Investigating the Antibacterial Effects of Synthetic Gamma-Lactam Heterocycles on Methicillin-Resistant Staphylococcus aureus Strains and Assessing the Safety and Effectiveness of Lead Compound MFM514
Abstract
1. Introduction
2. Results
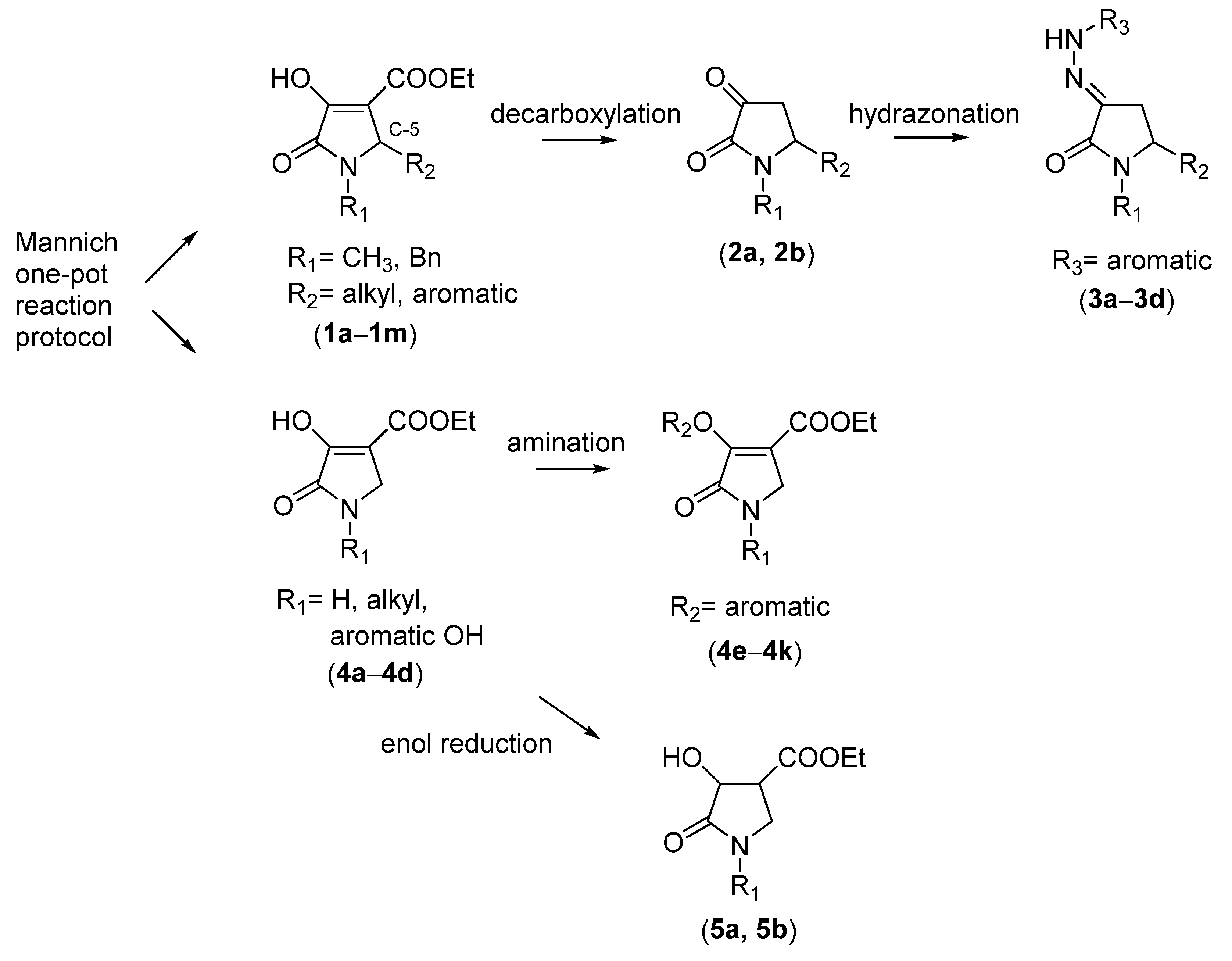
2.1. Synthesis of the Gamma-Lactams
2.2. Results of Antibacterial Test
2.3. Results of Cytotoxicity Test and Selectivity Index (SI) Values
2.4. Results of Oral Acute Toxicity Test
2.5. Mice Systemic Infection Test and Estimation of Mean Effective Dose (ED50)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of One-Pot Gamma-Lactam 1a–1l: See References [10,11,12]
4.3. Synthesis of Decarboxylated 2a,2b and Hydrazone Derivative 3a–3d
4.4. Synthesis of One-Pot Product 4a–4d
4.5. Synthesis of Enamine 4e–4k
4.6. Synthesis of Reduced Gamma-Lactam 5a, 5b: See Reference [13]
4.7. Antibacterial Activity Studies
4.8. Cytotoxicity Studies
4.9. Oral Acute Toxicity Studies
4.10. Mice Systemic Infection Assay
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajdács, M. The Continuing Threat of methicillin-resistant Staphylococcus aureus. Antibiotics 2019, 8, 52. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Zhao, H.; Wang, X.; Rao, L.; Ai, W.; Yu, J.; Guo, Y.; Wu, X.; Yu, F.; et al. In vitro activity of vancomycin, teicoplanin, linezolid and daptomycin against methicillin-resistant Staphylococcus aureus isolates collected from chinese hospitals in 2018–2020. Infect. Drug Resist. 2021, 14, 5449–5456. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25FebET_NM_WHO.pdf?ua=1 (accessed on 7 February 2023).
