Isolation and Characterization of One New Natural Compound with Other Potential Bioactive Secondary Metabolites from Glycosmis cyanocarpa (Blume) Spreng. (Family: Rutaceae)
Abstract
1. Introduction
2. Results
2.1. Isolated Phytochemicals from G. cyanocarpa
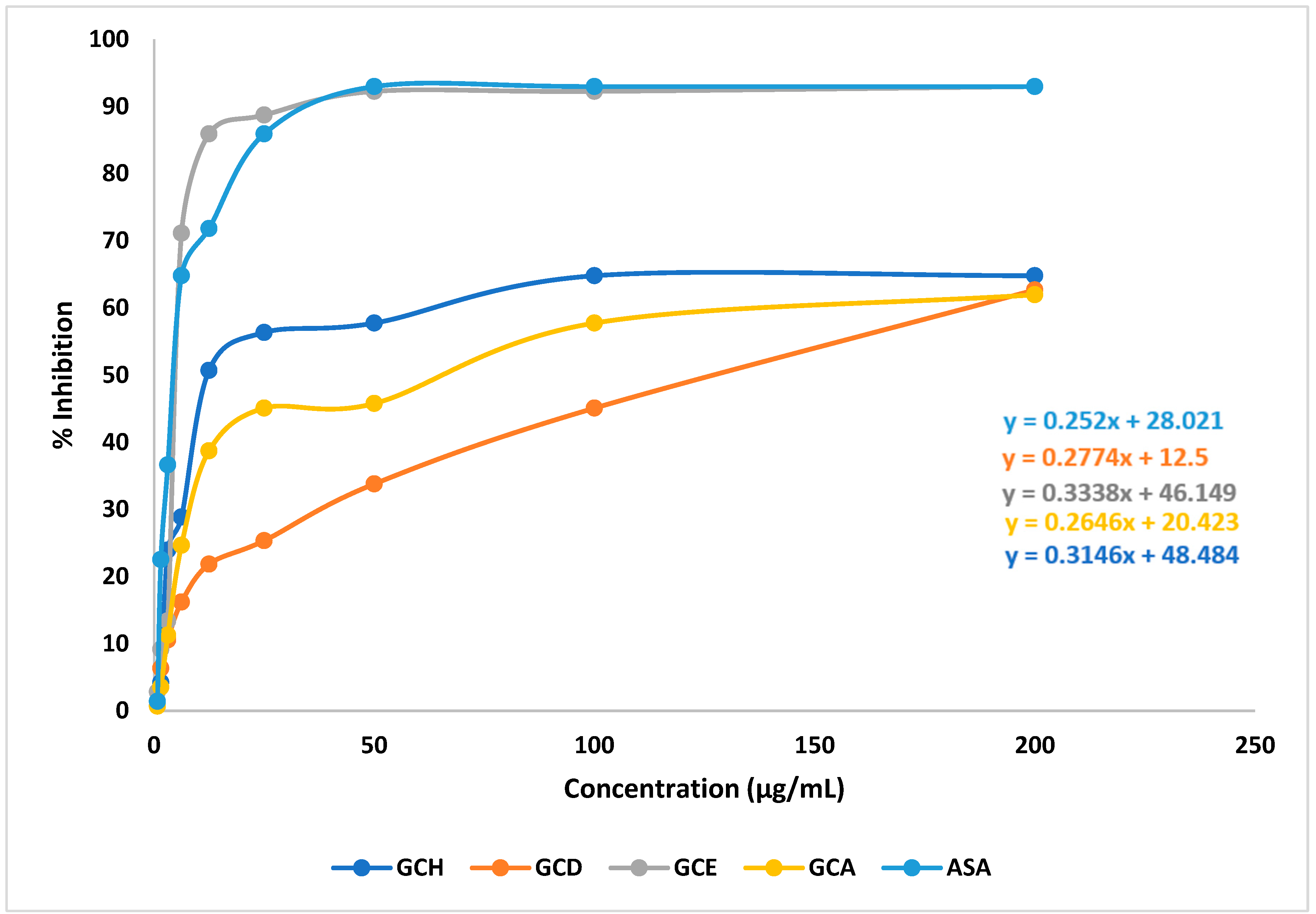
2.2. Effect of G. cyanocarpa Extracts on DPPH Free Radical Scavenging Activity
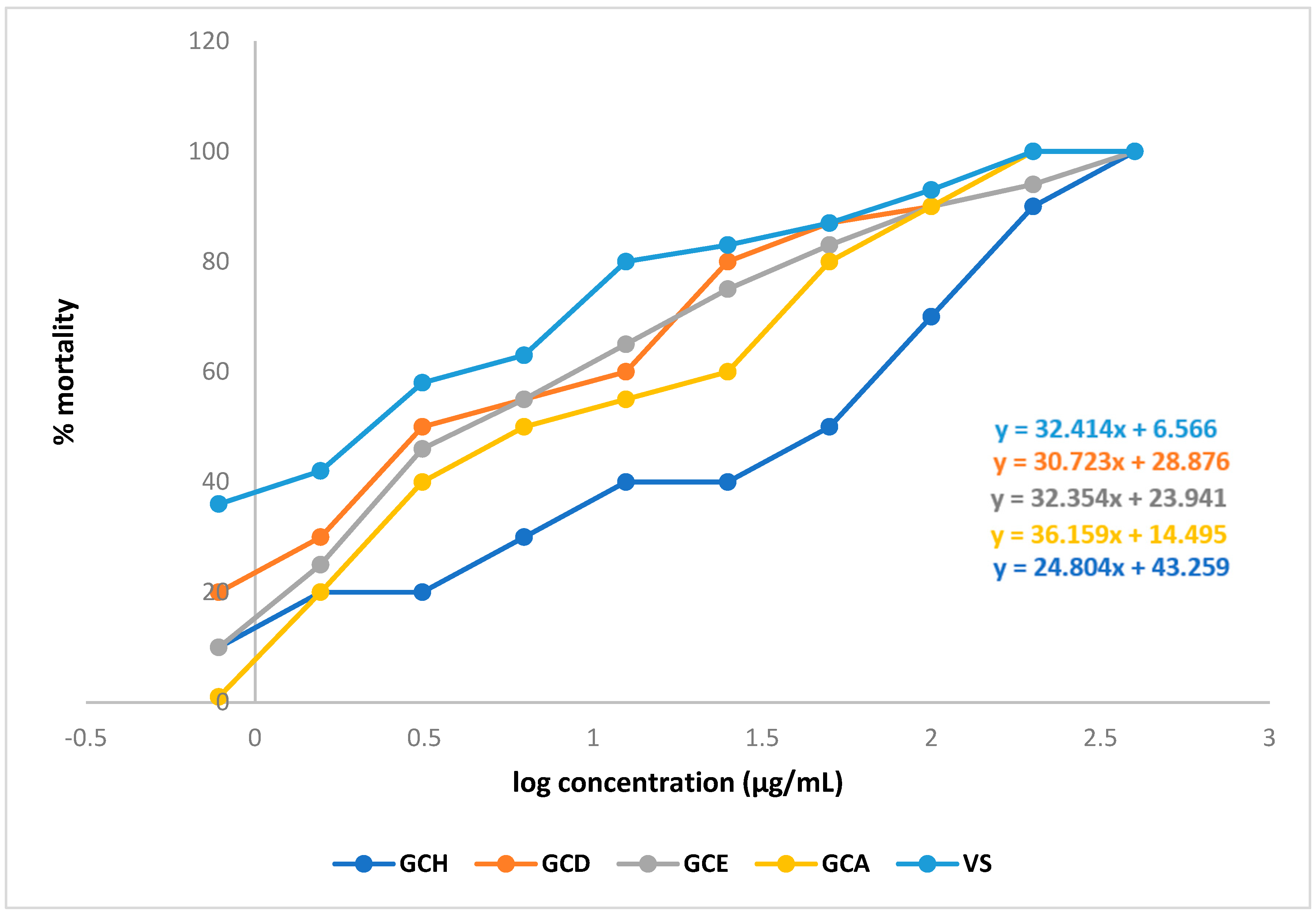
2.3. Effect of G. cyanocarpa Extracts on Brine Shrimp Lethality Bioassay
2.4. Effect of G. cyanocarpa Extracts on the Thrombolytic Activity
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of the Plant Material
4.2. Instrumentations, Drugs, and Chemicals
4.3. Experimental Design
4.3.1. Extraction of Plant Material
4.3.2. Isolation of Compounds
4.3.3. Preparation of Different Partitions for Biological Tests
4.3.4. Structural Identification of the Compounds
4.4. Antioxidant Assay
DPPH Free Radical Scavenging Assay
4.5. Cytotoxicity Assay
Brine Shrimp Lethality Bioassay
4.6. In Vitro Thrombolytic Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Harvey, A.L. Natural Products in Drug Discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Beniddir, M.A.; Le Borgne, E.; Iorga, B.I.; Loaëc, N.; Lozach, O.; Meijer, L.; Awang, K.; Litaudon, M. Acridone Alkaloids from Glycosmis Chlorosperma as DYRK1A Inhibitors. J. Nat. Prod. 2014, 77, 1117–1122. [Google Scholar] [CrossRef]
- Greger, H. Phytocarbazoles: Alkaloids with Great Structural Diversity and Pronounced Biological Activities. Phytochem. Rev. 2017, 16, 1095–1153. [Google Scholar] [CrossRef]
- Teja, P.K.; Patel, P.; Bhavsar, D.; Bindusri, C.; Jadhav, K.; Chauthe, S.K. Traditional Uses, Phytochemistry, Pharmacology, Toxicology and Formulation Aspects of Glycosmis Species: A Systematic Review. Phytochemistry 2021, 190, 112865. [Google Scholar] [CrossRef]
- Zohora, F.T.; Azam, A.T.M.Z.; Ahmed, S.; Rahman, K.M.; Halim, M.A.; Anwar, M.R.; Sohrab, M.H.; Tabassum, F.; Hasan, C.M.; Ahsan, M. Isolation and In Silico Prediction of Potential Drug-like Compounds with a New Dimeric Prenylated Quinolone Alkaloid from Zanthoxylum Rhetsa (Roxb.) Root Extracts Targeted against SARS-CoV-2 (Mpro). Molecules 2022, 27, 8191. [Google Scholar] [CrossRef]
