Artemisinin Confers Neuroprotection against 6-OHDA-Induced Neuronal Injury In Vitro and In Vivo through Activation of the ERK1/2 Pathway
Abstract
1. Introduction
2. Results
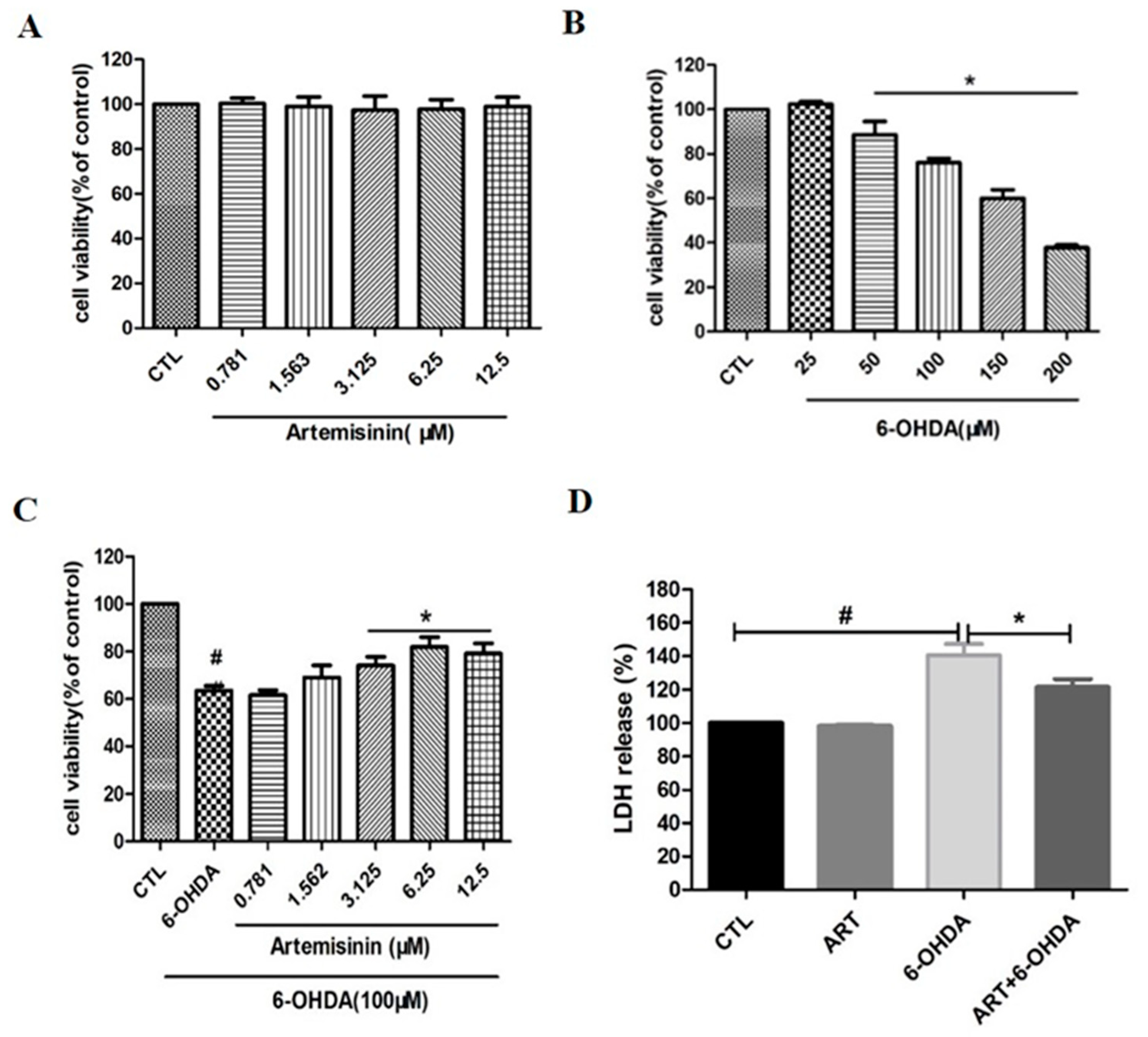
2.1. Artemisinin Attenuated the Decrease in Cell Viability and Cell Cytotoxicity Caused by 6-OHDA in PC12 Cells
2.2. Artemisinin Inhibited 6-OHDA-Induced ROS Accumulation, Loss of the Mitochondria Membrane Potential and Apoptosis in PC12 Cells
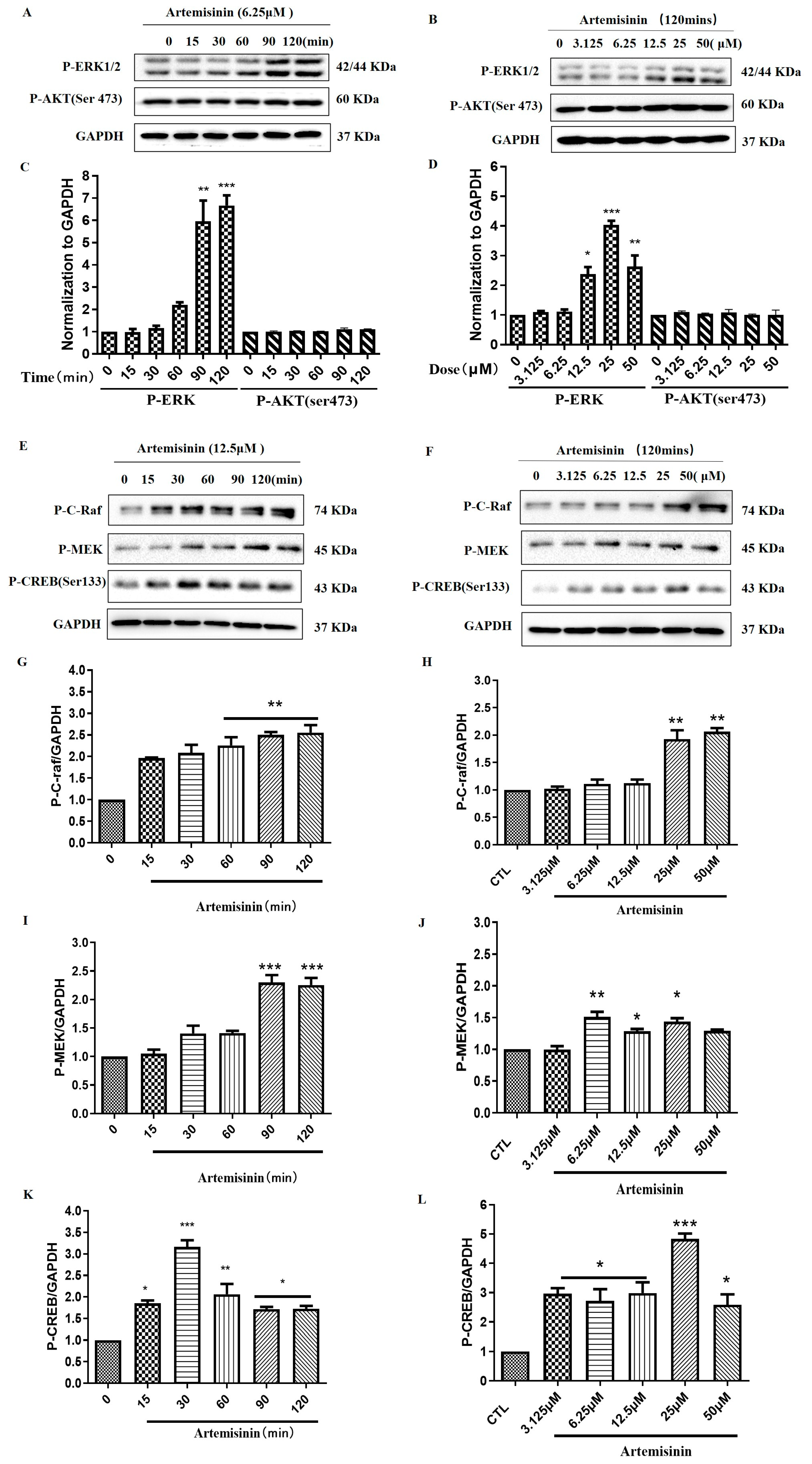
2.3. Artemisinin Increased the Phosphorylation/Activation of ERK1/2, and ERK’s Upstream Proteins Raf and MEK, and ERK’s Downstream Protein CREB in a Concentration- and Time-Dependent Manner in PC12 Cells
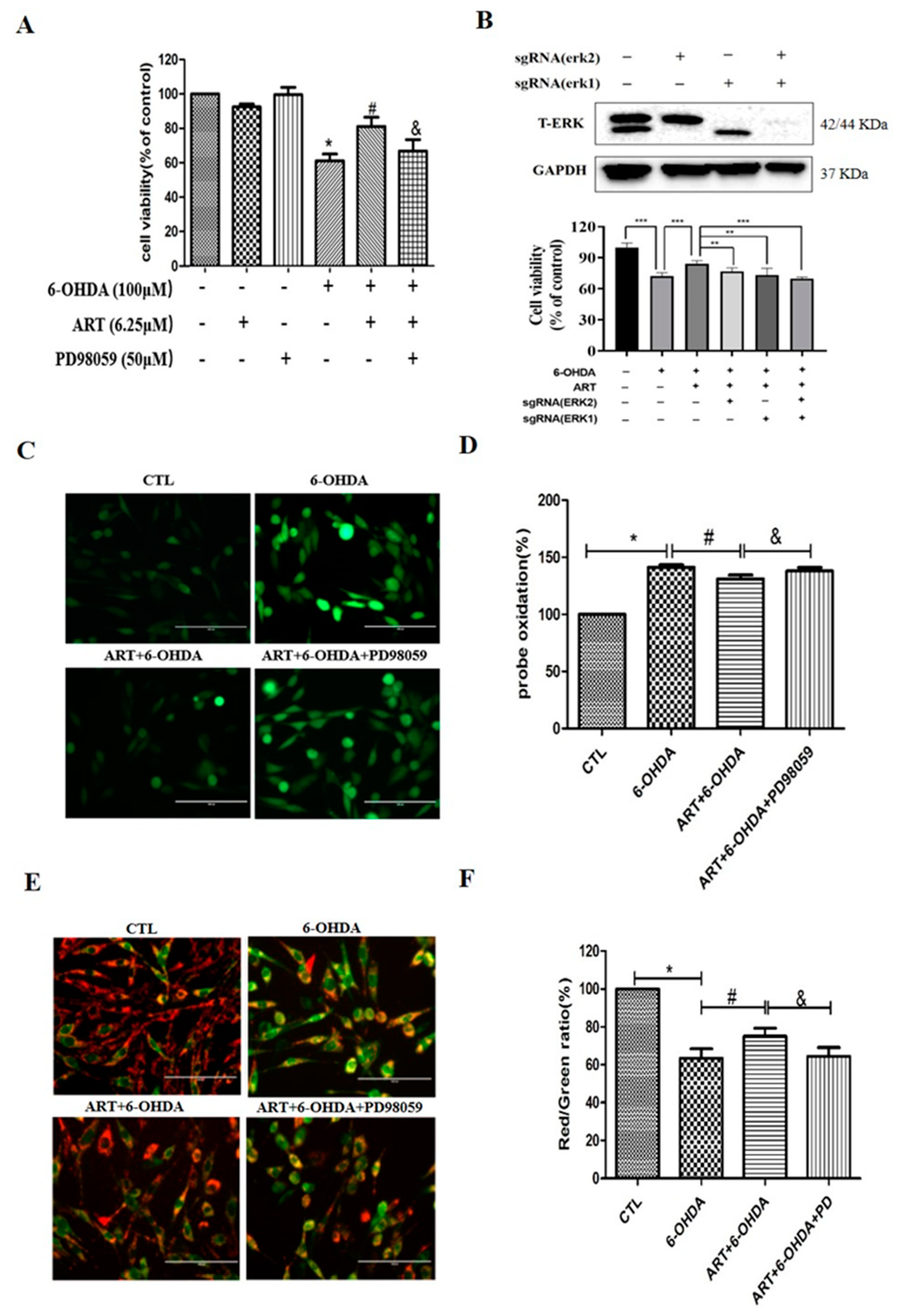
2.4. The ERK1/2 Pathway Inhibitor PD98059 or Silencing of ERK1/2 Blocked the Protective Effect of Artemisinin in PC12 Cells
2.5. Artemisinin Protected SH-SY5Y Cells and Primary Cultured Neuronal Cells against 6-OHDA and MPP+ Induced Cell Viability Loss
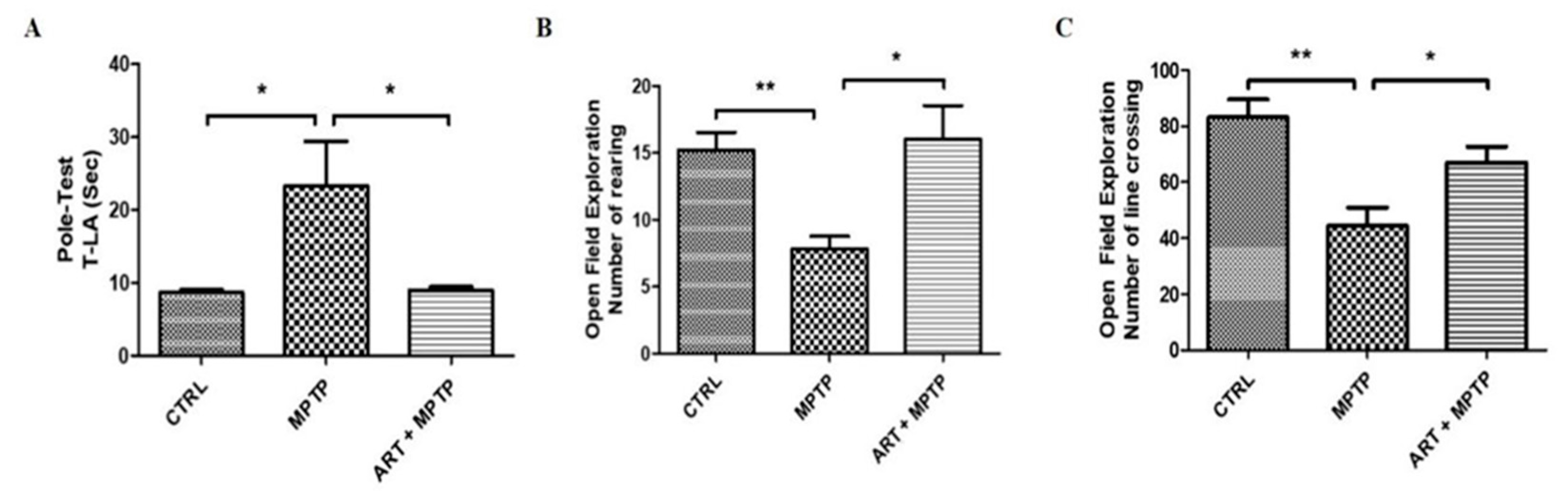
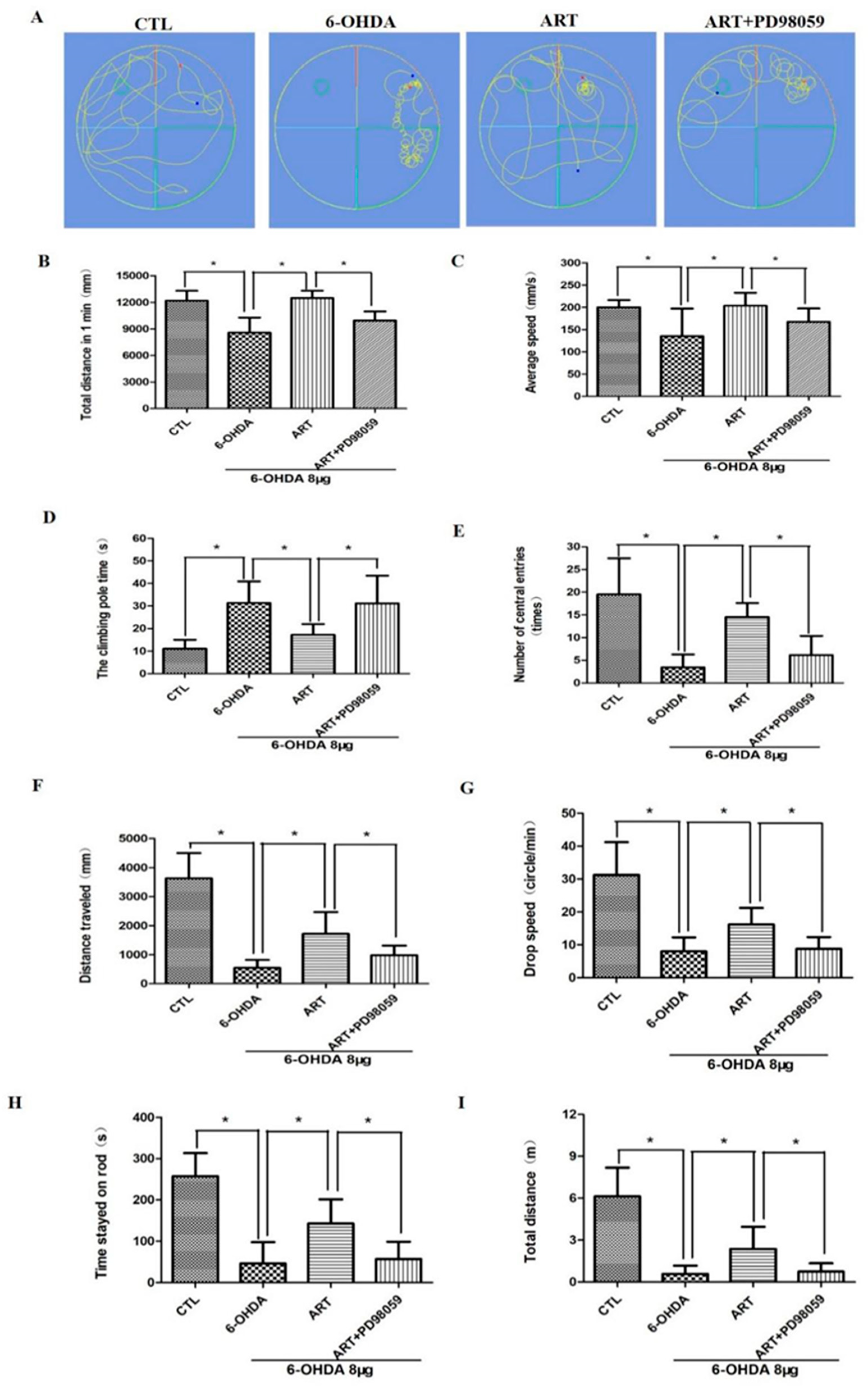
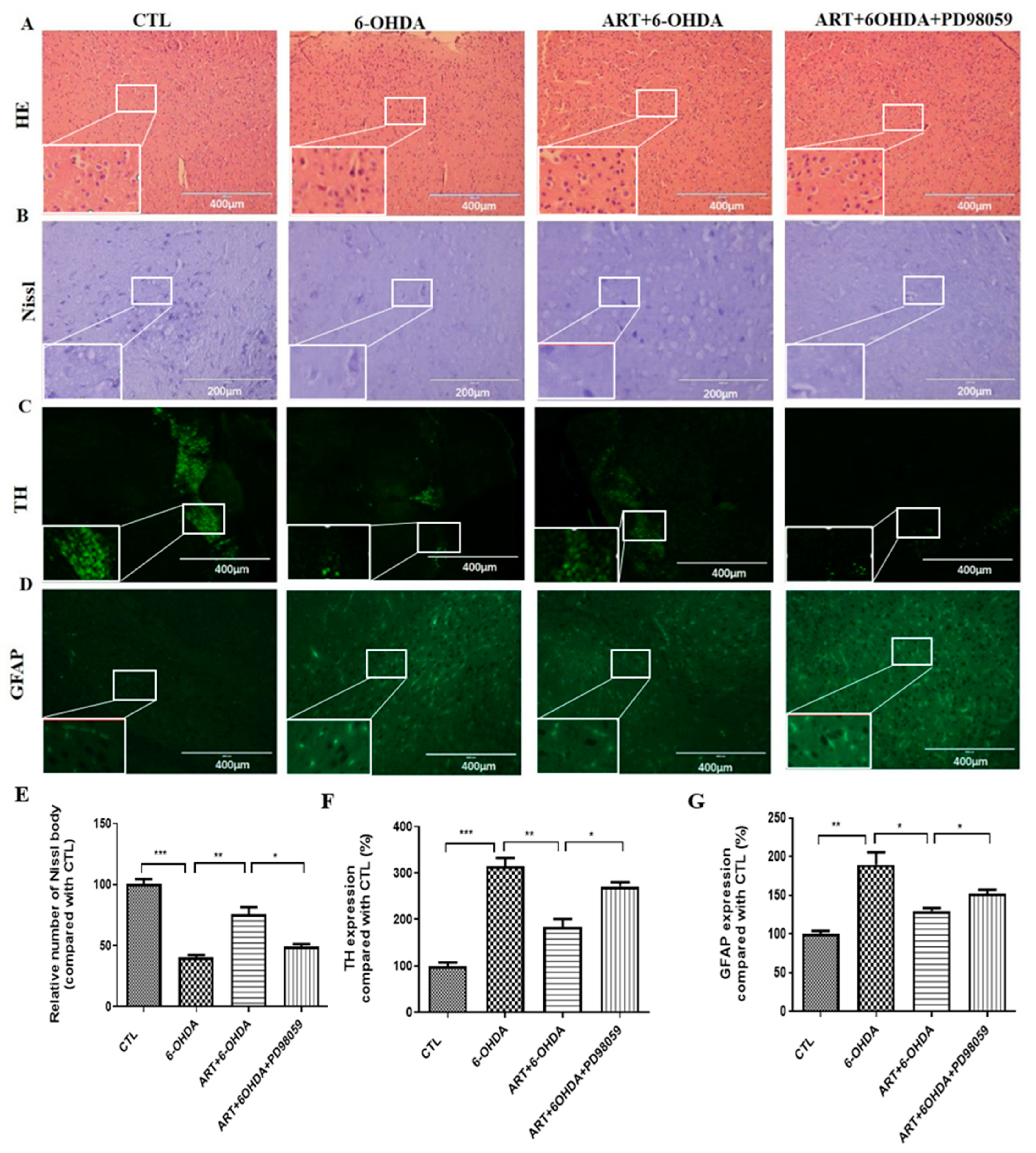
2.6. Artemisinin Attenuated the Behavioral Deficits in PD Mice Models Induced by 6-OHDA and MPTP
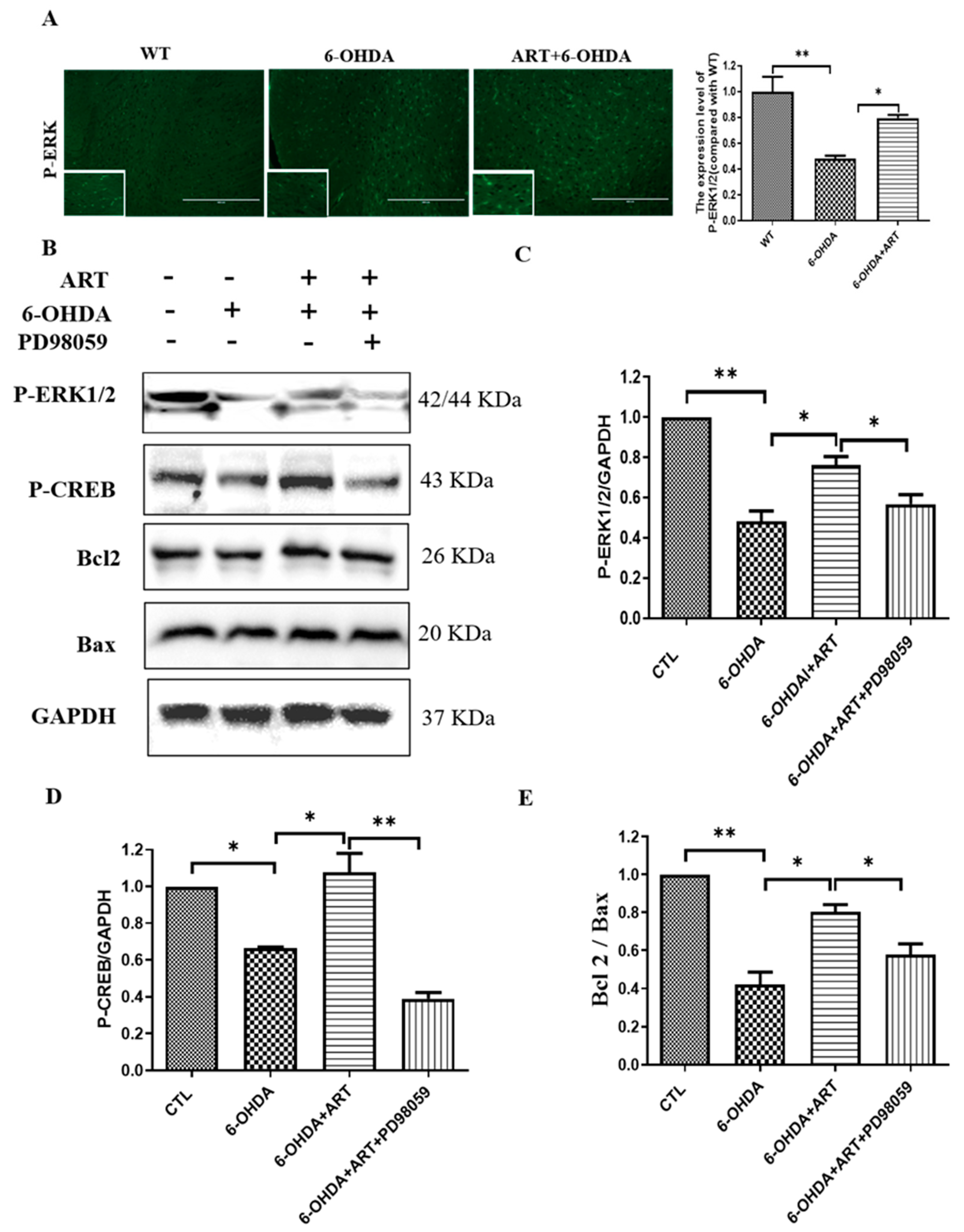
2.7. Artemisinin Treatment Stimulated the Phosphorylation of ERK1/2 in 6-OHDA-Induced PD Mice Model and This Effect Was Blocked by PD98059
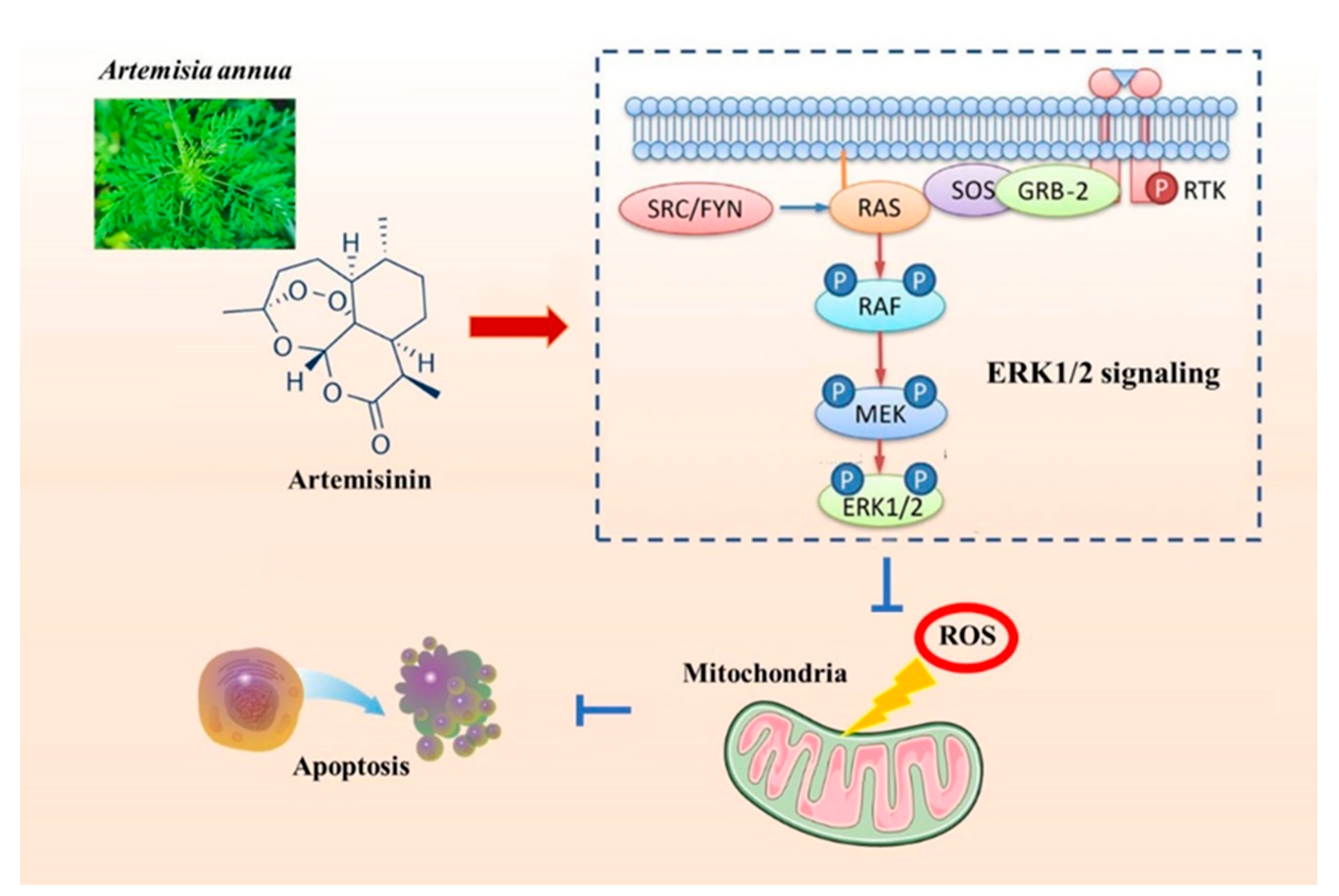
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatments
4.3. MTT Assay
4.4. LDH Assay
4.5. Measurement of Reactive Oxygen Species (ROS)
4.6. JC-1 Staining
4.7. Flow Cytometry
4.8. Western Blot Analysis of Cell Samples
4.9. CRISPR/Cas9 Knockout of ERK1/2 Gene
4.10. Hoechst 33342 Staining
4.11. Mouse Models of Parkinson’s Disease
4.11.1. Animals
4.11.2. Establishment and Grouping of the 6-OHDA-Induced Parkinson’s Disease Model