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 7 February 2023).
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baño, J. Impact of COVID-19 pandemic on multidrug resistant gram positive and gram negative pathogens: A systematic review. J. Infect. Public Health 2022, 16, 320–331. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Hogan, P.A.; Jones, R.N.; Sader, H.S.; Flamm, R.K. Surveillance for linezolid resistance via the Zyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) programme (2014): Evolving resistance mechanisms with stable susceptibility rates. J. Antimicrob. Chemother. 2016, 71, 1860–1865. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Ryzhkov, F.V.; Elinson, M.N.; Maslov, O.I.; Fakhrutdinov, A.N. One-Pot Solvent-Involved Synthesis of 5-O-Substituted 5H-Chromeno[2,3-b] pyridines. Molecules 2022, 28, 64. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef]
- Mohammat, M.F.; Shaameri, Z.; Hamzah, A.S. Synthesis of 2,3-Dioxo-5-(substituted)arylpyrroles and their 2-Oxo-5-aryl-3-hydrazone pyrrolidine derivatives. Molecules 2009, 14, 250–256. [Google Scholar] [CrossRef]
- Mohammat, M.F.; Najim, N.; Mansor, N.S.; Sarman, S.; Shaameri, Z.; Mat Zain, M.; Hamzah, A.S. Synthesis and bioactivity of some 2-oxo-5-aryl-3-hydrazone and 2-oxo-5-aryl-4-hydrazone pyrrolidine derivatives. Arkivoc 2011, 9, 429–438. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Sani, M.H.; Mohammat, M.F.; Mansor, N.S.; Shaameri, Z.; Teh, L.K.; Salleh, M.Z.; Hamzah, A.S. Antinociceptive activity of a synthetic oxopyrrolidine-based compound, ASH21374, and determination of its possible mechanisms. Can. J. Physiol. Pharmacol. 2013, 91, 1143–1153. [Google Scholar] [CrossRef]
- Mohammat, M.F.; Mansor, N.S.; Shaameri, Z.; Hamzah, A.S. Diastereoselective Reduction of 2,3-Dioxo-4-carboxy-5-substituted pyrrolidines using NaBH4/AcOH and heterogenous hydrogenation reactions. J. Korean Chem. Soc. 2015, 59, 31–34. [Google Scholar] [CrossRef][Green Version]
- Gibbons, S. Phytochemicals for bacterial resistance—Strengths, weaknesses and opportunities. Planta Med. 2008, 74, 594–602. [Google Scholar] [CrossRef]
- Otto, M. Community-associated MRSA: What makes them special? Int. J. Med. Microbiol. 2013, 303, 324–330. [Google Scholar] [CrossRef]
- Valdes, A.F.C.; Martinez, J.M.; Lizama, R.S.; Vermeersch, M.; Cos, P.; Maes, L. In vitro anti-microbial activity of the Cuban medicinal plants Simarouba glauca DC, Melaleuca leucadendron L and Artemisia absinthium L. Mem. Inst. Oswaldo Cruz 2008, 103, 615–618. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Bioorg. Med. Chem. 2003, 11, 2149–2156. [Google Scholar] [CrossRef]
- Karpin, G.W.; Morris, D.M.; Ngo, M.T.; Merola, J.S.; Falkinham, J.O., III. Transition metal diamine complexes with antimicrobial activity against Staphylococcus aureus and methicillin-resistant S. aureus (MRSA). Med. Chem. Com. 2015, 6, 1471–1478. [Google Scholar] [CrossRef]
- Jindal, H.M.; Le, C.F.; Mohd Yusof, M.Y.; Velayuthan, R.D.; Lee, V.S.; Zain, S.M.; Isa, D.M.; Sekaran, S.D. Antimicrobial activity of novel synthetic peptides derived from indolicidin and ranalexin against Streptococcus pneumoniae. PLoS ONE 2015, 10, e0128532. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Available online: https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/ST-SG-AC10-30-Rev4e.pdf (accessed on 7 February 2023).
