(n, m) Distribution of Single-Walled Carbon Nanotubes Grown from a Non-Magnetic Palladium Catalyst
Abstract
1. Introduction
2. DFT Calculations on SWNT–Pd Interfacial Formation Energy
2.1. Computational Methods
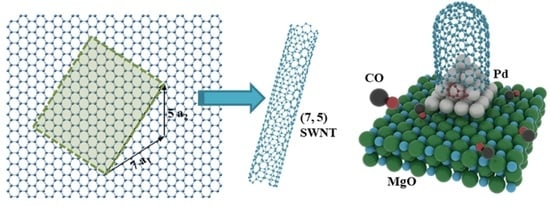
2.2. Model Construction
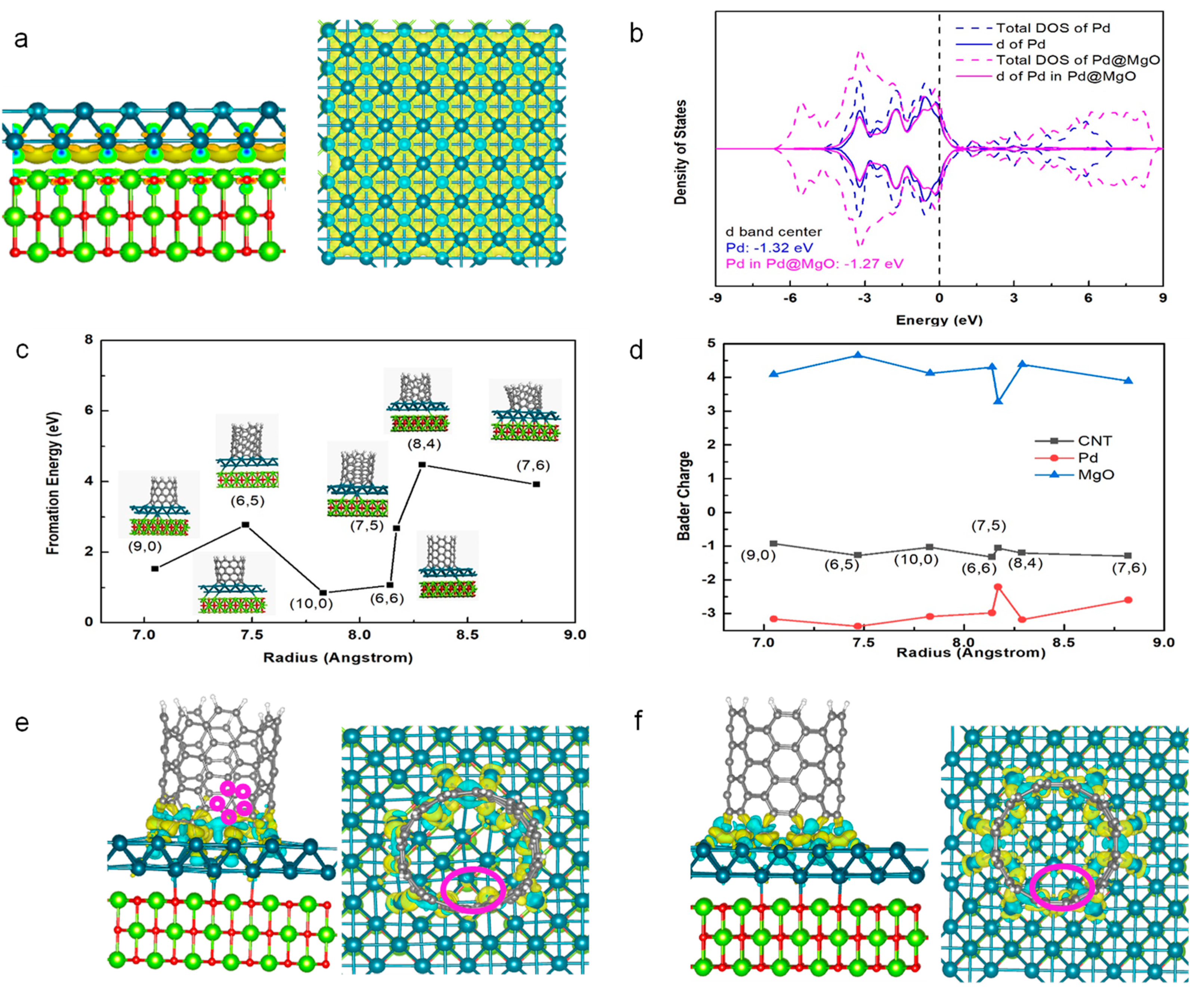
2.3. Interfacial Formation Energies of SWNTs on Pd@MgO Catalysts
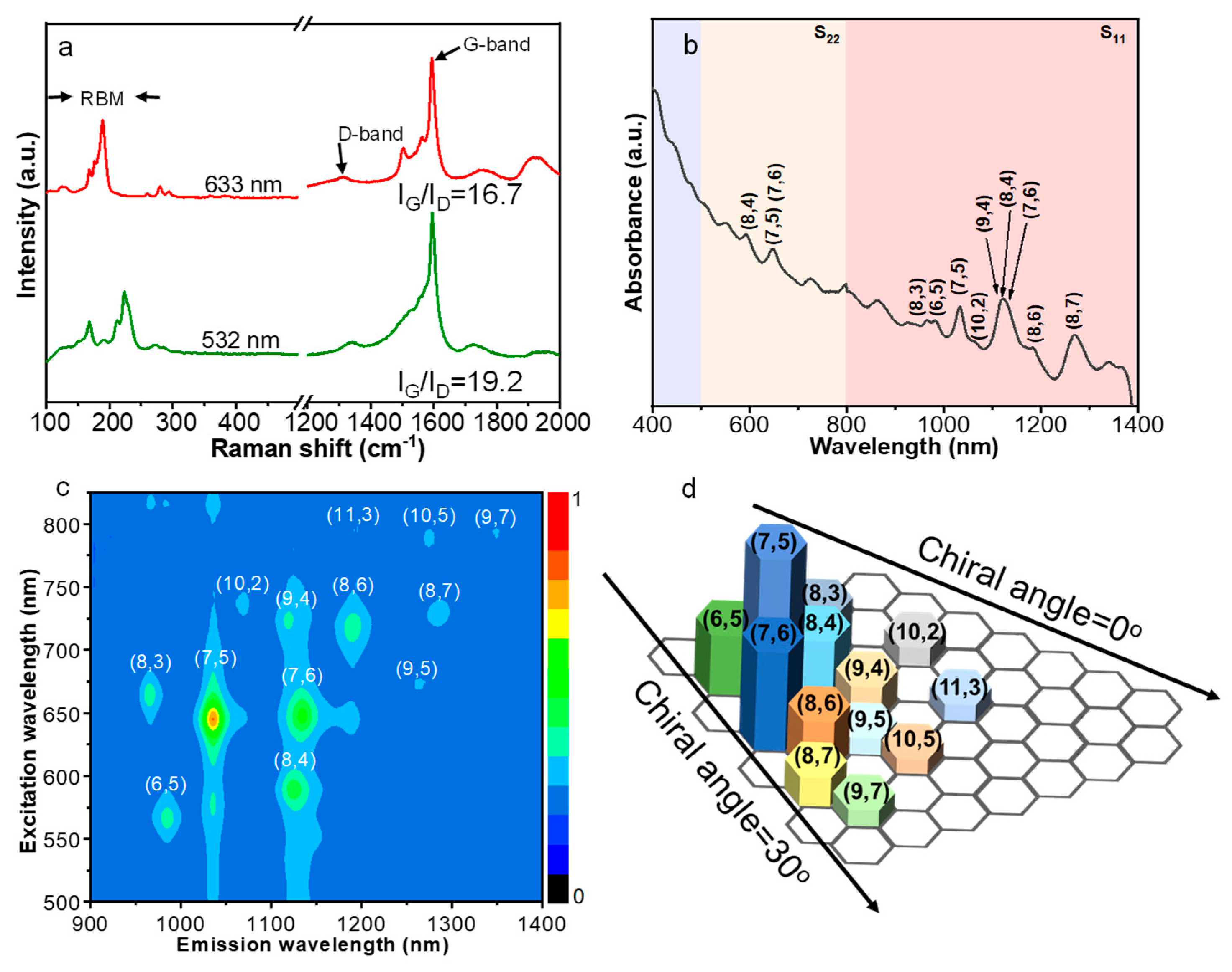
3. Results and Discussion
3.1. Calculation Results
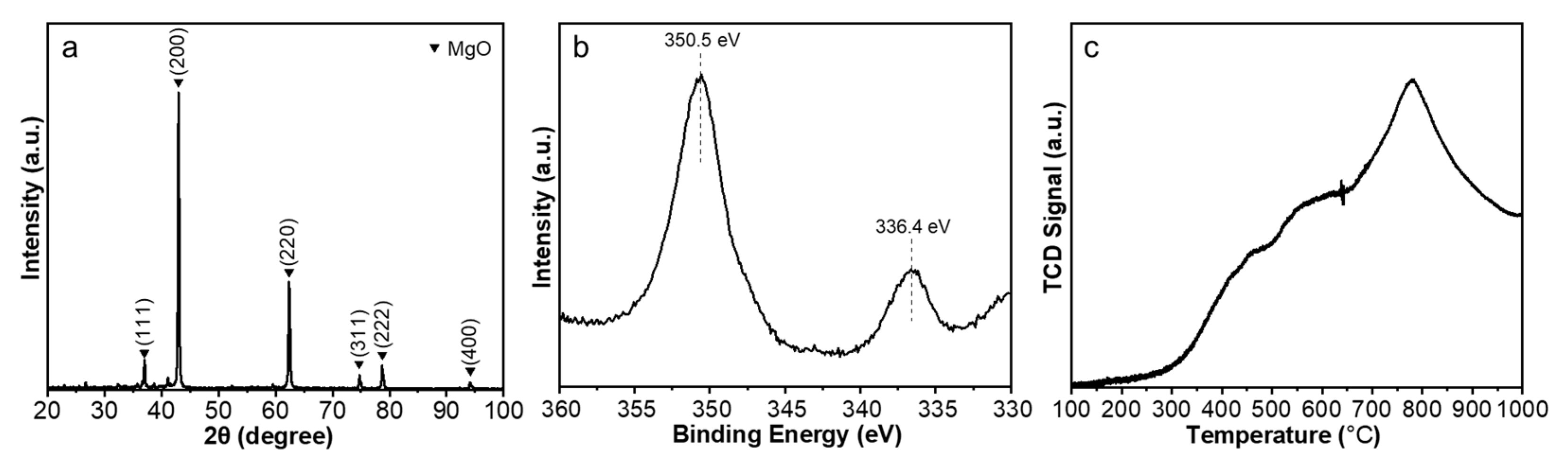
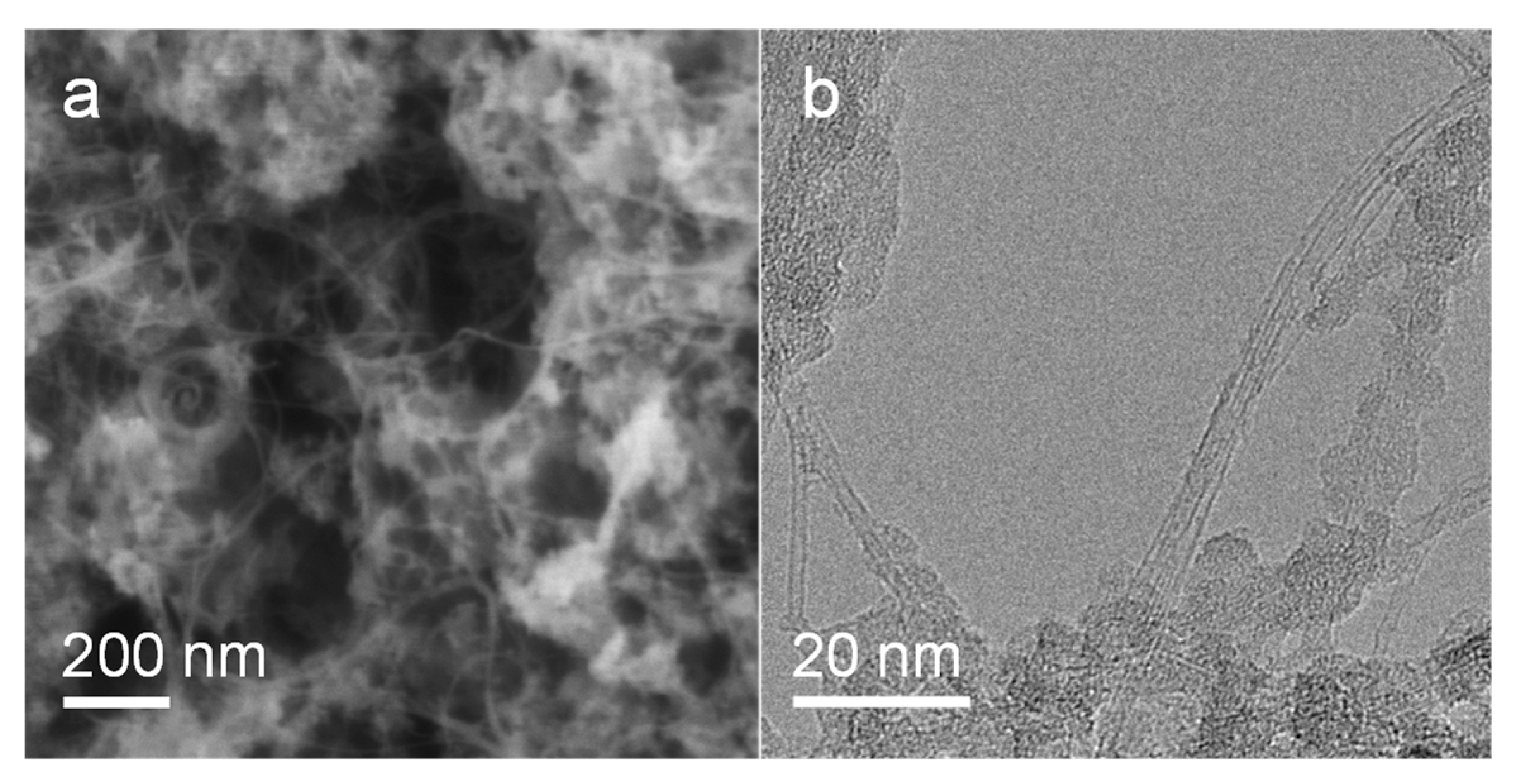
3.2. Experimental Results
4. Materials and Methods
4.1. Preparation of Pd@MgO Catalyst
4.2. CVD Growth of Carbon Nanotubes
4.3. Characterizations of Catalyst and Carbon Nanotubes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, L.-M.; Zhang, Z.; Qiu, C. Carbon nanotube digital electronics. Nat. Electron. 2019, 2, 499–505. [Google Scholar] [CrossRef]
- Liu, L.; Han, J.; Xu, L.; Zhou, J.; Zhao, C.; Ding, S.; Shi, H.; Xiao, M.; Ding, L.; Ma, Z.; et al. Aligned, high-density semiconducting carbon nanotube arrays for high-performance electronics. Science 2020, 368, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, Y.; Wang, K.; Zhang, Z.; Streit, J.K.; Fagan, J.A.; Tang, J.; Zheng, M.; Yang, C.; Zhu, Z.; et al. DNA-directed nanofabrication of high-performance carbon nanotube field-effect transistors. Science 2020, 368, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Hills, G.; Lau, C.; Wright, A.; Fuller, S.; Bishop, M.D.; Srimani, T.; Kanhaiya, P.; Ho, R.; Amer, A.; Stein, Y.; et al. Modern microprocessor built from complementary carbon nanotube transistors. Nature 2019, 572, 595–602. [Google Scholar] [CrossRef]
