Pickering Emulsions Based in Inorganic Solid Particles: From Product Development to Food Applications
Abstract
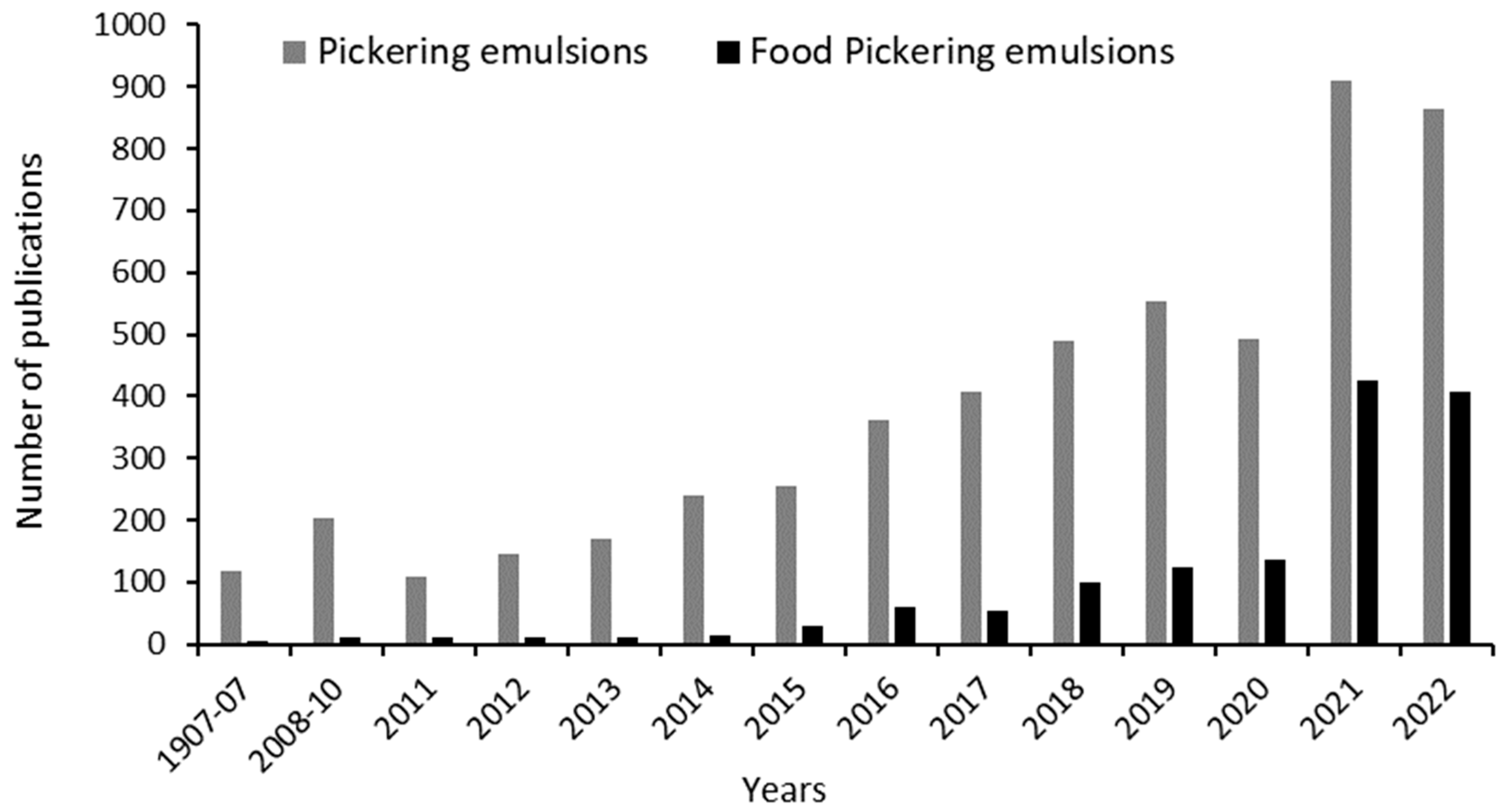
1. Introduction to Pickering Emulsions
2. Mechanisms and Parameters Influencing Pickering Emulsion Stability
2.1. Pickering Emulsion Formation Mechanism
2.2. Pickering Emulsion Parameters—Particle Properties
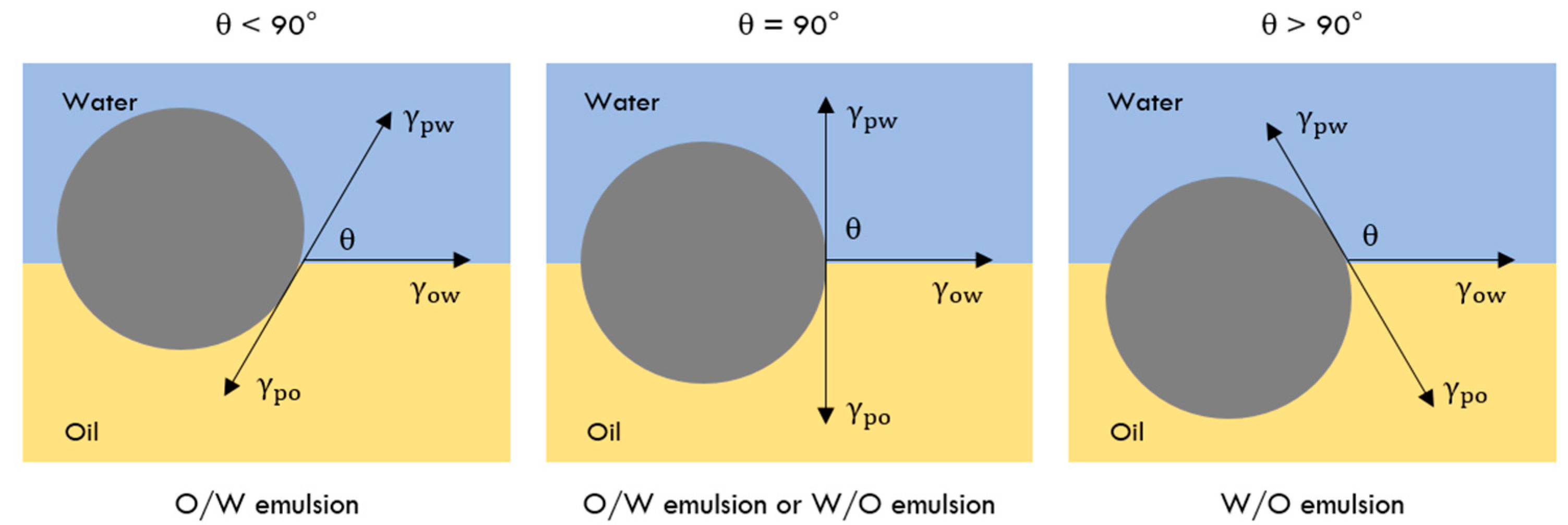
2.2.1. Particle Wettability
2.2.2. Solid Particle Concentration
2.2.3. Particle Size
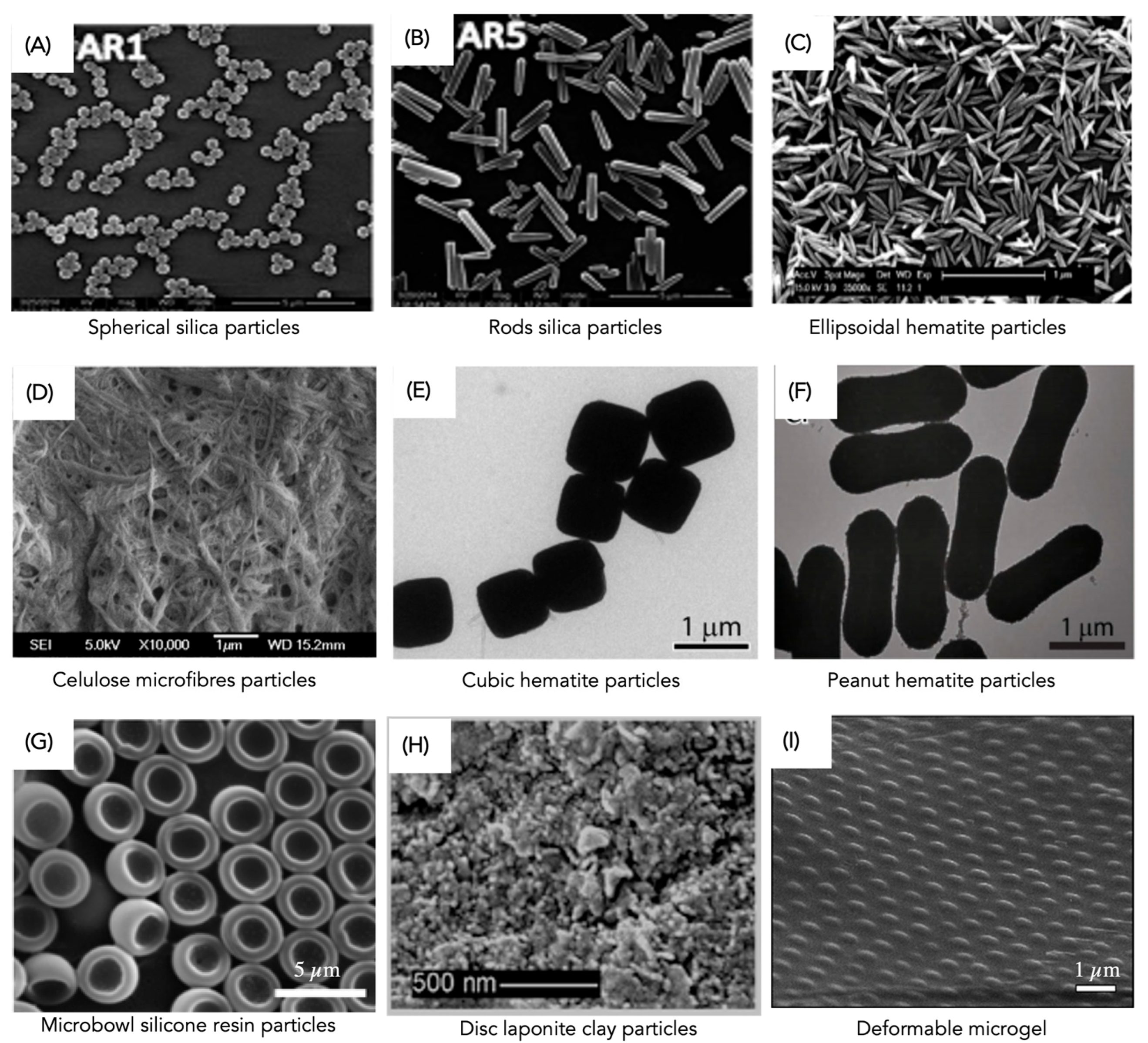
2.2.4. Particle Shape
2.3. Pickering Emulsion Parameters—Aqueous Phase Properties
2.4. Pickering Emulsion Parameters—Oil Phase Properties
3. Inorganic Solid Particles as Pickering Stabilisers
3.1. Types of Inorganic Solid Particles
3.2. Hydroxyapatite as Pickering Stabiliser
4. Preparation of Pickering Emulsions—Production Processes
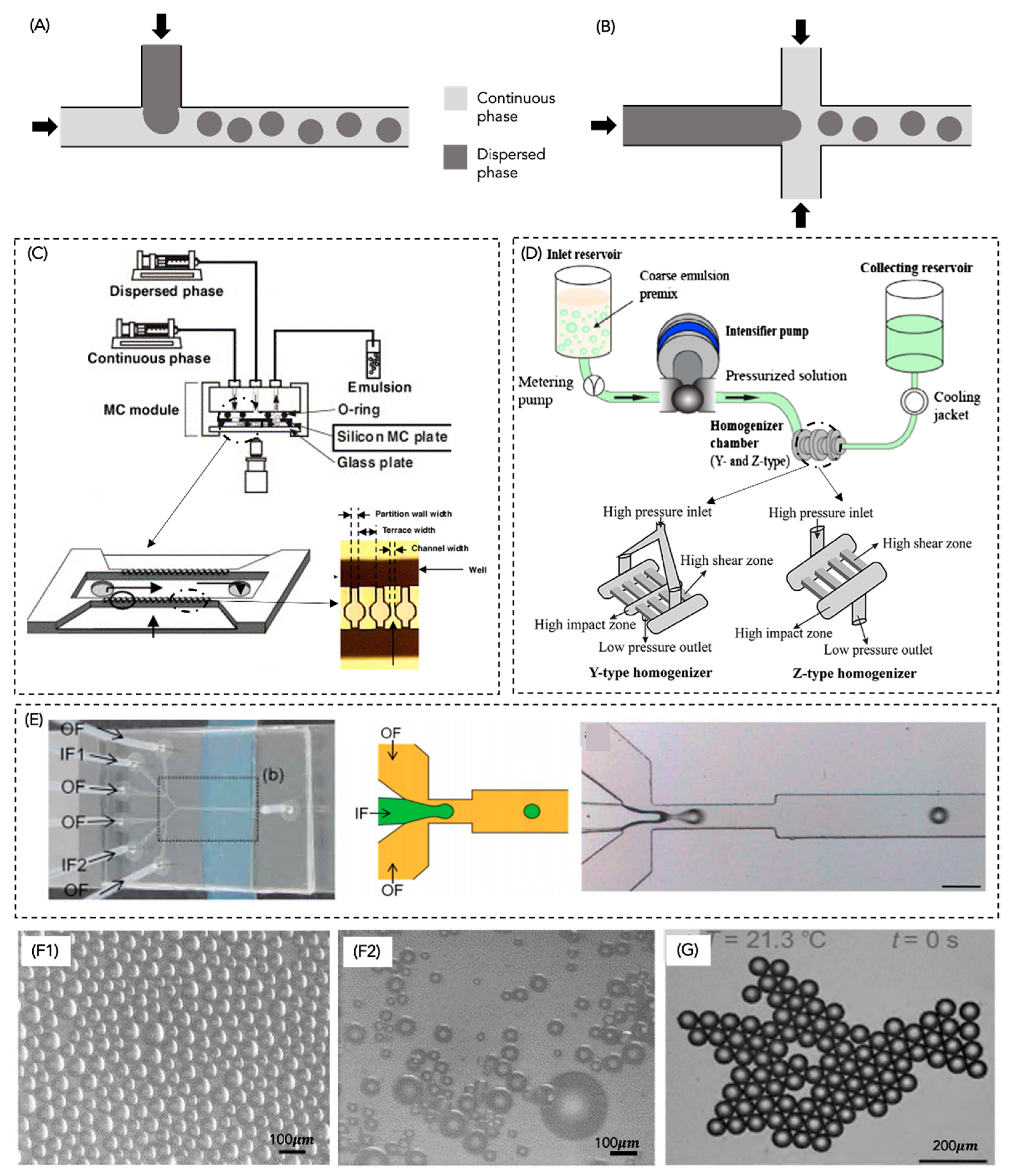
4.1. High-Shear Mixers
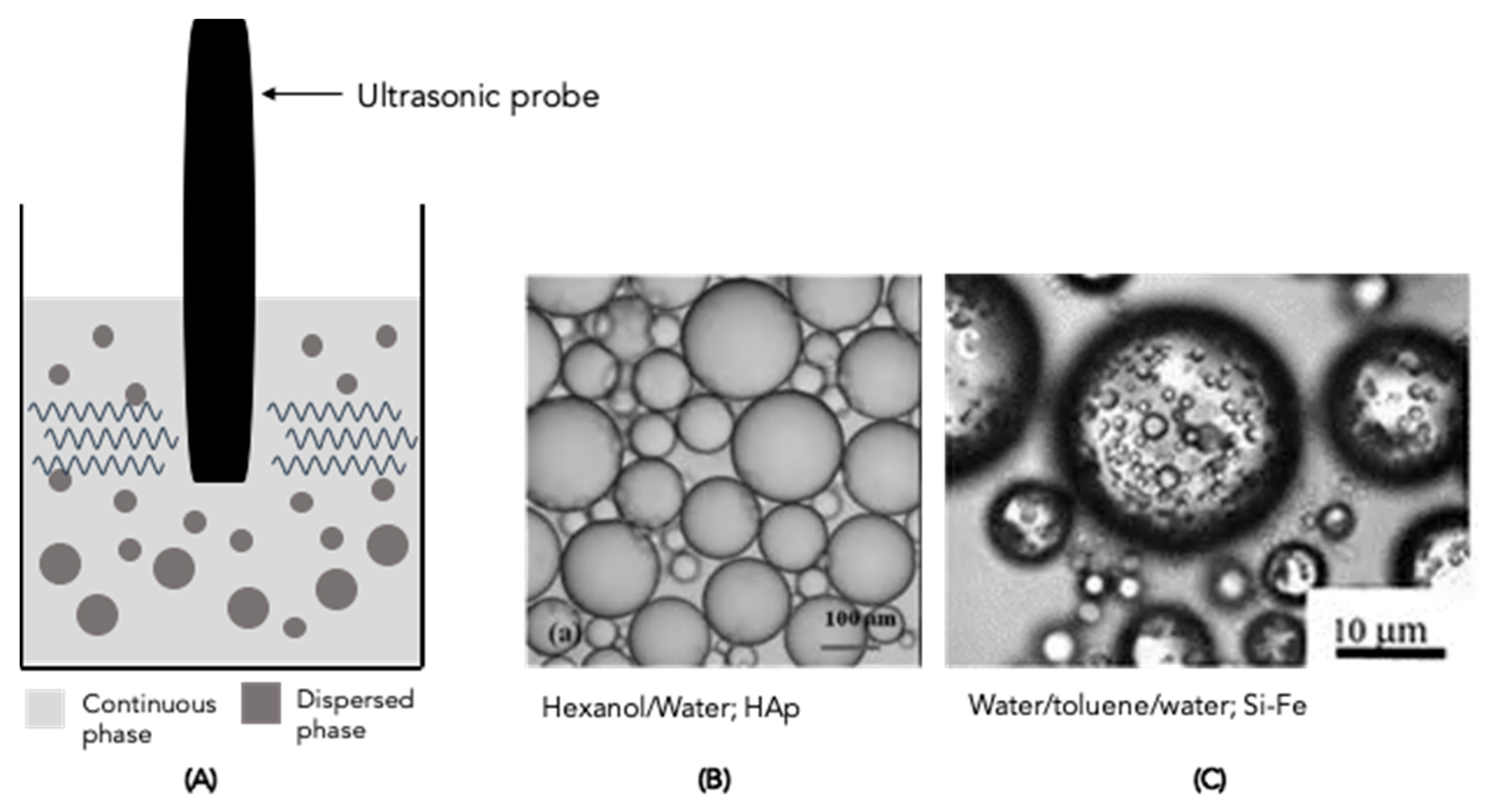
4.2. Ultrasonic Homogeniser
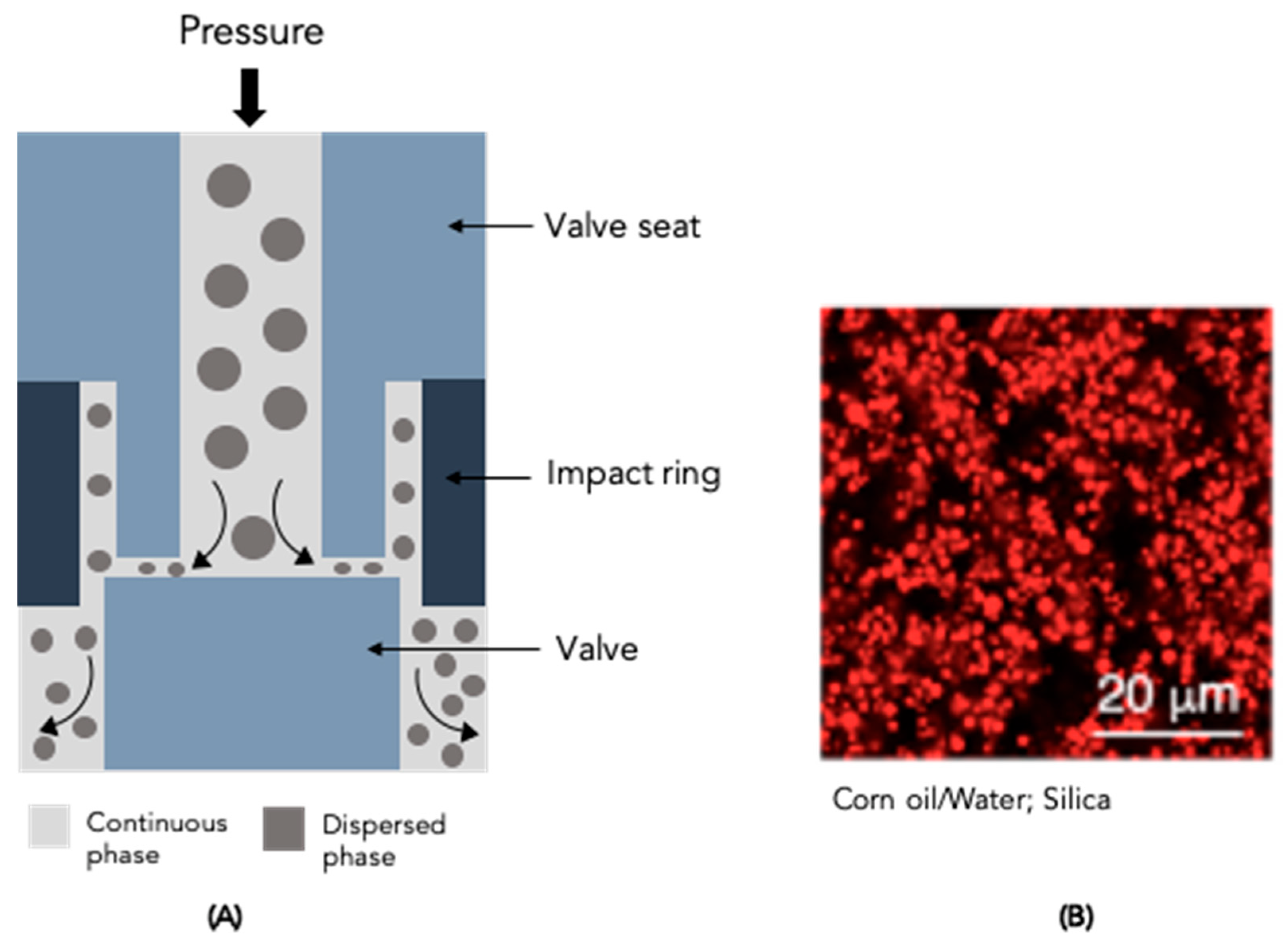
4.3. High-Pressure Homogeniser
4.4. Microfluidizers
4.5. Membrane Homogeniser
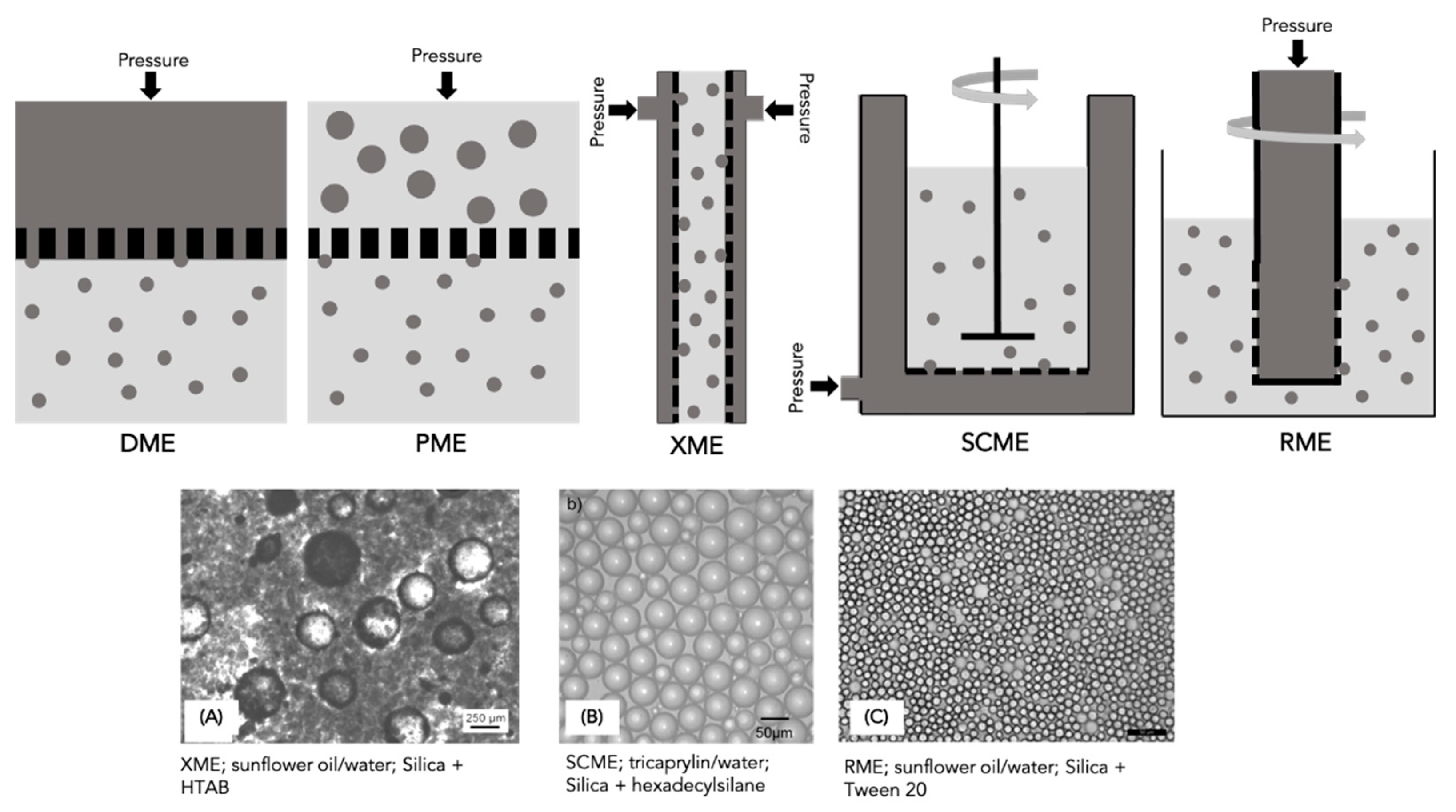
4.6. Static Mixers
5. Pickering Emulsions for Food Applications
5.1. Emulsifier Substitution in Food
5.2. Fat Reduction or Substitution
5.3. Encapsulation of Active Compounds and Development of Functional Foods
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: London, UK, 2016. [Google Scholar]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef] [PubMed]
- Son, H.A.; Yoon, K.Y.; Lee, G.J.; Cho, J.W.; Choi, S.K.; Kim, J.W.; Im, K.C.; Kim, H.T.; Lee, K.S.; Sung, W.M. The potential applications in oil recovery with silica nanoparticle and polyvinyl alcohol stabilized emulsion. J. Petrol. Sci. Eng. 2015, 126, 152–161. [Google Scholar] [CrossRef]
- Niakousari, M.; Damyeh, M.S.; Gahruie, H.H.; Bekhit, A.E.D.A.; Greiner, R.; Roohinejad, S. Conventional emulsions. In Emulsion-Based Systems for Delivery of Food Active Compounds, 1st ed.; Roohinejad, S., Greiner, R., Oey, I., Wen, J., Eds.; Wiley: Oxford, UK, 2018; pp. 1–28. [Google Scholar]
- Koubaa, M.; Roohinejad, S.; Sharma, P.; Nikmaran, N.; Hashemi, S.S.; Abbaspourrad, A.; Greiner, R. Multiple Emulsions. In Emulsion-Based Systems for Delivery of Food Active Compounds, 1st ed.; Roohinejad, S., Greiner, R., Oey, I., Wen, J., Eds.; Wiley: Oxford, UK, 2018; pp. 69–104. [Google Scholar]
- Wu, J.; Ma, G.-H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. T. 2015, 6, 263–297. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation)—Preliminary Account. Proc. R. Soc. Lond. 1903, 72, 156–164. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.-Emulsions. J. Chem. Soc. A 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Di Sotto, A.; Maffei, F.; Hrelia, P.; Di Giacomo, S.; Pagano, E.; Borrelli, F.; Mazzanti, G. Genotoxicity assessment of some cosmetic and food additives. Regul. Toxicol. Pharm. 2014, 68, 16–22. [Google Scholar] [CrossRef]
- Marefati, A.; Bertrand, M.; Sjöö, M.; Dejmek, P.; Rayner, M. Storage and digestion stability of encapsulated curcumin in emulsions based on starch granule Pickering stabilization. Food Hydrocoll. 2017, 63, 309–320. [Google Scholar] [CrossRef]
- Yuan, D.B.; Hu, Y.Q.; Zeng, T.; Yin, S.W.; Tang, C.H.; Yang, X.Q. Development of stable Pickering emulsions/oil powders and Pickering HIPEs stabilized by gliadin/chitosan complex particles. Food Funct. 2017, 8, 2220–2230. [Google Scholar] [CrossRef]
- Dokić, L.; Krstonošić, V.; Nikolić, I. Physicochemical characteristics and stability of oil-in-water emulsions stabilized by OSA starch. Food Hydrocoll. 2012, 29, 185–192. [Google Scholar] [CrossRef]
- Kargar, M.; Fayazmanesh, K.; Alavi, M.; Spyropoulos, F.; Norton, I.T. Investigation into the potential ability of Pickering emulsions (food-grade particles) to enhance the oxidative stability of oil-in-water emulsions. J. Colloid Interface Sci. 2012, 366, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Osborn, H.T.; Akoh, C.C. Effect of emulsifier type, droplet size, and oil concentration on lipid oxidation in structured lipid-based oil-in-water emulsions. Food Chem. 2004, 84, 451–456. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloid. Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.-A.; Pelletier, J.; Valour, J.-P.; Chevalier, Y. Topical delivery of lipophilic drugs from o/w Pickering emulsions. Int. J. Pharm. 2009, 371, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hiebler, K.; Lichtenegger, G.J.; Maier, M.C.; Park, E.S.; Gonzales-Groom, R.; Binks, B.P.; Gruber-Woelfler, H. Heterogeneous Pd catalysts as emulsifiers in Pickering emulsions for integrated multistep synthesis in flow chemistry. Beilstein J. Org. Chem. 2018, 14, 648–658. [Google Scholar] [CrossRef]
- Reed, K.M. Wettability of Solid Particles in Relation to Particle-Stabilised Foams and Emulsions. Ph.D. Thesis, University of Hull, Hull, UK, 2011. [Google Scholar]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef]
- Schröder, A.; Sprakel, J.; Schroën, K.; Spaen, J.N.; Berton-Carabin, C.C. Coalescence stability of Pickering emulsions produced with lipid particles: A microfluidic study. J. Food Eng. 2018, 234, 63–72. [Google Scholar] [CrossRef]
- Schröder, A.; Corstens, M.N.; Ho, K.K.H.Y.; Schroën, K.; Berton-Carabin, C.C. Pickering emulsions. In Emulsion-Based Systems for Delivery of Food Active Compounds, 1st ed.; Roohinejad, S., Greiner, R., Oey, I., Wen, J., Eds.; Wiley: Oxford, UK, 2018; pp. 29–68. [Google Scholar]
