Recent Advances in Imaging Agents Anchored with pH (Low) Insertion Peptides for Cancer Theranostics
Abstract
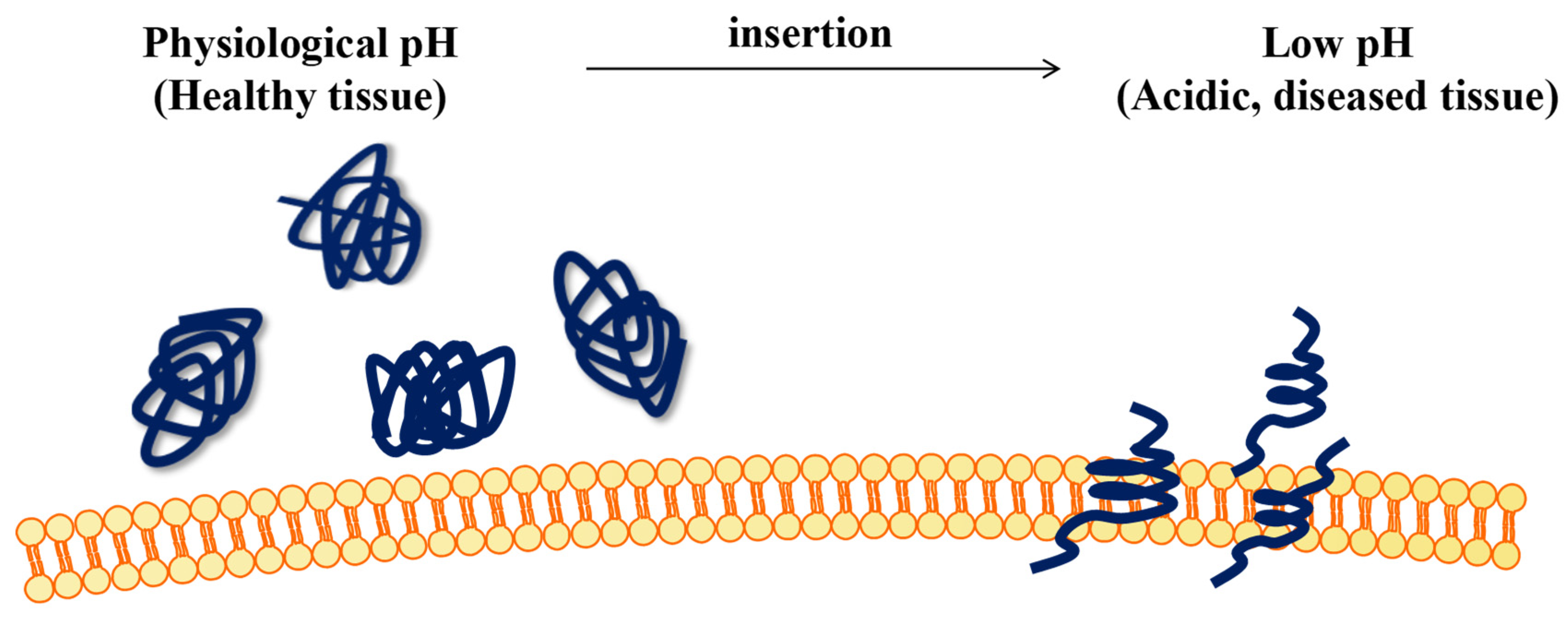
1. Introduction
2. pHLIP-Functionalized Imaging Agents
2.1. MRI
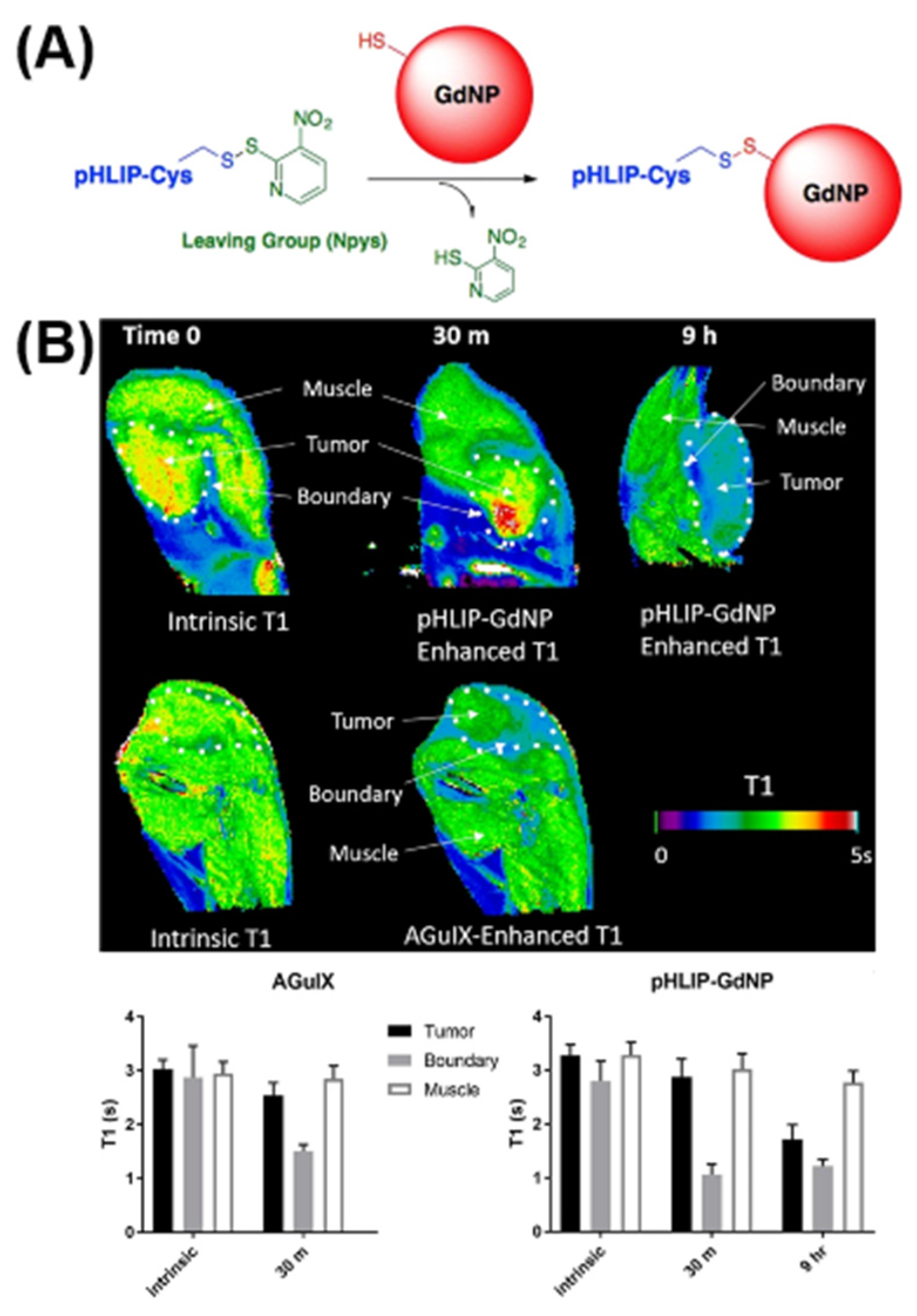
2.1.1. T1 Imaging
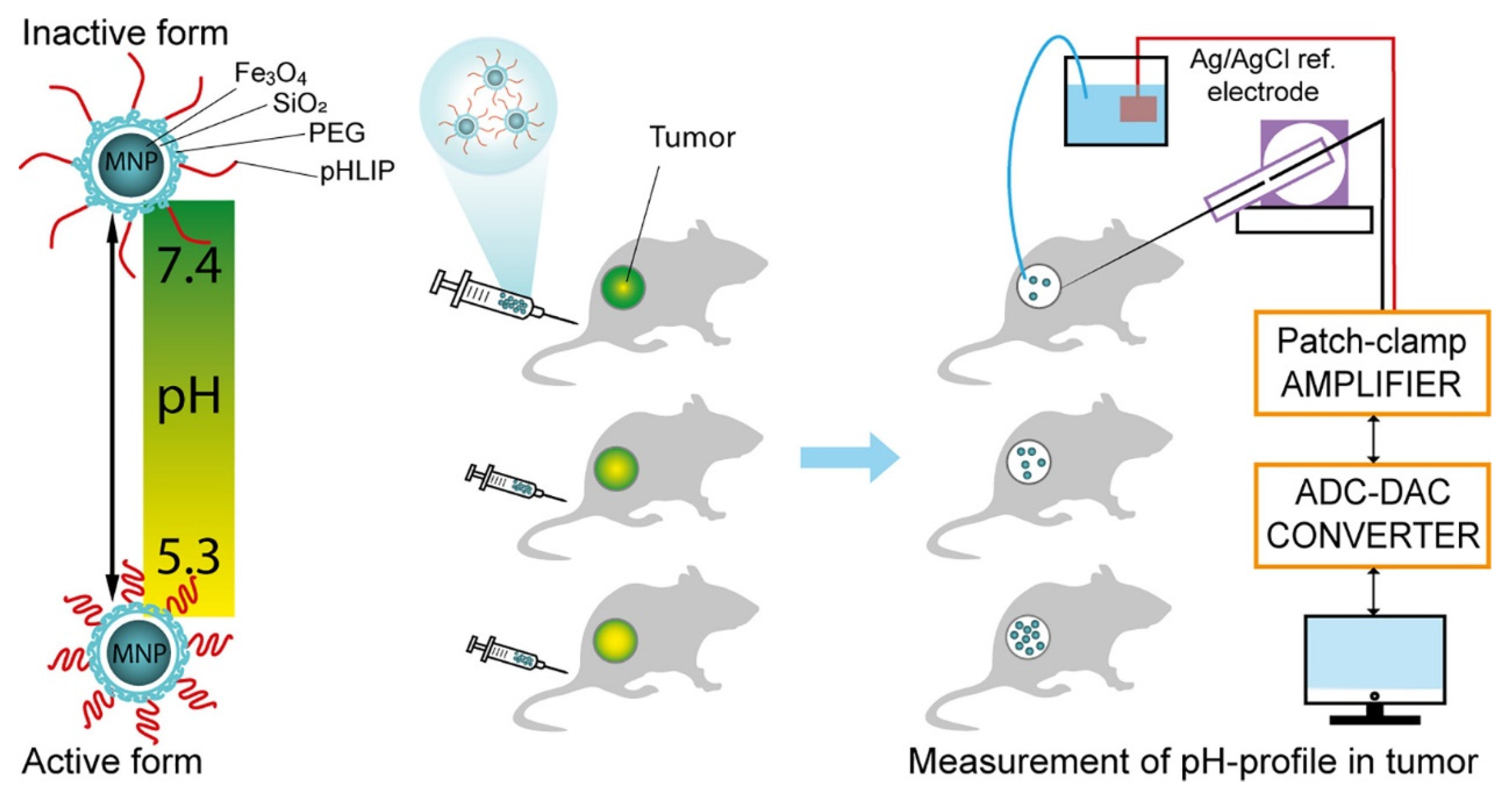
2.1.2. T2 Imaging
2.2. SPECT/PET
2.3. Fluorescence Imaging
2.3.1. Fluorescence Probes
2.3.2. Nanoparticle-Based Probe
2.4. Photoacoustic Imaging
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pspp, A.; Lo, B.; An, A.; Pah, C.; Mgk, D.; Psjp, E.; Pdl, F.; Pwg, B.; Pcyjs, E.; Ejk, G. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar]
- Nussbaum, S.; Shoukry, M.; Ashary, M.A.; Kasbi, A.A.; Baksh, M.; Gabriel, E. Advanced Tumor Imaging Approaches in Human Tumors. Cancers 2022, 14, 1549. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Grimaldi, S.; Ribeiro, M.J.; Frismand, S.; Guedj, E. Single Photon Emission Computed Tomography/Positron Emission Tomography Molecular Imaging for Parkinsonism: A Fast-Developing Field. Ann. Neurol. 2021, 90, 711–719. [Google Scholar] [CrossRef]
- Walker, S.M.; Lim, I.; Lindenberg, L.; Mena, E.; Choyke, P.L.; Turkbey, B. Positron emission tomography (PET) radiotracers for prostate cancer imaging. Abdom. Radiol. 2020, 45, 2165–2175. [Google Scholar] [CrossRef]