- Hersam, M.C. Progress towards monodisperse single-walled carbon nanotubes. Nat. Nanotechnol. 2008, 3, 387–394. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Zhang, D.; Yang, J.; Zheng, M.; Li, Y. Chirality pure carbon nanotubes: Growth, sorting, and characterization. Chem. Rev. 2020, 120, 2693–2758. [Google Scholar] [CrossRef]
- He, M.; Zhang, S.; Zhang, J. Horizontal single-walled carbon nanotube arrays: Controlled synthesis, characterizations, and applications. Chem. Rev. 2020, 120, 12592–12684. [Google Scholar] [CrossRef]
- Zhang, S.; Tong, L.; Zhang, J. The road to chirality-specific growth of single-walled carbon nanotubes. Natl. Sci. Rev. 2018, 5, 310–312. [Google Scholar] [CrossRef]
- Zhao, B.; Futaba, D.N.; Yasuda, S.; Akoshima, M.; Yamada, T.; Hata, K. Exploring advantages of diverse carbon nanotube forests with tailored structures synthesized by supergrowth from engineered catalysts. ACS Nano 2009, 3, 108–114. [Google Scholar] [CrossRef]
- Ahmad, S.; Liao, Y.; Hussain, A.; Zhang, Q.; Ding, E.-X.; Jiang, H.; Kauppinen, E.I. Systematic investigation of the catalyst composition effects on single-walled carbon nanotubes synthesis in floating-catalyst CVD. Carbon 2019, 149, 318–327. [Google Scholar] [CrossRef]
- Shchegolkov, A.V. Synthesis of carbon nanotubes using microwave radiation: Technology, properties, and structure. Russ. J. Gen. Chem. 2022, 92, 1168–1172. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Komarov, F.F.; Lipkin, M.S.; Milchanin, O.V.; Parfimovich, I.D.; Shchegolkov, A.V.; Velichko, A.V.; Chebotov, K.D.; Nokhaeva, V.A. Synthesis and study of cathode materials based on carbon nanotubes for lithium-ion batteries. Inorg. Mater. Appl. Res. 2021, 12, 1281–1287. [Google Scholar] [CrossRef]
