- Amerine, M.A.; Berg, H.W.; Kunkee, R.E.; Ough, C.S.; Singleton, V.L.; Webb, A.D. The Technology of Wine Making, 4th ed.; AVI Publishing: Westport, CT, USA, 1980. [Google Scholar]
- Amerine, M.A.; Ough, C.S. Wine and Must Analysis; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- OIV. Criteria for the Methods of Quantification of Potentially Allergenic Residues of Fining Agent Proteins in Wine. Resolution OIV/OENO 427/2010. Available online: http://www.oiv.int/oiv/info/enmethodesinternationalesvin (accessed on 20 October 2022).
- Chanthai, S.; Danvirutai, P. Chemical analysis of locally produced wines. J. Univ. Acad. Serv. Cent. 2004, 12, 23–29. [Google Scholar]
- Chanprasartsuk, O.; Pheanudomkitlert, K.; Toonwai, D. Pineapple wine fermentation with yeasts isolated from fruit as single and mixed starter cultures. Asian J. Food Agro-Ind. 2012, 5, 104–111. [Google Scholar]
- Boonsupa, W.; Kerdchan, K. Development of fermented prunus vinegar: Chemical characterization and antioxidant activity. Curr. Appl. Sci. Technol. 2021, 21, 78–87. [Google Scholar] [CrossRef]
- Birch, R.M.; Walker, G.M. Influence of magnesium ions on heat shock and ethanol stress responses of Saccharomyces cerevisiae. Enzym. Microb. Technol. 2000, 26, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Towantakavanit, K.; Park, Y.S.; Gorinstein, S. Quality properties of wine from Korean kiwifruit new cultivars. Food Res. Int. 2011, 44, 1364–1372. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Cvejić Hogervorst, J.; Torović, L. Phenolic compounds, chromatic characteristics and antiradical activity of plum wines. Int. J. Food Prop. 2017, 20, 2022–2033. [Google Scholar] [CrossRef]
- Stratil, P.; Kuban, V.; Fojtova, J. Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech J. Food Sci. 2008, 26, 242–253. [Google Scholar] [CrossRef]
- Niyomvong, N.; Sritawan, R.; Keabpimai, J.; Trakunjae, C.; Boondaeng, A. Comparison of the chemical properties of vinegar obtained via one-step fermentation and sequential fermentation from dragon fruit and pineapple. Beverages 2022, 8, 74. [Google Scholar] [CrossRef]
- Boondaeng, A.; Kasemsumran, S.; Ngowsuwan, K.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Janchai, P.; Jungtheerapanich, S.; Niyomvong, N. Fermentation condition and quality evaluation of pineapple fruit wine. Fermentation 2021, 8, 11. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Zambonelli, C.; Romano, P.; Suzzi, G. Microorganisms of wine. In Biotechnology Applications in Beverage Productions; Cantarelli, C., Lanzarini, G., Eds.; Elsevier: London, UK, 1989; pp. 17–30. [Google Scholar]
- Lema, C.; Garcia-Jares, C.; Orriols, I.; Angulo, L. Contribution of Saccharomyces and non-Saccharomyces populations to the production of some components of Albarino wine aroma. Am. J. Enol. Vitic. 1996, 47, 206–216. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jime´nez, M.; Huerta, T.; Pastor, A. Contribution of different yeasts isolated from musts of monastrell grapes to the aroma of wine. Int. J. Food Microbiol. 1991, 14, 153–160. [Google Scholar] [CrossRef]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The oenological potential of Hanseniaspora uvarum in simultaneous and sequential co-fermentation with Saccharomyces cerevisiae for industrial wine production. Front. Microbiol. 2016, 670. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behavior and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Erten, H. Relations between elevated temperatures and fermentation behaviour of Kloeckera apiculata and Saccharomyces cerevisiae associated with winemaking in mixed cultures. World J. Microbiol. Biotechnol. 2002, 18, 373–378. [Google Scholar] [CrossRef]
- Gil, M.; Cabellos, J.M.; Arroyo, T.; Prodanov, M. Characterization of the volatile fraction of young wines from the denomination of origin “Vinos de Madrid” (Spain). Anal. Chim. Acta. 2006, 563, 145–153. [Google Scholar] [CrossRef]
- Zironi, R.; Romano, P.; Suzzi, G.; Battistuta, F.; Comi, G. Volatile metabolites produced in wine by mixed and sequential cultures of Hanseniaspora guilliermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol. Lett. 1993, 15, 235–238. [Google Scholar] [CrossRef]
- Abdul, S.; Boli, G.; Xiaowen, Z.; Hussain, I.; Yimin, W. Recent Development in the Application of Analytical Techniques for the Traceability and Authenticity of Food of Plant Origin. Microchem. J. 2020, 152, 104295. [Google Scholar] [CrossRef]
- Cavdaroglu, C.; Ozen, B. Detection of Vinegar Adulteration with Spirit vinegar and acetic acid using uv–visible and fourier transform infrared spectroscopy. Food Chem. 2022, 379, 132150. [Google Scholar] [CrossRef]
- Rios-Reina, R.; Elcoroaristizaba, S.; Ocana-Gonzalez, J.A.; Garcia-Gonzalez, D.L.; Amigo, J.M.; Callejon, R.M. Characterization and authentication of Spanish PDO wine vinegars using multidimensional fluorescence and chemometrics. Food Chem. 2017, 23, 108–111. [Google Scholar] [CrossRef]
- Gulcin, I.; Kufrevioglu, O.I.; Oktay, M.; Buyukokuroglu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Soare, J.R.; Dinis, T.C.; Cunha, A.P.; Almeida, L. Antioxidant activities of some extracts of Thymus zygis. Free Radic. Res. 1997, 26, 469–478. [Google Scholar] [CrossRef]
- IFU. Determination of Titratable Acidity. IFU Analysis No. 3. 2007. Available online: https://www.ifu-fruitjuice.com/ (accessed on 20 October 2022).
