Abstract
The development of an efficient and straightforward method for cyanation of alcohols is of great value. However, the cyanation of alcohols always requires toxic cyanide sources. Herein, an unprecedented synthetic application of an isonitrile as a safer cyanide source in B(C6F5)3-catalyzed direct cyanation of alcohols is reported. With this approach, a wide range of valuable α-aryl nitriles was synthesized in good to excellent yields (up to 98%). The reaction can be scaled up and the practicability of this approach is further manifested in the synthesis of an anti-inflammatory drug, naproxen. Moreover, experimental studies were performed to illustrate the reaction mechanism.
1. Introduction
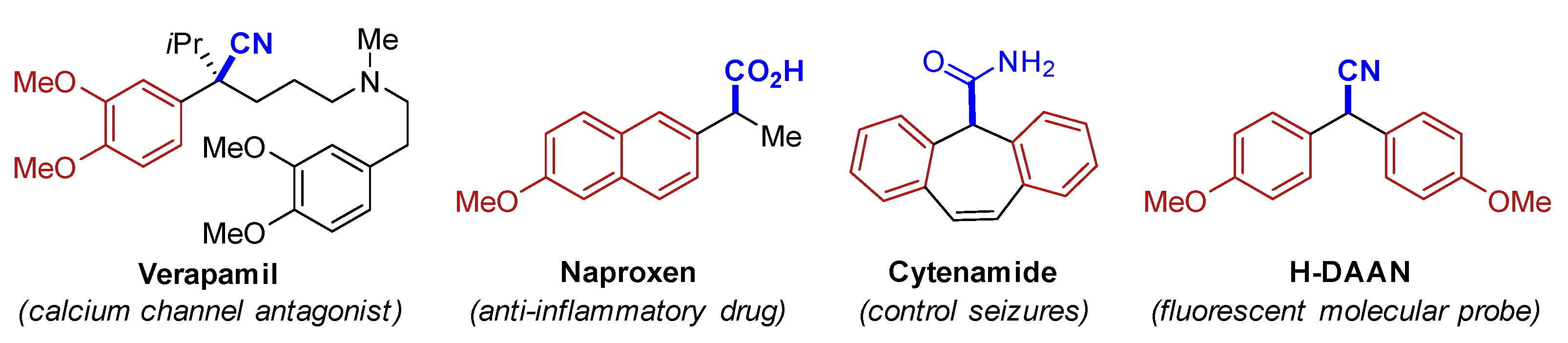
The need for the development of new reactions that are based on applying the atom-economy concept [1] and avoiding the use of toxic reagents has become a consensus. Alcohols are highly attractive starting materials for synthesis because they are stable, have low toxicity, and are available. Direct nucleophilic substitution of an alcohol is attractive since water is, in principle, the only by-product [2,3,4,5]. However, this reaction is difficult because hydroxide is such a poor leaving group and therefore alcohols are classically derivatized to halides or pseudohalides prior to substitution, which results in the formation of vast amounts of waste. Thus, the development of new catalytic methodologies for dehydrative substitutions of alcohols was considered a central issue, as demonstrated by the inclusion of the “direct substitution of alcohols” in the ACS Green Chemistry Institute® Pharmaceutical Roundtable’s 2018 update on key green chemistry research areas [6]. In this context, the deoxygenative cyanation of readily available benzyl alcohols represent one of the most powerful methods for preparing α-aryl nitriles [7,8,9,10,11,12,13,14,15,16,17,18,19,20], an important class of core structures found in bioactive moleculars [21] and functional materials [22], and precursors that have applications in the synthesis of well-known drugs such as verapamil [23], naproxen [24], and cytenamide [25] as shown in Figure 1.
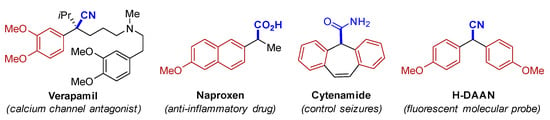
Figure 1.
Verapamil, H-DAAN, and some derivatives of α-aryl nitriles.
As early as 1967, the one-pot method for the conversion of alcohols into cyanides based on the concept of the Mitsunobu reaction using NaCN as the cyanide source has been described [26]. Subsequently, there are a few reports on one-pot transformations of alcohols to α-aryl nitrile using Me3SiCl/NaI/NaCN [27], PPh3/nBu4NCN/DDQ [28], N-(p-toluenesulfonyl)imidazole (TsIm)/NaCN [29], and PPh3/DEAD/acetone cyanohydrin [30].
However, these methods suffer from major disadvantages such as the presence of hazardous and toxic cyanide sources and the use of stoichiometric activating reagents.
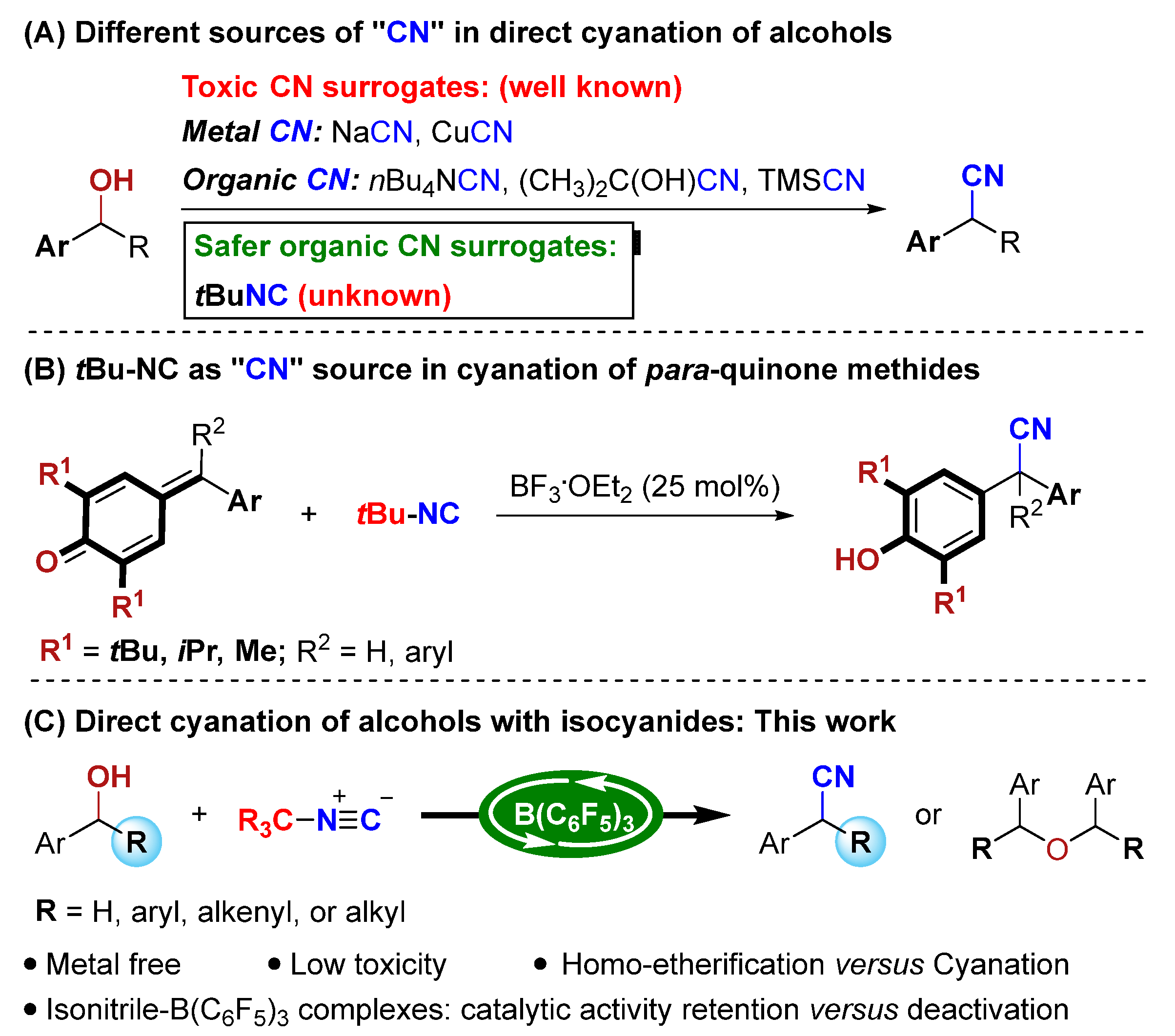
Recently, catalytic synthesis of α-aryl nitrile from benzyl alcohols was successfully developed. Ding’s group performed pioneering work on the direct cyanation of α-aryl alcohols with trimethylsilyl cyanide (TMSCN) by indium halide catalysis [31]. Later, other Lewis acids, such as FeCl3·6H2O [32], Zn(OTf)2 [33], Brønsted acid montmorillonite catalysts [34], and others [35] were also used to catalyze this transformation. Besides these, ruthenium-catalyzed cyanation of benzyl alcohol with cuprous cyanide (CuCN) was also reported [36]. Again, these methods are typically plagued by notorious toxic cyanide source issues. To solve the severe safety issues associated with the handling of traditional cyanide sources, such as metal cyanides, ketone cyanohydrins [37,38,39], or TMSCN, several safer alternatives have been introduced, including: DMF [40], DMSO/ammonium ion [41], azobisisobutyronitrile (AIBN) [42], TsN(Ph)CN [43], isocyanides [44,45,46,47,48,49,50,51], and so on [52,53]. However, no direct cyanation of alcohols has been described so far for the synthesis of α-aryl nitriles from these safer organic CN surrogates (Scheme 1A).
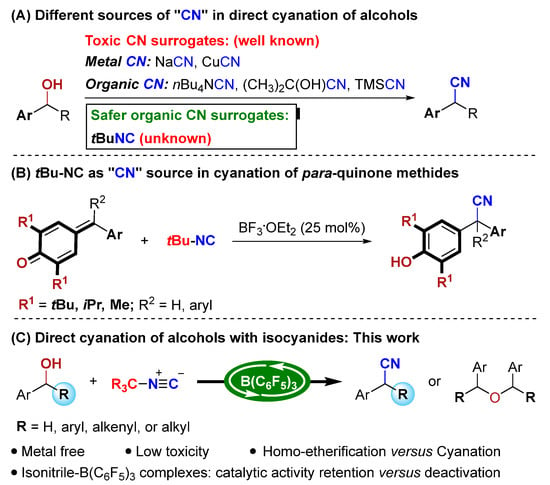
Scheme 1.
Our catalytic strategy to access α-aryl nitriles and its scientific context.
