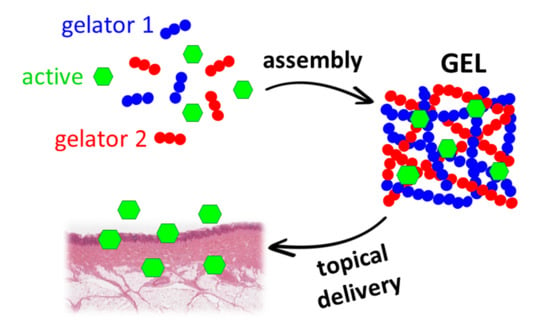
Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels
Abstract
1. Introduction
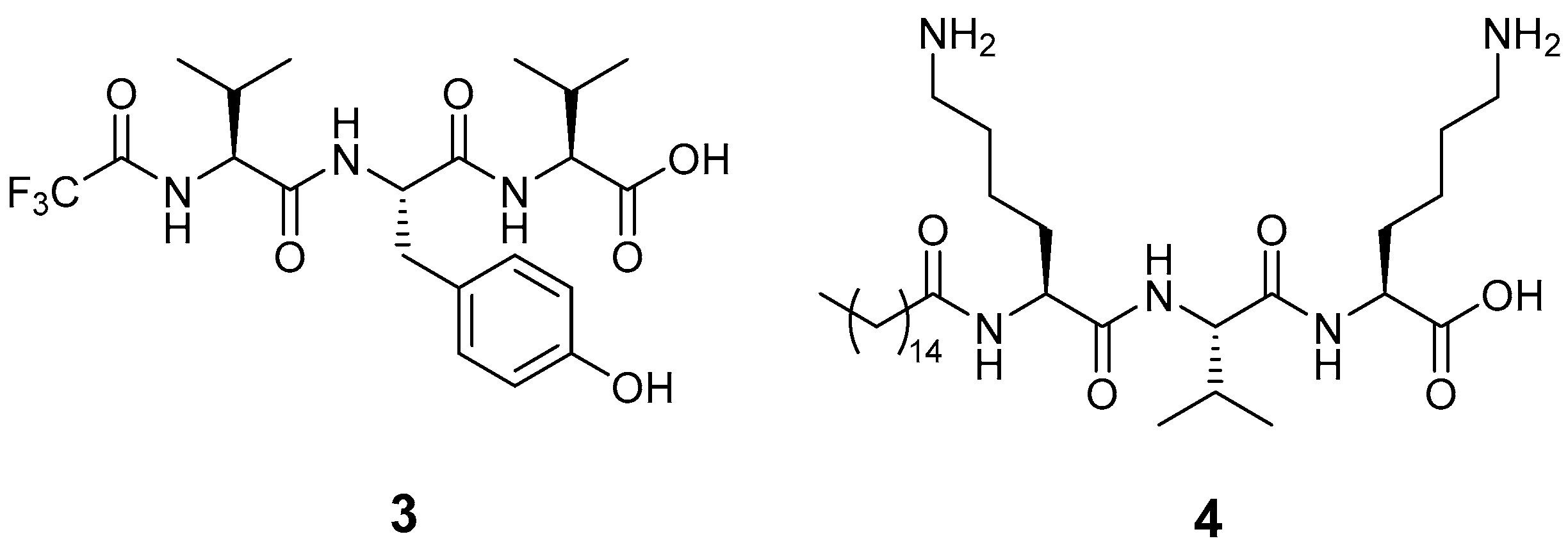
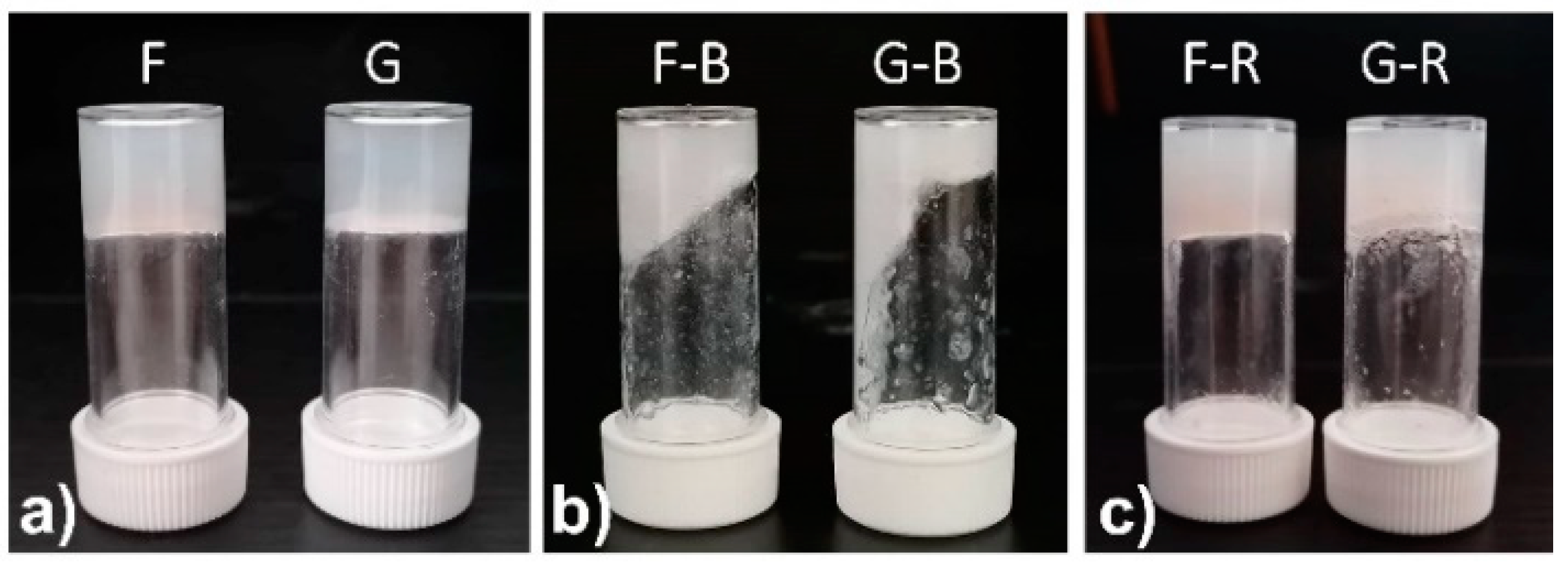
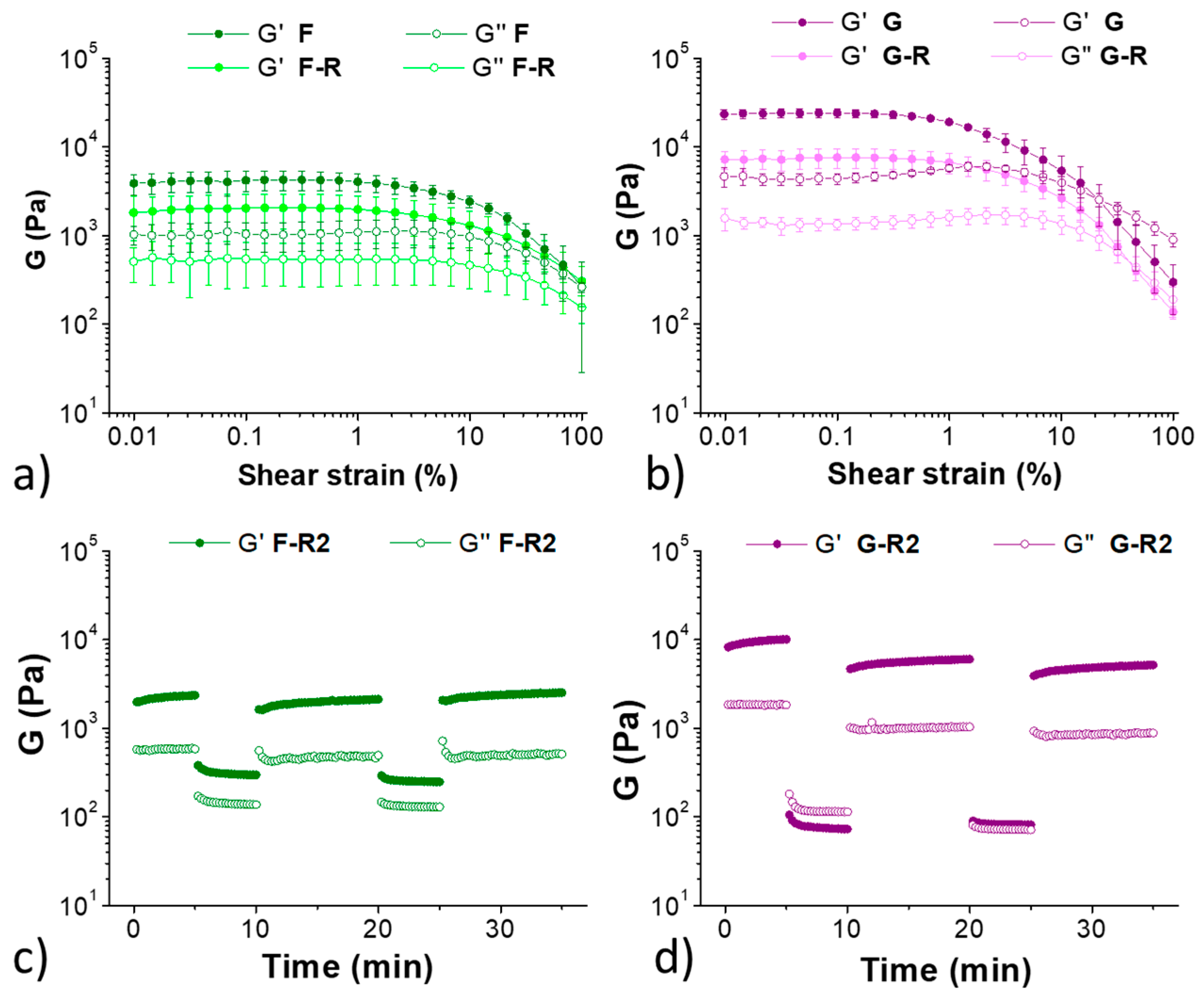
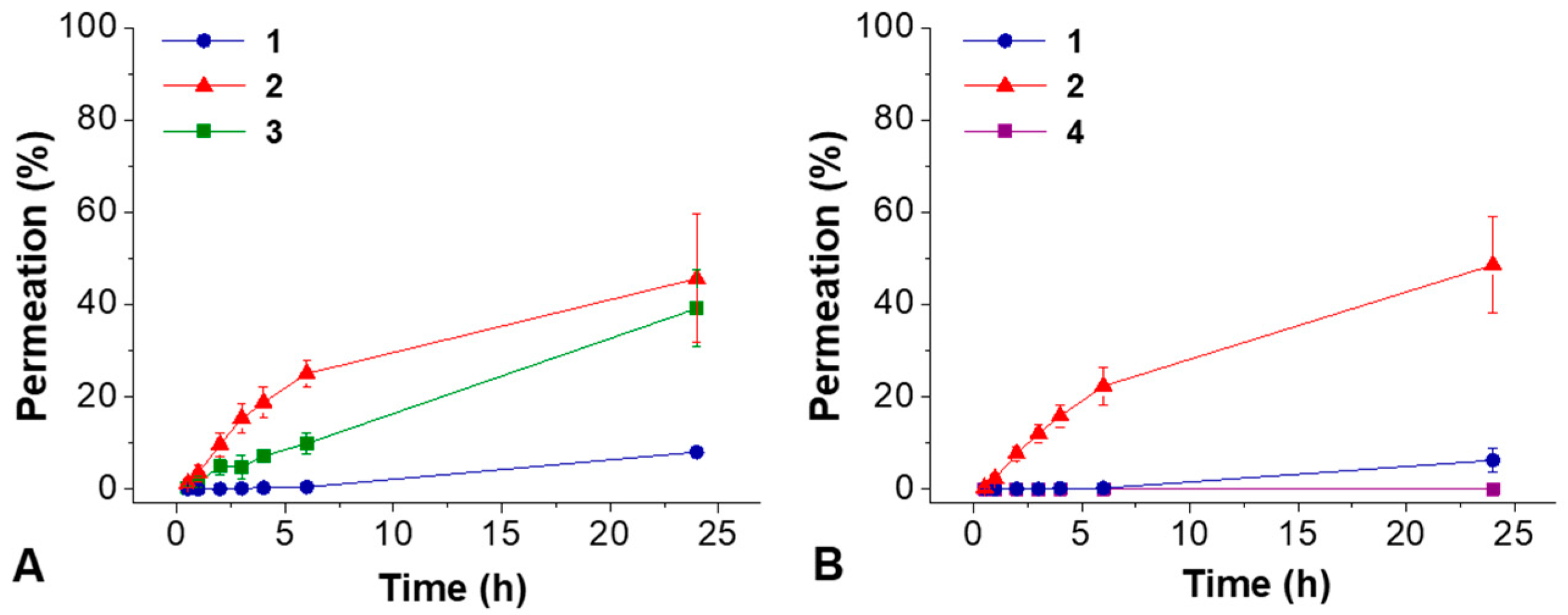
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef] [PubMed]
- Mayr, J.; Saldías, C.; Díaz Díaz, D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Tomasini, C.; Castellucci, N. Peptides and peptidomimetics that behave as low molecular weight gelators. Chem. Soc. Rev. 2013, 42, 156–172. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390. [Google Scholar] [CrossRef]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A new method for maintaining homogeneity during liquid–hydrogel transitions using low molecular weight hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- Xian, S.; Webber, M.J. Temperature-responsive supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef]
- Chen, L.; Pont, G.; Morris, K.; Lotze, G.; Squires, A.; Serpell, L.C.; Adams, D.J. Salt-induced hydrogelation of functionalised-dipeptides at high pH. Chem. Commun. 2011, 47, 12071–12073. [Google Scholar] [CrossRef]