4.11.3. Establishment and Grouping of the MPTP-Induced Parkinson’s Disease Model
4.12. Neurobehavioral Observation Method
4.12.1. Open Field Test
4.12.2. Pole Test
4.12.3. Swimming Test
4.12.4. Rotarod Test
4.13. H&E, Nissl Staining, and Immunofluorescence
4.14. Western Blot Analysis of Brain Tissue
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Martinez, B.A.; Petersen, D.A.; Gaeta, A.L.; Stanley, S.P. Dysregulation of the Mitochondrial Unfolded Protein Response Induces Non-Apoptotic Dopaminergic Neurodegeneration in C. elegans Models of Parkinson’s Disease. J. Neurosci. 2017, 37, 11085–11100. [Google Scholar] [PubMed]
- Pang, S.Y.; Ho, P.W.; Liu, H.F.; Leung, C.T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.L. The Interplay of Aging, Genetics and Environmental Factors in the Pathogenesis of Parkinson’s Disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M. The Many Roads to Mitochondrial Dysfunction in Neuroimmune and Neuropsychiatric Disorders. BMC Med. 2015, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Sun, X.; Liao, X.; Zhang, X.; Zarabi, S.; Schimmer, A.; Hong, Y.; Ford, C.; Luo, Y.; Qi, X. Alpha-Synuclein Suppresses Mitochondrial Protease Clpp to Trigger Mitochondrial Oxidative Damage and Neurotoxicity. Acta Neuropathol. 2019, 137, 939–960. [Google Scholar] [CrossRef]
- Ding, Y.; Xin, C.; Zhang, C.W.; Lim, K.L.; Zhang, H.; Fu, Z.; Li, L.; Huang, W. Natural Molecules from Chinese Herbs Protecting against Parkinson’s Disease via Anti-Oxidative Stress. Front. Aging Neurosci. 2018, 10, 246. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative Stress and Cellular Pathologies in Parkinson’s Disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to Dopaminergic Neurons by Oxidative Stress in Parkinson’s Disease (Review). Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef]