- Thorek, D.; Chen, A.K.; Czupryna, J.; Tsourkas, A. Superparamagnetic Iron Oxide Nanoparticle Probes for Molecular Imaging. Ann. Biomed. Eng. 2006, 34, 23–38. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Z. Targeting Peptide-Based Probes for Molecular Imaging and Diagnosis. Adv. Mater. 2019, 31, 1804827. [Google Scholar] [CrossRef]
- Crooke, S.T.; Witztum, J.L.; Bennett, C.F.; Baker, B.F. RNA-targeted therapeutics. Cell Metab. 2018, 27, 714–739. [Google Scholar] [CrossRef]
- Li, H.; Wan, A. Fluorescent probes for real-time measurement of nitric oxide in living cells. Analyst 2015, 140, 7129–7141. [Google Scholar] [CrossRef]
- Cao, R.; Liu, H.; Cheng, Z. Radiolabeled peptide probes for liver cancer imaging. Curr. Med. Chem. 2020, 27, 6968–6986. [Google Scholar] [CrossRef]
- Sakamoto, K.; Akishiba, M.; Iwata, T.; Murata, K.; Mizuno, S.; Kawano, K.; Imanishi, M.; Sugiyama, F.; Futaki, S. Optimizing charge switching in membrane lytic peptides for endosomal release of biomacromolecules. Angew. Chem.-Int. Ed. 2020, 59, 19990–19998. [Google Scholar] [CrossRef]
- Demin, A.M.; Pershina, A.G.; Minin, A.S.; Brikunova, O.Y.; Murzakaev, A.M.; Perekucha, N.A.; Romashchenko, A.V.; Shevelev, O.B.; Uimin, M.A.; Byzov, I.V. Smart design of a pH-responsive system based on pHLIP-modified magnetite nanoparticles for tumor MRI. ACS Appl. Mater. Interfaces 2021, 13, 36800–36815. [Google Scholar] [CrossRef]
- Otieno, S.A.; Qiang, W. Roles of key residues and lipid dynamics reveal pHLIP-membrane interactions at intermediate pH. Biophys. J. 2021, 120, 4649–4662. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.L.; Westerfield, J.M.; Barrera, F.N. Determination of the membrane translocation pK of the pH-low insertion peptide. Biophys. J. 2017, 113, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Engelman, D.M.; Reshetnyak, Y.K.; Andreev, O.A. Mapping pH at Cancer Cell Surfaces. Mol. Imaging Biol. 2019, 21, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.D.; Chakraborty, H.; Chaudhuri, A.; Chattopadhyay, A. Differential sensitivity of pHLIP to ester and ether lipids. Chem. Phys. Lipids 2020, 226, 104849. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, Y.; Zhao, Y.; Zhang, Y.; Su, S.; Wang, J.; Wu, M.; Shi, Q.; Anderson, G.J.; Thomsen, J. pHLIP-mediated targeting of truncated tissue factor to tumor vessels causes vascular occlusion and impairs tumor growth. Oncotarget 2015, 6, 23523. [Google Scholar] [CrossRef] [PubMed]
- Ataka, K.; Drauschke, J.; Stulberg, V.; Koksch, B.; Heberle, J. pH-induced insertion of pHLIP into a lipid bilayer: In-situ SEIRAS characterization of a folding intermediate at neutral pH. Biochim. Biophys. Acta-Biomembr. 2022, 1864, 183873. [Google Scholar] [CrossRef]
- Gilli, P.; Pretto, L.; Bertolasi, V.; Gilli, G. Predicting hydrogen-bond strengths from acid− base molecular properties. The p K a slide rule: Toward the solution of a long-lasting problem. Acc. Chem. Res. 2009, 42, 33–44. [Google Scholar] [CrossRef]
- Vasquez-Montes, V.; Gerhart, J.; Thévenin, D.; Ladokhin, A.S. Divalent Cations and Lipid Composition Modulate Membrane Insertion and Cancer-Targeting Action of pHLIP. J. Mol. Biol. 2019, 431, 5004–5018. [Google Scholar] [CrossRef]