- Castellucci, N.; Falini, G.; Angelici, G.; Tomasini, C. Formation of gels in the presence of metal ions. Amino Acids 2011, 41, 609–620. [Google Scholar] [CrossRef]
- Pramanik, B.; Ahmed, S. Peptide-Based Low Molecular Weight Photosensitive Supramolecular Gelators. Gels 2022, 8, 533. [Google Scholar] [CrossRef]
- Bardelang, D.; Camerel, F.; Margeson, J.C.; Leek, D.M.; Schmutz, M.; Zaman, M.B.; Yu, K.; Soldatov, D.V.; Ziessel, R.; Ratcliffe, C.I.; et al. Unusual sculpting of dipeptide particles by ultrasound induces gelation. J. Am. Chem. Soc. 2008, 130, 3313–3315. [Google Scholar] [CrossRef]
- Poustchi, F.; Amani, H.; Ahmadian, Z.; Niknezhad, S.V.; Mehrabi, S.; Santos, H.A.; Shahbazi, M.A. Combination Therapy of Killing Diseases by Injectable Hydrogels: From Concept to Medical Applications. Adv. Healthc. Mater. 2021, 10, e2001571. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Valls, A.; Isabel Burguete, M.; Kuret, L.; Altava, B.; Luis, S.V. Open chain pseudopeptides as hydrogelators with reversible and dynamic responsiveness to pH, temperature and sonication as vehicles for controlled drug delivery. J. Mol. Liq. 2022, 348, 118051. [Google Scholar] [CrossRef]
