Effect of Immunomodulating Extract and Some Isolates from Etlingera rubroloba A.D. Poulsen Fruits on Diabetic Patients with Tuberculosis
Abstract
1. Introduction
2. Results
2.1. Phagocytosis Activity and Levels of Interleukin-12 Extracts and Fractions In Vivo
2.2. Isolation and Purification of Fraction C
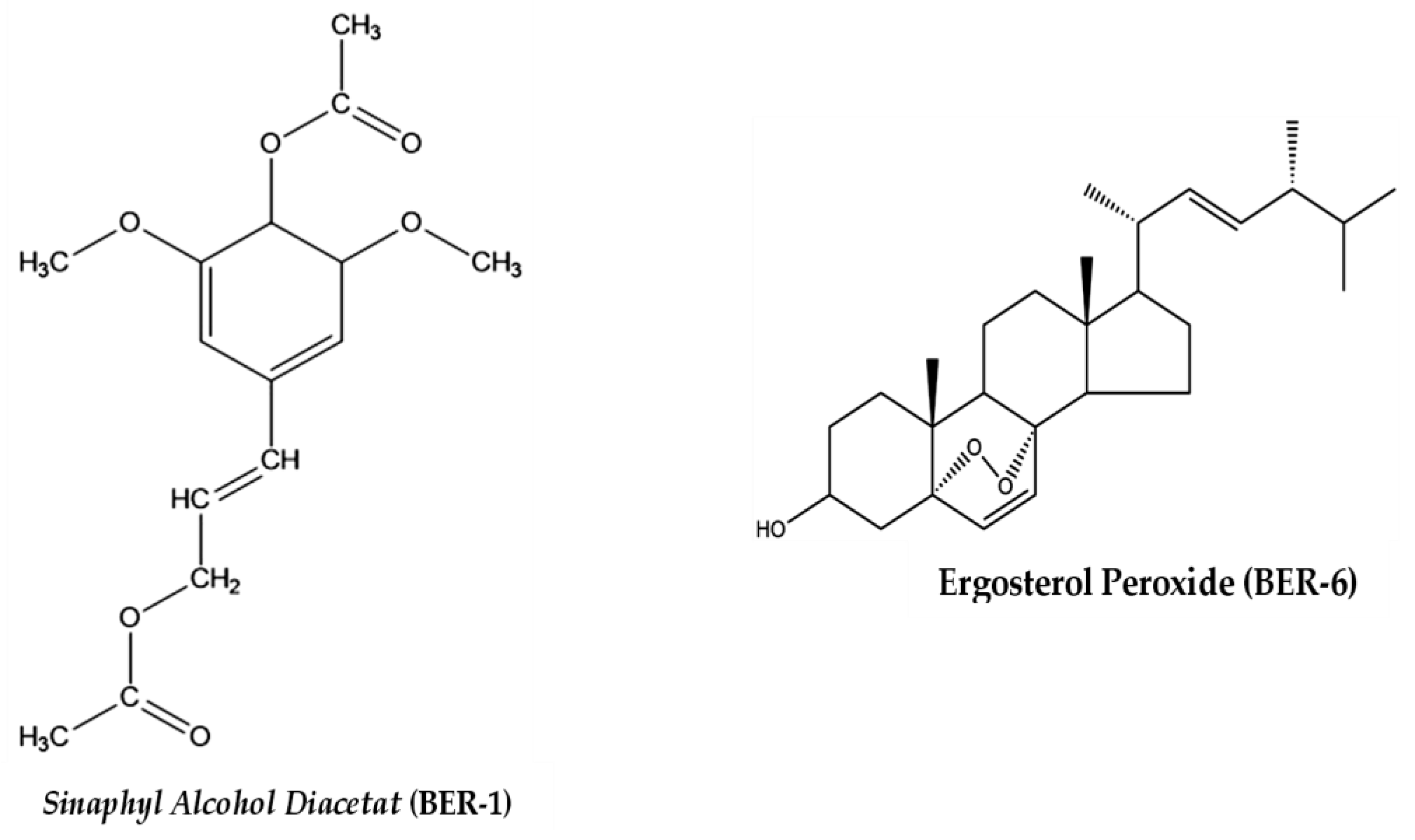
2.3. Identification of Isolated Compound Structures
2.4. Immunomodulating Effects of E. rubroloba Fruit In Vitro
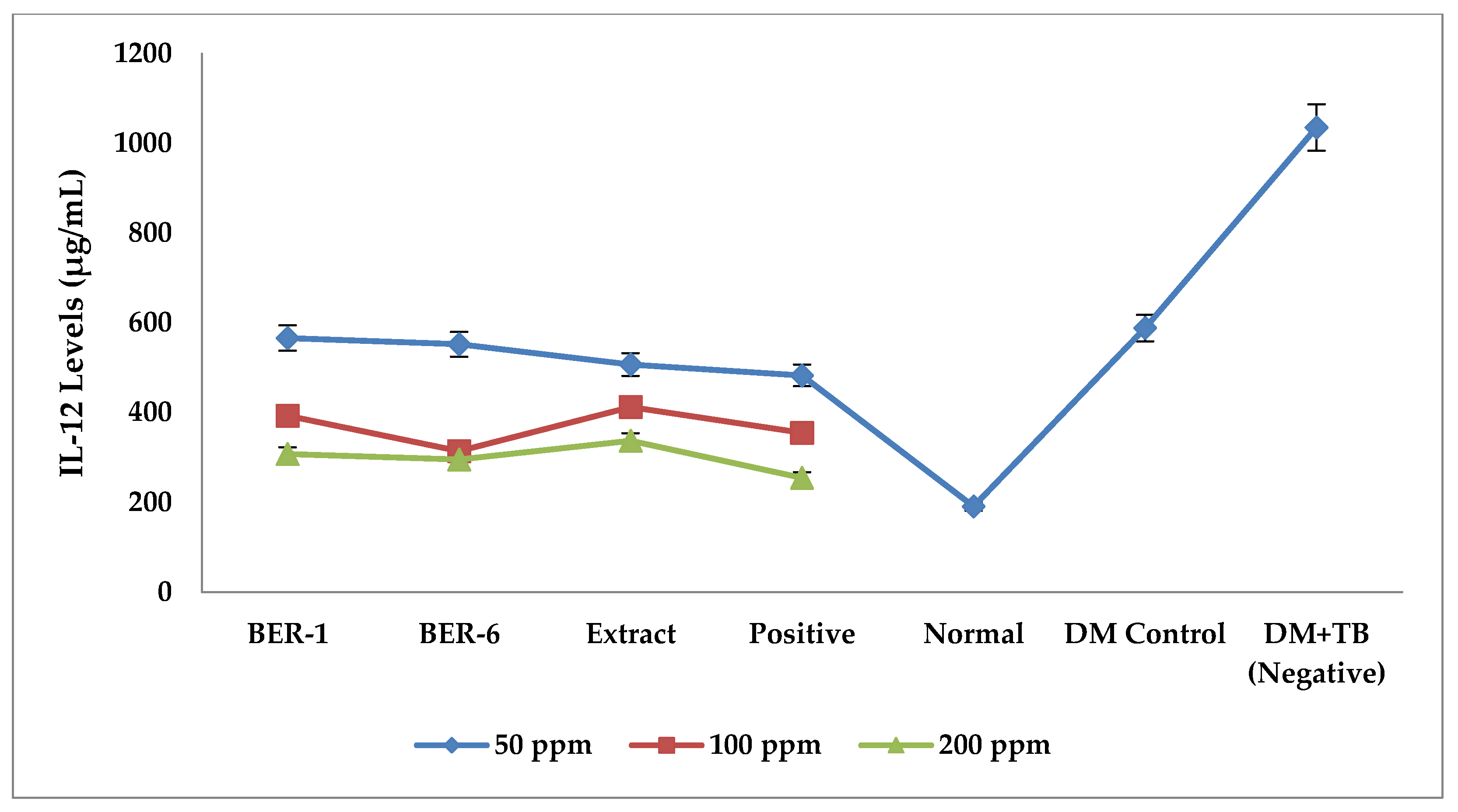
Effect of Extracts and Isolate Compounds on IL-12 Levels
2.5. Effects of Extracts and Isolate Compounds on TLR-2 Protein Expression
2.6. Effects of Extracts and Isolate Compounds on HLA-DR Protein Expression
3. Discussion
4. Materials and Methods
4.1. Sampling and Preparation of Samples
4.2. Extraction and Fractionation
4.3. Screening Test of Phagocytosis Activity and Levels of Interleukin-12 (IL-12) Extracts and Fractions In Vivo
- FA group: BALB/c mice were given fraction A at a dose of 0.1 mg/g bw;
- FB group: BALB/c mice were given fraction B at a dose of 0.1 mg/g bw;
- FC group: BALB/c mice were given fraction C at a dose of 0.1 mg/g bw;
- FD group: BALB/c mice were given fraction D at a dose of 0.1 mg/g bw;
- FE group: BALB/c mice were given fraction E at a dose of 0.1 mg/g bw;
- FF group: BALB/c mice were given fraction F at a dose of 0.1 mg/g bw;
- FEA group: BALB/c mice were given fraction Ethyl acetate at a dose of 0.1 mg/g bw;
- BER-X group: BALB/c mice were given E. rubroloba fruit extract at a dose of 0.1 mg/g bw;
- K+ group: BALB/c mice were given a commercial meniran (Phyllanthus niruri Linn.) extract suspension at a dose of 0.101 mg/g bw;
- Group K-: BALB/c mice were given 0.5% Na-CMC suspension;
- KN group: the control group of normal test animals was only given standard feed.
4.4. Isolation and Purification of E. rubroloba Fruit Isolates
4.5. Identification of Isolate Compound Structures
4.6. Immunomodulation Test of Extracts and Isolates in an In Vitro DM Model Stimulated with TB Antigen
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Report on Diabetes 2016; WHO Library Cataloguing-in-Publication Data: Geneva, Switzerland. Available online: http://www.who.int (accessed on 15 January 2023).
