Biomolecular Screening of Pimpinella anisum L. for Antioxidant and Anticholinesterase Activity in Mice Brain
Abstract
1. Introduction
2. Results
2.1. Frasctionation by Column Chromatography
2.2. In Vitro Testing of Purified Fractions
2.3. Results of GC-MS Analysis
2.4. Findings of Behavioral Studies
2.5. Findings of Biochemical Studies
2.6. Acute Toxicity
3. Discussion
4. Materials and Methods
4.1. Extraction and Fractionation via Column Chromatography
4.1.1. Botanical Material
4.1.2. Extraction and Fractionation
4.1.3. Column Chromatography
4.2. In Vitro Anti-Cholinesterase Activity
4.3. GC-MS Analysis
4.4. Animals
4.5. In Vivo Behavioral and Biochemical Studies
4.6. Choline Acetyltransferase Activity (ChAT)
4.7. Acute Toxicity Study
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, K.N.; Hovland, A.R.; Cole, W.C.; Prasad, K.C.; Nahreini, P.; Edwards-Prasad, J.; Andreatta, C.P. Multiple antioxidants in the prevention and treatment of Alzheimer disease: Analysis of biologic rationale. Clin. Neuropharmacol. 2000, 23, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.; Khani, M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomised, placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863–866. [Google Scholar] [CrossRef]
- Umare, M.; Wankhede, N.; Bajaj, K.; Trivedi, R.; Taksande, B.; Umekar, M.; Mahore, J.; Kale, M. Interweaving of reactive oxygen species and major neurological and psychiatric disorders. Ann. Pharm. Fr. 2022, 80, 409–425. [Google Scholar] [CrossRef]
- Amina, B.; Soumeya, B.; Salim, B.; Mahieddine, B.; Sakina, B.; Chawki, B.; Francesca, N.; Marzia, V.; Carmine, N. Chemical profiling, antioxidant, enzyme inhibitory and in silico modeling of Rosmarinus officinalis L. and Artemisia herba alba Asso. essential oils from Algeria. S. Afr. J. Bot. 2022, 147, 501–510. [Google Scholar] [CrossRef]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Hoa, V.V.; Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T. Acetylcholinesterase inhibitory activities of essential oils from Vietnamese traditional medicinal plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.-H.; Zhang, J.; Yu, B.-Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agr. Food Chem. 2008, 56, 3876–3883. [Google Scholar] [CrossRef]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, E. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef]