Isocyanides, which are isoelectronic with carbon monoxide, have emerged as powerful C1 building blocks in organic synthesis [54,55,56,57,58,59,60]. The distinctive reactivity of isocyanides makes them well-known in Passerini and Ugi multicomponent reactions and others [61]. In 1982, Saegusa and co-workers performed seminal work on the conjugate hydrocyanation and 1,2-addition reactions with tert-butyl isocyanide in the presence of stoichiometric Lewis acids [44]. Subsequently, commercially available tert-butyl isocyanide as a safer cyanide alternative in C-H cyanation [45,46,47,48], noble-metal-catalyzed cyanation of aryl iodides [49], and cyanothiolation of alkynes [50] has been reported, which further broadened the application of isocyanides in organic synthesis. To date, the catalytic method to obtain valuable α-aryl nitriles relied upon the usage of isocyanides as a cyanide source; however, these are rarely known. As a rare example, Muthukrishnan and co-workers reported a BF3·OEt2-catalyzed 1,6-conjugate addition reaction of p-quinone methides (p-QMs) with tert-butyl isocyanide for synthesis of α-diaryl and α-triaryl nitriles (Scheme 1B) [51]. Nonetheless, the substrates of the reaction are limited to p-QMs that feature bulky tert-butyl substituents at the 2- and 6-positions. Therefore, the development of new reactions with isocyanides for synthesis of α-aryl nitriles is highly desirable.
In recent years, B(C6F5)3 as a non-metallic Lewis acid has received widespread attention because of its strong Lewis acidity, commercial availability, and environmental friendliness [62,63,64,65,66,67,68]. Although still limited in its success, it mainly involves the B(C6F5)3-catalyzed activation of hydroxyl groups, as reported by Meng, Zhao and Chan [69,70], Marek [71], Tang [72], Maji [73], Gevorgyan [74], and Moran [75]. Inspired by these reports and building on our ongoing interest in the developing atom-economic reactions [76,77,78,79], we questioned if the direct cyanation of alcohols with isocyanides in the presence of B(C6F5)3 could be realized to meet the requirements of atom economy and green chemistry (Scheme 1C). However, this hypothesis may face considerable challenges, such as the following: (a) the catalyst should be stable in wet and Lewis basic conditions, and (b) tert-butylisocyanide/B(C6F5)3 and nitrile/B(C6F5)3 adducts can be easily formed, as reported by Berke and Erker and co-workers [80]. It is unknown whether B(C6F5)3 can maintain its catalytic activity during the current cyanation reaction, and (c) the catalyst should be able to dissociate from nitrile products. Finally, (d) another challenge is to suppress the B(C6F5)3-catalyzed homo-etherification of alcohols reported by Chan and co-workers [70].
2. Results
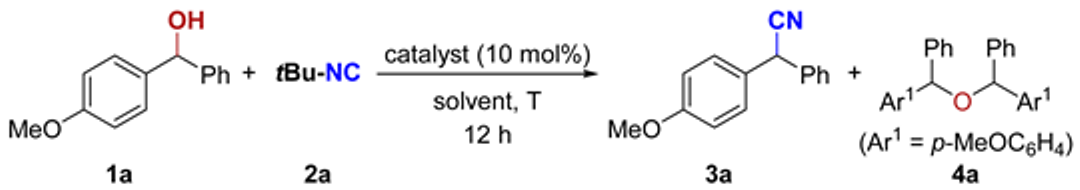
We initiated our investigation with the optimization of the reaction of benzhydryl alcohol (1a) and tert-butyl isocyanide (2a). Initially, 1a and 1.5 equivalents of 2a were subjected to a solution of FeCl3 (10 mol%) in toluene at 100 °C (Table 1, entry 1). However, FeCl3 showed almost no catalytic activity. Other commonly used Lewis acids, including AlCl3, Cu(OTf)2, AgClO4, and BF3·(OEt)2 were also tested, but led to low yield (see entries 2–5 in Table 1). Of note, using AlCl3, BF3·(OEt)2, or TsOH·H2O as a catalyst, a mixture of 3a and some homo-etherification side product 4a was obtained (entries 2, 5 and 7). With the use of Brønsted acid Tf2NH, the cyanation provided the desired 3a in 72% NMR yield. Interestingly, the reaction favors the formation of etherification product 4a rather than 3a when using diphenyl phosphate as the catalyst (entry 8 versus 9). Gratifyingly, we found that B(C6F5)3 affords the desired α-aryl nitrile 3a in >99% NMR yield at 100 °C (entry 6). No reaction occurred when B(C6F5)3 was used as the catalyst at 50 °C (entry 11). Increasing the temperature to 80 °C improved both the yield of 3a (20%) and ether 4a (30%). Different solvents such as 1,2-dichloroethane (DCE), THF, and hexafluoroisopropanol (HFIP) (entries 12–15) were also tested, and the results revealed that toluene was superior to other solvents.

Table 1.
Optimization of reaction conditions [a].
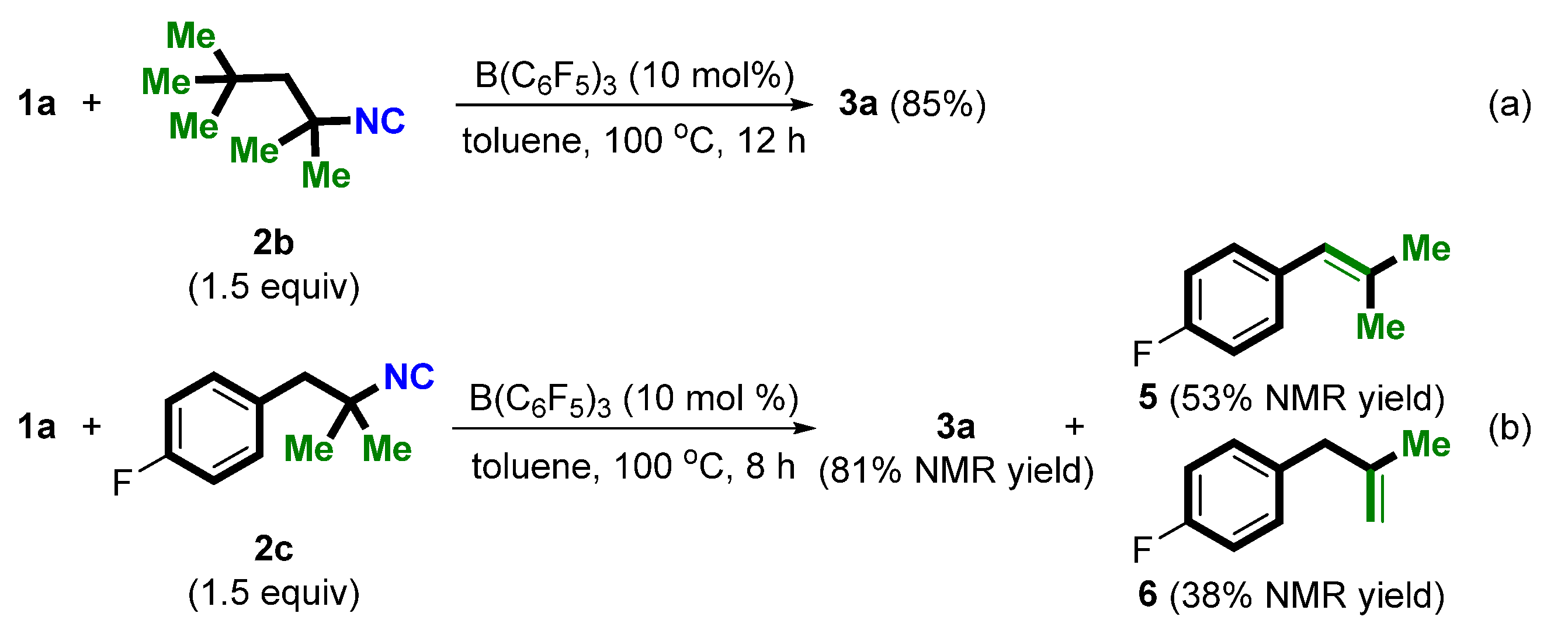
With the optimized conditions identified, we then proceeded to explore the scope of isocyanides (Scheme 2). Besides tBu-NC (2a), other tertiary amine-derived isocyanides, such as 2b and 2c, can also be used as a novel cyano source in the current direct cyanation of α-aryl alcohols. In contrast, when secondary amine- and aniline-derived isocyanides were used as substrates, only the corresponding ether products were obtained (not shown). Of note, treatment of 1a and 2c with the standard conditions afforded 3a in 81% NMR yield together with internal alkene 5 in 53% NMR yield and terminal alkene 6 in 38% NMR yield (Scheme 2b), indicating a tertiary carbon cation might be an intermediate.
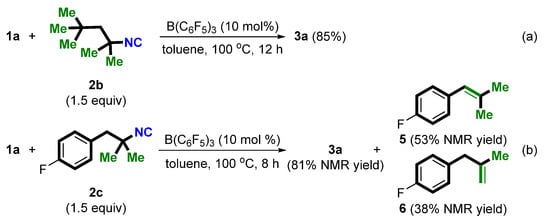
Scheme 2.
Survey of the scope of isocyanides (a,b).
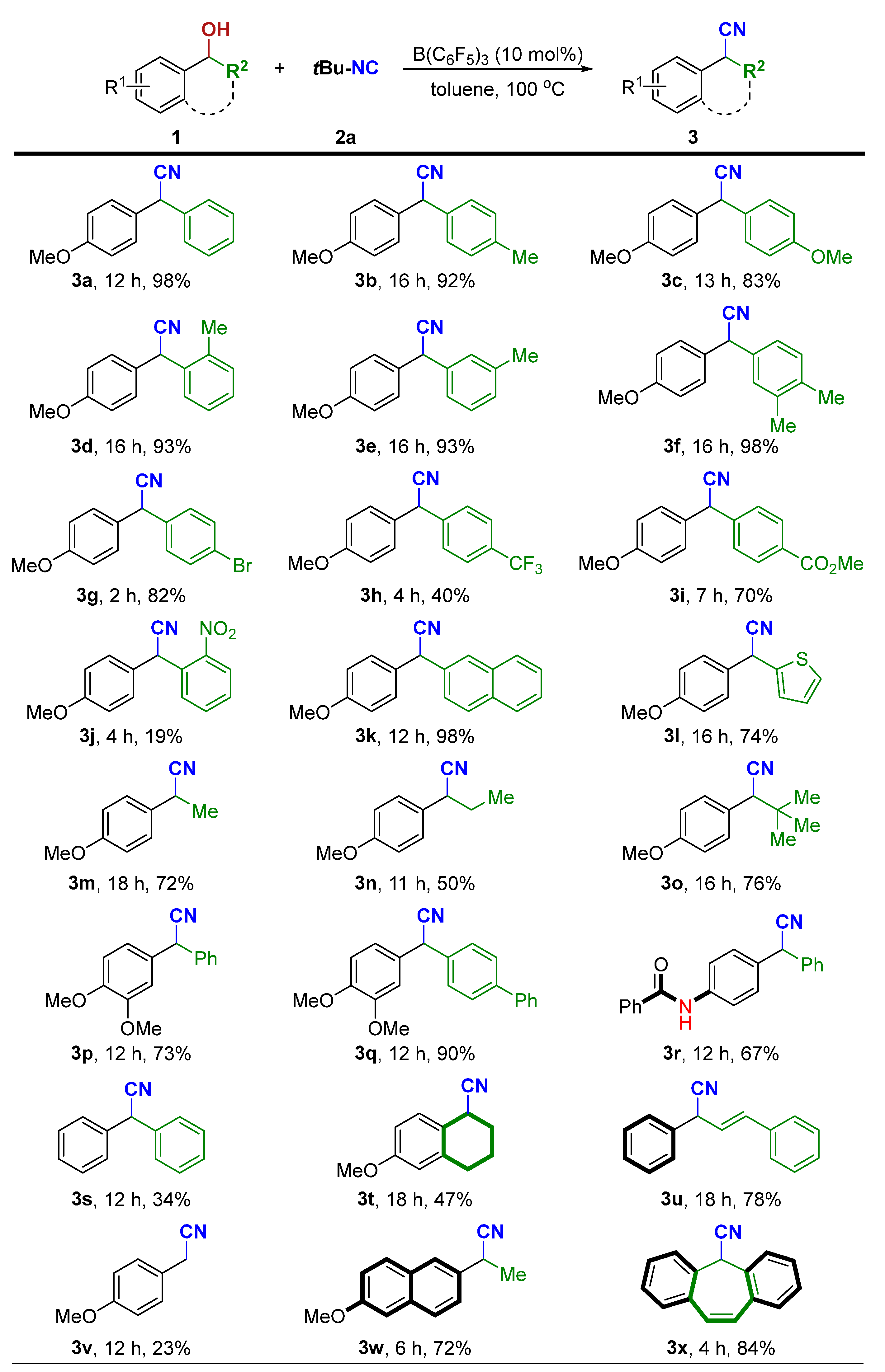
Next, we turned to explore the generality of this cyanidation reaction with a variety of alcohols with tBu-NC (2a). As shown in Scheme 3, a wide range of benzylic alcohols can smoothly react with 2a under the optimized conditions, giving the corresponding α-aryl nitriles in good to excellent yields. Diarylsubstituted alcohols (R2 = aryl) underwent reaction with tBu-NC to furnish the corresponding products (3a–3k) in 19−98% yields.