- Sari, A.H.; Khazali, A.; Parhizgar, S.S. Synthesis and characterization of long-CNTs by electrical arc discharge in deionized water and NaCl solution. Inter. Nano Lett. 2018, 8, 19–23. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.-Q.; Xu, Z.; Peng, F.; et al. Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 2014, 510, 522–524. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, L.; Wang, X.; Tong, L.; Yang, L.; Wang, Z.; Qi, K.; Deng, S.; Li, Q.; Bai, X.; et al. Arrays of horizontal carbon nanotubes of controlled chirality grown using designed catalysts. Nature 2017, 543, 234–238. [Google Scholar] [CrossRef]
- He, M.; Wang, X.; Zhang, S.; Jiang, H.; Cavalca, F.; Cui, H.; Wagner, J.B.; Hansen, T.W.; Kauppinen, E.; Zhang, J.; et al. Growth kinetics of single-walled carbon nanotubes with a (2n, n) chirality selection. Sci. Adv. 2019, 5, eaav9668. [Google Scholar] [CrossRef]
- Ding, F.; Harutyunyan, A.R.; Yakobson, B.I. Dislocation theory of chirality-controlled nanotube growth. Proc. Natl. Acad. Sci. USA 2009, 106, 2506–2509. [Google Scholar] [CrossRef]
- Artyukhov, V.I.; Penev, E.S.; Yakobson, B.I. Why nanotubes grow chiral? Nat. Commun. 2014, 5, 4892. [Google Scholar] [CrossRef]
- Wang, X.; Ding, F. How a solid catalyst determines the chirality of the single-wall carbon nanotube grown on it. J. Phys. Chem. Lett. 2019, 10, 735–741. [Google Scholar] [CrossRef]
- Qiu, L.; Ding, F. Understanding single-walled carbon nanotube growth for chirality controllable synthesis. Acc. Chem. Res. 2021, 2, 828–841. [Google Scholar] [CrossRef]
- He, M.; Zhang, S.; Wu, Q.; Xue, H.; Xin, B.; Wang, D.; Zhang, J. Designing catalysts for chirality-selective synthesis of single-walled carbon nanotubes: Past success and future opportunity. Adv. Mater. 2019, 31, 1800805. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, Y.; Wei, L.; Goh, K.; Yu, D.; Chen, Y. Catalysts for chirality selective synthesis of single-walled carbon nanotubes. Carbon 2015, 81, 1–19. [Google Scholar] [CrossRef]