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Segura-Cerda, C.A.; López-Romero, W.; Flores-Valdez, M.A. Changes in Host Response to Mycobacterium tuberculosis Infection Associated With Type 2 Diabetes: Beyond Hyperglycemia. Front. Cell. Infect. Microbiol. 2019, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Harries, A.D.; Jeon, C.Y.; Hart, J.E.; Kapur, A.; Lönnroth, K.; Ottmani, S.-E.; Goonesekera, S.D.; Murray, M.B. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011, 9, 81. [Google Scholar] [CrossRef]
- Workneh, M.H.; Bjune, G.A.; Yimer, S.A. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: A systematic review. PLoS ONE 2017, 12, e0175925. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019; Licence: CC BY-NC-SA 3.0 IGO; Available online: http://apps.who.int/iris (accessed on 15 January 2023).
- Kumar Nathella, P.; Babu, S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017, 152, 13–24. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The Immune Response in Tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Roat, C.; Ramawat, K.G. Morphactin and 2iP markedly enhance accumulation of stilbenes in cell cultures of Cayratia trifolia (L.) Domin. Acta Physiol. Plant. 2009, 31, 411–414. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Kaur, R.; Bhat, Z.A.; Gupta, V.K.; Kumar, V. A review of immunomodulators in the Indian traditional health care system. J. Microbiol. Immunol. Infect. 2012, 45, 165–184. [Google Scholar] [CrossRef]
- Ilyas, Y.M.; Diantini, A.; Ghozali, M.; Sahidin, I.; Fristiohady, A. Immunomodulatory Potency Etlingera rubroloba A.D. Poulsen Fruit Ethanol extract against Macrophage Phagocytic Activity and CD4 Levels in Wistar Male Rats. Res. J. Pharm. Technol. 2022, 15, 4067–4072. [Google Scholar] [CrossRef]
- Jabbar, A.; Wahyuono, S.; Sahidin, I.; Puspitasari, I. Xanthine Oxidase Inhibitory Activity and DPPH radical scavenging Assay of isolated compound from Etlingera rubroloba (Blume) A.D Poulsen stem. Int. J. Pharm. Res. 2020, 13, 2020. [Google Scholar]
- Jabbar, A.; Wahyuono, S.U.B.A.G.U.S.; Puspitasari, I.; Sahidin, I. Free radical scavenging activity of methanol extract and compounds isolated from stems of Etlingera rubroloba A.D. Poulsen. Int. J. Pharm. Res. 2021, 13, 1099–1105. [Google Scholar]
- Jabbar, A.; Sahidin, I.; Monstavevi, S.; Malaka, M.; Malik, F.; Ilyas, Y. Antioxidant and Anti-Inflammatory Activity of Ethanol Extract Stem of Etlingera rubroloba A.D. Poulsen. Pak. J. Biol. Sci. 2022, 25, 885–891. [Google Scholar] [CrossRef]