- Soto-Otero, R.; Méndez-Alvarez, E.; Hermida-Ameijeiras, A.; Muñoz-Patiño, A.M.; Labandeira-Garcia, J.L. Autoxidation and Neurotoxicity of 6-Hydroxydopamine in the Presence of Some Antioxidants: Potential Implication in Relation to the Pathogenesis of Parkinson’s Disease. J. Neurochem. 2000, 74, 1605–1612. [Google Scholar] [CrossRef]
- Zheng, W.; Chong, C.-M.; Wang, H.; Zhou, X.; Zhang, L.; Wang, R.; Meng, Q.; Lazarovici, P.; Fang, J. Artemisinin Conferred Erk Mediated Neuroprotection to Pc12 Cells and Cortical Neurons Exposed to Sodium Nitroprusside-Induced Oxidative Insult. Free Radic. Biol. Med. 2016, 97, 158–167. [Google Scholar] [CrossRef]
- Chong, C.M.; Zheng, W. Artemisinin Protects Human Retinal Pigment Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Damage through Activation of Erk/Creb Signaling. Redox Biol. 2016, 9, 50–56. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, J.; Zheng, W. Artemisinin Protects Pc12 Cells against Beta-Amyloid-Induced Apoptosis through Activation of the Erk1/2 Signaling Pathway. Redox Biol. 2017, 12, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s Disease: A Target for Neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Giannoccaro, M.P.; La Morgia, C.; Rizzo, G.; Carelli, V. Mitochondrial DNA and Primary Mitochondrial Dysfunction in Parkinson’s Disease. Mov. Disord. 2017, 32, 346–363. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, a Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Ellis, A.C. Dietary Antioxidants and Parkinson’s Disease. Antioxidants 2020, 9, 570. [Google Scholar] [CrossRef]