- Takagi, D.; Homma, Y.; Hibino, H.; Suzuki, S.; Kobayashi, Y. Single-walled carbon nanotube growth from highly activated metal nanoparticles. Nano Lett. 2006, 6, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Ding, L.; Chu, H.; Feng, Y.; McNicholas, T.P.; Liu, J. Horizontally aligned single-walled carbon nanotube on quartz from a large variety of metal catalysts. Nano Lett. 2008, 8, 2576–2579. [Google Scholar] [CrossRef]
- Ding, L.P.; McLean, B.; Xu, Z.; Kong, X.; Hedman, D.; Qiu, L.; Page, A.J.; Ding, F. Why carbon nanotubes grow. J. Am. Chem. Soc. 2022, 144, 5606–5613. [Google Scholar] [CrossRef]
- Robertson, J. Heterogeneous catalysis model of growth mechanisms of carbon nanotubes, graphene and silicon nanowires. J. Mater. Chem. 2012, 22, 19858–19862. [Google Scholar] [CrossRef]
- Maruyama, T. Current status of single-walled carbon nanotube synthesis from metal catalysts by chemical vapor deposition. Mater. Express. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Nørskov, J.K. Covalent effects in the effective-medium theory of chemical binding: Hydrogen heats of solution in the 3d metals. Phys. Rev. B 1982, 26, 2875–2885. [Google Scholar] [CrossRef]
- Silvearv, F.; Larsson, P.; Jones, S.L.; Ahuja, R.; Larsson, J.A. Establishing the most favorable metal-carbon bond strength for carbon nanotube catalysts. J. Mater. Chem. C 2015, 3, 3422–3427. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, L.; Yao, X.; Zhang, L.; Wu, Q.; Li, D.; Ma, C.; Zhao, N.; Xin, L.; Liu, C.; et al. Solid supported ruthenium catalyst for growing single-walled carbon nanotubes with narrow chirality distribution. Carbon 2022, 193, 35–41. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Zhang, L.; Qian, L.; Zhao, Y.; Tian, Y.; Wu, Q.; Li, D.; Zhao, N.; Zhang, X.; et al. Bulk growth and separation of single-walled carbon nanotubes from rhenium catalyst. Nano Res. 2022, 15, 5575–5780. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Ding, F.; Larsson, P.; Larsson, J.A.; Ahuja, R.; Duan, H.; Rosen, A.; Bolton, K. The importance of strong carbon-metal adhesion for catalytic nucleation of single-walled carbon nanotubes. Nano Lett. 2008, 8, 463–468. [Google Scholar] [CrossRef]
- Lv, S.; Wu, Q.; Xu, Z.; Yang, T.; Jiang, K.; He, M. Chirality distribution of single-walled carbon nanotubes grown from gold nanoparticles. Carbon 2022, 192, 259–264. [Google Scholar] [CrossRef]
- Hammer, B.; Morikawa, Y.; Nørskov, J.K. CO chemisorption at metal surfaces and overlayers. Phys. Rev. Lett. 1996, 76, 2141. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, X.; Zhang, L.; Wu, Q.; Song, X.; Chernov, A.I.; Fedotov, P.V.; Obraztsova, E.D.; Sainio, J.; Jiang, H.; et al. Anchoring effect of Ni2+ in stabilizing reduced metallic particles for growing single-walled carbon nanotubes. Carbon 2018, 128, 249–256. [Google Scholar] [CrossRef]
- Yu, Q.; Pan, H.; Zhao, M.; Liu, Z.; Wang, J.; Chen, Y.; Gong, M. Influence of calcination temperature on the performance of Pd-Mn/SiO2-Al2O3 catalysts for ozone decomposition. J. Hazard. Mater. 2009, 172, 631–634. [Google Scholar] [CrossRef]
- Qin, C.; Guo, Q.; Guo, J.; Chen, P. Atomically dispersed Pd atoms on a simple MgO support with an ultralow loading for selective hydrogenation of acetylene to ethylene. Chem. Asian J. 2021, 16, 1225–1228. [Google Scholar] [CrossRef]
- Babucci, M.; Guntida, A.; Gates, B.C. Atomically dispersed metals on well-defined supports including zeolites and metal-organic frameworks: Structure, bonding, reactivity, and catalysis. Chem. Rev. 2020, 120, 11956–11985. [Google Scholar] [CrossRef]