- Tabassum, F.; Hasan, C.; Masud, M.; Jamshidi, S.; Rahman, K.M.; Ahsan, M. Indole Alkaloids from the Leaves of Ravenia Spectabilis Engl. with Activity against Pancreatic Cancer Cell Line. Elsevier 2021, 186, 112744. [Google Scholar] [CrossRef]
- Yasir, M.; Tripathi, M.K.; Singh, P.; Shrivastava, R. The Genus Glycosmis [Rutaceae]: A Comprehensive Review on Its Phytochemical and Pharmacological Perspectives. Nat. Prod. J. 2018, 9, 98–124. [Google Scholar] [CrossRef]
- Wurz, G.; Hofer, O.; Greger, H. Structure and Synthesis of Phenaglydon, a New Quinolone Derived Phenanthridine Alkaloid from Glycosmis Cyanocarpa. Nat. Prod. Lett. 2006, 3, 177–182. [Google Scholar] [CrossRef]
- Sarkar, M.; Kundu, S.; Chakraborty, D.P. Glycarpine, a New Alkaloid from Glycosmis Cyanocarpa. Phytochemistry 1978, 17, 2145–2146. [Google Scholar] [CrossRef]
- Greger, H.; Hofer, O.; Kählig, H.; Wurz, G. Sulfur Containing Cinnamides with Antifungal Activity from Glycosmis Cyanocarpa. Tetrahedron 1992, 48, 1209–1218. [Google Scholar] [CrossRef]
- Greger, H.; Hadacek, F.; Hofer, O.; Wurz, G.; Zechner, G. Different Types of Sulphur-Containing Amides from Glycosmis Cf. Chlorosperma. Phytochemistry 1993, 32, 933–936. [Google Scholar] [CrossRef]
- Greger, H.; Zechner, G.; Hofer, O.; Hadacek, F.; Wurz, G. Sulphur-Containing Amides from Glycosmis Species with Different Antifungal Activity. Phytochemistry 1993, 34, 175–179. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Zare Marzouni, H. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid.-Based Integr. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- Uddin Emon, N.; Jahan, I.; Mohammed Sayeed, A. Investigation of Antinociceptive, Anti-Inflammatory and Thrombolytic Activity of Caesalpinia Digyna (Rottl.) Leaves by Experimental and Computational Approaches. Adv. Tradit. Med. 2020, 20, 451–459. [Google Scholar] [CrossRef]