- Li, S.; Chaudhary, S.C.; Zhao, X.; Gaur, U.; Fang, J.; Yan, F.; Zheng, W. Artemisinin Protects Human Retinal Pigmented Epithelial Cells against Hydrogen Peroxide-Induced Oxidative Damage by Enhancing the Activation of Amp-Active Protein Kinase. Int. J. Biol. Sci. 2019, 15, 2016–2028. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, X.; Li, S.; Xing, X.; Wang, H.; Lazarovici, P.; Zheng, W. Protective Mechanism of Artemisinin on Rat Bone Marrow-Derived Mesenchymal Stem Cells against Apoptosis Induced by Hydrogen Peroxide via Activation of C-Raf-Erk1/2-P90(Rsk)-Creb Pathway. Stem Cell Res. Ther. 2019, 10, 312. [Google Scholar] [CrossRef]
- Fang, J.; Silva, M.; Lin, R.; Zhou, W.; Chen, Y.; Zheng, W. Artemisinin Reverses Glucocorticoid-Induced Injury in Bone Marrow-Derived Mesenchymal Stem Cells through Regulation of Erk1/2-Creb Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5574932. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Gaur, U.; Zheng, W. Artemisinin Improved Neuronal Functions in Alzheimer’s Disease Animal Model 3xtg Mice and Neuronal Cells via Stimulating the Erk/Creb Signaling Pathway. Aging Dis. 2020, 11, 801–819. [Google Scholar] [CrossRef]
- Lin, S.P.; Li, W.; Winters, A.; Liu, R.; Yang, S.H. Artemisinin Prevents Glutamate-Induced Neuronal Cell Death via Akt Pathway Activation. Front. Cell. Neurosci. 2018, 12, 108. [Google Scholar] [CrossRef]
- Macdonald, R.; Barnes, K.; Hastings, C.; Mortiboys, H. Mitochondrial Abnormalities in Parkinson’s Disease and Alzheimer’s Disease: Can Mitochondria Be Targeted Therapeutically? Biochem. Soc. Trans. 2018, 46, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, O.; Zhu, X.; Perry, G.; Smith, M.A. Mitochondrial Abnormalities and Oxidative Imbalance in Neurodegenerative Disease. Sci. Aging Knowl. Environ. 2002, 2002, pe16. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Huang, L.; Min, Z.; Sun, S.; Yan, W. Insulin Like Growth Factor-1 Prevents 1-Mentyl-4-Phenylphyridinium-Induced Apoptosis in Pc12 Cells through Activation of Glycogen Synthase Kinase-3beta. Toxicology 2010, 271, 5–12. [Google Scholar]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The Sh-Sy5y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Yan, J.; Ma, H.; Lai, X.; Wu, J.; Liu, A.; Huang, J.; Sun, W.; Shen, M.; Zhang, Y. Artemisinin attenuated oxidative stress and apoptosis by inhibiting autophagy in MPP+-treated SH-SY5Y cells. J. Biol. Res. 2021, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The Mitochondrial Pathways of Apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial Dysfunction and Oxidative Stress in Parkinson’s Disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef]
- Marcondes, N.A.; Terra, S.R.; Lasta, C.S.; Hlavac, N.R.C.; Dalmolin, M.L.; Lacerda, L.A.; Faulhaber, G.A.M.; González, F.H.D. Comparison of Jc-1 and Mitotracker Probes for Mitochondrial Viability Assessment in Stored Canine Platelet Concentrates: A Flow Cytometry Study. Cytom. A 2019, 95, 214–218. [Google Scholar] [CrossRef]
- Yan, F.; Wang, H.; Gao, Y.; Xu, J.; Zheng, W. Artemisinin Protects Retinal Neuronal Cells against Oxidative Stress and Restores Rat Retinal Physiological Function from Light Exposed Damage. ACS Chem. Neurosci. 2017, 8, 1713–1723. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in Sh-Sy5y and Hippocampal Neurons via the Activation of Ampk Pathway. Int. J. Mol. Sci. 2019, 20, 2680. [Google Scholar] [CrossRef]
- Gerlach, M.; Riederer, P.; Przuntek, H.; Youdim, M. Mptp Mechanisms of Neurotoxicity and Their Implications for Parkinson’s Disease. Eur. J. Pharmacol. Mol. Pharmacol. 1991, 208, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhu, J.; Wang, P.; Liu, T.; Yuan, J.; Yin, H.; Lan, Y.; Sun, Q.; Zhang, Z.; Ding, G.; et al. Artemisinin exerts a protective effect in the MPTP mouse model of Parkinson’s disease by inhibiting microglial activation via the TLR4/Myd88/NF-KB pathway. CNS Neurosci. Ther. 2023, 29, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- William, L.J. The Mptp Story. J. Park. Dis. 2017, 7, S11–S19. [Google Scholar]