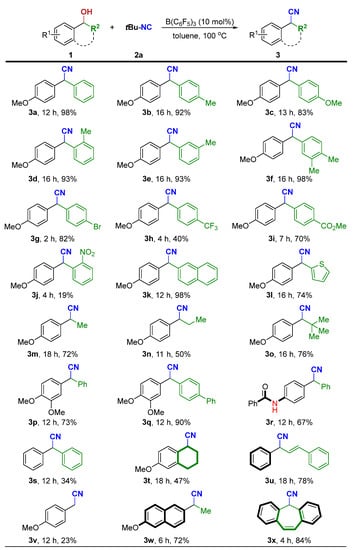
Scheme 3.
Survey of the scope of α-aryl alcohols [a,b]. [a] Standard conditions: 1 (0.20 mmol), 2a (0.30 mmol, 1.5 equiv), B(C6F5)3 (10 mol%), toluene (2 mL), at 100 °C for 2–18 h. [b] Isolated yield of 3.
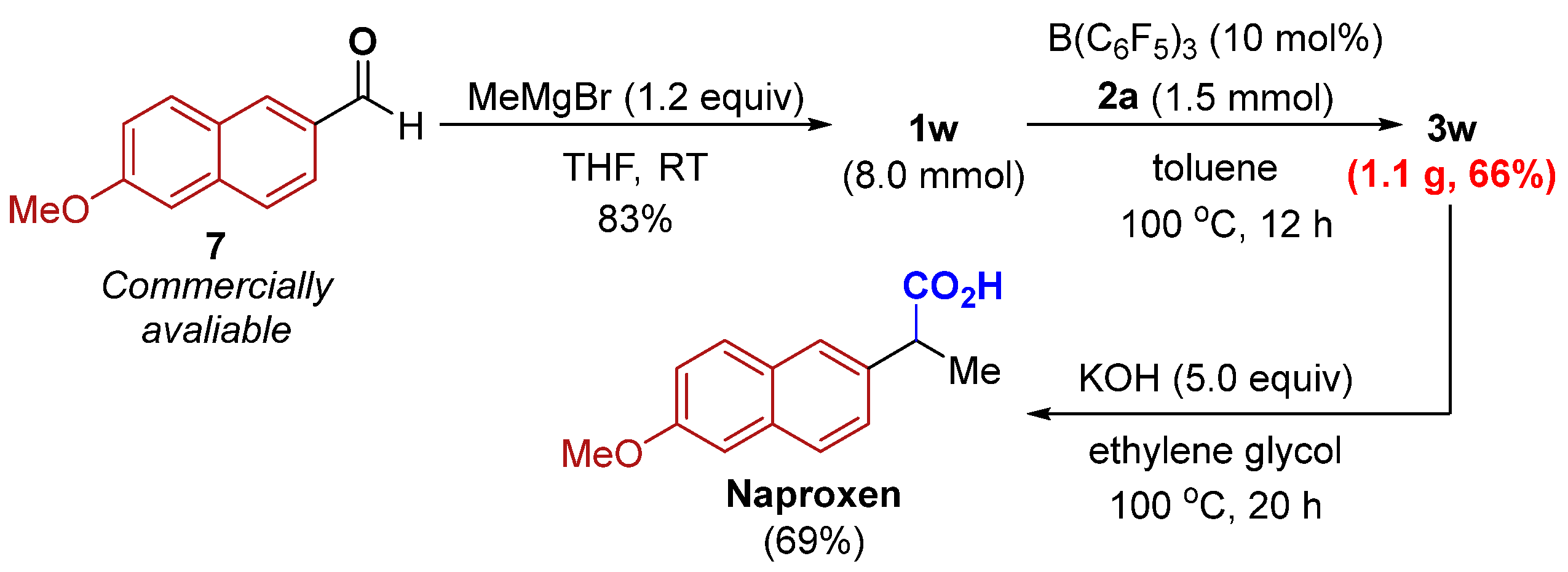
Diarylsubstituted alcohols bearing electron-donating (methoxy, alkyl) groups at the para-, ortho- or meta-positions of the benzene rings reacted smoothly (1a–1f). A variety of electron-withdrawing functional groups at the para-positions of the benzene rings such as -Br, -CF3, and -CO2Me were tolerated, affording the desired products in moderate to good yields (1g–1i). However, a low yield of 3j was isolated from 1j having an ortho-nitro group on the aromatic substituent. The naphthyl-containing alcohol 1k and heteroaryl-containing alcohol 1l also afforded the corresponding products in good to excellent yields. In addition, alkyl-substituted alcohols (R2 = methyl, ethyl, and tert-butyl) were also well-tolerated to afford the desired products 3m–3o. Of note, a competitive side reaction encountered in the reaction with 1m or 1n is the formation of styrene derivatives via the dehydration reactions of alcohols. Besides R1 = methoxy (1p–1q), the substituent R1 can be benzamide (1r). Benzhydrol 1s was tested but afforded the corresponding product 3s in low yield together with (oxybis(methanetriyl))tetrabenzene in 64% NMR yield. However, 1,2,3,4-tetrahydronaphthalen-1-ol (1t) and allylic alcohol (1u) underwent smooth cyanation. It is worth noting that the reported indium halide-catalyzed protocol with TMSCN as the cyanide source is not amenable to primary alcohol 1v for cyanation reaction [31]. Our system, however, gives reasonable yield for the same substrate. To our delight, the precursors for naproxen and cytenamide, respectively, can also be efficiently obtained in high yields by this protocol (3w and 3x).
To disclose the synthetic practicability of the developed method, we studied a gram-scale reaction, and 66% yield of 3w was obtained, which might provide potential value in synthetic chemistry. Having established a protocol for the efficient synthesis of α-aryl nitrile 3w, (±)-naproxen, a nonsteroidal anti-inflammatory drug [24], was prepared in three steps from commercially available materials (Scheme 4).
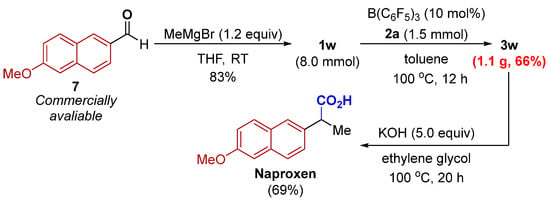
Scheme 4.
Scale-up synthesis and synthetic transformations.
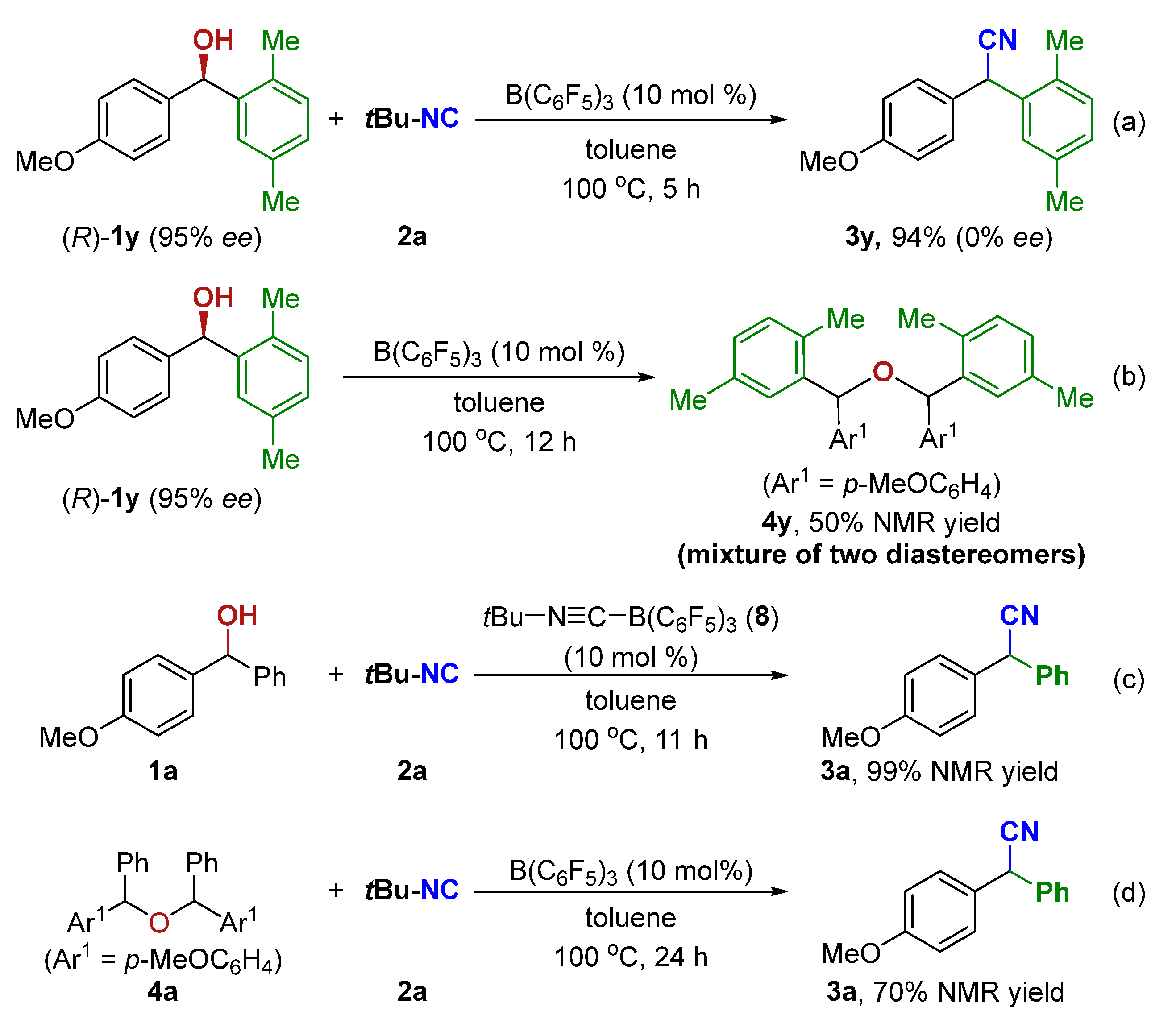
To gain insight into the reaction mechanism, several control experiments were conducted. When an enantiomerically pure sample of (R)-1y was subjected to standard conditions, the resulting nitrile 3y was obtained in racemic form (Scheme 5a). Moreover, (R)-1y was also employed to perform the etherification reaction under the standard condition. The 1H NMR spectrum showed the ether 4y was obtained in 50% NMR yield with a 48/52 ratio of the two diastereomers (Scheme 5b). These control experiments support an SN1 pathway and rule out a concerted SN2 mechanism. The tert-Butylisocyanide-B(C6F5)3 adduct (8) was easily prepared [80] and used as a catalyst in the current reaction, affording the corresponding product 3a in 99% NMR yield (Scheme 5c). As shown in Table 1, entry 10, the reaction did form 3a in 20% NMR yield along with ether 4a in 30% NMR yield at 80 °C. Treatment of 4a with the standard setup then gave the desired 3a in 70% NMR yield (Scheme 5d).