- de Medeiros da Silva, C.; Bolzan, A.A.; Mallmann, C.A.; Pozzatti, P.; Alves, S.H.; Heinzmann, B.M. Sesquiterpenoids of Senecio Bonariensis Hook. & Arn., Asteraceae. Rev. Bras. Farmacogn. 2010, 20, 87–92. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Islam, A.; Emon, N.U.; Islam, Q.S.; Ahsan, M. Chemico-Biological Profiling of Blumea Lacera (Burm.f.) DC. (Family: Asteraceae) Provides New Insights as a Potential Source of Antioxidant, Cytotoxic, Antimicrobial, and Antidiarrheal Agents. Evid.-Based Complement. Altern. Med. 2022, 2022, 2293415. [Google Scholar] [CrossRef] [PubMed]
- Bang, M.H.; Soo, Y.C.; Jang, T.O.; Sang, K.K.; Kwon, O.S.; Kang, T.C.; Moo, H.W.; Park, J.; Baek, N.I. Phytol, SSADH Inhibitory Diterpenoid OfLactuca Sativa. Arch. Pharmacal Res. 2002, 25, 643–646. [Google Scholar] [CrossRef]
- Chhetri, H.; Yogol, N.; Sherchan, J.; Anupa, K.C.; Mansoor, S.; Thapa, P. Phytochemical and Antimicrobial Evaluations of Some Medicinal Plants of Nepal. Kathmandu Univ. J. Sci. Eng. Technol. 2008, 4, 49–54. [Google Scholar] [CrossRef]
- Samid, D.; Hudgins, W.R.; Shack, S.; Liu, L.; Prasanna, P.; Myers, C.E. Phenylacetate and Phenylbutyrate as Novel, Nontoxic Differentiation Inducers. Adv. Exp. Med. Biol. 1997, 400, 501–505. [Google Scholar] [CrossRef]
- Qi, Z.; Li, Z.; Zhu, M.; Zhang, X.; Zhang, G.; Zhuang, T.; Chen, Y.; Huang, L. Design, Synthesis, and Evaluation of Phenylpiperazine-Phenylacetate Derivatives as Rapid Recovery Hypnotic Agents. Bioorg. Med. Chem. Lett. 2022, 57, 128497. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Badisa, V.L.D.; Latinwo, L.M.; Cooperwood, J.; Sinclair, A.; Abdullah, A. Cytotoxic Activity of New Acetoxycoumarin Derivatives in Cancer Cell Lines. Anticancer Res. 2011, 31, 2017–2022. [Google Scholar] [PubMed]
- Chávez, M.I.; Soto, M.; Taborga, L.; Díaz, K.; Olea, A.F.; Bay, C.; Peña-Cortés, H.; Espinoza, L. Synthesis and in Vitro Antifungal Activity against Botrytis Cinerea of Geranylated Phenols and Their Phenyl Acetate Derivatives. Int. J. Mol. Sci. 2015, 16, 19130–19152. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, D.; Cho, H. Effect of Thiazolidinedione Phenylacetate Derivatives on Wound-Healing Activity. Arch. Pharm. Res. 2019, 42, 790–814. [Google Scholar] [CrossRef]