- Kang, B.; Mu, S.; Yang, Q.; Guo, S.; Chen, X.; Guo, H. Ape1 Protects against Mpp+-Induced Neurotoxicity through Erk1/2 Signaling in Pc12 Cells. Neuroreport 2017, 28, 10–16. [Google Scholar] [CrossRef]
- Yan, J.; Qiao, L.; Wu, J.; Fan, H.; Sun, J.; Zhang, Y. Simvastatin Protects Dopaminergic Neurons against Mpp+-Induced Oxidative Stress and Regulates the Endogenous Anti-Oxidant System through Erk. Cell. Physiol. Biochem. 2018, 51, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Cho, H.N.; Soh, J.W.; Jhon, G.J.; Cho, C.K.; Chung, H.Y.; Bae, S.; Lee, S.J.; Lee, Y.S. Oxidative Stress-Induced Apoptosis Is Mediated by Erk1/2 Phosphorylation. Exp. Cell Res. 2003, 291, 251–266. [Google Scholar] [CrossRef]
- Tuo, Q.H.; Wang, C.; Yan, F.X.; Liao, D.F. Mapk Pathway Mediates the Protective Effects of Onychin on Oxidative Stress-Induced Apoptosis in Ecv304 Endothelial Cells. Life Sci. 2004, 76, 487–497. [Google Scholar] [CrossRef]
- Teng, L.; Kou, C.; Lu, C.; Xu, J.; Xie, J.; Lu, J.; Liu, Y.; Wang, Z.; Wang, D. Involvement of the Erk Pathway in the Protective Effects of Glycyrrhizic Acid against the Mpp+-Induced Apoptosis of Dopaminergic Neuronal Cells. Int. J. Mol. Med. 2014, 34, 742–748. [Google Scholar] [CrossRef]
- Xiao, H.; Lv, F.; Xu, W.; Zhang, L.; Jing, P.; Cao, X. Deprenyl Prevents Mpp(+)-Induced Oxidative Damage in Pc12 Cells by the Upregulation of Nrf2-Mediated Nqo1 Expression through the Activation of Pi3k/Akt and Erk. Toxicology 2011, 290, 286–294. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.; Zhang, S.; Iyaswamy, A.; Sun, J.; Wang, J.; Yang, C. Novel Insight into Functions of Transcription Factor EB (TFEB) in Alzheimer’s Disease and Parkinson’s Disease. Aging Dis. 2023, 14, 652–669. [Google Scholar] [CrossRef]