- Ribeiro, A.; Manrique, Y.A.; Ferreira, I.C.F.R.; Barreiro, F.; Lopes, J.C.B.; Dias, M.M. Nanohydroxyapatite (n-HAp) as a Pickering stabilizer in oil-in-water (O/W) emulsions: A stability study. J. Dispers. Sci. Technol. 2020, 43, 814–826. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Li, J.; Song, N.; Song, Y.; He, R. Factors influencing the stability and type of hydroxyapatite stabilized Pickering emulsion. Mater. Sci. Eng. C 2017, 70, 396–404. [Google Scholar] [CrossRef]
- Okada, M.; Maeda, H.; Fujii, S.; Nakamura, Y.; Furuzono, T. Formation of Pickering Emulsions Stabilized via Interaction between Nanoparticles Dispersed in Aqueous Phase and Polymer End Groups Dissolved in Oil Phase. Langmuir 2012, 28, 9405–9412. [Google Scholar] [CrossRef]
- Ortiz, D.G.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Tech. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef]
- Lou, F.; Ye, L.; Kong, M.; Yang, Q.; Li, G.; Huang, Y. Pickering emulsions stabilized by shape-controlled silica microrods. RSC Adv. 2016, 6, 24195–24202. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Huang, F.; Liang, Y.; He, Y. On the Pickering emulsions stabilized by calcium carbonate particles with various morphologies. Colloid. Surf. A 2019, 580, 123722. [Google Scholar] [CrossRef]
- Marefati, A.; Sjöö, M.; Timgren, A.; Dejmek, P.; Rayner, M. Fabrication of encapsulated oil powders from starch granule stabilized W/O/W Pickering emulsions by freeze-drying. Food Hydrocoll. 2015, 51, 261–271. [Google Scholar] [CrossRef]
- Pawlik, A.K.; Norton, I.T. Bridging benchtop research and industrial processed foods: Structuring of model food emulsions. Food Struct. 2014, 1, 24–38. [Google Scholar] [CrossRef]
- Grodzka, J.; Pomianowski, A. Wettability versus hydrophilicity. Physicochem. Probl. Miner. Process. 2006, 40, 5–18. [Google Scholar]
- Finkle, P.; Draper, H.D.; Hildebrand, J.H. The theory of emulsification1. J. Am. Chem. Soc. 1923, 45, 2780–2788. [Google Scholar] [CrossRef]
- Xiao, M.; Xu, A.; Zhang, T.; Hong, L. Tailoring the Wettability of Colloidal Particles for Pickering Emulsions via Surface Modification and Roughness. Front. Chem. 2018, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Tsabet, È.; Fradette, L. Effect of the properties of oil, particles, and water on the production of Pickering emulsions. Chem. Eng. Res. Des. 2015, 97, 9–17. [Google Scholar] [CrossRef]
- Monteillet, H.; Workamp, M.; Appel, J.; Kleijn, J.M.; Leermakers, F.A.M.; Sprakel, J. Ultrastrong Anchoring Yet Barrier-Free Adsorption of Composite Microgels at Liquid Interfaces. Adv. Mater. Interfaces 2014, 1, 1300121. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y. Tuning Amphiphilicity of Particles for Controllable Pickering Emulsion. Materials 2016, 9, 903. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Pirolt, F.; Glatter, O. Submicrometer-Sized Pickering Emulsions Stabilized by Silica Nanoparticles with Adsorbed Oleic Acid. Langmuir 2013, 29, 6004–6012. [Google Scholar] [CrossRef]
- Santini, E.; Guzmán, E.; Ferrari, M.; Liggieri, L. Emulsions stabilized by the interaction of silica nanoparticles and palmitic acid at the water–hexane interface. Colloid. Surf. A 2014, 460, 333–341. [Google Scholar] [CrossRef]
- Björkegren, S.; Nordstierna, L.; Törncrona, A.; Palmqvist, A. Hydrophilic and hydrophobic modifications of colloidal silica particles for Pickering emulsions. J. Colloid Interface Sci. 2017, 487, 250–257. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, Y. Surface modification of zein colloidal particles with sodium caseinate to stabilize oil-in-water pickering emulsion. Food Hydrocoll. 2016, 56, 292–302. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, I.-S. Microbial metabolism of prenylated apigenin derivatives by Mucor hiemalis. Phytochem. Lett. 2016, 16, 197–202. [Google Scholar] [CrossRef]
- Cui, Z.G.; Cui, C.F.; Zhu, Y.; Binks, B.P. Multiple Phase Inversion of Emulsions Stabilized by in Situ Surface Activation of CaCO3 Nanoparticles via Adsorption of Fatty Acids. Langmuir 2012, 28, 314–320. [Google Scholar] [CrossRef]
- Ribeiro, A.; Manrique, Y.A.; Lopes, J.C.B.; Dias, M.M.; Barreiro, M.F. Development of water-in-oil Pickering emulsions from sodium oleate surface-modified nano-hydroxyapatite. Surf. Interfaces 2022, 29, 101759. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Tech. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Quinoa starch: Structure, properties, and applications. Carbohydr. Polym. 2018, 181, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Azfaralariff, A.; Fazial, F.F.; Sontanosamy, R.S.; Nazar, M.F.; Lazim, A.M. Food-grade particle stabilized pickering emulsion using modified sago (Metroxylon sagu) starch nanocrystal. J. Food Eng. 2020, 280, 109974. [Google Scholar] [CrossRef]
- Li, C.; Sun, P.; Yang, C. Emulsion stabilized by starch nanocrystals. Starch–Stärke 2012, 64, 497–502. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, K.; Niu, C.; Liu, C.; Li, Y.; Wang, P.; Binks, B.P. Triglyceride–water emulsions stabilised by starch-based nanoparticles. Food Hydrocoll. 2014, 36, 70–75. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, K.; Liu, C.; Li, Y.; Lu, C.; Wang, P. Fabrication of starch-based nanospheres to stabilize pickering emulsion. Carbohydr. Polym. 2012, 88, 1358–1363. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.-A.; Chevalier, Y. Effects of solid particle content on properties of o/w Pickering emulsions. J. Colloid Interface Sci. 2010, 351, 348–356. [Google Scholar] [CrossRef]
- Dickinson, E. Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci. Tech. 2012, 24, 4–12. [Google Scholar] [CrossRef]
- Gould, J.; Vieira, J.; Wolf, B. Cocoa particles for food emulsion stabilisation. Food Funct. 2013, 4, 1369–1375. [Google Scholar] [CrossRef]