- Wu, J.J.; Huang, J.W.; Deng, W.L. Phenylacetic Acid and Methylphenyl Acetate from the Biocontrol Bacterium Bacillus Mycoides BM02 Suppress Spore Germination in Fusarium Oxysporum f. Sp. Lycopersici. Front. Microbiol. 2020, 11, 3022. [Google Scholar] [CrossRef]
- Xiong, L.; Gao, Y.; Niu, C.; Wang, H.B.; Li, W.H. Synthesis and in Vitro Anticancer Activity of Novel 2-((3-Thioureido) Carbonyl) Phenyl Acetate Derivatives. Lett. Drug Des. Discov. 2014, 11, 132–137. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Balaneva, N.N.; Bode, A.M.; Stonik, V.A.; Dong, Z. Evaluation of Cancer-Preventive Activity and Structure-Activity Relationships of 3-Demethylubiquinone Q2, Isolated from the Ascidian Aplidium Glabrum, and Its Synthetic Analogs. Pharm. Res. 2006, 23, 70–81. [Google Scholar] [CrossRef]
- De Rosa, S.; De Giulio, A.; Iodice, C. Biological Effects of Prenylated Hydroquinones: Structure-Activity Relationship Studies in Antimicrobial, Brine Shrimp, and Fish Lethality Assays. J. Nat. Prod. 1994, 57, 1711–1716. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids; Academic Press: Cambridge, MA, USA, 2017; pp. 233–266. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and Anti-Inflammatory Activity of Caryophyllene Oxide from Annona squamosa L. Bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene Oxide Inhibits Growth and Induces Apoptosis through the Suppression of PI3K/AKT/MTOR/S6K1 Pathways and ROS-Mediated MAPKs Activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, S.K.; Cheng, X.L.; Tian, X.W.; Wang, J. Caryophyllene Oxide Exhibits Anti-Cancer Effects in MG-63 Human Osteosarcoma Cells via the Inhibition of Cell Migration, Generation of Reactive Oxygen Species and Induction of Apoptosis. Bangladesh J. Pharmacol. 2016, 11, 817–823. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and Antifungal Chemicals Produced by Plants: A Review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.P.; Ferreira, P.B.; De Sousa, D.P.; Jordan, J.; Freitas, R.M. Anticonvulsant Effect of Phytol in a Pilocarpine Model in Mice. Neurosci. Lett. 2012, 523, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Rajab, M.S.; Cantrell, C.L.; Franzblau, S.G.; Fischer, N.H. Antimycobacterial Activity of (E)-Phytol and Derivatives: A Preliminary Structure-Activity Study. Planta Med. 1998, 64, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An Insight into the Cytotoxic Activity of Phytol at in Vitro Conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Carolina De Menezes, C.; Santos, P.; Salvadori, M.S.; Gomes Mota, V.; Muratori Costa, L.; Cardoso De Almeida, A.A.; Lopes De Oliveira, G.A.; Pereira Costa, J.; Pergentino De Sousa, D.; Mendes De Freitas, R.; et al. Antinociceptive and Antioxidant Activities of Phytol in Vivo and in Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A Review of Biomedical Activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Pelletier, S.W.; Chokshi, H.P.; Desai, H.K. Separation of Diterpenoid Alkaloid Mixtures Using Vacuum Liquid Chromatography. J. Nat. Prod. 1986, 49, 892–900. [Google Scholar] [CrossRef]
- VanWagenen, B.C.; Larsen, R.; Cardellina, J.H.; Randazzo, D.; Lidert, Z.C.; Swithenbank, C. Ulosantoin, a Potent Insecticide from the Sponge Ulosa Ruetzleri. J. Org. Chem. 1993, 58, 335–337. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Emon, N.U.; Ahsan, M. Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis Cyanocarpa (Blume) Spreng. Molecules 2022, 27, 5972. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Singh, O.M. Recent Progress in Biological Activities of Indole and Indole Alkaloids. Mini-Rev. Med. Chem. 2017, 18, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Süzen, S. Antioxidant Activities of Synthetic Indole Derivatives and Possible Activity Mechanisms. In Bioactive Heterocycles V. Topics in Heterocyclic Chemistry; Springer: Berlin/Heidelberg, Germany, 2007; pp. 145–178. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, Antioxidant and Cytoprotective Activity Evaluation of C-3 Substituted Indole Derivatives. Sci. Rep. 2021, 11, 15425. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Rahman, M.S. Preliminary Assessment of Free Radical Scavenging, Thrombolytic and Membrane Stabilizing Capabilities of Organic Fractions of Callistemon Citrinus (Curtis.) Skeels Leaves. BMC Complement. Altern. Med. 2016, 16, 247. [Google Scholar] [CrossRef]