- Ilyas, Y.M.; Diantini, A.; Halimah, E.L.I.; Amalia, R. Potential Immunomodulator Fraction Fruit Of Etlingera rubroloba A.D Poulsen Against Macrophage Phagocytosis And Interleukin-12 Levels In BCG-Stimulated Balb/C Mice. Int. J. Pharm. Res. 2021, 13, 3262–3269. [Google Scholar]
- Diantini, A.; Halimah, E.; Amalia, R.; Ghozali, M.; Julaeha, E.; Sahidin, I.; Jabbar, A. Phytochemical Analysis and Immunomodulatory Potential on Diabetic-Infected Tuberculosis by Fruit Etlingera rubroloba A.D. Poulsen. Pak. J. Biol. Sci. 2022, 25, 669–675. [Google Scholar] [CrossRef]
- Sabilu, Y.; Mukaddin, A.; Bittikaka, Y.; Tawa, R.A.; Paddo, J.; Saptaputra, S.K. Advances in Environmental Biology The Utilization of Sikala (Etlingera elatior) As Traditional Medicine in Porehu District, North Kolaka Regency, Southeast Sulawesi Province, Indonesia. Adv. Environ. Biol. 2017, 11, 5–9. [Google Scholar]
- Poulsen, A. Etlingera of Sulawesi; Natural History Publications (Borneo): Kinabalu, SBH, Malaysia, 2012; ISBN 978-983-812-138-5. [Google Scholar]
- Lachumy, S.J.T.; Sasidharan, S.; Sumathy, V.; Zuraini, Z. Pharmacological activity, phytochemical analysis and toxicity of methanol extract of Etlingera elatior (torch ginger) flowers. Asian Pac. J. Trop. Med. 2010, 3, 769–774. [Google Scholar] [CrossRef]
- Wegner, M.; Winiarska, H.; Bobkiewicz-kozłowska, T.; Dworacka, M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas Cytokine IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine 2008, 42, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.; Ho, K.; Tsao, C.; Chang, Y.; Shiau, M. Role of Cyotkines in Metabolism and Type 2 Diabetes Mellitus. Int. J. Biomed. Lab. Sci. 2013, 2, 1–6. [Google Scholar]
- Ali, M.; Mali, V.; Haddox, S.; AbdelGhany, S.M.; El-Deek, S.E.; Abulfadl, A.; Matrougui, K.; Belmadani, S. Essential Role of IL-12 in Angiogenesis in Type 2 Diabetes. Am. J. Pathol. 2017, 187, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Essone, P.N.; Leboueny, M.; Maloupazoa Siawaya, A.C.; Alame-Emane, A.K.; Aboumegone Biyogo, O.C.; Dapnet Tadatsin, P.H.; Mveang Nzoghe, A.; Essamazokou, D.U.; Mvoundza Ndjindji, O.; Padzys, G.S.; et al. M. tuberculosis infection and antigen specific cytokine response in healthcare workers frequently exposed to tuberculosis. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Robinson, C.M.; Nau, G.J. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J. Infect. Dis. 2008, 198, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, J.; Fieschi, C.; Doffinger, R.; Feinberg, M.; Leclerc, T.; Boisson-Dupuis, S.; Picard, C.; Bustamante, J.; Chapgier, A.; Filipe-Santos, O.; et al. Bacillus Calmette Guérin triggers the IL-12/IFN-γ axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur. J. Immunol. 2004, 34, 3276–3284. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, Z.; Kiani, Z.; Nasiri, A.A.; Kohan, F. Toll-like receptor 2 and type 2 diabetes. Cell. Mol. Biol. Lett. 2016, 21, 2. [Google Scholar] [CrossRef]
- Thuong, N.T.T.; Hawn, T.R.; Thwaites, G.; Chau, T.T.H.; Lan, N.T.N.; Quy, H.T.; Hieu, N.T.; Aderem, A.; Hien, T.T.; Farrar, J.; et al. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 2007, 8, 422–428. [Google Scholar] [CrossRef]
- Jin, C.; Henao-Mejia, J.; Flavell, R.A. Innate Immune Receptors: Key Regulators of Metabolic Disease Progression. Cell Metab. 2013, 17, 873–882. [Google Scholar] [CrossRef]
- Aghamiri, S.H.; Komlakh, K.; Ghaffari, M. The crosstalk among TLR2, TLR4 and pathogenic pathways; a treasure trove for treatment of diabetic neuropathy. Inflammopharmacology 2022, 30, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Padhi, A.; Pattnaik, K.; Biswas, M.; Jagadeb, M.; Behera, A.; Sonawane, A. Mycobacterium tuberculosis LprE Suppresses TLR2-Dependent Cathelicidin and Autophagy Expression to Enhance Bacterial Survival in Macrophages. J. Immunol. 2019, 203, 2665–2678. [Google Scholar] [CrossRef]
- Kohanawa, M.; Zhao, S.; Ozaki, M.; Haga, S.; Nan, G.; Kuge, Y.; Tamaki, N. Contribution of Toll-Like Receptor 2 to the Innate Response against Staphylococcus aureus Infection in Mice. PLoS ONE 2013, 8, e74287. [Google Scholar]
- Jialal, I.; Kaur, H. The Role of Toll-Like Receptors in Diabetes-Induced Inflammation: Implications for Vascular Complications. Curr. Diabetes Rep. 2012, 12, 172–179. [Google Scholar] [CrossRef]
- Morris, J.; Williams, N.; Rush, C.; Govan, B.; Sangla, K.; Norton, R.; Ketheesan, N. Burkholderia pseudomallei triggers altered inflammatory profiles in a whole-blood model of type 2 diabetes-melioidosis comorbidity. Infect. Immun. 2012, 80, 2089–2099. [Google Scholar] [CrossRef]
- Durinovic-Bello, I. Autoimmune Diabetes: The Role of T Cells, MHC Molecules and Autoantigens. Autoimmunity 1998, 27, 159–177. [Google Scholar] [CrossRef]
- Kust, S.A.; Streltsova, M.A.; Panteleev, A.V.; Karpina, N.L.; Lyadova, I.V.; Sapozhnikov, A.M.; Kovalenko, E.I. HLA-DR-Positive NK Cells Expand in Response to Mycobacterium tuberculosis Antigens and Mediate Mycobacteria-Induced T Cell Activation. Front. Immunol. 2021, 12, 662128. [Google Scholar] [CrossRef]