- Govindarajan, R.; Vijayakumar, M.; Pushpangadan, P. Antioxidant approach to disease management and the role of ‘Rasayana’ herbs of Ayurveda. J. Ethnopharmacol. 2005, 99, 165–178. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; de Aguiar Andrade, E.H.; de Oliveira, M.S. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- de Torre, M.P.; Cavero, R.Y.; Calvo, M.I. Anticholinesterase activity of selected medicinal plants from Navarra region of Spain and a detailed phytochemical investigation of Origanum vulgare L. ssp. vulgare. Molecules 2022, 27, 7100. [Google Scholar] [CrossRef]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef]
- Bakhshi, M.; Kamalinejad, M.; Shokri, M.; Forouzani, G.; Heidari, F.; Tofangchiha, M. In vitro antibacterial effect of Pimpinella anisum essential oil on Enterococcus faecalis, Lactobacillus casei, Actinomyces naeslundii, and Aggregatibacter actinomycetemcomitans. Folia Med. 2022, 64, 799–806. [Google Scholar] [CrossRef]
- Shojaii, A.; Abdollahi Fard, M. Review of pharmacological properties and chemical constituents of Pimpinella anisum. ISRN Pharm. 2012, 2012, 510795. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Hosseini, M.; Mangeng, D.; Alavi, H.; Hassanzadeh, G.R.; Bayat, M.; Jafarian, M.; Kazemi, H.; Gorji, A. Anticonvulsant and neuroprotective effects of Pimpinella anisum in rat brain. BMC Complement. Altern. Med. 2012, 12, 76. [Google Scholar] [CrossRef]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J. Agr. Food Chem. 2009, 57, 4313–4318. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Dubey, N.K. Nanostructured Pimpinella anisum essential oil as novel green food preservative against fungal infestation, aflatoxin B1 contamination and deterioration of nutritional qualities. Food Chem. 2021, 344, 128574. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Memory enhancing effect of anise (Pimpinella anisum) with respect to its antioxidant activity in albino mice. J. Anim. Plant Sci. 2019, 29, 602–610. [Google Scholar]
- Wang, M.; Liu, T.; Chen, S.; Wu, M.; Han, J.; Li, Z. Design and synthesis of 3-(4-pyridyl)-5-(4-sulfamido-phenyl)-1, 2, 4-oxadiazole derivatives as novel GSK-3β inhibitors and evaluation of their potential as multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2021, 209, 112874. [Google Scholar] [CrossRef] [PubMed]
- Gobec, M.; Tomasic, T.; Markovic, T.; Mlinaric-Rascan, I.; Dolenc, M.S.; Jakopin, Z. Antioxidant and anti-inflammatory properties of 1, 2, 4-oxadiazole analogs of resveratrol. Chem. Biol. Interact. 2015, 240, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Chawla, G.; Kumar, U.; Bawa, S.; Kumar, J. Syntheses and evaluation of anti-inflammatory, analgesic and ulcerogenic activities of 1, 3, 4-oxadiazole and 1, 2, 4-triazolo [3, 4-b]-1, 3, 4-thiadiazole derivatives. J. Enzyme Inhib. Med. Chem. 2012, 27, 658–665. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Eraky, S.M.; Ramadan, N.M.; El-Magd, N.F.A. Ameliorative effects of bromelain on aluminum-induced Alzheimer’s disease in rats through modulation of TXNIP pathway. Int. J. Biol. Macromol. 2022, 227, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.B.; Choi, H.J.; Kim, J.E.; Park, J.W.; Kang, M.J.; Bae, S.J.; Lee, Y.J.; Choi, Y.S.; Kim, K.S.; Jung, Y.-S. Comparison of scopolamine-induced cognitive impairment responses in three different ICR stocks. Lab. Anim. Res. 2018, 34, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Papas, M.; Catalan, J.; Barranco, I.; Arroyo, L.; Bassols, A.; Yeste, M.; Miró, J. Total and specific activities of superoxide dismutase (SOD) in seminal plasma are related with the cryotolerance of jackass spermatozoa. Cryobiology 2020, 92, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tsosura, T.V.S.; Dos Santos, R.M.; Neto, A.H.C.; Chiba, F.Y.; Carnevali, A.C.N.; Mattera, M.S.d.L.C.; Belardi, B.E.; Cintra, L.T.Â.; da Silva Machado, N.E.; Matsushita, D.H. Maternal apical periodontitis increases insulin resistance and modulates the antioxidant defense system in the gastrocnemius muscle of adult offspring. J. Endod. 2021, 47, 1126–1131. [Google Scholar] [CrossRef]
- Mohammadi-Khanaposhtani, M.; Mahdavi, M.; Saeedi, M.; Sabourian, R.; Safavi, M.; Khanavi, M.; Foroumadi, A.; Shafiee, A.; Akbarzadeh, T. Design, synthesis, biological evaluation, and docking study of acetylcholinesterase inhibitors: New acridone-1, 2, 4-oxadiazole-1, 2, 3-triazole Hybrids. Chem. Biol. Drug Des. 2015, 86, 1425–1432. [Google Scholar] [CrossRef]
- Elghazawy, N.H.; Zaafar, D.; Hassan, R.R.; Mahmoud, M.Y.; Bedda, L.; Bakr, A.F.; Arafa, R.K. Discovery of New 1, 3, 4-Oxadiazoles with dual activity targeting the cholinergic pathway as effective anti-Alzheimer agents. ACS Chem. Neurosci. 2022, 13, 1187–1205. [Google Scholar] [CrossRef]
- Bárez-Lopez, S.; Montero-Pedrazuela, A.; Bosch-García, D.; Venero, C.; Guadano-Ferraz, A. Increased anxiety and fear memory in adult mice lacking type 2 deiodinase. Psychoneuroendocrinology 2017, 84, 51–60. [Google Scholar] [CrossRef]
- Alegiry, M.H.; Hajrah, N.H.; Alzahrani, N.A.Y.; Shawki, H.H.; Khan, M.; Zrelli, H.; Atef, A.; Kim, Y.; Alsafari, I.A.; Arfaoui, L. Attitudes toward psychological disorders and alternative medicine in Saudi participants. Front. Psychiatry 2021, 12, 577103. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Gohar, U.F.; Ahmad, M.; Marc, R.A.; Mureşan, C.C.; Irimie, M.; Bobescu, E. Biomolecular evaluation of Lavandula stoechas L. for nootropic activity. Plants 2021, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Jaffuel, G.; Chappuis, L.; Guillarme, D.; Turlings, T.C.; Glauser, G. Improved separation by at-column dilution in preparative hydrophilic interaction chromatography. J. Chromatogr. A 2018, 1532, 136–143. [Google Scholar] [CrossRef]