- Cayman Chemicals. Amikacin Safety Data Sheet. Available online: https://www.caymanchem.com/msdss/15405m.pdf (accessed on 7 February 2023).
- Cayman Chemicals. Ofloxacin Safety Data Sheet. Available online: https://www.caymanchem.com/msdss/22891m.pdf (accessed on 7 February 2023).
- Ntchapda, F.; Abakar, D.; Kom, B.; Nana, P.; Hamadjida, A.; Dimo, T. Acute and sub-chronic oral toxicity assessment of the aqueous extract leaves of Ficus glumosa Del. (Moraceae) in rodents. J. Intercult. Ethnopharmacol. 2014, 3, 206–213. [Google Scholar] [CrossRef]
- Chaotham, C.; Chivapat, S.; Chaikitwattana, A.; De-Eknamkul, W. Acute and chronic oral toxicity of a partially purified plaunotol extract from Croton stellatopilosus Ohba. Biomed Res. Int. 2013, 2013, 303162. [Google Scholar] [CrossRef] [PubMed]
- Im, W.B.; Choi, S.H.; Park, J.Y.; Choi, S.H.; Finn, J.; Yoon, S.H. Discovery of torezolid as a novel 5-hydroxymethyl-oxazolidinone antibacterial agent. Eur. J. Med. Chem. 2011, 46, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.M.; Zakaria, A.S.; Edward, E.A.; Abdel-Bary, A. In vitro and in vivo activity of zabofloxacin and other fluoroquinolones against MRSA isolates from a university hospital in Egypt. Pol. J. Microbiol. 2019, 68, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Banevicius, M.A.; Kaplan, N.; Hafkin, B.; Nicolau, D.P. Pharmacokinetics, pharmacodynamics and efficacy of novel FabI inhibitor AFN-1252 against MSSA and MRSA in the murine thigh infection model. J. Chemother. 2019, 25, 26–31. [Google Scholar] [CrossRef]
- Wittke, F.; Vincent, C.; Chen, J.; Heller, B.; Kabler, H.; Overcash, J.S.; Leylavergne, F.; Dieppois, G. Afabicin, a first-in-class antistaphylococcal antibiotic, in the treatment of acute bacterial skin and skin structure infections: Clinical noninferiority to vancomycin/linezolid. Antimicrob. Agents Chemother. 2020, 64, e00250-20. [Google Scholar] [CrossRef]
- Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506. [Google Scholar] [CrossRef]
- Hamamoto, H.; Kurokawa, K.; Kaito, C.; Kamura, K.; Manitra Razanajatovo, I.; Kusuhara, H.; Santa, T.; Sekimizu, K. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents Chemother. 2004, 48, 774–779. [Google Scholar] [CrossRef]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-Spectrum Antibacterial Agents. Medchemcomm 2018, 9, 12–21. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.; Avent, M. Oral or intravenous antibiotics? Aust. Prescr. 2020, 43, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development. OECD Guideline for the Testing of Chemicals, Revised Draft Test Guideline 420, Acute Oral Toxicity—Fixed Dose Procedure. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl420.pdf (accessed on 7 February 2023).
| Code | Structure | Code | Structure | Code | Structure |
|---|---|---|---|---|---|
| 1a | 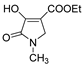 | MFM514 |  | 4d | 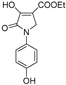 |
| 1b | 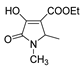 | 1l | 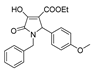 | 4e | 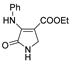 |
| 1c | 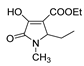 | 2a | 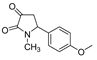 | 4f | 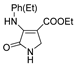 |
| 1d | 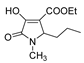 | 2b | 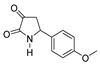 | 4g | 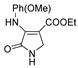 |
| 1e | 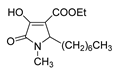 | 3a | 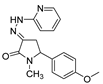 | 4h | 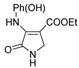 |
| 1f |  | 3b | 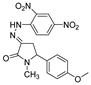 | 4i | 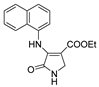 |
| 1g | 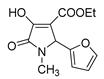 | 3c | 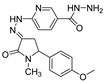 | 4j |  |