- Giuri, D.; Marshall, L.J.; Dietrich, B.; McDowall, D.; Thomson, L.; Newton, J.Y.; Wilson, C.; Schweins, R.; Adams, D.J. Exploiting and controlling gel-to-crystal transitions in multicomponent supramolecular gels. Chem. Sci. 2021, 12, 9720–9725. [Google Scholar] [CrossRef]
- Okesola, B.O.; Mata, A. Multicomponent self-assembly as a tool to harness new properties from peptides and proteins in material design. Chem. Soc. Rev. 2018, 47, 3721–3736. [Google Scholar] [CrossRef]
- Genio, F.A.F.; Paderes, M.C. Functional Supramolecular Gels Comprised of Bis-Urea Compounds and Cosmetic Solvents. ChemistrySelect 2021, 6, 7906–7911. [Google Scholar] [CrossRef]
- Uzan, S.; Barış, D.; Çolak, M.; Aydın, H.; Hoşgören, H. Organogels as novel carriers for dermal and topical drug delivery vehicles. Tetrahedron 2016, 72, 7517–7525. [Google Scholar] [CrossRef]
- Palomo, M.C.; Prieto, V.M.; Kirilov, P. Colloidal dispersions of gelled lipid nanoparticles (Gln): Concept and potential applications. Gels 2017, 3, 33. [Google Scholar] [CrossRef]
- Nicastro, G.; Black, L.M.; Ravarino, P.; D’Agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, A.; Coelho, S.G.; Hearing, V.J. Effects of cosmetic formulations containing hydroxyacids on sun-exposed skin: Current applications and future developments. Dermatol. Res. Pract. 