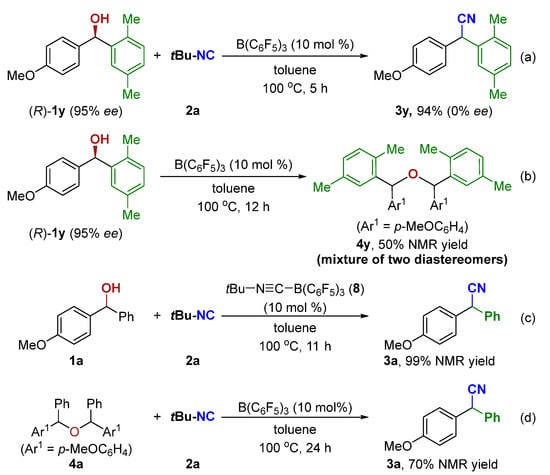
Scheme 5.
Control experiments (a–d).
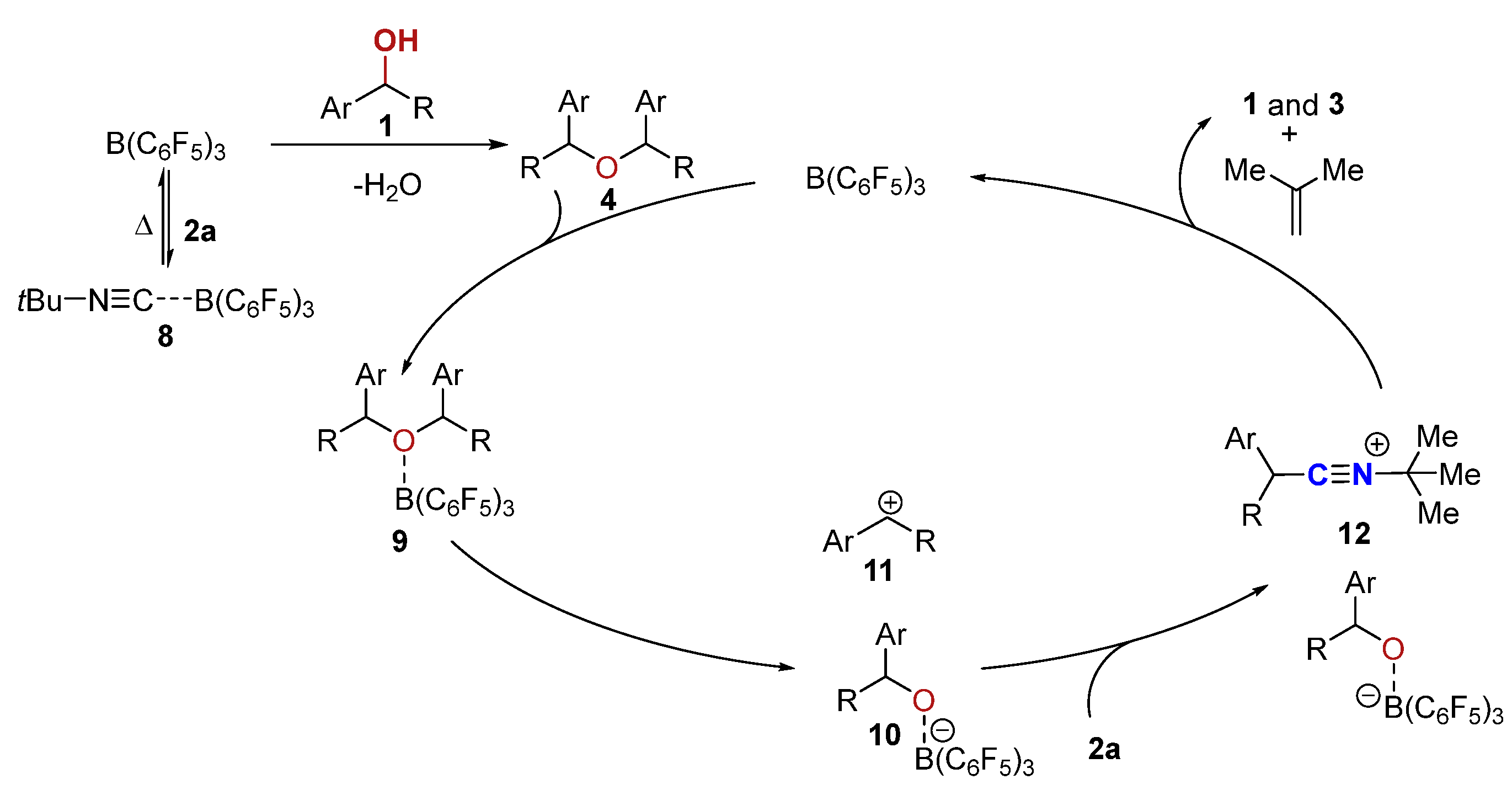
Based on the results above, a plausible mechanism is proposed in Scheme 6. First, tert-butylisocyanide 2a forms a reversibly Lewis adduct 8 with B(C6F5)3 [80]. The homo-etherification of alcohol in the presence of the B(C6F5)3 quickly delivers the ether 4 [70], which could furnish an adduct 9 with B(C6F5)3 through the oxygen center. Subsequently, the adduct 9 could break into an intermediate 10 and carbocation 11. However, an alternative reaction path for the formation of the carbocation 11 directly from alcohol in the presence of the in situ-generated strong Brønsted acid B(C6F5)3·nH2O or boron Lewis acid B(C6F5)3 (not shown, see Supporting Information for details) cannot be ruled out [69,70]. The carbocation 11 could then be intercepted instantaneously by the tBu-NC (2a) to afford an intermediate 12 with a borate anion 10 as the counteranion. The stability of the tertiary carbon cation is the driving force to break the C-N bond in 12, leading to the α-aryl nitrile 3 and 2-methylpropene by proton elimination via a tert-butyl carbon cation intermediate (supported by Scheme 2b) [81]. The borate anion 10, on the other hand, transforms into alcohol 1 with the regeneration of the B(C6F5)3 catalyst.
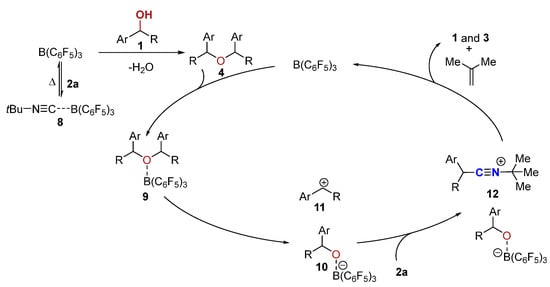
Scheme 6.
Proposed mechanism.
3. Materials and Methods
3.1. General Information
All reactions were performed in flame-dried glassware using conventional Schlenk techniques under a static pressure of nitrogen unless otherwise stated. Liquids and solutions were transferred with syringes. The known alcohols 1 [31,35] and tBu-NC-B(C5F5)3 adduct 8 [80] were prepared according to reported procedures. (R)-1y was prepared in 80% yield according to the known procedure [82] (95% ee of (R)-1y was determined by HPLC: OJ-H Column, 5/95 iPrOH/hexane, 0.5 mL/min, 254 nm, 35 °C; retention time = 75.36 min (minor), 81.66 min (major)). Tris(pentafluorophenyl)borane (B(C5F5)3, 98%, Energy Chemical) and tert-butyl isocyanate (97%, Energy Chemical) were purchased from commercial suppliers and used as received. Other commercially available reagents were purchased from Sigma-Adrich, Leyan and Bide Chemical Company. All solvents (tetrahydrofuran, toluene, and 1,2-dichloroethane etc.) were dried and purified following standard procedures. Technical grade solvents for extraction or chromatography (petroleum ether, CH2Cl2, and ethyl acetate) were distilled prior to use. Analytical thin layer chromatography (TLC) was performed on silica gel 60 F254 glass plates by Merck. Flash column chromatography was performed on silica gel 60 (40–63 μm, 230–400 mesh, ASTM) by Grace using the indicated solvents. 1H, 13C NMR spectra (Supplementary Materials) were recorded in CDCl3 on Bruker AV400 instruments. Chemical shifts are reported in parts per million (ppm) and are referenced to the residual solvent resonance as the internal standard (CHCl3: δ = 7.26 ppm for 1H NMR and CDCl3: δ = 77.0 ppm for 13C NMR). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplett, q = quartet, m = multiplet), coupling constants (Hz), and integration. Mass spectra were recorded on a THERMO FINNIGAN LTQ-XL. The MS inlet capillary temp was always maintained at 275 °C and capillary voltage at 5 kV. No other source gases were used when digestion was performed in microdroplets. The samples were dissolved in 1:1 methanol:water.
3.2. Typical Procedure for Direct Cyanation of Alcohols
In a glove box, alcohol 1 (0.2 mmol), isocyanide 2 (0.3 mmol, 1.5 equiv), B(C6F5)3 (10.2 mg, 20 μmol, 10 mol%), and toluene (2.0 mL) were added to an oven-dried 10 mL pressure vial. The vial was sealed and removed from the glove box. The reaction mixture was stirred at 100 °C (oil bath) for 2–18 h. After the reaction was completed, the reaction mixture was purified by silica gel column chromatography by using petroleum ether/ethyl acetate mixture to obtain the desired nitrile 3.