- Nelson, N.C.; Chen, L.; Meira, D.; Kovarik, L.; Szanyi, J. In situ dispersion of palladium on TiO2 during reverse water-gas shift reaction: Formation of atomically dispersed palladium. Angew. Chem. Int. Ed. 2020, 59, 17657–17663. [Google Scholar] [CrossRef]
- He, M.; Magnin, Y.; Jiang, H.; Amara, H.; Kauppinen, E.I.; Loiseau, A.; Bichara, C. Growth modes and chiral selectivity of single-walled carbon nanotubes. Nanoscale 2018, 10, 6744–6750. [Google Scholar] [CrossRef]
- He, M.; Magnin, Y.; Amara, H.; Jiang, H.; Cui, H.; Fossard, F.; Castan, A.; Kauppinen, E.; Loiseau, A.; Bichara, C. Linking growth mode to lengths of single-walled carbon nanotubes. Carbon 2017, 113, 231–236. [Google Scholar] [CrossRef]
- Diaz, M.C.; Balbuena, P.B. On the role of surface oxygen during nascent single-walled carbon nanotube cap spreading and tube nucleation on iron catalysts. Carbon 2021, 184, 470–478. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Yu, Z.; Brus, L.E. (n, m) Structural assignments and chirality dependence in single-wall carbon nanotube Raman scattering. J. Phys. Chem. B 2001, 105, 6831–6837. [Google Scholar] [CrossRef]
- Wu, Q.; Qiu, L.; Zhang, L.; Liu, H.; Ma, R.; Xie, P.; Liu, R.; Hou, P.; Ding, F.; Liu, C.; et al. Temperature-dependent selective nucleation of single-walled carbon nanotubes from stabilized catalyst nanoparticles. Chem. Eng. J. 2022, 431, 133487. [Google Scholar] [CrossRef]
- Han, F.; Qian, L.; Wu, Q.; Li, D.; Hao, S.; Feng, L.; Xin, L.; Yang, T.; Zhang, J.; He, M. Narrow-chirality distributed single-walled carbon nanotube synthesized from oxide promoted Fe–SiC catalyst. Carbon 2022, 191, 146–152. [Google Scholar] [CrossRef]
- Bachilo, S.M.; Strano, M.S.; Kittrell, C.; Hauge, R.H.; Smalley, R.E.; Weisman, R.B. Structure-assigned optical spectra of single-walled carbon nanotubes. Science 2002, 298, 2361–2366. [Google Scholar] [CrossRef]