- Yuan, Q.; Cayre, O.J.; Manga, M.; Williams, R.A.; Biggs, S. Preparation of particle-stabilized emulsions using membrane emulsification. Soft Matter 2010, 6, 1580–1588. [Google Scholar] [CrossRef]
- Köhler, K.; Santana, A.S.; Braisch, B.; Preis, R.; Schuchmann, H.P. High pressure emulsification with nano-particles as stabilizing agents. Chem. Eng. Sci. 2010, 65, 2957–2964. [Google Scholar] [CrossRef]
- Madivala, B.; Vandebril, S.; Fransaer, J.; Vermant, J. Exploiting particle shape in solid stabilized emulsions. Soft Matter 2009, 5, 1717–1727. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Pickering emulsions containing cellulose microfibers produced by mechanical treatments as stabilizer in the food industry. Appl. Sci. 2019, 9, 359–374. [Google Scholar] [CrossRef]
- Folter, J.W.J.; Hutter, E.M.; Castillo, S.I.R.; Klop, K.E.; Philipse, A.P.; Kegel, W.K. Particle shape anisotropy in pickering emulsions: Cubes and peanuts. Langmuir 2014, 30, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, Y.; Kobayashi, N.; Nakagawa, N. Multiple Pickering Emulsions Stabilized by Microbowls. Langmuir 2011, 27, 4557–4562. [Google Scholar] [CrossRef]
- Bippus, L.; Jaber, M.; Lebeau, B. Laponite and hybrid surfactant/laponite particles processed as spheres by spray-drying. New J. Chem. 2009, 33, 1116–1126. [Google Scholar] [CrossRef]
- Destribats, M.; Lapeyre, V.; Wolfs, M.; Sellier, E.; Leal-Calderon, F.; Ravaine, V.; Schmitt, V. Soft microgels as Pickering emulsion stabilisers: Role of particle deformability. Soft Matter 2011, 7, 7689–7698. [Google Scholar] [CrossRef]
- Creighton, M.A.; Ohata, Y.; Miyawaki, J.; Bose, A.; Hurt, R.H. Two-Dimensional Materials as Emulsion Stabilizers: Interfacial Thermodynamics and Molecular Barrier Properties. Langmuir 2014, 30, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Dugyala, V.R.; Daware, S.V.; Basavaraj, M.G. Shape anisotropic colloids: Synthesis, packing behavior, evaporation driven assembly, and their application in emulsion stabilization. Soft Matter 2013, 9, 6711–6725. [Google Scholar] [CrossRef]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Manrique, Y.A.; Barreiro, M.F.; Lopes, J.C.B.; Dias, M.M. Effect of temperature, pH and ionic strength on hydroxyapatite stabilised Pickering emulsions produced in batch and continuous mode. Food Biophys. 2022, 17, 422–436. [Google Scholar] [CrossRef]
- Ashby, N.P.; Binks, B.P. Pickering emulsions stabilised by Laponite clay particles. Phys. Chem. Chem. Phys. 2000, 2, 5640–5646. [Google Scholar] [CrossRef]
- Yang, F.; Liu, S.; Xu, J.; Lan, Q.; Wei, F.; Sun, D. Pickering emulsions stabilized solely by layered double hydroxides particles: The effect of salt on emulsion formation and stability. J. Colloid Interface Sci. 2006, 302, 159–169. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, X.; Perez Gonzalez, A.J.; Huang, Q. Kafirin nanoparticles-stabilized Pickering emulsions: Microstructure and rheological behavior. Food Hydrocoll. 2016, 54, 30–39. [Google Scholar] [CrossRef]
- Rayner, M. Current status on novel ways for stabilizing food dispersions by oleosins, particles and microgels. Curr. Opin. Food Sci. 2015, 3, 94–109. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Effects of oil type and aqueous phase composition on oil–water mixtures containing particles of intermediate hydrophobicity. Phys. Chem. Chem. Phys. 2000, 2, 2959–2967. [Google Scholar] [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 1. Formation and stability. Food Hydrocoll. 2019, 96, 699–708. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Catastrophic Phase Inversion of Water-in-Oil Emulsions Stabilized by Hydrophobic Silica. Langmuir 2000, 16, 2539–2547. [Google Scholar] [CrossRef]
- Rousseau, D. Trends in structuring edible emulsions with Pickering fat crystals. Curr. Opin. Colloid Interface Sci. 2013, 18, 283–291. [Google Scholar] [CrossRef]
- Rousseau, D.; Hodge, S.M. Stabilization of water-in-oil emulsions with continuous phase crystals. Colloid. Surf. A 2005, 260, 229–237. [Google Scholar] [CrossRef]
- Yusoff, A.; Murray, B.S. Modified starch granules as particle-stabilizers of oil-in-water emulsions. Food Hydrocoll. 2011, 25, 42–55. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-based Pickering emulsions and their applications: A review. Carbohydr. Polym. 2020, 250, 116885. [Google Scholar] [CrossRef] [PubMed]
- Destribats, M.; Rouvet, M.; Gehin-Delval, C.; Schmitt, C.; Binks, B.P. Emulsions stabilised by whey protein microgel particles: Towards food-grade Pickering emulsions. Soft Matter 2014, 10, 6941–6954. [Google Scholar] [CrossRef]
- Pichot, R.; Spyropoulos, F.; Norton, I.T. O/W emulsions stabilised by both low molecular weight surfactants and colloidal particles: The effect of surfactant type and concentration. J. Colloid Interface Sci. 2010, 352, 128–135. [Google Scholar] [CrossRef]
- Skelhon, T.S.; Grossiord, N.; Morgan, A.R.; Bon, S.A.F. Quiescent water-in-oil Pickering emulsions as a route toward healthier fruit juice infused chocolate confectionary. J. Mater. Chem. 2012, 22, 19289–19295. [Google Scholar] [CrossRef]
- European_Parliament. Council_Directive European Parliament and Council Directive No 95/2/EC of 20 February 1995 on Food Additives Other than Colours and Sweeteners. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31995L0002 (accessed on 12 July 2022).
- Regulation Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008R1333 (accessed on 12 July 2022).
- Binks, B.P.; Muijlwijk, K.; Koman, H.; Poortinga, A.T. Food-grade Pickering stabilisation of foams by in situ hydrophobisation of calcium carbonate particles. Food Hydrocoll. 2017, 63, 585–592. [Google Scholar] [CrossRef]