- Clement, C.C.; Becerra, A.; Yin, L.; Zolla, V.; Huang, L.; Merlin, S.; Follenzi, A.; Shaffer, S.A.; Stern, L.J.; Santambrogio, L. The Dendritic Cell Major Histocompatibility Complex II (MHC II) Peptidome Derives from a Variety of Processing Pathways and Includes Peptides with a Broad Spectrum of HLA-DM Sensitivity. J. Biol. Chem. 2016, 291, 5576–5595. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Vomund, A.N.; Peterson, O.J.; Chervonsky, A.V.; Lichti, C.F.; Unanue, E.R. The MHC-II peptidome of pancreatic islets identifies key features of autoimmune peptides. Nat. Immunol. 2020, 21, 455–463. [Google Scholar] [CrossRef]
- Tuomilehto-Wolf, E.; Tuomilehto, J.; Hitman, G.A.; Nissinen, A.; Stengard, J.; Pekkanen, J.; Kivinen, P.; Kaarsalo, E.; Karvonen, M.J. Genetic susceptibility to non-insulin dependent diabetes mellitus and glucose intolerance are located in HLA region. BMJ 1993, 307, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, V.V.; Rakh, S.S.; Radha, B.A.; Priya, V.H.S.; Pantula, V.; Jasti, S.; Latha, G.S.; Murthy, K. Role of HLA-B51 and HLA-B52 in susceptibility to pulmonary tuberculosis. Infect. Genet. Evol. 2006, 6, 436–439. [Google Scholar] [CrossRef]
- Fernando, J.; Figueiredo, D.C.; De Lourdes, M.; Rodrigues, V. HLA Profile in Patients with AIDS and Tuberculosis. Braz. J. Infect. Dis. 2008, 12, 278–280. [Google Scholar]
- Çelik, A.; Yaman, H.; Turan, S.; Kara, A.; Kara, F.; Zhu, B.; Qu, X.; Tao, Y.; Zhu, Z.; Dhokia, V.; et al. Isolation, Elucidation & Cancer Chemoprevention Mechhanisms of Secondary Metabolites from Etlingera coccinea (TUHAU). J. Mater. Process. Technol. 2018, 1, 1–8. [Google Scholar]
- Yuliastri, W.O.; Diantini, A.; Ghozali, M.; Sahidin, I.; Isrul, M. Immunomodulatory activity and phytochemical analysis of Hibiscus sabdariffa L. flower fractions. J. Appl. Pharm. Sci. 2021, 11, 131–140. [Google Scholar] [CrossRef]
- Wahyuni, W.; Diantini, A.; Ghozali, M.; Subarnas, A.; Julaeha, E.; Amalia, R.; Fristiohady, A.; Sundowo, A.; Fajriah, S.; Hadisaputri, Y.E.; et al. In-Vitro Anticancer Activity of Chemical Constituents from Etlingera alba Poulsen against Triple Negative Breast Cancer and in silico Approaches towards Matrix metalloproteinase-1 Inhibition. Indones. J. Sci. Technol. 2022, 7, 251–278. [Google Scholar] [CrossRef]