- IFU. Determination of Volatile Acids. IFU Analysis No. 5. 2005. Available online: https://www.ifu-fruitjuice.com/ (accessed on 20 October 2022).
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Boondaeng, A.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Kongtud, W. Statistical approach for optimization of ethanol production from fast-growing trees: Acacia mangium and Acacia hybrid. Bioresour. Technol. 2015, 10, 3154–3168. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Vidal-Gutiérrez, M.; Robles-Zepeda, R.E.; Vilegas, W.; Gonzalez-Aguilar, G.A.; Torres-Moreno, H.; López-Romero, J.C. Phenolic composition and antioxidant activity of Bursera microphylla A. Gray. Ind. Crop. Prod. 2020, 152, 112412. [Google Scholar] [CrossRef]
- Augustine, S.K.; Bhavsar, S.P.; Kapadnis, B.P. A non-polyene antifungal antibiotic from Streptomyces albidoflavus PU 23. J. Biosci. 2005, 30, 201–211. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]

| Yeast Species | TP (mg GAE/L) | DPPH (µg TE/g) | ||
|---|---|---|---|---|
| Day 0 | Day 14 | Day 0 | Day 14 | |
| S. cerevisiae var burgundy | 361.04 ± 75.32 | 469.84 a ± 6.95 | 272.50 ± 41.63 | 304.36 a ± 6.24 |
| H. thailandica Zal1 | 331.48 c ± 16.24 | 241.65 c ± 0.94 | ||
| EC1118 | 418.27 b ± 3.40 | 288.2 b ± 7.9 | ||
| Assignment Compounds | Retention Time | % Area | % Similarity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | H | EC | B | H | EC | B | H | EC | |
| Ethanol | 1.287 ± 0.005 | 1.292 ± 0.002 | 1.292 ± 0.005 | 82.18 ± 2.19 | 71.97 ± 0.644 | 85.52 ± 0.300 | 95.7 ± 1.15 | 98.0 ± 0.0 | 95.0 ± 0.0 |
| Acetic acid | 1.407 ± 0.020 | 1.545 ± 0.000 | - | 0.47 ± 0.11 | 0.24 ± 0.000 | - | 90.0 ± 0.0 | 96.0 ± 0.0 | - |
| Acetic acid ethyl ester | 1.539 ± 0.002 | 1.545 ± 0.001 | 1.545 ± 0.000 | 8.51 ± 1.41 | 16.23 ± 0.008 | 7.37 ± 0.312 | 96.0 ± 0.0 | 96.0 ± 0.0 | 96.0 ± 0.0 |
| Isobutylalcohol | 1.585 ± 0.000 | - | 1.59 ± 0.000 | 0.35 ± 0.00 | - | 0.54 ± 0.087 | 96.0 ± 0.0 | - | 96.0 ± 0.0 |
| Isopentyl alcohol | 2.023 ± 0.001 | 2.029 ± 0.001 | 2.029 ± 0.000 | 8.81 ± 0.70 | 11.05 ± 0.036 | 6.58 ± 0.061 | 96.0 ± 0.0 | 96.0 ± 0.0 | 96.0 ± 0.0 |
| Propanoic acid, 2-methyl-, ethyl ester | - | 2.185 ± 0.000 | - | - | 0.37 ± 0.000 | - | - | 84.0 ± 0.0 | - |
| 2,3-Butanediol | 2.334 ± 0.000 | - | - | 0.21 ± 0.00 | - | - | 96.0 ± 0.0 | - | - |
| 1-Butanol, 3-methyl-, acetate | - | 3.535 ± 0.003 | - | - | 0.55 ± 0.015 | - | - | 97.0 ± 0.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niyomvong, N.; Trakunjae, C.; Boondaeng, A. Fermentation Characteristics and Aromatic Profiles of Plum Wines Produced with Hanseniaspora thailandica Zal1 and Common Wine Yeasts. Molecules 2023, 28, 3009. https://doi.org/10.3390/molecules28073009
Niyomvong N, Trakunjae C, Boondaeng A. Fermentation Characteristics and Aromatic Profiles of Plum Wines Produced with Hanseniaspora thailandica Zal1 and Common Wine Yeasts. Molecules. 2023; 28(7):3009. https://doi.org/10.3390/molecules28073009
Chicago/Turabian StyleNiyomvong, Nanthavut, Chanaporn Trakunjae, and Antika Boondaeng. 2023. "Fermentation Characteristics and Aromatic Profiles of Plum Wines Produced with Hanseniaspora thailandica Zal1 and Common Wine Yeasts" Molecules 28, no. 7: 3009. https://doi.org/10.3390/molecules28073009
APA StyleNiyomvong, N., Trakunjae, C., & Boondaeng, A. (2023). Fermentation Characteristics and Aromatic Profiles of Plum Wines Produced with Hanseniaspora thailandica Zal1 and Common Wine Yeasts. Molecules, 28(7), 3009. https://doi.org/10.3390/molecules28073009