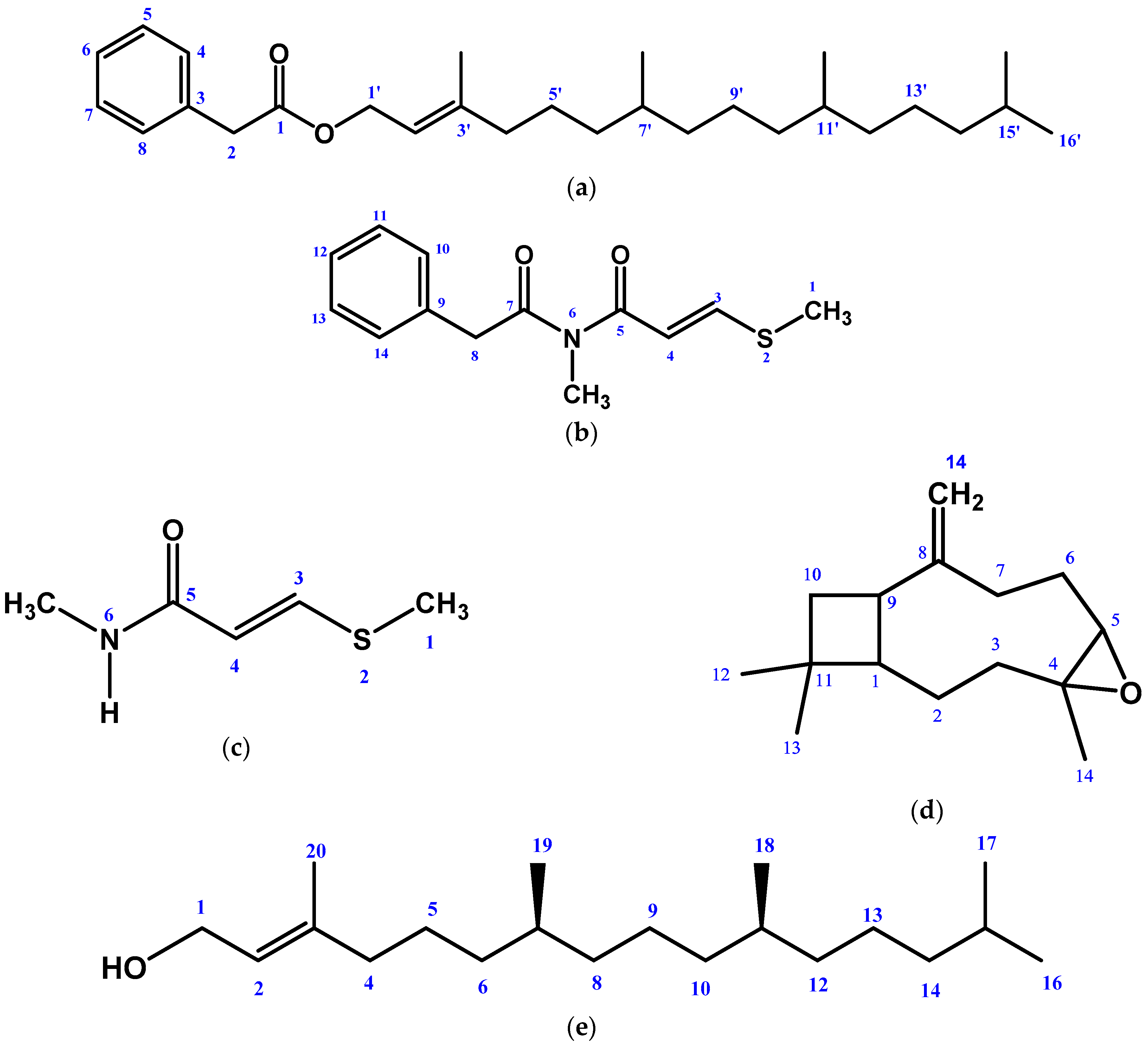
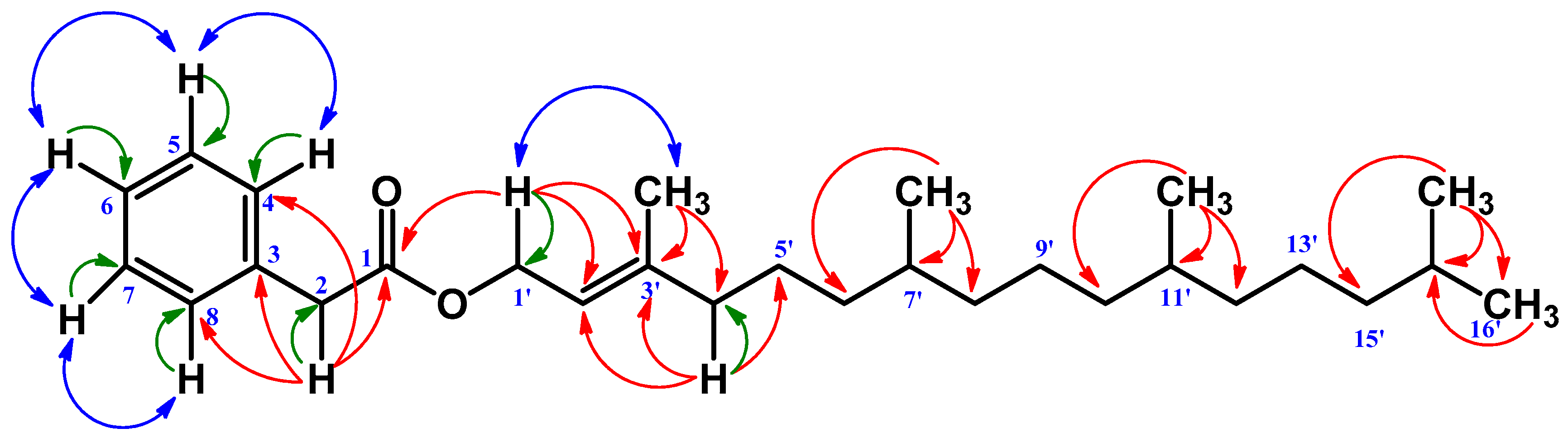
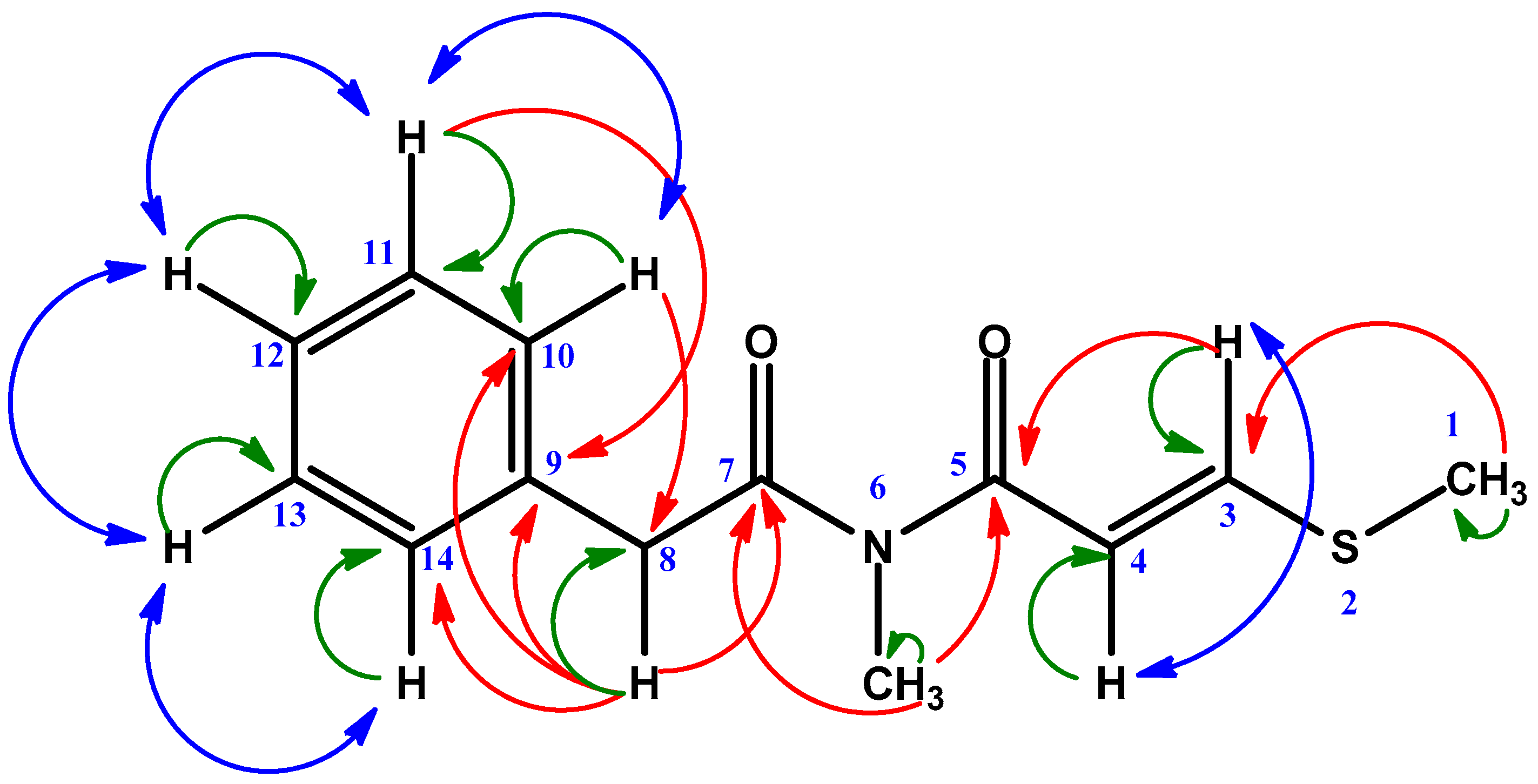
| Position | δC | δH | HMBC |
|---|---|---|---|
| 1 | 171.7 | --- | --- |
| 2 | 41.4 | 3.62 2H s | 171.7 (C-1), 134.2 (C-3), 129.3 (C-4) |
| 3 | 134.2 | --- | --- |
| 4,8 | 129.3 | 7.24–7.30 m | --- |
| 5,7 | 128.5 | 7.24–7.30 m | --- |
| 6 | 127.0 | 7.3 m | --- |
| 1′ | 61.9 | 4.61 2H d (J = 7.2 Hz) | 171.7 (C-1), 143.0 (C-3′), 117.9 (C-2′) |
| 2′ | 117.9 | 5.32 t (J = 7.2 Hz) | --- |
| 3′ | 143.0 | --- | --- |
| 4′ | 39.9 | 1.99 2H t (J = 7.4 Hz) | 143.0 (C-3′), 117.9 (C-2′) |
| 5′ | 25.1 | 1.35 m, 1.24 m | --- |
| 6′ | 37.5 | 1.28 m, 1.19 m | --- |
| 7′ | 32.8 | 1.36 m | --- |
| 8′ | 37.4 | 1.28 m, 1.19 m | --- |
| 9′ | 24.8 | 1.35 m, 1.24 m | --- |
| 10′ | 37.3 | 1.08 m, 1.00 m | --- |
| 11′ | 32.7 | 1.36 m | --- |
| 12′ | 37.3 | 1.08 m, 1.00 m | --- |
| 13′ | 24.5 | 1.14 m, 1.07 m | --- |
| 14′ | 39.4 | 1.16 m, 1.10 m | 22.6* (C-16), 22.7* (15′-CH3) |
| 15′ | 28.0 | 1.52 1H sep (J = 6.8 Hz) | 22.6* (C-16), 22.7* (15′-CH3) |
| 16′ | 22.7* | 0.86 3H d (J = 6.8 Hz) | |
| CH3-3′ | 16.4 | 1.66 3Hs | 143.0 (C-3′), 117.9 (C-2′), 39.9 (C-4′) |
| CH3-7′ | 19.8 | 0.84 3H d (J = 6.8 Hz) | 32.7 (C-11′), 32.8 (C-7′) |
| CH3-11 | 19.7 | 0.84 3H d (J = 6.8 Hz) | 37.3 (C-10′), 37.4 (C-8′) |
| CH3-15 | 22.6* | 0.86 3H d (J = 6.8 Hz) | 39.4 (C-14′), 28.0 (C-15′) |
| Position | δC | δH | HMBC |
|---|---|---|---|
| 3 | 149.6 | 7.90 d (J = 14.4 Hz) | 14.9 (S-Me), 167.3 (C-5) |
| 4 | 115.1 | 6.42 d (J = 14.4 Hz) | 149.6 (C-3) |
| 5 | 167.3 | --- | --- |
| 7 | 174.2 | --- | --- |
| 8 | 44.3 | 4.06 2H s | 128.6 (C-10,14)) 174.2(C-7) |
| 9 | 134.4 | --- | |
| 10 | 129.4 | 7.24–7.33 m | 44.3 (C-8) |
| 11 | 128.6 | 7.33 m | --- |
| 12 | 127.1 | 7.24–7.33 m | --- |