- Yusuf, M.I.; Susanty, S.; Fawwaz, M. Antioxidant and Antidiabetic Potential of Galing Stem Extract (Cayratia trifolia Domin). Pharmacogn. J. 2018, 10, 686–690. [Google Scholar] [CrossRef]
- Sabahi, Z.; Khoshnoud, M.J.; Hosseini, S.; Khoshraftar, F.; Rashedinia, M. Syringic Acid Attenuates Cardiomyopathy in Streptozotocin-Induced Diabetic Rats. Adv. Pharmacol. Pharm. Sci. 2021, 2021, e5018092. [Google Scholar] [CrossRef] [PubMed]
- Qinna, N.; Badwan, A. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des. Dev. Ther. 2015, 9, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Diantini, A.; Ghozali, M.; Subarnas, A.; Julaeha, E.; Amalia, R.; Sahidin, I. Phytochemical Screening, Toxicity Activity and Antioxidant Capacity of Ethanolic Extract of Etlingera alba Rhizome. Pakistan J. Biol. Sci. PJBS 2021, 24, 807–814. [Google Scholar]
- Lundanes, E.; Reubsaet, L.; Greibrokk, T. Chromatography: Basic Principles, Sample Preparations and Related Methods; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Fristiohady, A.; Sadarun, B.; Bafadal, M.; Andriani, R.; Purnama, L.O.M.J.; Malik, F.; Ilyas, M.; Malaka, M.H.; Sahidin, I. Pharmacological activity of compounds isolated from methanolic extract marine sponge xestospongia sp. Against Escherichia coli and Staphylococcus aureus. J. Phys. Conf. Ser. 2021, 1899, 012048. [Google Scholar] [CrossRef]
- Grosick, R.; Alvarado-Vazquez, P.A.; Messersmith, A.R.; Romero-Sandoval, E.A. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J. Pain Res. 2018, II, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, R.Y.; Tambunan, B.A.; Nugraha, J.; Srioetami, F. IFN-γ Expression by CD4+ and CD8+ T Cells After Stimulation of ESAT-6-CFP-10 Fusion Antigen in Active Pulmonary Tuberculosis Patients. Bul. Penelit. Kesehat. 2017, 45, 223–226. [Google Scholar]
- Abeles, D.; McPhail, M.J.; Sowter, D.; Antoniades, C.G.; Vergis, N.; Vijay, G.K.M.; Xystrakis, E.; Khamri, W.; Shawcross, D.L.; Ma, Y.; et al. CD14, CD16 and HLA-DR Reliably Identifies Human Monocytes and Their Subsets in the Context of Pathologically Reduced HLA-DR Expression by CD14 hi/CD16 neg Monocytes: Expansion of CD14 hi / CD16 pos and Contraction of CD14 lo/CD16 pos Monocytes in Ac. Cytom. Part A 2012, 81A, 823–834. [Google Scholar] [CrossRef]