- Commission_Regulation Commission Regulation (EU) No. 1129/2011 of 11 November 2011 Amending Annexx II to Regulation (EC) No. 1333/2008 of the European Parliament and of the Council by Establishing a Union List of food Additives Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1129 (accessed on 12 July 2022).
- Xu, Q.Y.; Nakajima, M.; Binks, B.P. Preparation of particle-stabilized oil-in-water emulsions with the microchannel emulsification method. Colloid. Surf. A 2005, 262, 94–100. [Google Scholar] [CrossRef]
- Eskandar, N.G.; Simovic, S.; Prestidge, C.A. Synergistic effect of silica nanoparticles and charged surfactants in the formation and stability of submicron oil-in-water emulsions. Phys. Chem. Chem. Phys. 2007, 9, 6426–6434. [Google Scholar] [CrossRef] [PubMed]
- Pichot, R.; Spyropoulos, F.; Norton, I.T. Mixed-emulsifier stabilised emulsions: Investigation of the effect of monoolein and hydrophilic silica particle mixtures on the stability against coalescence. J. Colloid Interface Sci. 2009, 329, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Whitby, C.P.; Fornasiero, D.; Ralston, J. Effect of adding anionic surfactant on the stability of Pickering emulsions. J. Colloid Interface Sci. 2009, 329, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Pichot, R.; Spyropoulos, F.; Norton, I.T. Competitive adsorption of surfactants and hydrophilic silica particles at the oil–water interface: Interfacial tension and contact angle studies. J. Colloid Interface Sci. 2012, 377, 396–405. [Google Scholar] [CrossRef]
- Gautier, F.; Destribats, M.; Perrier-Cornet, R.; Dechézelles, J.-F.; Giermanska, J.; Héroguez, V.; Ravaine, S.; Leal-Calderon, F.; Schmitt, V. Pickering emulsions with stimulable particles: From highly- to weakly-covered interfaces. Phys. Chem. Chem. Phys. 2007, 9, 6455–6462. [Google Scholar] [CrossRef]
- Kargar, M.; Spyropoulos, F.; Norton, I.T. The effect of interfacial microstructure on the lipid oxidation stability of oil-in-water emulsions. J. Colloid Interface Sci. 2011, 357, 527–533. [Google Scholar] [CrossRef]
- Manga, M.S.; Cayre, O.J.; Williams, R.A.; Biggs, S.; York, D.W. Production of solid-stabilised emulsions through rotational membrane emulsification: Influence of particle adsorption kinetics. Soft Matter 2012, 8, 1532–1538. [Google Scholar] [CrossRef]
- Manga, M.S.; York, D.W. Production of concentrated Pickering emulsions with narrow size distributions using stirred cell membrane emulsification. Langmuir 2017, 33, 9050–9056. [Google Scholar] [CrossRef]
- Kumar, G.; Kakati, A.; Mani, E.; Sangwai, J.S. Stability of nanoparticle stabilized oil-in-water Pickering emulsion under high pressure and high temperature conditions: Comparison with surfactant stabilized oil-in-water emulsion. J. Dispers. Sci. Technol. 2020, 42, 1–14. [Google Scholar] [CrossRef]
- Tikekar, R.V.; Pan, Y.; Nitin, N. Fate of curcumin encapsulated in silica nanoparticle stabilized Pickering emulsion during storage and simulated digestion. Food Res. Int. 2013, 51, 370–377. [Google Scholar] [CrossRef]
- Drelich, A.; Gomez, F.; Clausse, D.; Pezron, I. Evolution of water-in-oil emulsions stabilized with solid particles: Influence of added emulsifier. Colloid. Surf. A 2010, 365, 171–177. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, J.; Liu, K.; Cui, Z.; Binks, B.P. Switchable Pickering Emulsions Stabilized by Silica Nanoparticles Hydrophobized in Situ with a Conventional Cationic Surfactant. Langmuir 2015, 31, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Arkoumanis, P.G.; Norton, I.T.; Spyropoulos, F. Pickering particle and emulsifier co-stabilised emulsions produced via rotating membrane emulsification. Colloid. Surf. A 2019, 568, 481–492. [Google Scholar] [CrossRef]
- Yuan, Q.; Williams, R.A. CO-stabilisation mechanisms of nanoparticles and surfactants in Pickering Emulsions produced by membrane emulsification. J. Membr. Sci. 2016, 497, 221–228. [Google Scholar] [CrossRef]
- Binks, B.P.; Yin, D. Pickering emulsions stabilized by hydrophilic nanoparticles: In situ surface modification by oil. Soft Matter 2016, 12, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Wang, C.; Gao, Q.; Tong, Z. Surfactant-Free Multiple Pickering Emulsions Stabilized by Combining Hydrophobic and Hydrophilic Nanoparticles. J. Dispers. Sci. Technol. 2013, 34, 173–181. [Google Scholar] [CrossRef]
- Alison, L.; Rühs, P.A.; Tervoort, E.; Teleki, A.; Zanini, M.; Isa, L.; Studart, A.R. Pickering and Network Stabilization of Biocompatible Emulsions Using Chitosan-Modified Silica Nanoparticles. Langmuir 2016, 32, 13446–13457. [Google Scholar] [CrossRef]
- Zheng, B.; Zheng, B.; Carr, A.J.; Yu, X.; McClements, D.J.; Bhatia, S.R. Emulsions stabilized by inorganic nanoclays and surfactants: Stability, viscosity, and implications for applications. Inorg. Chim. Acta 2020, 508, 119566. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, W.; Cao, J.; Liu, W.; Zhu, S. Preparation of core–shell CaCO3 capsules via Pickering emulsion templates. J. Colloid Interface Sci. 2012, 372, 24–31. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X. Binding, stability, and antioxidant activity of quercetin with soy protein isolate particles. Food Chem. 2015, 188, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Frasch-Melnik, S. Fat Crystal-Stabilised Double Emulsions. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2011. [Google Scholar]
- Frasch-Melnik, S.; Spyropoulos, F.; Norton, I.T. W1/O/W2 double emulsions stabilised by fat crystals–Formulation, stability and salt release. J. Colloid Interface Sci. 2010, 350, 178–185. [Google Scholar] [CrossRef]