- Richmond, A.; Umashanker, N.; Puneet, K. Repurposing artemisinins as neuroprotective agents: A focus on the PI3k/Akt signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 593–605. [Google Scholar]
- Fu, C.; Shi, H.; Chen, H.; Zhang, K.; Wang, M.; Qiu, F. Oral Bioavailability Comparison of Artemisinin, Deoxyartemisinin, and 10-Deoxoartemisinin Based on Computer Simulations and Pharmacokinetics in Rats. ACS Omega 2020, 6, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Schober, A. Classic Toxin-Induced Animal Models of Parkinson’s Disease: 6-Ohda and Mptp. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef]
- Salari, S.; Bagheri, M. In Vivo, in Vitro and Pharmacologic Models of Parkinson’s Disease. Physiol. Res. 2019, 68, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.S.; Goes, A.T.; Boeira, S.P.; Prigol, M.; Jesse, C.R. Protective Effect of Hesperidin in a Model of Parkinson’s Disease Induced by 6-Hydroxydopamine in Aged Mice. Nutrition 2014, 30, 1415–1422. [Google Scholar] [CrossRef]
- Yan, T.; Sun, Y.; Gong, G.; Li, Y.; Fan, K.; Wu, B.; Bi, K.; Jia, Y. The Neuroprotective Effect of Schisandrol a on 6-Ohda-Induced Pd Mice May Be Related to Pi3k/Akt and Ikk/Iκbα/Nf-Κb Pathway. Exp. Gerontol. 2019, 128, 110743. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Lazarovici, P.; Zheng, W. Artemether Activation of Ampk/Gsk3beta(Ser9)/Nrf2 Signaling Confers Neuroprotection towards Beta-Amyloid-Induced Neurotoxicity in 3xtg Alzheimer’s Mouse Model. Oxid. Med. Cell. Longev. 2019, 2019, 1862437. [Google Scholar] [CrossRef]
- Li, S.; Peng, T.; Zhao, X.; Silva, M.; Liu, L.; Zhou, W.; Chen, L.; Zheng, W. Artemether Confers Neuroprotection on Cerebral Ischemic Injury through Stimulation of the Erk1/2-P90rsk-Creb Signaling Pathway. Redox Biol. 2021, 46, 102069. [Google Scholar] [CrossRef]