- Andrade, B.B.; Singh, A.; Narendran, G.; Schechter, M.E.; Nayak, K.; Subramanian, S.; Anbalagan, S.; Jensen, S.M.R.; Porter, B.O.; Antonelli, L.R.; et al. Mycobacterial Antigen Driven Activation of CD14++CD16− Monocytes Is a Predictor of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. PLoS Pathog. 2014, 10, e1004433. [Google Scholar] [CrossRef]



| Groups | Phagocytic Activity Average ± SEM (%) | IL-12 Levels Average ± SEM (ng/mL) |
|---|---|---|
| FA (Fraction A 0.1 mg/g bw) | 52.25 ± 2.30 *a | 22.37 ± 0.18 *a |
| FB (Fraction B 0.1 mg/g bw) | 50.55 ± 2.00 *a | 23.11 ± 0.28 *a |
| FC (Fraction C 0.1 mg/g bw) | 59.35 ± 1.71 *a,*b | 19.09 ± 0.14 *a,*b |
| FD (Fraction D 0.1 mg/g bw) | 51.0 ± 2.10 *a | 21.02 ± 0.06 *a |
| FE (Fraction E 0.1 mg/g bw) | 49.25 ± 1.81 *a | 25.03 ± 0.12 *a |
| FF (Fraction F 0.1 mg/g bw) | 47.00 ± 2.72 *a | 28.05 ± 0.13 *a |
| FEA (Ethylacetate fraction 0.1 mg/g bw) | 60.25 ± 2.00 *a,*b | 21.22 ± 0.11 *a,*b |
| BER-X (Ethanol extract 0.1 mg/g bw) | 63.75 ± 2.15 *a,*b | 20.01 ± 0.11 *a,*b |
| K+ (Positive control 0.1 mg/g bw) | 51.25 ± 2.16 *a | 22.22 ± 0.65 *a |
| K- (Negative control/DM+TB) | 29.67 ± 2.06 | 31.51 ± 0.40 |
| Normal | 0 | 15.467 ± 0.41 |
| No.C | BER-1 | BER-6 | ||||
|---|---|---|---|---|---|---|
| δ C | δ H | (∑H, m, J (Hz)) | δ C | δ H | (∑H, m, J (Hz)) | |
| 1 | 134.73 | - | - | 37.0 | - | - |
| 2 | 103.30 | 6.63 | (1H, s) | 30.2 | - | - |
| 3 | 152.26 | - | - | 66.5 | 3.95 | (1H, m) |
| 4 | 128.63 | - | - | 51.2 | - | - |
| 5 | 152.26 | - | - | 79.4 | - | - |
| 6 | 103.30 | 6.63 | (1H, s) | 130.7 | 6.23 | (1H, d, 8.0) |
| 7 | 134.06 | 6.57 | (1H, d, 15.85) | 135.2 | 6.49 | (1H, d, 8.0) |
| 8 | 123.71 | 6.22 | (1H, dt,15.87, 6.71) | 82.1 | - | - |
| 9 | 64.97 | 4.70 | (2H, dd, 6.10, 1.22) | 51.7 | - | - |
| 10 | 170.97 | - | - | 34.7 | - | - |
| 11 | 21.12 | 2.32 | (3H, s) | 20.9 | - | - |
| 12 | 168.85 | - | - | 39.4 | - | - |
| 13 | 20.57 | 2.10 | (3H, s) | 44.6 | - | - |
| 14 | 56.21 | 3.82 | (6H, s) | 37.0 | - | - |
| 15 | 56.21 | - | - | 23.4 | - | - |
| 16 | - | - | - | 28.6 | - | - |
| 17 | - | - | - | 56.3 | - | - |
| 18 | - | - | - | 12.9 | 0.8 | (3H, s) |
| 19 | - | - | - | 18.2 | 0.87 | (3H, s) |
| 20 | - | - | - | 39.7 | - | - |
| 21 | - | - | - | 19.6 | 0.98 | (3H, d, 7.0) |
| 22 | - | - | - | 135.4 | 5.12 | (1H, dd, 15.5, 8.5) |
| 23 | - | - | - | 132.4 | 5.20 | (1H, ddd, 7.5, 15.2) |
| 24 | - | - | - | 42.8 | - | - |
| 25 | - | - | - | 23.1 | - | - |
| 26 | - | - | - | 20.6 | 0.82 | (3H, d, 7.0) |
| 27 | - | - | - | 19.9 | 0.81 | (3H, d, 6.5) |