- Matos, M.; Timgren, A.; Sjöö, M.; Dejmek, P.; Rayner, M. Preparation and encapsulation properties of double Pickering emulsions stabilized by quinoa starch granules. Colloid. Surf. A 2013, 423, 147–153. [Google Scholar] [CrossRef]
- Xiao, J.; Lu, X.; Huang, Q. Double emulsion derived from kafirin nanoparticles stabilized Pickering emulsion: Fabrication, microstructure, stability and in vitro digestion profile. Food Hydrocoll. 2017, 62, 230–238. [Google Scholar] [CrossRef]
- Ribeiro, A.; Gonçalves, R.F.S.; Pinheiro, A.C.; Manrique, Y.A.; Barreiro, M.F.; Lopes, J.C.B.; Dias, M.M. In vitro digestion and bioaccessibility studies of vitamin E-loaded nanohydroxyapatite Pickering emulsions and derived fortified foods. LWT 2022, 154, 112706. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; McClements, D.J. Encapsulation of vitamin D3 in pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum-stabilized Pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Low, L.E.; Tan, L.T.H.; Goh, B.H.; Tey, B.T.; Ong, B.H.; Tang, S.Y. Magnetic cellulose nanocrystal stabilized Pickering emulsions for enhanced bioactive release and human colon cancer therapy. Int. J. Biol. Macromol. 2019, 127, 76–84. [Google Scholar] [CrossRef]
- Silva, V.M.T.M.; Quadros, P.A.; Laranjeira, P.E.M.S.C.; Dias, M.M.; Lopes, J.C.B. A Novel Continuous Industrial Process for Producing Hydroxyapatite Nanoparticles. J. Dispers. Sci. Technol. 2008, 29, 542–547. [Google Scholar] [CrossRef]
- Canillas, M.; Pena, P.; de Aza, A.H.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín De La Soc. Española De Cerámica Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Epple, M. Synthetic hydroxyapatite as a biomimetic oral care agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Omelon, S.; Ariganello, M.; Bonucci, E.; Grynpas, M.; Nanci, A. A Review of Phosphate Mineral Nucleation in Biology and Geobiology. Calcif. Tissue Int. 2013, 93, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.C.B.; Dias, M.M.; Silva, V.M.M.; Quadros, P.A.; Monteiro, F.J.; Gomes, P.J.; Mateus, A.Y. Production Method for Calcium Phosphate Nano-Particles with High Purity and Their Use. U.S. Patent Application No. 12/159,696, 22 October 2009. [Google Scholar]
- Chan, G.R. Development of Hydroxyapatite-Based Hybrid Materials for Biomedical Applications. Ph.D.Thesis., Faculdade de Engenharia–Universidade do Porto, Porto, Protugal, 2016. [Google Scholar]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Fujii, S.; Okada, M.; Nishimura, T.; Maeda, H.; Sugimoto, T.; Hamasaki, H.; Furuzono, T.; Nakamura, Y. Hydroxyapatite-armored poly(ε-caprolactone) microspheres and hydroxyapatite microcapsules fabricated via a Pickering emulsion route. J. Colloid Interface Sci. 2012, 374, 1–8. [Google Scholar] [CrossRef]
- Schoepf, J.J.; Bi, Y.; Kidd, J.; Herckes, P.; Hristovski, K.; Westerhoff, P. Detection and dissolution of needle-like hydroxyapatite nanomaterials in infant formula. NanoImpact 2017, 5, 22–28. [Google Scholar] [CrossRef]
- FDA Select Committee on GRAS Substances (SCOGS) Opinion: Phosphates. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS (accessed on 12 July 2022).
- Ramis, J.M.; Coelho, C.C.; Córdoba, A.; Quadros, P.A.; Monjo, M. Safety assessment of nano-hydroxyapatite as an oral care ingredient according to the EU cosmentics regulation. Cosmetics 2018, 5, 53–66. [Google Scholar] [CrossRef]
- Fujii, S.; Okada, M.; Nishimura, T.; Sugimoto, T.; Maeda, H.; Hamasaki, H.; Furuzono, T.; Nakamura, Y. Hydroxyapatite-coated poly(ϵ-caprolactone) microspheres fabricated via a Pickering emulsion route: Effect of fabrication parameters on diameter and chemical composition. Compos. Interfaces 2013, 20, 45–56. [Google Scholar] [CrossRef]
- Samanta, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Hydroxyapatite stabilized pickering emulsions of poly(ε-caprolactone) and their composite electrospun scaffolds. Colloid. Surf. A 2017, 533, 224–230. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Li, X.; Hu, X.; Zhou, W.; Dong, X.; Wang, C.; Yang, Z.; Binks, B.P. Facile preparation of bioactive nanoparticle/poly(ε-caprolactone) hierarchical porous scaffolds via 3D printing of high internal phase Pickering emulsions. J. Colloid Interface Sci. 2019, 545, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Okada, M.; Maeda, H.; Fujii, S.; Furuzono, T. Hydroxyapatite/biodegradable poly(l-lactide–co-ε-caprolactone) composite microparticles as injectable scaffolds by a Pickering emulsion route. Acta Biomater. 2011, 7, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zou, S.; Chen, W.; Tong, Z.; Wang, C. Mineralization and drug release of hydroxyapatite/poly(l-lactic acid) nanocomposite scaffolds prepared by Pickering emulsion templating. Colloid. Surf. B 2014, 122, 559–565. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, S.; Yang, Z.; Zhou, W.; Du, Z.; Huang, J.; Yi, H.; Wang, C. Facile fabrication of poly(L-lactic acid) microsphere-incorporated calcium alginate/hydroxyapatite porous scaffolds based on Pickering emulsion templates. Colloid. Surf. B 2016, 140, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, A.; Li, J.; Song, N. Effect of stearic acid modified HAp nanoparticles in different solvents on the properties of Pickering emulsions and HAp/PLLA composites. Mater. Sci. Eng. C 2017, 79, 255–261. [Google Scholar] [CrossRef]
- Tham, C.Y.; Chow, W.S. Poly(lactic acid) microparticles with controllable morphology by hydroxyapatite stabilized pickering emulsions: Effect of pH, salt, and amphiphilic agents. Colloid. Surf. A 2017, 533, 275–285. [Google Scholar] [CrossRef]