| Antibody | Cat.NO | Source | Dilution Ratio | Company |
|---|---|---|---|---|
| Phospho-Erk1/2 (Thr202/Tyr204) | 9101S | Rabbit | 1:1000 | CST |
| ERK 1/2 Polyclonal | 40902 | Rabbit | 1:1000 | SAB |
| P-AKT (S473) | 4060 | Rabbit | 1:1000 | CST |
| Phospho-c-Raf (Ser259) | 9421 | Rabbit | 1:1000 | CST |
| Phospho-MEK1/2 (Ser217/221) (41G9) | 9154 | Rabbit | 1:1000 | CST |
| Phospho-CREB (Ser133) (87G3) | 9198 | Rabbit | 1:1000 | CST |
| Bax | 34260-2 | Rabbit | 1:1000 | SAB |
| Bcl-2 | 32012 | Rabbit | 1:1000 | SAB |
| Anti-rabbit IgG, HRP-linked Antibody | 7074 | 1:5000 | CST |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, S.; Fang, J.; Yang, C.; Zhao, X.; Wang, Q.; Zhou, W.; Zheng, W. Artemisinin Confers Neuroprotection against 6-OHDA-Induced Neuronal Injury In Vitro and In Vivo through Activation of the ERK1/2 Pathway. Molecules 2023, 28, 5527. https://doi.org/10.3390/molecules28145527
Li Q, Li S, Fang J, Yang C, Zhao X, Wang Q, Zhou W, Zheng W. Artemisinin Confers Neuroprotection against 6-OHDA-Induced Neuronal Injury In Vitro and In Vivo through Activation of the ERK1/2 Pathway. Molecules. 2023; 28(14):5527. https://doi.org/10.3390/molecules28145527
Chicago/Turabian StyleLi, Qin, Shuai Li, Jiankang Fang, Chao Yang, Xia Zhao, Qing Wang, Wenshu Zhou, and Wenhua Zheng. 2023. "Artemisinin Confers Neuroprotection against 6-OHDA-Induced Neuronal Injury In Vitro and In Vivo through Activation of the ERK1/2 Pathway" Molecules 28, no. 14: 5527. https://doi.org/10.3390/molecules28145527
APA StyleLi, Q., Li, S., Fang, J., Yang, C., Zhao, X., Wang, Q., Zhou, W., & Zheng, W. (2023). Artemisinin Confers Neuroprotection against 6-OHDA-Induced Neuronal Injury In Vitro and In Vivo through Activation of the ERK1/2 Pathway. Molecules, 28(14), 5527. https://doi.org/10.3390/molecules28145527