| 28 | - | - | - | 17.5 | 0.89 | (3H, d, 7.0) |
| Groups | IL-12 Levels Average ± SEM (µg/mL) |
|---|---|
| BER-1 | 422.32 ± 58 *a,*b |
| BER-6 | 454.04 ± 47 *a,*b |
| Extract | 485.65 ± 36 *a,*b |
| Positive | 272.12 ± 20 *a |
| Normal | 190.91 ± 46.08 *a |
| DM control | 763.33 ± 96.37 *a |
| DM+TB (negative) | 1082.12 ± 178.17 |
| Groups | TLR-2 Expression Average ± SEM (%) |
|---|---|
| BER-1 | 5.31 ± 1.38 *a |
| BER-6 | 4.39 ± 0.41 *a |
| Extract | 3.52 ± 0.72 *a |
| Positive | 5.04 ± 0.85 *a |
| Normal | 1.47 ± 0.49 *a |
| DM control | 7.68 ± 0.42 *a |
| DM+TB (negative) | 17.89 ± 1.10 |
| Groups | HLA-DR Expression Average ± SEM (%) |
|---|---|
| BER-1 | 5.6 ± 0.77 *a |
| BER-6 | 3.74 ± 0.65 *a,*b |
| Extract | 9.57 ± 0.38 *a,*b |
| Positive | 7.37 ± 0.73 *a |
| Normal | 1.47 ± 0.49 *a |
| DM control | 1.8 ± 0.34 *a |
| DM+TB (negative) | 0.72 ± 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas Y., M.; Sahidin, I.; Jabbar, A.; Yodha, A.W.M.; Diantini, A.; Pradipta, I.S.; Amalia, R.; Febrianti, R.M.; Hadisaputri, Y.E.; Ghozali, M.; et al. Effect of Immunomodulating Extract and Some Isolates from Etlingera rubroloba A.D. Poulsen Fruits on Diabetic Patients with Tuberculosis. Molecules 2023, 28, 2401. https://doi.org/10.3390/molecules28052401
Ilyas Y. M, Sahidin I, Jabbar A, Yodha AWM, Diantini A, Pradipta IS, Amalia R, Febrianti RM, Hadisaputri YE, Ghozali M, et al. Effect of Immunomodulating Extract and Some Isolates from Etlingera rubroloba A.D. Poulsen Fruits on Diabetic Patients with Tuberculosis. Molecules. 2023; 28(5):2401. https://doi.org/10.3390/molecules28052401
Chicago/Turabian StyleIlyas Y., Muhammad, Idin Sahidin, Asriullah Jabbar, Agung W. M. Yodha, Ajeng Diantini, Ivan Surya Pradipta, Riezki Amalia, Raden Maya Febrianti, Yuni Elsa Hadisaputri, Mohammad Ghozali, and et al. 2023. "Effect of Immunomodulating Extract and Some Isolates from Etlingera rubroloba A.D. Poulsen Fruits on Diabetic Patients with Tuberculosis" Molecules 28, no. 5: 2401. https://doi.org/10.3390/molecules28052401
APA StyleIlyas Y., M., Sahidin, I., Jabbar, A., Yodha, A. W. M., Diantini, A., Pradipta, I. S., Amalia, R., Febrianti, R. M., Hadisaputri, Y. E., Ghozali, M., & Julaeha, E. (2023). Effect of Immunomodulating Extract and Some Isolates from Etlingera rubroloba A.D. Poulsen Fruits on Diabetic Patients with Tuberculosis. Molecules, 28(5), 2401. https://doi.org/10.3390/molecules28052401