| 1h | 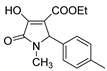 | 3d | 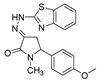 | 4k | 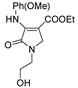 |
| 1i | 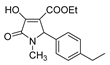 | 4a | 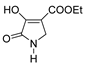 | 5a |  |
| 1j |  | 4b | 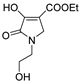 | 5b | 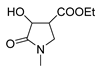 |
| 1k | 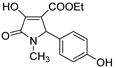 | 4c | 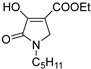 |
| Compounds | MIC Values (µg/mL) | |
|---|---|---|
| MRSA (ATCC 33591) | MSSA (ATCC 25923) | |
| 1a–1d | >500 | >500 |
| 1e | 125 | 250 |
| 1f–1l | >500 | >500 |
| MFM514 | 15.6 | 31.3 |
| 2a–5b | >500 | >500 |
| S. aureus Isolates | MIC Values (µg/mL) |
|---|---|
| MRSA isolates | |
| A1, A2, A3, A4, A7, A8, BAA-1556, C1, C4, C5, C8, HN1, HN3, HN4, HN5, HN13, HN14, HS3178, HS770, HS3175, N441, N391, N829, N850, N1406, U949 | 15.6 |
| BAA-1688, D3, HN7 | 7.8 |
| MSSA isolates | |
| B1, UM9, ATCC 6538, HN6, HN11, A5, A6, C6, ATCC 35556 | 15.6 |
| HN8, HN9, HN10 | 7.8 |
| Compounds | Cell Lines | IC50 | MIC | SI Values (IC50/MIC) |
|---|---|---|---|---|
| (µg/mL) | ||||
| MFM514 | 3T3, Vero and WRL-68 | >625 | 7.8 | 80.1 |
| 15.6 | 40.1 | |||
| Paclitaxel | 3T3 | 0.012 ± 0.01 | ND 1 | ND |
| Vero | 0.0055 ± 0.02 | |||
| WRL-68 | 0.0027 ± 0.06 | |||
| Mice Group | Cell Lines | % of Weight Change | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | Day 10 | Day 14 | ||
| Untreated (5% Tween 80) | 26.3 ± 1.2 | 27.2 ± 1.7 | 27.8 ± 1.4 | 28.2 ± 1.8 | 29.5 ± 1.2 | 11.0 ± 3.2 |
| MFM514 | 26.4 ± 1.6 | 27.9 ± 3.0 | 28.8 ± 3.1 | 28.5 ± 1.9 | 29.7 ± 2.3 | 11.1 ± 2.3 |
| Mice Groups | Untreated/Treated Mice | Total of Mice Survive | % Survive |
|---|---|---|---|
| Untreated mice | MRSA adjuvant only | 0/8 | 0 |
| MRSA adjuvant + 25 mg/kg linezolid | 8/8 | 100 | |
| MHB + 5% mucin only | 8/8 | 100 | |
| Healthy and untreated mice | 8/8 | 100 | |
| Mice treated with MFM514 | 125 mg/kg | 7/8 | 87.5 |
| 62.5 mg/kg | 5/8 | 62.5 | |
| 31.3 mg/kg | 4/8 | 50 | |
| 15.6 mg/kg | 3/8 | 37.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johari, S.A.; Mohtar, M.; Mohammat, M.F.; Abdul Rashid, F.N.A.; Bacho, M.Z.; Mohamed, A.; Mohamad Ridhwan, M.J.; Syed Mohamad, S.A. Investigating the Antibacterial Effects of Synthetic Gamma-Lactam Heterocycles on Methicillin-Resistant Staphylococcus aureus Strains and Assessing the Safety and Effectiveness of Lead Compound MFM514. Molecules 2023, 28, 2575. https://doi.org/10.3390/molecules28062575
Johari SA, Mohtar M, Mohammat MF, Abdul Rashid FNA, Bacho MZ, Mohamed A, Mohamad Ridhwan MJ, Syed Mohamad SA. Investigating the Antibacterial Effects of Synthetic Gamma-Lactam Heterocycles on Methicillin-Resistant Staphylococcus aureus Strains and Assessing the Safety and Effectiveness of Lead Compound MFM514. Molecules. 2023; 28(6):2575. https://doi.org/10.3390/molecules28062575
Chicago/Turabian StyleJohari, Saiful Azmi, Mastura Mohtar, Mohd Fazli Mohammat, Fatin Nur Ain Abdul Rashid, Muhamad Zulfaqar Bacho, Azman Mohamed, Mohamad Jemain Mohamad Ridhwan, and Sharifah Aminah Syed Mohamad. 2023. "Investigating the Antibacterial Effects of Synthetic Gamma-Lactam Heterocycles on Methicillin-Resistant Staphylococcus aureus Strains and Assessing the Safety and Effectiveness of Lead Compound MFM514" Molecules 28, no. 6: 2575. https://doi.org/10.3390/molecules28062575
APA StyleJohari, S. A., Mohtar, M., Mohammat, M. F., Abdul Rashid, F. N. A., Bacho, M. Z., Mohamed, A., Mohamad Ridhwan, M. J., & Syed Mohamad, S. A. (2023). Investigating the Antibacterial Effects of Synthetic Gamma-Lactam Heterocycles on Methicillin-Resistant Staphylococcus aureus Strains and Assessing the Safety and Effectiveness of Lead Compound MFM514. Molecules, 28(6), 2575. https://doi.org/10.3390/molecules28062575