- Song, N.; Wang, A.-J.; Li, J.-M.; Zhu, Z.; Shi, H.; Ma, X.-L.; Sun, D. Study on influencing factors of Pickering emulsions stabilized by hydroxyapatite nanoparticles with nonionic surfactants. Soft Matter 2018, 14, 3889–3901. [Google Scholar] [CrossRef]
- Hu, B.; Zhao, C.; Jin, X.; Wang, H.; Xiong, J.; Tan, J. Antagonistic effect in pickering emulsion stabilized by mixtures of hydroxyapatite nanoparticles and sodium oleate. Colloid. Surf. A 2015, 484, 278–287. [Google Scholar] [CrossRef]
- Rodríguez, K.; Villalta, M.; Marín, E.; Briceño, M.; León, G.; Montero, M.L. Physical characteristics of nano-Hydroxyapatite Pickering-emulsions and their adjuvant activity on the antibody response towards the Bothros asper snake venom. Mater. Sci. Eng., C 2019, 100, 23–29. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Ning, Y.; Wang, C.; Tong, Z. Facile preparation of artemisia argyi oil-loaded antibacterial microcapsules by hydroxyapatite-stabilized Pickering emulsion templating. Colloid. Surf. B 2013, 112, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, S.; Yu, L.; Shangguan, S.; Song, C.; Li, Q.; Chen, K.; Sun, J.; Li, M.; Hou, H. Protocells self-assembled by hydroxyapatite nanoparticles: Highly efficient and selective enrichment of chlorophenols in an aqueous environment. Chemosphere 2019, 233, 1–8. [Google Scholar] [CrossRef]
- Ribeiro, A.; Manrique, Y.A.; Barreiro, F.; Lopes, J.C.B.; Dias, M.M. Continuous production of hydroxyapatite Pickering emulsions using a mesostructured reactor. Colloid. Surf. A 2021, 616, 126365. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Yin, S.-W.; Zhu, J.-H.; Qi, J.-R.; Guo, J.; Wu, L.-Y.; Tang, C.-H.; Yang, X.-Q. Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles. Food Hydrocoll. 2016, 61, 300–310. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Lee, S.J.; Yuan, Q.; Teo, A.; Goh, K.K.T.; Wong, M. Namoemulsions. In Emulsion-Based Systems for Delivery of Food Active Compounds: Formation, Application, Health and Safety, 1st ed.; Roohinejad, S., Shahin, R., Oey, I., Wen, J., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 181–230. [Google Scholar]
- Suslick, K.S.; McNamara, W.B.; Didenko, Y. Hot Spot Conditions During Multi-bubble Cavitation. In Sonochemistry and Sonoluminescence; Crum, L.A., Mason, T.J., Reisse, J.L., Suslick, K.S., Eds.; Kluwer Publishers: Dordrecht, The Netherlands, 1999; pp. 191–204. [Google Scholar]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; He, F.; Chen, Z.-H.; Xie, R.; Ju, X.-J.; Liua, Z.; Chu, L.-Y. On-chip thermo-triggered coalescence of controllable Pickering emulsion droplet pairs. RSC Adv. 2016, 6, 64182–64192. [Google Scholar] [CrossRef]
- Bai, L.; McClements, D.J. Development of microfluidization methods for efficient production of concentrated nanoemulsions: Comparison of single- and dual-channel microfluidizers. J. Colloid Interface Sci. 2016, 466, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Qi, F.; Wu, J.; Ma, G.; Ngai, T. Preparation of Uniform Particle-Stabilized Emulsions Using SPG Membrane Emulsification. Langmuir 2014, 30, 7052–7056. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Lemenand, T.; Della Valle, D.; Peerhossaini, H. Static mixers: Mechanisms, applications, and characterization methods–A review. Chem. Eng. Res. Des. 2014, 92, 205–228. [Google Scholar] [CrossRef]
- Muruganandam, L.; Kunal, D.; Melwyn, G.O. Studies on Droplet Size Distribution of Oil-in-Water Emulsion in SMX Static Mixer. J. Appl. Fluid Mech. 2018, 11, 107–117. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Encapsulation of Vitamin D3 in Pickering Emulsion Stabilized by Nanofibrillated Mangosteen Cellulose: Effect of Environmental Stresses. J. Food Sci. 2019, 84, 3213–3221. [Google Scholar] [CrossRef]
- Zembyla, M.; Murray, B.S.; Radford, S.J.; Sarkar, A. Water-in-oil Pickering emulsions stabilized by an interfacial complex of water-insoluble polyphenol crystals and protein. J. Colloid Interface Sci. 2019, 548, 88–99. [Google Scholar] [CrossRef]
- Luo, Z.; Murray, B.S.; Ross, A.-L.; Povey, M.J.W.; Morgan, M.R.A.; Day, A.J. Effects of pH on the ability of flavonoids to act as Pickering emulsion stabilizers. Colloid. Surf. B 2012, 92, 84–90. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Marshall, L.J.; Akhtar, M.; Murray, B.S. Structure and oxidative stability of oil in water emulsions as affected by rutin and homogenization procedure. Food Chem. 2012, 134, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. Soc. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Shimoni, G.; Shani Levi, C.; Levi Tal, S.; Lesmes, U. Emulsions stabilization by lactoferrin nano-particles under in vitro digestion conditions. Food Hydrocoll. 2013, 33, 264–272. [Google Scholar] [CrossRef]
- Nagpal, R.; Behare, P.; Rana, R.; Kumar, A.; Kumar, M.; Arora, S.; Morotta, F.; Jain, S.; Yadav, H. Bioactive peptides derived from milk proteins and their health beneficial potentials: An update. Food Funct. 2011, 2, 18–27. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, S.; Ye, F.; Zhou, G.; Gao, R.; Qin, D.; Zhao, G. A novel cholesterol-free mayonnaise made from Pickering emulsion stabilized by apple pomace particles. Food Chem. 2021, 353, 129418. [Google Scholar] [CrossRef]
- Akcicek, A.; Karasu, S.; Bozkurt, F.; Kayacan, S. Egg Yolk-Free Vegan Mayonnaise Preparation from Pickering Emulsion Stabilized by Gum Nanoparticles with or without Loading Olive Pomace Extracts. ACS Omega 2022, 7, 26316–26327. [Google Scholar] [CrossRef]