| 13 | 128.6 | 7.33 m | --- |
| 14 | 129.4 | 7.24–7.33 m | 44.3 (C-8) |
| N-Me | 32.1 | 3.26 3H s | 167.3 (C-5), 174.2(C-7) |
| S-Me | 14.9 | 2.36 3H s | 149.6 (C-3) |
| 5-CO | 167.3 | --- | --- |
| 7-CO | 174.2 | --- | --- |
| Position | Compound-3 | Penangin [11] | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 2.33 s | 14.7 | 2.32 s | 14.6 |
| 2 | --- | --- | --- | --- |
| 3 | 7.61 d | 142.9 | 7.62 d | 142.6 |
| 4 | 5.6 d | 115.6 | 5.61 d | 115.7 |
| 5 | --- | 165.2 | --- | 165.2 |
| 6 | 5.4 br q | --- | 5.3 br q | --- |
| N-Me | 2.89 d | 26.4 | 2.88 | 26.3 |
| Position | Compound-4 | β-Caryophyllene Oxide [16] | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | 50.8 | 1.76 m | 50.9 | 1.76 t (J = 9.3 Hz) |
| 2 | 27 | 1.64 m, 1.42 m | 27.1 | 1.65 m, 1.43 m |
| 3 | 39.2 | 0.96 m, 2.08 m | 39.2 | 0.98 m, 2.11 m |
| 4 | 59.6 | --- | 59.7 | --- |
| 5 | 63.8 | 2.87 dd (J = 10.5, 4.1) | 63.7 | 2.87 dd (J = 10.6,4.2) |
| 6 | 31.9 | 2.24 m, 1.32 m | 31.8 | 2.24 m 1.30 m |
| 7 | 30.2 | 2.34 m, 2.12 m | 30.2 | 2.33 ddd (J = 4.6, 7.7, 12.4 Hz) 2.11 m |
| 8 | 151.7 | ---- | 151.6 | ---- |
| 9 | 48.8 | 2.60 q (J = 9.4) | 48.7 | 2.61 q (J = 9.3) |
| 10 | 39.8 | 1.63–1.69 2H m | 39.8 | 1.65 2H m |
| 11 | 33.9 | --- | 34.0 | --- |
| 12 | 21.7 | 1.00 3H s | 21.6 | 1.01 3H s |
| 13 | 29.7 | 0.98 3H s | 29.8 | 0.98 3H s |
| 14 | 17.0 | 1.20 3H s | 17.0 | 1.20 3H s |
| 15 | 112.7 | 4.96 s, 4.84 s | 112.7 | 4.98, 4.86 s |
| Position | Compound 5 | Phytol [18] |
|---|---|---|
| δH | δH | |
| H-1 | 4.15, 2H d (J = 6.8 Hz) | 4.14, 2H d (J = 6.8 Hz) |
| H-2 | 5.42, 1H t (J = 6.8 Hz) | 5.40, 1H dq (J = 6.8 Hz, 1.4 Hz) |
| H-4 | 1.98, 2H t (J = 7.0 Hz) | 1.99, 2H t (J = 7.0 Hz) |
| H-16 | 0.86, 3H (J = 6.4 Hz) | 0.87, 6H d (J = 6.3 Hz) |
| H-17 | 0.86, 3H d (J = 6.4 Hz) | 0.87, 6H d (J = 6.3 Hz) |
| H-18 | 0.85, 3H d (J = 6.0 Hz) | 0.85, 3H d (J = 6.1 Hz) |
| H-19 | 0.84, 3H d (J = 6.8Hz) | 0.84, 3H d (J = 6.6 Hz) |
| H-20 | 1.66, 3H s | 1.66, 3H s |
| Test Sample | LC50 (µg/mL) |
|---|---|
| Vincristine Sulphate (VS) | 0.272 |
| GCH | 1.339 |
| GCD | 0.687 |