3.3. Procedure for the Preparation of Naproxen
To a solution of 6-methoxy-2-naphthaldehyde (1.86 g, 10.0 mmol, 1.0 equiv) in THF (20 mL, 0.5 M), methylmagnesium bromide (4.0 mL, 12 mmol, 3.0 M, 1.2 equiv) was added. When the reaction was judged to have reached completion (as determined by TLC), sat. NH4Cl was added slowly at 0 °C, and the mixture was extracted with EtOAc. The combined organic layers were washed with brine, dried over MgSO4, and purified by column chromatography on silica gel to obtain 1w (1.60 g, 80% yield).
To an oven-dried 200 mL Schlenk flask containing a magnetic stir bar under an atmosphere of nitrogen, alcohol 1w (1.62 g, 8.0 mmol), isocyanide 2a (1.0 g, 12 mmol, 1.5 equiv), B(C6F5)3 (0.41 g, 0.8 mmol, 10 mol%), and toluene (80 mL) were added. The reaction mixture was stirred at 100 °C (oil bath) for 12 h. After the reaction was completed, the reaction mixture was purified by silica gel column chromatography by using petroleum ether/ethyl acetate mixture to obtain the desired nitrile 3w (1.12 g, 66% yield).
To a suspension of 3w (42.3 mg, 0.2 mmol, 1.0 equiv) in ethylene glycol (0.6 mL), KOH (0.1 mL, 10 M solution in H2O, 5.0 equiv) was added. The reaction vessel was sealed with a rubber septum and submerged in an oil bath at 100 °C for 20 h. We then added 1M HCl (2.5 mL) dropwise, and the mixture was extracted with EtOAc. The combined organic layers were washed with brine, dried over MgSO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel to obtain Naproxen (31.8 mg, 69% yield).
3.4. Characterization Data of the Products
2-(4-methoxyphenyl)-2-phenylacetonitrile (3a) [83]. White solid (43.8 mg, 98% yield); mp 130–132 °C; Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.39–7.29 (m, 5H), 7.25 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 8.4 Hz, 2H), 5.09 (s, 1H), 3.79 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.4, 136.2, 129.1, 128.9, 128.1, 127.9, 127.6, 119.9, 114.5, 55.3, 41.8 ppm.
2-(4-methoxyphenyl)-2-(p-tolyl)acetonitrile (3b) [83]. White solid (43.9 mg, 92% yield); mp 85–87 °C; Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.25–7.15 (m, 6H), 6.87 (d, J = 8.8 Hz, 2H), 5.05 (s, 1H), 3.79 (s, 3H), 2.33 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 137.9, 133.2, 129.8, 128.8, 128.2, 127.5, 120.0, 114.4, 55.3, 41.4, 21.0 ppm.
2,2-bis(4-methoxyphenyl)acetonitrile (3c) [83]. White solid (41.9 mg, 83% yield); mp 156–158 °C; Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.23 (d, J = 8.4 Hz, 4H), 6.88 (d, J = 8.8 Hz, 4H), 5.04 (s, 1H), 3.79 (s, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 128.7, 128.2, 120.1, 114.4, 55.3, 41.0 ppm.
2-(4-methoxyphenyl)-2-(o-tolyl)acetonitrile (3d) [83]. Colorless oil (44.4 mg, 93% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.40–7.38 (m, 1H), 7.25 (t, J = 4.8 Hz, 2H), 7.20–7.17 (m, 3H), 6.87 (d, J = 8.8 Hz, 2H), 5.23 (s, 1H), 3.78 (s, 3H), 2.26 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 135.8, 133.8, 131.2, 128.9, 128.5, 128.4, 126.8, 126.7, 119.8, 114.4, 55.3, 39.1, 19.4 ppm. MS (ESI) m/z: [M+H]+ calcd. for C16H16NO: 238.12; found: 238.18.
2-(4-methoxyphenyl)-2-(m-tolyl)acetonitrile (3e) [83]. Colorless oil (44.4 mg, 93% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.25–7.22 (m, 3H), 7.14–7.10 (m, 3H), 6.88 (d, J = 8.4 Hz, 2H), 5.04 (s, 1H), 3.78 (s, 3H), 2.33 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 139.0, 136.1, 128.9, 128.84, 128.81, 128.2, 128.0, 124.6, 120.0, 114.4, 55.3, 41.7, 21.3 ppm. MS (ESI) m/z: [M+H]+ calcd. for C16H16NO: 238.12; found: 238.82.
2-(3,4-dimethylphenyl)-2-(4-methoxyphenyl)acetonitrile (3f) [83]. Colorless oil (55.1 mg, 98% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.24 (d, J = 6.8 Hz, 2H), 7.12–7.03 (m, 3H), 6.87 (d, J = 6.4 Hz, 2H), 5.02 (s, 1H), 3.78 (s, 3H), 2.24 (s, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 137.5, 136.6, 133.6, 130.2, 128.8, 128.7, 128.3, 124.9, 120.1, 114.4, 55.3, 41.4, 19.8, 19.4 ppm. MS (ESI) m/z: [M+H]+ calcd. for C17H18NO: 252.14; found: 252.18.
2-(4-bromophenyl)-2-(4-methoxyphenyl)acetonitrile (3g) [83]. Colorless oil (49.4 mg, 82% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.48 (d, J = 8.4 Hz, 2H), 7.20 (t, J = 7.5 Hz, 4H), 6.88 (d, J = 8.4 Hz, 2H), 5.04 (s, 1H), 3.79 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.6, 135.3, 132.2, 129.2, 128.8, 127.2, 122.2, 119.3, 114.6, 55.3, 41.2 ppm. MS (ESI) m/z: [M+H]+ calcd. for C15H13BrNO: 302.02; found: 302.36.
2-(4-methoxyphenyl)-2-(4-(trifluoromethyl)phenyl)acetonitrile (3h). Colorless oil (23.2 mg, 40% yield); Rf = 0.40 (petroleum ether/EtOAc = 10/1). 1H NMR (400 MHz, CDCl3): δ 7.63 (d, J = 8.4 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H), 6.91 (d, J = 8.4 Hz, 2H), 5.14 (s, 1H), 3.80 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.8, 140.1, 130.6 (q, J = 32.8 Hz), 128.9, 128.0, 126.9, 126.1 (q, J = 3.7 Hz), 123.8 (q, J = 270.5 Hz, 1H), 119.1, 114.8, 55.3, 41.6 ppm. 19F NMR (376 MHz, CDCl3) δ −62.75 (s) ppm. HRMS (MALDI-TOF/TOF) for C16H13F3NO [M+H]+: calculated 292.0944, found 292.0947.
Methyl 4-(cyano(4-methoxyphenyl)methyl)benzoate (3i). Yellow oil (39.4 mg, 70% yield); Rf = 0.50 (petroleum ether/EtOAc = 5/1). 1H NMR (400 MHz, CDCl3): δ 8.03 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 8.2 Hz, 2H), 7.23 (d, J = 8.6 Hz, 2H), 6.89 (d, J = 8.6 Hz, 2H), 5.14 (s, 1H), 3.91 (s, 3H), 3.79 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 166.3, 159.6, 141.0, 130.4, 130.1, 128.9, 127.6, 127.1, 119.2, 114.7, 55.3, 52.2, 41.7 ppm. HRMS (ESI) for C17H16NO3 [M+H]+: calculated 282.1125, found 282.1126.
2-(4-methoxyphenyl)-2-(2-nitrophenyl)acetonitrile (3j) [84]. Yellow oil (10.1 mg, 19% yield); Rf = 0.40 (petroleum ether/EtOAc = 10/1). 1H NMR (400 MHz, CDCl3): δ 8.04 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.69 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.22 (d, J = 8.4 Hz, 2H), 6.87 (d, J = 8.4 Hz, 2H), 6.11 (s, 1H), 3.79 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.8, 147.7, 134.0, 130.9, 130.7, 129.5, 129.1, 126.0, 125.7, 118.8, 114.7, 55.3, 37.6 ppm.
2-(4-methoxyphenyl)-2-(naphthalen-2-yl)acetonitrile (3k) [85]. Colorless oil (53.5 mg, 98% yield). Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.87–7.81 (m, 4H), 7.53–7.48 (m, 2H), 7.34 (d, J = 8.4 Hz, 1H), 7.29 (d, J = 8.8 Hz, 2H), 6.89 (d, J = 8.4 Hz, 2H), 5.25 (s, 1H), 3.79 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.5, 133.4, 133.2, 132.7, 129.2, 129.0, 128.0, 127.8, 127.7, 126.7, 126.6, 126.5, 125.2, 119.8, 114.5, 55.3, 42.0 ppm.
2-(4-methoxyphenyl)-2-(thiophen-2-yl)acetonitrile (3l). Colorless oil (33.8 mg, 74% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.31 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 5.2 Hz, 1H), 7.06 (d, J = 3.2 Hz, 1H), 6.97–6.95 (m, 1H), 6.91 (d, J = 8.4 Hz, 2H), 5.30 (s, 1H), 3.80 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.7, 139.1, 128.7, 127.5, 127.0, 126.5, 126.3, 119.0, 114.5, 55.3, 37.2 ppm. HRMS (MALDI-TOF/TOF) for C13H12NOS [M+H]+: calculated 230.0634, found 230.0631.
2-(4-methoxyphenyl)propanenitrile (3m) [48]. Colorless oil (23.3 mg, 72% yield); Rf = 0.70 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.27 (d, J = 8.4 Hz, 2H), 6.90 (d, J = 8.4 Hz, 2H), 3.85 (q, J = 7.6 Hz, 1H), 3.81 (s, 3H), 1.61 (d, J = 7.3 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.2, 129.0, 127.8, 121.8, 114.4, 55.3, 30.4, 21.5 ppm.
2-(4-methoxyphenyl)butanenitrile (3n) [86]. Colorless oil (17.5 mg, 50% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.23 (d, J = 8.4 Hz, 2H), 6.90 (d, J = 8.4 Hz, 2H), 3.81 (s, 3H), 3.68 (t, J = 7.2 Hz, 1H), 1.95–1.86 (m, 2H), 1.06 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 128.4, 127.7, 121.0, 114.3, 55.3, 38.1, 29.2, 11.4 ppm.
2-(4-methoxyphenyl)-3,3-dimethylbutanenitrile (3o) [87]. White solid (31.1 mg, 76% yield); mp 50–52 °C; Rf = 0.70 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.19 (d, J = 6.8 Hz, 2H), 6.88 (d, J = 8.4 Hz, 2H), 3.82 (s, 3H), 3.51 (s, 1H), 1.04 (s, 9H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 130.4, 125.4, 120.6, 113.6, 55.3, 48.9, 35.1, 27.3 ppm.
2-(3,4-dimethoxyphenyl)-2-phenylacetonitrile (3p) [88]. Colorless oil (37.0 mg, 73% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.38–7.31 (m, 5H), 6.90–6.80 (m, 3H), 5.10 (s, 1H), 3.86 (s, 3H), 3.84 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 149.4, 148.9, 136.0, 129.1, 128.13, 128.12, 127.5, 120.1, 119.8, 111.3, 110.7, 55.89, 55.88, 42.1 ppm.