2012, 2012, 710893. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Underhill, C.B.; Lakkakorpi, J.; Ditre, C.M.; Uitto, J.; Yu, R.J.; Van Scott, E. Citric acid increases viable epidermal thickness and glycosaminoglycan content of sun-damaged skin. Dermatol. Surg. 1997, 23, 689–694. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Bartosova, L.; Bajgar, J. Transdermal Drug Delivery In Vitro Using Diffusion Cells. Curr. Med. Chem. 2012, 19, 4671–4677. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular Hydrogels with Properties Tunable by Calcium Ions: A Bio-Inspired Chemical System. ACS Appl. Bio Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef]
- Di Filippo, M.F.; Giuri, D.; Marchiori, G.; Maglio, M.; Pagani, S.; Fini, M.; Tomasini, C.; Panzavolta, S. Self-assembling of fibers inside an injectable calcium phosphate bone cement: A feasibility study. Mater. Today Chem. 2022, 24, 100991. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech 2007, 8, 91–98. [Google Scholar] [CrossRef]
- Giuri, D.; D’Agostino, S.; Ravarino, P.; Faccio, D.; Falini, G.; Tomasini, C. Water Remediation from Pollutant Agents by the Use of an Environmentally Friendly Supramolecular Hydrogel. ChemNanoMat 2022, 8, e202200093. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in skincare-the fascinating world of skin-brain connection: A review to explore ingredients, commercial products for skin aging, and cosmetic regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical peptide treatments with effective anti-aging results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Yuan, L.; Alexander, P.B.; Wang, X.-F. Cellular senescence: From anti-cancer weapon to anti-aging target. Sci. China. Life Sci. 2020, 63, 332–342. [Google Scholar] [CrossRef]
- Han, F.; Luo, D.; Qu, W.; Chen, D.; Hong, Y.; Sheng, J.; Yang, X.; Liu, W. Nanoliposomes codelivering bioactive peptides produce enhanced anti-aging effect in human skin. J. Drug Deliv. Sci. Technol. 2020, 57, 101693. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef]
- Yan, C.; Altunbas, A.; Yucel, T.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable solid hydrogel: Mechanism of shear-thinning and immediate recovery of injectable β-hairpin peptide hydrogels. Soft Matter 2010, 6, 5143–5156. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef]
- Limpongsa, E.; Umprayn, K. Preparation and evaluation of diltiazem hydrochloride diffusion-controlled transdermal delivery system. AAPS PharmSciTech 2008, 9, 464–470. [Google Scholar] [CrossRef]
- Clément, P.; Laugel, C.; Marty, J.P. Influence of three synthetic membranes on the release of caffeine from concentrated W/O emulsions. J. Control. Release 2000, 66, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, H. A review of cosmetic skin delivery. J. Cosmet. Dermatol. 2021, 20, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Health and Consumers. Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients; European Union: Brussels, Belgium, 2010; Volume SCCS/1358. [Google Scholar] [CrossRef]
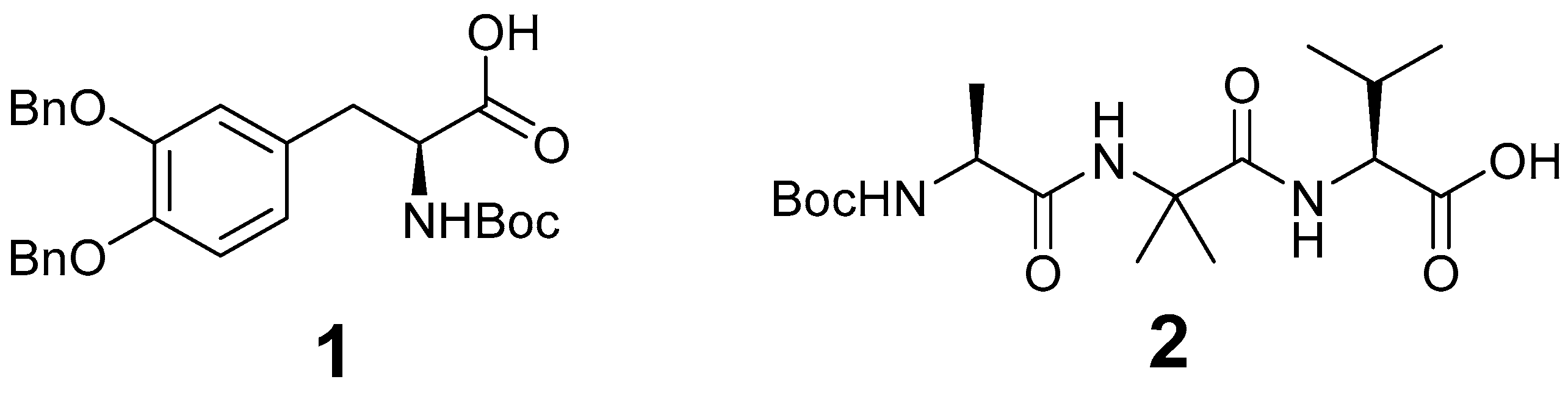
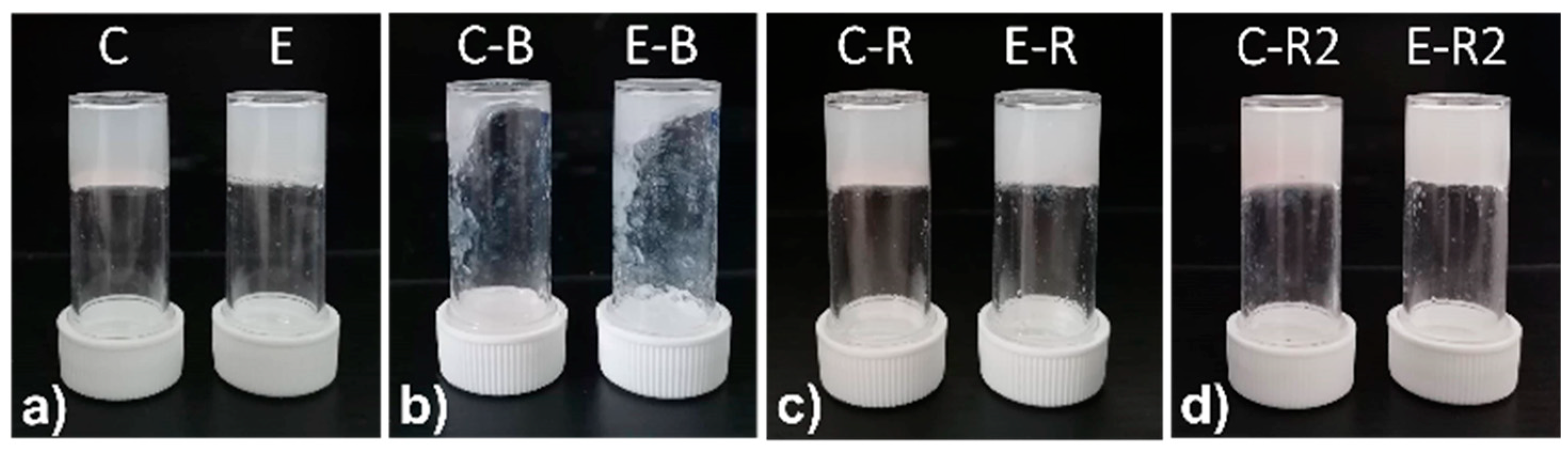


| Gel | 1 | 2 | Citric Acid | T (°C) | Result | pH |
|---|---|---|---|---|---|---|
| A | 0.65% | 0.35% | - | 23 | Gel | 7.60 |
| B | 0.65% | 0.50% | - | 23 | Gel | 7.30 |
| C | 0.35% | 0.65% | - | 23 | Gel | 6.80 |
| D | 0.35% | 0.65% | 0.38% | 23 | Sol | 5.84 |
| E | 0.35% | 0.65% | 0.38% | 60 | Gel | 5.55 |
| Gel | 1 | 2 | Active Peptide | T (°C) | Result | pH |
|---|---|---|---|---|---|---|
| F | 0.35% | 0.65% | 3 (0.1%) | 40 | Gel | 6.70 |
| G | 0.35% | 0.65% | 4 (0.1%) | 40 | Gel | 6.72 |
| Gel | 1 (mg) | 1 M NaOH (µL) | H2O (mL) | 2 (mg) | PB 0.1 M (mL) | Tot V (mL) |