- Ghirro, L.C.; Rezende, S.; Ribeiro, A.S.; Rodrigues, N.; Carocho, M.; Pereira, J.A.; Barros, L.; Demczuk, B.; Barreiro, M.-F.; Santamaria-Echart, A. Pickering Emulsions Stabilized with Curcumin-Based Solid Dispersion Particles as Mayonnaise-like Food Sauce Alternatives. Molecules 2022, 27, 1250. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiao, B.; Meng, S.; Fu, W.; Faisal, S.; Li, X.; Liu, H.; Wang, Q. Edible mayonnaise-like Pickering emulsion stabilized by pea protein isolate microgels: Effect of food ingredients in commercial mayonnaise recipe. Food Chem. 2022, 376, 131866. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Gao, Z.-M.; Yang, X.-Q.; Wu, N.-N.; Wang, L.-J.; Wang, J.-M.; Guo, J.; Yin, S.-W. Protein-Based Pickering Emulsion and Oil Gel Prepared by Complexes of Zein Colloidal Particles and Stearate. J. Agric. Food. Chem. 2014, 62, 2672–2678. [Google Scholar] [CrossRef]
- Okuro, P.K.; Gomes, A.; Costa, A.L.R.; Adame, M.A.; Cunha, R. L Formation and stability of W/O-high internal phase emulsions (HIPEs) and derived O/W emulsions stabilized by PGPR and lecithin. Food Res. Int. 2019, 122, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Weiss, J.; Ahmad, T.; Zhang, C.; Zhang, H. A review of recent progress on high internal-phase Pickering emulsions in food science. Trends Food Sci. Techol. 2020, 106, 91–103. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Vitamin E bioaccessibility: Influence of carrier oil type on digestion and release of emulsified α-tocopherol acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef]
- Leal-Calderon, F.; Schmitt, V. Solid-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2008, 13, 217–227. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, S.; Bei, W.; Zahi, M.R.; Yuan, Q.; Liang, H. Preparation and antimicrobial activity of oregano essential oil Pickering emulsion stabilized by cellulose nanocrystals. Int. J. Biol. Macromol. 2018, 112, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Stratulat, I.; Britten, M.; Salmieri, S.; Fustier, P.; St-Gelais, D.; Champagne, C.P.; Lacroix, M. Enrichment of cheese with vitamin D3 and vegetable omega-3. J. Funct. Foods 2015, 13, 300–307. [Google Scholar] [CrossRef]


| Particle Characterisation | Emulsion Characterisation | Production | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Solid Particle | Surface Modification | Shape | Size | Water Phase | Oil Phase | Emulsion Type | Homogeniser | Rate Time Pressure Cycles | |
| Silica | n.a. | Spherical | 30 nm | Water | Tricaprylin | O/W | Microfluidizer | n.d. | [89] |
| Silica | Lecithin or oleylamine | Spherical | 7 nm | Water | Miglyol | O/W | High-pressure | 500 1000 bar 5 cycles | [90] |
| Silica | Monoolein | Spherical | 150 nm | Water | Vegetable oil | O/W | High-shear | 8000 rpm 5 min | [91] |
| Silica | Sodium dodecyl sulphate | Spherical | 12 nm | Water | n-dodecane | O/W | Rotor-stator | 13,000 rpm 2 min | [92] |
| Silica | n.a. | Spherical | 80 nm or 800 nm | Water | Ethyl acetate | O/W | XME; RME | n.a. | [59] |
| Silica | Tween 60; sodium caseinate; lecithin | Spherical | 150 nm | Water | Vegetable oil | O/W | High-shear | 8000 rpm 5 min | [83,93] |
| Silica | n.a. | Spherical | 145 nm | Water | Hexadecane | O/W | Hand shaking | n.a. | [94] |
| Silica | n.a. | Spherical | 100 nm | Water | Toluene | O/W | Ultrasonic | 40% amplitude | [56] |
| Silica | n.a. | Spherical | 12 nm or 200 nm | Water | Corn oil | O/W | High-pressure | 350–1000 bar 1 cycle | [60] |
| Silica | n.a. | Spherical | 12 nm | Water | Sunflower oil | O/W | Rotor-stator | 7 min | [95] |
| Silica | n.a. | Spherical | 800 nm | Water | Tricaprylin oil | O/W | RME | n.a. | [96] |
| Silica | n.a. | Spherical | 10–12 nm | Water | Tricaprylin oil | O/W | SCME | n.a. | [97] |
| Silica | n.a. | Spherical | 15 nm | Water | n-dodecane | O/W | Rotor-stator | 13,000 rpm 3 min | [98] |
| Silica | n.a. | n.d. | 8 nm | Water | Canola oil | O/W | High-pressure | 600 bar 3 cycles | [99] |
| Silica | Sorbitan monooleate | Spherical | 12 nm | Water | Paraffin oil | O/W | Rotor-stator | 25,000 rpm 5 min | [100] |
| Silica | mPEG silanes; organosilanes | Spherical | 13–70 nm | Water | Exxsol D60 | O/W; W/O | Rotor-stator | 10,000–20,000 rpm 4 min | [44] |
| Silica | Palmitic acid | Spherical | 15 nm | Water | Hexane | O/W | Rotor-stator | 10,000 rpm 10 min | [43] |
| Silica | Oleic acid | Spherical | 5–10 nm | Water | Paraffin oil | O/W | Magnetic stirrer | 2500 rpm 2 min | [42] |
| Silica | CTAB | Spherical | 20 nm | Water | n-dodecane | O/W | Rotor-stator | 7000 rpm 2 min | [101] |
| Silica; hydroxyl methyl cellulose | Tween 20; whey protein | n.d. | n.d. | Water | Sunflower oil | O/W | RME | n.a. | [102] |
| Silica + PS latex | SDS; HTAB; Tween 20 | n.d. | n.d. | Water | Paraffin oil; ethyl acetate; sunflower oil | O/W | XME; RME | n.a. | [103] |
| Silica (1) or zirconia (2) | Dipropyl adipate | Spherical; n.d. | 5–30 nm (1); 5–10 nm (2) | Water | n-dodecane | O/W | Rotor-stator | 13,000 rpm 2 min | [104] |
| Clay (1); silica (2); Fe2O3 (3); oleic acid-coated Fe2O3 (4); microgel (5) | n.a. | Platelets (1); spherical (2,3,4); microgel (5) | 1 × 30 nm (1); 5–30 nm (2); 5 nm (3,4); 220 nm (5) | Water | Styrene; toluene | W/O/W; O/W/O | Ultrasonic; Hand shaking | 2 min | [105] |
| Silica/ chitosan | n.a. | n.d. | n.d. | Water | Sunflower oil; cocoa butter | W/O | Rotor-stator | 11,000 rpm 2 min | [84] |
| Silica/ chitosan | n.a. | n.d. | n.d. | Water | Corn oil | O/W | High-pressure | 1380 bar 7 cycles 2760 bar 1 cycle | [106] |
| Clay | SDS; DTAB; Pluronic | Spherical | 9–50 nm | Water | Mineral oil | O/W | Rotor-stator | 11,000 rpm 5 min | [107] |
| Calcium carbonate | n.a. | Cubic | ~1 µm | Buffer solution | Sunflower oil | O/W | Rotor-stator | 6000 rpm 2 min | [108] |