- Yuan, Q.; Ding, F. How a zigzag carbon nanotube grows. Angew. Chem. 2015, 127, 6022–6026. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Yao, F.; He, M.; Lin, D.; Ma, H.; Sun, Y.; Zhao, Q.; Liu, K.; Ding, F.; et al. Controllable growth of (n, n−1) family of semiconducting carbon nanotubes. Chem 2019, 5, 1182–1193. [Google Scholar] [CrossRef]
- Hao, S.; Qian, L.; Wu, Q.; Li, D.; Han, F.; Feng, L.; Xin, L.; Yang, T.; Wang, S.; Zhang, J.; et al. Subnanometer single-walled carbon nanotube growth from Fe-containing layered double hydroxides. Chem. Eng. J. 2022, 446, 137087. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Li, D.; Feng, L.; Wang, Y.; Zhang, L.; Qian, L.; Zhao, W.; Xu, N.; Chi, X.; Wang, S.; et al. (n, m) Distribution of Single-Walled Carbon Nanotubes Grown from a Non-Magnetic Palladium Catalyst. Molecules 2023, 28, 2453. https://doi.org/10.3390/molecules28062453
Qin X, Li D, Feng L, Wang Y, Zhang L, Qian L, Zhao W, Xu N, Chi X, Wang S, et al. (n, m) Distribution of Single-Walled Carbon Nanotubes Grown from a Non-Magnetic Palladium Catalyst. Molecules. 2023; 28(6):2453. https://doi.org/10.3390/molecules28062453
Chicago/Turabian StyleQin, Xiaofan, Dong Li, Lihu Feng, Ying Wang, Lili Zhang, Liu Qian, Wenyue Zhao, Ningning Xu, Xinyan Chi, Shiying Wang, and et al. 2023. "(n, m) Distribution of Single-Walled Carbon Nanotubes Grown from a Non-Magnetic Palladium Catalyst" Molecules 28, no. 6: 2453. https://doi.org/10.3390/molecules28062453
APA StyleQin, X., Li, D., Feng, L., Wang, Y., Zhang, L., Qian, L., Zhao, W., Xu, N., Chi, X., Wang, S., & He, M. (2023). (n, m) Distribution of Single-Walled Carbon Nanotubes Grown from a Non-Magnetic Palladium Catalyst. Molecules, 28(6), 2453. https://doi.org/10.3390/molecules28062453