- Monadi, T.; Azadbakht, M.; Ahmadi, A.; Chabra, A. A Comprehensive Review on the Ethnopharmacology, Phytochemistry, Pharmacology, and Toxicology of the Mandragora Genus; from Folk Medicine to Modern Medicine. Curr. Pharm. Des. 2021, 27, 3609–3637. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Lavandula stoechas (L) a Very Potent Antioxidant Attenuates Dementia in Scopolamine Induced Memory Deficit Mice. Front. Pharmacol. 2018, 9, 1375. [Google Scholar] [CrossRef]
- Chao, L.-P.; Wolfgram, F. Spectrophotometric assay for choline acetyltransferase. Anal. Biochem. 1972, 46, 114–118. [Google Scholar] [CrossRef] [PubMed]
- El Hilaly, J.; Israili, Z.H.; Lyoussi, B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol. 2004, 91, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Akhila, J.S.; Shyamjith, D.; Alwar, M. Acute toxicity studies and determination of median lethal dose. Curr. Sci. 2007, 97, 917–920. Available online: http://www.jstor.org/stable/24099255 (accessed on 21 January 2023).
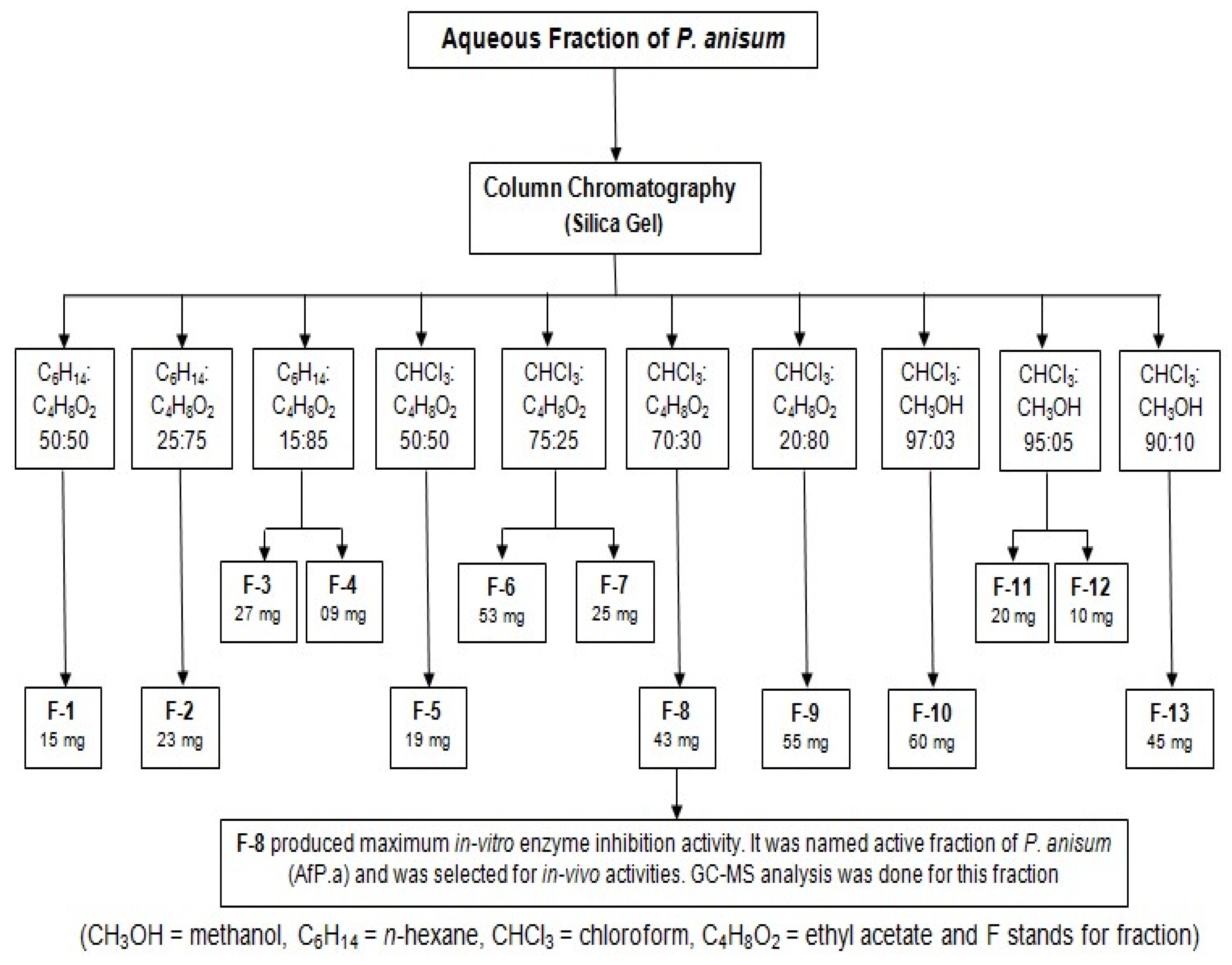

| Fractions | Color of Solution | AChE Inhibition |
|---|---|---|
| F-1 | Purple | No |
| F-2 | Purple | No |
| F-3 | Purple | No |
| F-4 | Purple | No |
| F-5 | Purple | No |
| F-6 | Purple | No |
| F-7 | Purple | No |
| F-8 | Colorless | Yes |
| F-9 | Purple | No |
| F-10 | Purple | No |
| F-11 | Purple | No |
| F-12 | Purple | No |
| F-13 | Purple | No |
| Compound Name | Molecular Formula | Molecular Weight (g/mol) | Mass Peak | Retention Time (min) |
|---|---|---|---|---|
| 1-Benzylbenzimidazole 3-oxide | C14H12N2O | 224 | 43 | 2.683 |
| Apiol | C12H14O4 | 222 | 146 | 20.158 |
| Cyclohexanone | C6H10O | 98 | 34 | 4.567 |
| 1,2,5 oxadiazole | C2H2N2O | 70 | 26 | 2.992 |
| Elevated Plus Maze | Light/Dark Paradigm | Hole-Board | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Day-1 | Day-2 | I.R | Time Spent on Day-1 | Time Spent on Day-2 | Day-1 | Day-2 | ||
| I1 (s) | I2 (s) | L.Cmpt (s) | D.Cmpt (s) | L.Cmpt (s) | D.Cmpt (s) | n.Pok/5 min | n.Pok/5 min | ||
| G-1 | 23.16 ± 1.17 | 16.66 ± 0.98 | 0.26 ± 0.07 | 50.83 ± 2.42 | 249.17 ± 2.42 | 41.50 ± 1.76 | 258.50 ± 1.76 | 48.33 ± 1.33 | 43.33 ± 1.74 |
| G-2 | 68.11 ± 2.39 a | 81.02 ± 2.78 a | ࢤ0.18 ± 0.05 a | 173.22 ± 6.99 a | 126.78 ± 7.20 a | 179.00 ± 5.89 a | 121.00 ± 5.88 a | 21.00 ± 1.13 a | 29.01 ± 1.81 a |
| G-3 | 20.83 ± 0.87 b,σ | 17.16 ± 1.07 b,σ | 0.17 ± 0.04 b,σ | 41.66 ± 4.41 b,σ | 258.34 ± 4.41 b,σ | 31.67 ± 2.47 b,σ | 268.33 ± 2.47 b,σ | 55.50 ± 2.21 b,σ | 45.50 ± 1.80 b,σ |