2-([1,1′-biphenyl]-4-yl)-2-(3,4-dimethoxyphenyl)acetonitrile (3q) [48]. Colorless oil (59.3 mg, 90% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.60–7.55 (m, 4H), 7.45–7.39 (m, 4H), 7.37–7.33 (m, 1H), 6.92 (d, J = 8.4 Hz, 1H), 6.85 (d, J = 7.2 Hz, 2H), 5.13 (s, 1H), 3.86 (s, 3H), 3.85 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 149.5, 149.0, 141.1, 140.1, 135.0, 128.8, 128.1, 128.0, 127.8, 127.6, 127.0, 120.2, 119.8, 111.4, 110.7, 56.0, 55.9, 41.8 ppm. HRMS (MALDI-TOF/TOF) for C22H20NO2 [M+H]+: calculated 330.1489, found 330.1386.
N-(4-(cyano(phenyl)methyl)phenyl)benzamide (3r). White solid (41.6 mg, 67% yield); mp 196–198 °C; Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, DMSO-d6): δ 10.35 (s, 1H), 7.98 (d, J = 7.6 Hz, 2H), 7.85 (d, J = 8.4 Hz, 2H), 7.65–7.61 (m, 1H), 7.58–7.55 (m, 2H), 7.48–7.39 (m, 7H), 5.81 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 166.5, 139.8, 137.7, 135.6, 132.5, 130.1, 129.3, 128.8, 128.7, 128.5, 128.3, 121.8, 121.3, 41.2 ppm. HRMS (ESI) m/z: [M+H]+ calcd. for C21H17N2O: 313.1336; found: 313.1334.
2,2-diphenylacetonitrile (3s) [83]. White solid (13.1 mg, 34% yield); mp 70–72 °C; Rf = 0.50 (petroleum ether/EtOAc = 15/1). 1H NMR (400 MHz, CDCl3): δ 7.39–7.30 (m, 10H), 5.14 (s, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 135.9, 129.2, 128.2, 127.7, 119.6, 42.6 ppm.
6-methoxy-1,2,3,4-tetrahydronaphthalene-1-carbonitrile (3t) [48]. Colorless oil (17.5 mg, 47% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.26 (d, J = 8.8 Hz, 1H), 6.78–6.75 (m, 1H), 6.64 (s, 1H), 3.92 (t, J = 6.4 Hz, 1H), 3.78 (s, 3H), 2.87–2.71 (m, 2H), 2.13–2.09 (m, 2H), 2.07–1.96 (m, 1H), 1.87–1.77 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.1, 137.7, 129.9, 121.9, 114.2, 112.8, 55.2, 30.1, 28.7, 27.5, 20.7 ppm.
(E)-2,4-diphenylbut-3-enenitrile (3u) [89]. Colorless oil (34.0 mg, 78% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.42–7.26 (m, 10H), 6.81 (d, J = 15.6 Hz, 1H), 6.19 (dd, J = 16.0, 6.4 Hz, 1H), 4.69 (d, J = 6.4 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 135.4, 134.5, 133.2, 129.2, 128.7, 128.4, 127.5, 126.7, 123.2, 118.8, 40.0 ppm.
2-(4-methoxyphenyl)acetonitrile (3v) [90]. Colorless oil (17.0 mg, 23% yield, 0.5 mmol scale); Rf = 0.50 (petroleum ether/EtOAc = 15/1). 1H NMR (400 MHz, CDCl3): δ 7.23 (d, J = 8.4 Hz, 2H), 6.90 (d, J = 8.4 Hz, 2H), 3.80 (s, 3H), 3.68 (s, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 129.0, 121.8, 118.1, 114.5, 55.3, 22.8 ppm.
2-(6-methoxynaphthalen-2-yl)propanenitrile (3w) [91]. White solid (42.3 mg, 72% yield); mp 72–74 °C; Rf = 0.70 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.76–7.72 (m, 3H), 7.38 (dd, J = 8.4, 2.0 Hz, 1H), 7.18 (dd, J = 8.8, 2.4 Hz, 1H), 7.13 (d, J = 2.4 Hz, 1H), 4.02 (q, J = 7.2 Hz, 1H), 3.92 (s, 3H), 1.71 (d, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 158.1, 134.0, 132.0, 129.3, 128.8, 127.9, 125.4, 124.9, 121.7, 119.6, 105.7, 55.3, 31.2, 21.4 ppm.
5H-dibenzo[a,d][7]annulene-5-carbonitrile (3x) [25]. Colorless oil (36.6 mg, 84% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.68 (s, 2H), 7.40 (t, J = 7.2 Hz, 2H), 7.35–7.28 (m, 4H), 7.11 (s, 2H), 4.72 (s, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 133.9, 132.3, 131.3, 129.2, 128.6, 127.8, 125.3, 118.3, 41.1 ppm.
2-(2,5-dimethylphenyl)-2-(4-methoxyphenyl)acetonitrile (3y) [92]. Colorless oil (47.2 mg, 94% yield); Rf = 0.50 (petroleum ether/EtOAc = 20/1). 1H NMR (400 MHz, CDCl3): δ 7.22–7.17 (m, 3H), 7.07 (s, 2H), 6.87 (d, J = 8.8 Hz, 2H), 5.20 (s, 1H), 3.79 (s, 3H), 2.33 (s, 3H), 2.21 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 159.3, 136.4, 133.6, 132.6, 131.1, 129.2, 129.1, 128.9, 127.1, 120.0, 114.4, 55.3, 39.1, 21.0, 18.9 ppm.
2,2′-(oxybis((4-methoxyphenyl)methylene))bis(1,4-dimethylbenzene) (4y). Colorless oil (17.9 mg, 38% yield); Rf = 0.50 (petroleum ether/EtOAc = 15/1). 1H NMR (400 MHz, CDCl3): δ 7.46 (s, 1H), 7.39 (s, 1H), 7.20–7.18 (m, 4H), 6.98 (d, J = 10.8 Hz, 4H), 6.82 (t, J = 6.4 Hz, 4H), 5.46 (s, 1H), 5.45 (s, 1H), 3.77 (s, 6H), 2.33 (s, 3H), 2.31 (s, 3H), 1.96 (s, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ 158.8, 158.6, 139.9, 139.6, 135.3, 134.0, 133.8, 133.0, 132.3, 130.4, 130.2, 129.1, 128.7, 128.3, 128.0, 127.8, 127.5, 113.6, 113.5, 76.9, 76.5, 55.2, 21.2, 21.2, 18.9, 18.8 ppm. HRMS (ESI) for C32H34O3Na [M+Na]+: calculated 489.2400, found 489.2414.
2-(6-methoxynaphthalen-2-yl)propanoic acid (Naproxen) [24]. White solid (31.8 mg, 69% yield); mp 156–158 °C; Rf = 0.50 (petroleum ether/EtOAc/MeOH = 5/2/1). 1H NMR (400 MHz, CDCl3): δ 7.70–7.67 (m, 3H), 7.40 (d, J = 8.4 Hz, 1H), 7.14–7.10 (m, 2H), 3.90–3.85 (m, 4H), 1.58 (d, J = 6.8 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 179.9, 157.7, 135.1, 133.8, 129.3, 128.9, 127.2, 126.2, 126.1, 118.9, 105.7, 55.3, 45.3, 18.2 ppm.
4. Conclusions
In conclusion, by taking advantage of isonitriles as low-toxic CN surrogates in the metal-free cyanation of alcohols, an efficient and green method for the direct catalytic synthesis of α-aryl nitriles was developed (up to 98% yield). To the best of our knowledge, this is the first B(C6F5)3-catalyzed transformation of isonitriles. Control experiments support an SN1 pathway and rule out a concerted SN2 mechanism. The in situ-generated ether 4 can be converted to the desired α-aryl nitriles under the current catalytic system via cleavage of the C-O bond. The use of readily available starting materials, low catalyst loading, a broad substrate scope, ease of scale-up, and application in the synthesis of the precursors for naproxen and cytenamide make this approach very practical and attractive. With these advantages, we expect that this method will find wide applications in organic synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28052174/s1, 1H, 13C, 19F and HPLC spectra.
Author Contributions
Conceptualization, J.-J.F.; methodology, T.-T.X. and J.-J.F.; investigation, T.-T.X., J.-L.Z., G.-Y.C. and J.-Y.-H.S.; data curation, S.-Q.W. and Y.M.; writing—original draft preparation, T.-T.X. and J.-J.F.; writing—review and editing, T.-T.X. and J.-J.F.; project administration, J.-J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Fundamental Research Funds for the Central Universities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors also thank the Fundamental Research Funds for the Central Universities. We thank Zhongyan Zhou and Lei Yue (Hunan University, China) for experimental assistance and spectroscopic characterization.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3 are available from the authors.