| GCE | 0.805 |
| GCA | 0.982 |
| Sample | Weight of Empty Eppendorf Tube (W1) g | Weight before Clot Disruption (W2) g | Weight after Clot Disruption (W3) g | Weight before Clot Lysis (W4 = W2 − W1) g | Weight of Lysis Clot (W5 = W2 − W3) g | % Lysis (W5/W4) × 100 |
|---|---|---|---|---|---|---|
| GCH | 0.8176 | 1.139 | 1.1207 | 0.3214 | 0.0183 | 5.69 |
| GCD | 0.8284 | 1.0879 | 1.0453 | 0.2595 | 0.0426 | 16.42 |
| GCE | 0.8256 | 1.2122 | 1.1993 | 0.3866 | 0.0129 | 3.34 |
| GCA | 0.8241 | 1.152 | 1.1319 | 0.3279 | 0.0201 | 6.13 |
| Blank | 0.8436 | 1.8425 | 1.8422 | 0.9989 | 0.0003 | 0.03 |
| SK | 0.798 | 1.427 | 1.012 | 0.629 | 0.415 | 65.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; Ashrafi, S.; Rahman, K.M.; Ahmed, S.; Molla Jamal, A.H.M.S.I.; Ahsan, M. Isolation and Characterization of One New Natural Compound with Other Potential Bioactive Secondary Metabolites from Glycosmis cyanocarpa (Blume) Spreng. (Family: Rutaceae). Molecules 2023, 28, 2207. https://doi.org/10.3390/molecules28052207
Islam MA, Ashrafi S, Rahman KM, Ahmed S, Molla Jamal AHMSI, Ahsan M. Isolation and Characterization of One New Natural Compound with Other Potential Bioactive Secondary Metabolites from Glycosmis cyanocarpa (Blume) Spreng. (Family: Rutaceae). Molecules. 2023; 28(5):2207. https://doi.org/10.3390/molecules28052207
Chicago/Turabian StyleIslam, Md. Ariful, Sania Ashrafi, Khondaker Miraz Rahman, Shamim Ahmed, A. H. M. Shofiul Islam Molla Jamal, and Monira Ahsan. 2023. "Isolation and Characterization of One New Natural Compound with Other Potential Bioactive Secondary Metabolites from Glycosmis cyanocarpa (Blume) Spreng. (Family: Rutaceae)" Molecules 28, no. 5: 2207. https://doi.org/10.3390/molecules28052207
APA StyleIslam, M. A., Ashrafi, S., Rahman, K. M., Ahmed, S., Molla Jamal, A. H. M. S. I., & Ahsan, M. (2023). Isolation and Characterization of One New Natural Compound with Other Potential Bioactive Secondary Metabolites from Glycosmis cyanocarpa (Blume) Spreng. (Family: Rutaceae). Molecules, 28(5), 2207. https://doi.org/10.3390/molecules28052207