| G-4 | 35.50 ± 0.92 b,α | 25.66 ± 1.30 b,β | 0.29 ± 0.02 b,σ | 62.50 ± 2.14 b,σ | 237.50 ± 2.14 b,σ | 49.17 ± 2.38 b,σ | 250.83 ± 2.38 b,σ | 44.00 ± 1.82 b,σ | 42.66 ± 1.60 b,σ |
| G-5 | 46.16 ± 1.68 b,α | 41.16 ± 1.85 b,α | 0.10 ± 0.03 b,σ | 66.00 ± 5.63 b,σ | 234.00 ± 5.63 b,σ | 64.00 ± 4.47 b,σ | 236.00 ± 4.47 b,α | 37.84 ± 2.70 b,β | 39.50 ± 1.47 b,σ |
| G-6 | 41.00 ± 1.59 b,α | 36.16 ± 1.85 b,α | 0.11 ± 0.03 b,σ | 55.83 ± 6.63 b,σ | 244.17 ± 6.63 b,σ | 53.33 ± 6.28 b,σ | 246.67 ± 6.28 b,σ | 39.66 ± 1.76 b,γ | 43.33 ± 1.70 b,σ |
| G-7 | 36.00 ± 1.59 b,α | 31.50 ± 1.92 b,α | 0.12 ± 0.04 b,σ | 52.50 ± 5.73 b,σ | 247.5 ± 5.73 b,σ | 51.66 ± 3.80 b,σ | 248.34 ± 3.80 b,σ | 41.00 ± 1.52 b,σ | 44.16 ± 1.24 b,σ |
| Groups | AChE μmol/min/mg | MDA nmol/h/g | SOD U/mg of Homogenate | Catalase U/mg of Homogenate | GSH μmol/mg |
|---|---|---|---|---|---|
| Group-1 | 3.79 ± 0.21 | 1.52 ± 0.11 | 25.91± 0.61 | 1.91 ± 0.17 | 40.21 ± 1.10 |
| Group-2 | 8.01 ± 0.31 a | 6.91 ± 0.40 a | 7.61 ± 0.24 a | 0.56 ± 0.04 a | 17.92 ± 0.33 a |
| Group-3 | 3.52 ± 0.30 b,σ | 1.40 ± 0.08 b,σ | 25.11 ± 0.89 b,σ | 2.01 ± 0.06 b,σ | 46.99 ± 0.89 b,γ |
| Group-4 | 4.61 ± 0.23 b,σ | 2.60 ± 0.11 b,β | 22.02 ± 0.61 b,α | 1.40 ± 0.07 b,β | 39.99 ± 1.23 b,σ |
| Group-5 | 6.31 ± 0.31 b,α | 2.29 ± 0.19 b,σ | 20.03 ± 0.61 b,α | 1.10 ± 0.07 c,α | 37.02 ± 1.89 b,σ |
| Group-6 | 5.91 ± 0.19 b,α | 1.99 ± 0.13 b,σ | 20.21 ± 0.71 b,α | 1.30 ± 0.07 b,β | 36.99 ± 1.69 b,σ |
| Group-7 | 4.40 ± 0.31 b,σ | 1.88 ± 0.17 b,σ | 21.14 ± 0.49 b,α | 1.71 ± 0.06 b,σ | 40.07 ± 1.39 b,σ |
| Groups | Treatment | ChAT (μmol/min/mg) |
|---|---|---|
| G-A | Normal Control | 12.10 ± 0.89 |
| G-B | Amnesic Control | 6.99 ± 0.81 * |
| G-C | Test Control-A | 10.92 ± 0.71 ns |
| G-D | Test Control-B | 10.44 ± 1.31 ns |
| G-E | Test Control-C | 8.81 ± 0.94 ns |
| Behavioral Changes | Number of Days | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | |
| Ataxia | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Strabo Tail | √ | √ | √ | √ | √ | √ | √ | √ | √ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Blanching | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Secretions | √ | √ | √ | √ | √ | √ | √ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Convulsions | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Salivation | √ | √ | √ | √ | √ | √ | √ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Hyperactivity | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Rigidity | √ | √ | √ | √ | √ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Hypnosis | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Ptosis | √ | √ | √ | √ | √ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Irritability | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Pilo erection | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Muscle Spasm | √ | √ | √ | √ | √ | √ | √ | √ | √ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Loss of Traction | √ | √ | √ | √ | √ | √ | √ | √ | √ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Groups | Treatment from Day 1–7 |
|---|---|
| G-1 (Normal Control) | Normal saline 10 mL/Kg/p.o. |
| G-2 (Amnesic Control) | 5% CMC 10 mL/Kg/p.o. |
| G-3 (Standard Control-A) | Piracetam 200 mg/Kg/p.o. |
| G-4 (Standard Control-B) | Piracetam 200 mg/Kg/p.o. |
| G-5 (Experimental Control-I) | P.aAF 3.5 mg/Kg/p.o. |
| G-6 (Experimental Control-II) | P.aAF 7 mg/Kg/p.o. |
| G-7 (Experimental Control-III) | P.aAF 7 mg/Kg/p.o. |
| Groups | Treatment |
|---|---|
| G-A (Normal Control) | Normal saline 10 mL/Kg/p.o. for 7 days |
| G-B (Amnesic Control) | 5% CMC 10 mL/Kg/p.o. for 6 days then Scopolamine 10 mg/Kg/p.o on 7th day. |
| G-C (Experimental Control-I) | P.aAF 7 mg/Kg/p.o. for 7 days consecutively |
| G-D (Experimental Control-II) | Scopolamine 10 mg/Kg/p.o on 1st day then P.aAF 7 mg/Kg/p.o. from day 2 to 7. |
| G-E (Experimental Control-III) | P.aAF 7 mg/Kg/p.o. for 6 days then Scopolamine 10 mg/Kg/p.o on 7th day. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushtaq, A.; Habib, F.; Manea, R.; Anwar, R.; Gohar, U.F.; Zia-Ul-Haq, M.; Ahmad, M.; Gavris, C.M.; Chicea, L. Biomolecular Screening of Pimpinella anisum L. for Antioxidant and Anticholinesterase Activity in Mice Brain. Molecules 2023, 28, 2217. https://doi.org/10.3390/molecules28052217
Mushtaq A, Habib F, Manea R, Anwar R, Gohar UF, Zia-Ul-Haq M, Ahmad M, Gavris CM, Chicea L. Biomolecular Screening of Pimpinella anisum L. for Antioxidant and Anticholinesterase Activity in Mice Brain. Molecules. 2023; 28(5):2217. https://doi.org/10.3390/molecules28052217
Chicago/Turabian StyleMushtaq, Aamir, Fatima Habib, Rosana Manea, Rukhsana Anwar, Umar Farooq Gohar, Muhammad Zia-Ul-Haq, Mobasher Ahmad, Claudia Mihaela Gavris, and Liana Chicea. 2023. "Biomolecular Screening of Pimpinella anisum L. for Antioxidant and Anticholinesterase Activity in Mice Brain" Molecules 28, no. 5: 2217. https://doi.org/10.3390/molecules28052217
APA StyleMushtaq, A., Habib, F., Manea, R., Anwar, R., Gohar, U. F., Zia-Ul-Haq, M., Ahmad, M., Gavris, C. M., & Chicea, L. (2023). Biomolecular Screening of Pimpinella anisum L. for Antioxidant and Anticholinesterase Activity in Mice Brain. Molecules, 28(5), 2217. https://doi.org/10.3390/molecules28052217