| Calcium carbonate | n.a. | Spherical; cubic; rod-like | ~5 µm | Water | Soybean oil | O/W | Hand shaking | 30 s | [33] |
| Calcium carbonate | Fatty acids | Spherical | 80–100 nm | Water | Toluene | O/W; W/O | Rotor-stator | 5000 rpm 2 min | [47] |
| Silicone resin | n.a. | Microbowl | 2–2.5 µm | Water | n-dodecane | O/W | Vortex mixer | n.d. 2 min | [64] |
| Particle Characterisation | Emulsion Characterisation | Production | Use | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Surface Modification | Shape | Size | Water Phase | Oil Phase | Emulsion Type | Homogenizer | Speed/Time Pressure/Cycles | ||
| PCL * | Rod-like | 30 nm | Water | DCM | O/W | Rotor-stator | 20,500 rpm 1 min | PE stabilisation | [127] |
| PCL * | Rod-like | 30 nm | Water | DCM | O/W | Rotor-stator | 14,500–30,000 rpm 1 min | PE stabilisation | [131] |
| PCL * | Fibril | 23 × 140 nm | Water | DCM and DMF | W/O | Rotor-stator | 15,000 rpm n.d. | Scaffolds fabrication | [132] |
| PCL * | Rod-like | 20–50 × 80–220 nm | Water | DCM | W/O | Vortex mixer | 3500 rpm n.d. | Scaffolds fabrication | [133] |
| P(LLA/CL) * | Spherical | 50 nm | Water | DCM | O/W | Rotor-stator | 20,450 rpm 3 min | Scaffolds fabrication | [134] |
| PLLA * | Spherical | 30–70 nm | Water | DCM | W/O | Rotor-stator | 12,000 rpm 1 min | Scaffolds fabrication | [135] |
| Alginate + PLLA * | Spherical | 20–70 nm | Water | DCM | O/W | Rotor-stator | 12,000 rpm 1.5 min | Scaffolds fabrication | [136] |
| Stearic acid + PLLA * | n.d. | n.d. | Water | DCM | O/W; W/O | Rotor-stator | 17,000 rpm 1 min | PE stabilisation | [137] |
| PLLA * | n.d. | n.d. | Water | DCM | O/W; W/O | Rotor-stator | 200–20,000 rpm 0.2–3 min | PE stabilisation | [26] |
| CTAB and PG + PLLA * | n.d. | 0.2–1.2 µm | Water | DCM | O/W | Ultrasonic | 250 W 5 min | PE stabilisation | [138] |
| Stearic acid; PLLA + Span 80 * | n.d. | n.d. | Water | DCM | O/W; W/O | Rotor-stator | 10,000–20,000 rpm 0.5–4 min | PE stabilisation | [139] |
| PS * | Spherical | 40 nm | Water | DCM | O/W | Vortex mixer | 3200 rpm 1 min | PE stabilisation | [27] |
| Sodium oleate | Rod-like | 23 × 70 nm | Water | Cy | W/O; O/W | Ultrasonic | 300 W 6 cycles | PE stabilisation | [140] |
| Stearic acid | n.d. | 30 nm | Water | n.d. | W/O | Magnetic stirrer | 12,000 rpm n.d. | PE stabilisation | [141] |
| PMF | Spherical | 30–70 nm | Water | Artemisia argyi oil | O/W | Rotor-stator | 10,000 rpm 2 min | PE stabilisation | [142] |
| DBP | Rod-like | n.d. | Water | Hexanol | O/W | Ultrasonic | n.d. | Protocells fabrication | [143] |
| n.a. | Rod-like | 50 nm | Water | Sunflower oil | O/W | Rotor-stator | 11,000 rpm 6 min | PE stabilisation | [25] |
| n.a. | Rod-like | 50 nm | Water | Sunflower oil | O/W | NETmix | 200–500 Reynolds number 1–35 cycles | PE stabilisation | [144] |
| n.a. | Rod-like | 50 nm | Water | Sunflower oil | O/W | NETmix | 300–400 Reynolds number 5–17 cycles | Vitamin E-loaded PE | [114] |
| Sodium oleate | Rod-like | 50 nm | Water | Sunflower oil | W/O | Rotor-stator | 11,000 rpm 2 min | PE stabilisation | [48] |
| Homogenizer Type | Throughput | Efficiency | Droplet Size | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|
| Control | Minimum | |||||
| High-shear | Batch | Low | Rotation speed Emulsification time | 2 µm | Easy set-up Quick processes Low operating cost Small amounts of the liquids Different apparatus available | Particle disruption Temperature increase Broad droplet size Limited energy input |
| Ultrasonic | Batch | Low | Ultrasound frequency Amplitude Emulsification time | 0.1 µm | Easy set-up Quick processes Small amounts of the liquids | Particle disruption Temperature increase Broad droplet size Probe degradation |
| High-pressure | Batch or continuous | High | Pressure value Number of homogenizing cycles | 0.1 µm | Quick processes Narrow droplet size | Particle disruption Temperature increase High energy consumption Difficult to clean |
| Membrane | Batch or continuous | Very high | Membrane pore size Injection rate Agitation speed | 0.3 µm | Particle integrity Temperature control Narrow droplet size Low energy consumption | Set-up Slow process Viscosity of the fluids |
| Microfluidizers | Continuous | High | Flow rate Microchannel geometry Number of cycles Phase viscosities | 0.1 µm | Particle integrity Temperature control Droplet size control Narrow droplet size Multiple emulsion production Low energy consumption | Viscosity of the fluids Set-up Slow process |
| Static mixers | Continuous | High | Flow rate Number of cycles | 0.3 µm | Particle integrity Mixing control Temperature control Droplet size control Low energy consumption | Viscosity of the fluids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.; Lopes, J.C.B.; Dias, M.M.; Barreiro, M.F. Pickering Emulsions Based in Inorganic Solid Particles: From Product Development to Food Applications. Molecules 2023, 28, 2504. https://doi.org/10.3390/molecules28062504
Ribeiro A, Lopes JCB, Dias MM, Barreiro MF. Pickering Emulsions Based in Inorganic Solid Particles: From Product Development to Food Applications. Molecules. 2023; 28(6):2504. https://doi.org/10.3390/molecules28062504
Chicago/Turabian StyleRibeiro, Andreia, José Carlos B. Lopes, Madalena M. Dias, and Maria Filomena Barreiro. 2023. "Pickering Emulsions Based in Inorganic Solid Particles: From Product Development to Food Applications" Molecules 28, no. 6: 2504. https://doi.org/10.3390/molecules28062504
APA StyleRibeiro, A., Lopes, J. C. B., Dias, M. M., & Barreiro, M. F. (2023). Pickering Emulsions Based in Inorganic Solid Particles: From Product Development to Food Applications. Molecules, 28(6), 2504. https://doi.org/10.3390/molecules28062504