References
- Trost, B.M. Atom Economy—A Challenge for Organic Synthesis: Homogeneous Catalysis Leads the Way. Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Estopina-Duran, S.; Taylor, J.E. Bronsted Acid-Catalysed Dehydrative Substitution Reactions of Alcohols. Chem. Eur. J. 2021, 27, 106–120. [Google Scholar] [CrossRef]
- Huy, P.H. Lewis Base Catalysis Promoted Nucleophilic Substitutions—Recent Advances and Future Directions. Eur. J. Org. Chem. 2020, 10–27. [Google Scholar] [CrossRef]
- Moran, J.; Dryzhakov, M.; Richmond, E. Recent Advances in Direct Catalytic Dehydrative Substitution of Alcohols. Synthesis 2016, 48, 935–959. [Google Scholar] [CrossRef]
- Emer, E.; Sinisi, R.; Capdevila, M.G.; Petruzziello, D.; De Vincentiis, F.; Cozzi, P.G. Direct Nucleophilic SN1-Type Reactions of Alcohols. Eur. J. Org. Chem. 2011, 647–666. [Google Scholar] [CrossRef]
- Bryan, M.C.; Dunn, P.J.; Entwistle, D.; Gallou, F.; Koenig, S.G.; Hayler, J.D.; Hickey, M.R.; Hughes, S.; Kopach, M.E.; Moine, G.; et al. Key Green Chemistry Research Areas from a Pharmaceutical Manufacturers’ Perspective Revisited. Green Chem. 2018, 20, 5082–5103. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; McCann, S.D.; Wang, D.; Chen, P.; Stahl, S.S.; Liu, G. Enantioselective Cyanation of Benzylic C–H Bonds Via Copper-Catalyzed Radical Relay. Science 2016, 353, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, N.; Chen, P.; Lin, Z.; Liu, G. Enantioselective Decarboxylative Cyanation Employing Cooperative Photoredox Catalysis and Copper Catalysis. J. Am. Chem. Soc. 2017, 139, 15632–15635. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, J.; Zhang, J. Cu-Catalyzed Enantioselective Decarboxylative Cyanation Via the Synergistic Merger of Photocatalysis and Electrochemistry. Chem. Sci. 2023, 14, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Culkin, D.A.; Hartwig, J.F. Palladium-Catalyzed α-Arylation of Carbonyl Compounds and Nitriles. Acc. Chem. Res. 2003, 36, 234–245. [Google Scholar] [CrossRef]
- Wu, G.; Deng, Y.; Wu, C.; Zhang, Y.; Wang, J. Synthesis of α-Aryl Esters and Nitriles: Deaminative Coupling of α-Aminoesters and α-Aminoacetonitriles with Arylboronic Acids. Angew. Chem. Int. Ed. 2014, 53, 10510–10514. [Google Scholar] [CrossRef]
- Falk, A.; Goderz, A.L.; Schmalz, H.G. Enantioselective Nickel-Catalyzed Hydrocyanation of Vinylarenes Using Chiral Phosphine-Phosphite Ligands and TMS-CN as a Source of HCN. Angew. Chem. Int. Ed. 2013, 52, 1576–1580. [Google Scholar] [CrossRef]
- Singh, D.K.; Prasad, S.S.; Kim, J.; Kim, I. One-Pot, Three-Component Approach to Diarylacetonitriles. Org. Chem. Front. 2019, 6, 669–673. [Google Scholar] [CrossRef]
- Tan, J.-P.; Chen, Y.; Ren, X.; Guo, Y.; Yi, B.; Zhang, H.; Gao, G.; Wang, T. In Situ Phosphonium-Containing Lewis Base-Catalyzed 1,6-Cyanation Reaction: A Facile Way to Obtain α-Diaryl and α-Triaryl Acetonitriles. Org. Chem. Front. 2022, 9, 156–162. [Google Scholar] [CrossRef]
- Kadunce, N.T.; Reisman, S.E. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling between Heteroaryl Iodides and α-Chloronitriles. J. Am. Chem. Soc. 2015, 137, 10480–10483. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Chee, K.W.; Zhou, J.S. Palladium-Catalyzed Asymmetric α-Arylation of Alkylnitriles. J. Am. Chem. Soc. 2016, 138, 16240–16243. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Y.; Bae, M.; Buchwald, S.L. Mechanistic Insight Facilitates Discovery of a Mild and Efficient Copper-Catalyzed Dehydration of Primary Amides to Nitriles Using Hydrosilanes. J. Am. Chem. Soc. 2018, 140, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yu, P.; Morandi, B. Catalytic Reversible Alkene-Nitrile Interconversion through Controllable Transfer Hydrocyanation. Science 2016, 351, 832–836. [Google Scholar] [CrossRef]
- Goswami, P.; Singh, G.; Vijaya Anand, R. N-Heterocyclic Carbene Catalyzed 1,6-Conjugate Addition of Me3Si-CN to para-Quinone Methides and Fuchsones: Access to α-Arylated Nitriles. Org. Lett. 2017, 19, 1982–1985. [Google Scholar] [CrossRef] [PubMed]
- Michel, N.W.M.; Jeanneret, A.D.M.; Kim, H.; Rousseaux, S.A.L. Nickel-Catalyzed Cyanation of Benzylic and Allylic Pivalate Esters. J. Org. Chem. 2018, 83, 11860–11872. [Google Scholar] [CrossRef]
- Fleming, F.F.; Yao, L.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kato, S.; Aoki, D.; Otsuka, H. A Diarylacetonitrile as a Molecular Probe for the Detection of Polymeric Mechanoradicals in the Bulk State through a Radical Chain-Transfer Mechanism. Angew. Chem. Int. Ed. 2021, 60, 2680–2683. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A.; Prisant, L.M. Pharmacologic and Therapeutic Considerationsin Hypertension Therapy with Calcium Channel Blockers: Focus on Verapamil. J. Clin. Hypertens. 2007, 9, 1–22. [Google Scholar] [CrossRef]
- Harrington, P.J.; Lodewijk, E. Twenty Years of Naproxen Technology. Org. Process Res. Dev. 1997, 1, 72–76. [Google Scholar] [CrossRef]
- Bedford, C.T. An Efficient, Large-Scale Synthesis of Cytenamide. J. Chem. Res. 2018, 42, 153–155. [Google Scholar] [CrossRef]
- Brett, D.; Downie, I.M.; Lee, J.B. Sugars with Potential Antiviral Activity. Conversion of Hydroxy Compounds to Nitriles. J. Org. Chem. 1967, 32, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Untch, K.G. Direct One-Step Conversion of Alcohols into Nitriles. J. Org. Chem. 1981, 46, 2985–2987. [Google Scholar] [CrossRef]
- Iranpoor, N.; Firouzabadi, H.; Akhlaghinia, B.; Nowrouzi, N. Conversion of Alcohols, Thiols, and Trimethysilyl Ethers to Alkyl Cyanides Using Triphenylphosphine/2,3-Dichloro-5,6-Dicyanobenzoquinone/n-Bu4NCN. J. Org. Chem. 2004, 69, 2562–2564. [Google Scholar] [CrossRef]
- Soltani Rad, M.N.; Khalafi-Nezhad, A.; Behrouz, S.; Faghihi, M.A. A Simple One-Pot Procedure for the Direct Conversion of Alcohols into Alkyl Nitriles Using TsIm. Tetrahedron Lett. 2007, 48, 6779–6784. [Google Scholar] [CrossRef]
- Aesa, M.C.; Baán, G.; Novák, L.; Szántay, C. Preparation of Unsaturated Nitriles by the Modification of Mitsunobu-Wilk Procedure. Synth. Commun. 1995, 25, 1545–1550. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.; Wu, J.; Ding, K. Facile Preparation of α-Aryl Nitriles by Direct Cyanation of Alcohols with TMSCN under the Catalysis of InX3. Org. Lett. 2008, 10, 4573–4576. [Google Scholar] [CrossRef]
- Trillo, P.; Baeza, A.; Nájera, C. Direct Nucleophilic Substitution of Free Allylic Alcohols in Water Catalyzed by FeCl3⋅6 H2O: Which Is the Real Catalyst? ChemCatChem 2013, 5, 1538–1542. [Google Scholar] [CrossRef]
- Theerthagiri, P.; Lalitha, A. Zn(OTf)2—Catalyzed Direct Cyanation of Benzylic Alcohols—a Novel Synthesis of α-Aryl Nitriles. Tetrahedron Lett. 2012, 53, 5535–5538. [Google Scholar] [CrossRef]
- Wang, J.; Masui, Y.; Onaka, M. Direct Synthesis of Nitriles from Alcohols with Trialkylsilyl Cyanide Using Brønsted Acid Montmorillonite Catalysts. ACS Catal. 2011, 1, 446–454. [Google Scholar] [CrossRef]
- Rajagopal, G.; Kim, S.S. Synthesis of α-Aryl Nitriles through B(C6F5)3-Catalyzed Direct Cyanation of α-Aryl Alcohols and Thiols. Tetrahedron 2009, 65, 4351–4355. [Google Scholar] [CrossRef]
- Bhor, M.D.; Panda, A.G.; Nandurkar, N.S.; Bhanage, B.M. Synthesis of Alkyl Iodides/Nitriles from Carbonyl Compounds Using Novel Ruthenium Tris(2,2,6,6-Tetramethyl-3,5-Heptanedionate) as Catalyst. Tetrahedron Lett. 2008, 49, 6475–6479. [Google Scholar] [CrossRef]
- Yu, R.; Rajasekar, S.; Fang, X. Enantioselective Nickel-Catalyzed Migratory Hydrocyanation of Nonconjugated Dienes. Angew. Chem. Int. Ed. 2020, 59, 21436–21441. [Google Scholar] [CrossRef]
- Gao, J.; Jiao, M.; Ni, J.; Yu, R.; Cheng, G.J.; Fang, X. Nickel-Catalyzed Migratory Hydrocyanation of Internal Alkenes: Unexpected Diastereomeric-Ligand-Controlled Regiodivergence. Angew. Chem. Int. Ed. 2021, 60, 1883–1890. [Google Scholar] [CrossRef]
- Sun, F.; Wang, T.; Cheng, G.-J.; Fang, X. Enantioselective Nickel-Catalyzed Hydrocyanative Desymmetrization of Norbornene Derivatives. ACS Catal. 2021, 11, 7578–7583. [Google Scholar] [CrossRef]
- Ding, S.; Jiao, N. Direct Transformation of N,N-Dimethylformamide to −CN: Pd-Catalyzed Cyanation of Heteroarenes Via C–H Functionalization. J. Am. Chem. Soc. 2011, 133, 12374–12377. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, J.; Chen, F.; Cheng, J. The Palladium-Catalyzed Cyanation of Indole C-H Bonds with the Combination of NH4HCO3 and DMSO as a Safe Cyanide Source. Chem. Commun. 2011, 47, 6725–6727. [Google Scholar] [CrossRef]
- Fan, C.; Zhou, Q.-L. Nickel-Catalyzed Group Transfer of Radicals Enables Hydrocyanation of Alkenes and Alkynes. Chem. Catal. 2021, 1, 117–128. [Google Scholar] [CrossRef]
- Cui, J.; Song, J.; Liu, Q.; Liu, H.; Dong, Y. Transition-Metal-Catalyzed Cyanation by Using an Electrophilic Cyanating Agent, N-Cyano-N-Phenyl-p-Toluenesulfonamide (NCTS). Chem. Asian J. 2018, 13, 482–495. [Google Scholar] [CrossRef]
- Ito, Y.; Kato, H.; Imai, H.; Saegusa, T. A Novel Conjugate Hydrocyanation with TiCl4-tert-Butyl Isocyanide. J. Am. Chem. Soc. 1982, 104, 6449–6450. [Google Scholar] [CrossRef]
- Xu, S.; Huang, X.; Hong, X.; Xu, B. Palladium-Assisted Regioselective C–H Cyanation of Heteroarenes Using Isonitrile as Cyanide Source. Org. Lett. 2012, 14, 4614–4617. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, J.; Hu, Z.; Liang, D.; Huang, J.; Zhu, Q. Palladium-Catalyzed C(Sp2)–H Cyanation Using Tertiary Amine Derived Isocyanide as a Cyano Source. Org. Lett. 2012, 14, 4966–4969. [Google Scholar] [CrossRef]
- Dewanji, A.; van Dalsen, L.; Rossi-Ashton, J.A.; Gasson, E.; Crisenza, G.E.M.; Procter, D.J. A General Arene C-H Functionalization Strategy Via Electron Donor-Acceptor Complex Photoactivation. Nat. Chem. 2022, 15, 43–52. [Google Scholar] [CrossRef]