|---|---|---|---|---|---|---|
| A | 13 | 27 | 1.303 | 7 | 0.670 | 2 |
| B | 13 | 27 | 1.303 | 10 | 0.670 | 2 |
| C | 7 | 15 | 1.315 | 13 | 0.670 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shariati Pour, S.R.; Oddis, S.; Barbalinardo, M.; Ravarino, P.; Cavallini, M.; Fiori, J.; Giuri, D.; Tomasini, C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules 2023, 28, 2528. https://doi.org/10.3390/molecules28062528
Shariati Pour SR, Oddis S, Barbalinardo M, Ravarino P, Cavallini M, Fiori J, Giuri D, Tomasini C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules. 2023; 28(6):2528. https://doi.org/10.3390/molecules28062528
Chicago/Turabian StyleShariati Pour, Seyedeh Rojin, Sara Oddis, Marianna Barbalinardo, Paolo Ravarino, Massimiliano Cavallini, Jessica Fiori, Demetra Giuri, and Claudia Tomasini. 2023. "Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels" Molecules 28, no. 6: 2528. https://doi.org/10.3390/molecules28062528
APA StyleShariati Pour, S. R., Oddis, S., Barbalinardo, M., Ravarino, P., Cavallini, M., Fiori, J., Giuri, D., & Tomasini, C. (2023). Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules, 28(6), 2528. https://doi.org/10.3390/molecules28062528