- Tang, S.; Guillot, R.; Grimaud, L.; Vitale, M.R.; Vincent, G. Electrochemical Benzylic C-H Functionalization with Isocyanides. Org. Lett. 2022, 24, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.-M.; Zhang, Y.; Chen, Z.; Zhu, Y.-M.; Ji, S.-J. Synthesis of Aryl Nitriles by Palladium-Assisted Cyanation of Aryl Iodides Using tert-Butyl Isocyanide as Cyano Source. Tetrahedron 2015, 71, 4883–4887. [Google Scholar] [CrossRef]
- Higashimae, S.; Kurata, D.; Kawaguchi, S.I.; Kodama, S.; Sonoda, M.; Nomoto, A.; Ogawa, A. Palladium-Catalyzed Cyanothiolation of Internal Alkynes Using Organic Disulfides and tert-Butyl Isocyanide. J. Org. Chem. 2018, 83, 5267–5273. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Shinde, G.H.; Shaikh, A.C.; Muthukrishnan, M. Accessing α-Arylated Nitriles Via BF3.OEt2 Catalyzed Cyanation of para-Quinone Methides Using tert-Butyl Isocyanide as a Cyanide Source. J. Org. Chem. 2018, 83, 12305–12314. [Google Scholar] [CrossRef]
- Nauth, A.M.; Opatz, T. Non-Toxic Cyanide Sources and Cyanating Agents. Org. Biomol. Chem. 2019, 17, 11–23. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.J.; Chang, S. Synthesis of Aromatic Nitriles Using Nonmetallic Cyano-Group Sources. Angew. Chem. Int. Ed. 2012, 51, 11948–11959. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, T.; Ding, C.-H.; Xu, B. C(Sp3)–H Functionalization with Isocyanides. Org. Chem. Front. 2021, 8, 3525–3542. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Li, J.; Zhu, Q. Advances in Palladium-Catalysed Imidoylative Cyclization of Functionalized Isocyanides for the Construction of N-Heterocycles. Org. Biomol. Chem. 2021, 19, 6730–6745. [Google Scholar] [CrossRef]
- Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G.C.; Zhu, J. To Each His Own: Isonitriles for All Flavors. Functionalized Isocyanides as Valuable Tools in Organic Synthesis. Chem. Soc. Rev. 2017, 46, 1295–1357. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Xu, B. Metal-Catalyzed C-H Functionalization Involving Isocyanides. Chem. Soc. Rev. 2017, 46, 1103–1123. [Google Scholar] [CrossRef]
- Zhang, B.; Studer, A. Recent Advances in the Synthesis of Nitrogen Heterocycles via Radical Cascade Reactions Using Isonitriles as Radical Acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Bokach, N.A.; Luzyanin, K.V.; Kukushkin, V.Y. Metal-Mediated and Metal-Catalyzed Reactions of Isocyanides. Chem. Rev. 2015, 115, 2698–2779. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Ding, Q.; Wu, J. Recent Advances in Isocyanide Insertion Chemistry. Chem. Soc. Rev. 2013, 42, 5257–5269. [Google Scholar] [CrossRef]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Fang, H.; Oestreich, M. Defunctionalisation Catalysed by Boron Lewis Acids. Chem. Sci. 2020, 11, 12604–12615. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lou, S.J.; Hou, Z. Electron-Deficient Boron-Based Catalysts for C-H Bond Functionalisation. Chem. Soc. Rev. 2021, 50, 1945–1967. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Winfrey, L.; Kustiana, B.A.; Melen, R.L.; Morrill, L.C.; Pulis, A.P. Electron Deficient Borane-Mediated Hydride Abstraction in Amines: Stoichiometric and Catalytic Processes. Chem. Soc. Rev. 2021, 50, 3720–3737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Roy, S.; Chatterjee, I. Tris(Pentafluorophenyl)Borane Catalyzed C-C and C-Heteroatom Bond Formation. Org. Biomol. Chem. 2021, 19, 1230–1267. [Google Scholar] [CrossRef]
- Oestreich, M.; Hermeke, J.; Mohr, J. A Unified Survey of Si-H and H-H Bond Activation Catalysed by Electron-Deficient Boranes. Chem. Soc. Rev. 2015, 44, 2202–2220. [Google Scholar] [CrossRef]
- Melen, R.L. Applications of Pentafluorophenyl Boron Reagents in the Synthesis of Heterocyclic and Aromatic Compounds. Chem. Commun. 2014, 50, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Erker, G. Tris(Pentafluorophenyl)Borane: A Special Boron Lewis Acid for Special Reactions. Dalton Trans. 2005, 1883–1890. [Google Scholar] [CrossRef]
- Meng, S.-S.; Tang, X.; Luo, X.; Wu, R.; Zhao, J.-L.; Chan, A.S.C. Borane-Catalyzed Chemoselectivity-Controllable N-Alkylation and ortho C-Alkylation of Unprotected Arylamines Using Benzylic Alcohols. ACS Catal. 2019, 9, 8397–8403. [Google Scholar] [CrossRef]
- Meng, S.S.; Wang, Q.; Huang, G.B.; Lin, L.R.; Zhao, J.L.; Chan, A.S.C. B(C6F5)3 Catalyzed Direct Nucleophilic Substitution of Benzylic Alcohols: An Effective Method of Constructing C-O, C-S and C-C Bonds from Benzylic Alcohols. RSC Adv. 2018, 8, 30946–30949. [Google Scholar] [CrossRef]
- Chen, X.; Patel, K.; Marek, I. Stereoselective Construction of Tertiary Homoallyl Alcohols and Ethers by Nucleophilic Substitution at Quaternary Carbon Stereocenters. Angew. Chem. Int. Ed. 2023. [Google Scholar] [CrossRef]
- San, H.H.; Huang, J.; Lei Aye, S.; Tang, X.Y. Boron-Catalyzed Dehydrative Friedel-Crafts Alkylation of Arenes Using β-Hydroxyl Ketone as MVK Precursor. Adv. Synth. Catal. 2021, 363, 2386–2391. [Google Scholar] [CrossRef]
- Guru, M.M.; Thorve, P.R.; Maji, B. Boron-Catalyzed N-Alkylation of Arylamines and Arylamides with Benzylic Alcohols. J. Org. Chem. 2020, 85, 806–819. [Google Scholar] [CrossRef]
- Rubin, M.; Gevorgyan, V. B(C6F5)3-Catalyzed Allylation of Secondary Benzyl Acetates with Allylsilanes. Org. Lett. 2001, 3, 2705–2707. [Google Scholar] [CrossRef]
- Dryzhakov, M.; Hellal, M.; Wolf, E.; Falk, F.C.; Moran, J. Nitro-Assisted Bronsted Acid Catalysis: Application to a Challenging Catalytic Azidation. J. Am. Chem. Soc. 2015, 137, 9555–9558. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, L.; Xu, T.T.; Feng, J.J. Boron Lewis Acid Catalyzed Intermolecular Trans-Hydroarylation of Ynamides with Hydroxyarenes. Org. Lett. 2022, 24, 2619–2624. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Wu, H.-H.; Feng, J.-J.; Zhang, J. Design and Enantioselective Synthesis of β-Vinyl Tryptamine Building Blocks for Construction of Privileged Chiral Indole Scaffolds. ACS Catal. 2017, 7, 4047–4052. [Google Scholar] [CrossRef]
- Feng, J.-J.; Zhang, J. Rhodium-Catalyzed Stereoselective Intramolecular Tandem Reaction of Vinyloxiranes with Alkynes: Atom- and Step-Economical Synthesis of Multifunctional Mono-, Bi-, and Tricyclic Compounds. ACS Catal. 2017, 7, 1533–1542. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Feng, J.J.; Zhang, J. Rhodium(I)-Catalyzed Intermolecular Aza-[4+3] Cycloaddition of Vinyl Aziridines and Dienes: Atom-Economical Synthesis of Enantiomerically Enriched Functionalized Azepines. Angew. Chem. Int. Ed. 2017, 56, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.; Berke, H.; Döring, S.; Kehr, G.; Erker, G.; Fröhlich, R.; Meyer, O. Lewis Acid Properties of Tris(Pentafluorophenyl)Borane. Structure and Bonding in L−B(C6F5)3 Complexes. Organometallics 1999, 18, 1724–1735. [Google Scholar] [CrossRef]
- Tumanov, V.V.; Tishkov, A.A.; Mayr, H. Nucleophilicity Parameters for Alkyl and Aryl Isocyanides. Angew. Chem. Int. Ed. 2007, 46, 3563–3566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, Y.Q.; Zhang, K.; Yang, M.Y.; Chen, Y.B.; Li, Y.; Peng, Q.; Zhu, S.F.; Zhou, Q.L.; Ye, L.W. Stereoselective Synthesis of Medium Lactams Enabled by Metal-Free Hydroalkoxylation/Stereospecific [1,3]-Rearrangement. Nat. Commun. 2019, 10, 3234. [Google Scholar] [CrossRef] [PubMed]
- Nambo, M.; Yar, M.; Smith, J.D.; Crudden, C.M. The Concise Synthesis of Unsymmetric Triarylacetonitriles via Pd-Catalyzed Sequential Arylation: A New Synthetic Approach to Tri- and Tetraarylmethanes. Org. Lett. 2015, 17, 50–53. [Google Scholar] [CrossRef]
- Choi, I.; Chung, H.; Park, J.W.; Chung, Y.K. Active and Recyclable Catalytic Synthesis of Indoles by Reductive Cyclization of 2-(2-Nitroaryl)Acetonitriles in the Presence of Co-Rh Heterobimetallic Nanoparticles with Atmospheric Hydrogen under Mild Conditions. Org. Lett. 2016, 18, 5508–5511. [Google Scholar] [CrossRef]
- Asai, K.; Hirano, K.; Miura, M. Divergent Synthesis of Isonitriles and Nitriles by Palladium-Catalyzed Benzylic Substitution with TMSCN. J. Org. Chem. 2020, 85, 12703–12714. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Jiang, Y.Y.; Kang, L.; Yang, L. Ni-Catalyzed hydrocyanation of alkenes with formamide as the cyano source. Green Chem. 2020, 22, 2734–2738. [Google Scholar] [CrossRef]
- Weinreb, S.; Sengupta, R. A One-Pot Umpolung Method for Preparation of α-Aryl Nitriles from α-Chloro Aldoximes via Organocuprate Additions to Transient Nitrosoalkenes. Synthesis 2012, 44, 2933–2937. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Aksenov, N.A.; Dzhandigova, Z.V.; Aksenov, D.A.; Rubin, M. Nitroalkenes as surrogates for cyanomethylium species in a one-pot synthesis of non-symmetric diarylacetonitriles. RSC Adv. 2015, 5, 106492–106497. [Google Scholar] [CrossRef]
- Liu, S.W.; Meng, L.L.; Zeng, X.J.; Hammond, G.B.; Xu, B. Synthesis of Acrylonitriles via Mild Base Promoted Tandem Nucleophilic Substitution-Isomerization of α-Cyanohydrin Methanesulfonates. Chin. J. Chem. 2021, 39, 913–917. [Google Scholar] [CrossRef]
- Lindsay-Scott, P.J.; Clarke, A.; Richardson, J. Two-Step Cyanomethylation Protocol: Convenient Access to Functionalized Aryl- and Heteroarylacetonitriles. Org. Lett. 2015, 17, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, L.T.; Jiang, Y.W.; Ma, D.W. Assembly of α-(Hetero)aryl Nitriles via Copper-Catalyzed Coupling Reactions with (Hetero)aryl Chlorides and Bromides. Angew. Chem. Int. Ed. 2021, 60, 7082–7086. [Google Scholar] [CrossRef] [PubMed]
- Yuen, O.Y.; Chen, X.; Wu, J.; So, C.M. Palladium-Catalyzed Direct α-Arylation of Arylacetonitriles with Aryl Tosylates and Mesylates. Eur. J. Org. Chem. 2020, 1912–1916. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).