- Scott, H.L.; Heberle, F.A.; Katsaras, J.; Barrera, F.N. Phosphatidylserine asymmetry promotes the membrane insertion of a transmembrane helix. Biophys. J. 2019, 116, 1495–1506. [Google Scholar] [CrossRef]
- Westerfield, J.M.; Gupta, C.; Scott, H.L.; Ye, Y.; Cameron, A.; Mertz, B.; Barrera, F.N. Sodium Ions Hinder the Membrane Insertion of the pH-Low Insertion Peptide. Biophys. J. 2020, 118, 367a. [Google Scholar] [CrossRef]
- Gupta, C.; Mertz, B. Protonation enhances the inherent helix-forming propensity of pHLIP. ACS Omega 2017, 2, 8536–8542. [Google Scholar] [CrossRef]
- Vasquez-Montes, V.; Gerhart, J.; King, K.E.; Thévenin, D.; Ladokhin, A.S. Comparison of lipid-dependent bilayer insertion of pHLIP and its P20G variant. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 534–543. [Google Scholar] [CrossRef]
- Vaughan, J.T., Jr. Radio frequency coils are the ‘lenses’ of the MRI system. NMR Biomed. 2009, 22, 907. [Google Scholar] [CrossRef]
- Furman, G.; Meerovich, V.; Sokolovsky, V.; Xia, Y. Spin-lattice relaxation in liquid entrapped in a nanocavity. J. Magn. Reson. 2020, 311, 106669. [Google Scholar] [CrossRef] [PubMed]
- Rotkopf, L.; Wehrse, E.; Kampf, T.; Vogel, P.; Schlemmer, H.-P.; Ziener, C.H. Spin echo formation in muscle tissue. Phys. Rev. E 2021, 104, 034419. [Google Scholar] [CrossRef] [PubMed]
- Wansapura, J.P.; Holland, S.K.; Dunn, R.S.; Ball, W.S., Jr. NMR relaxation times in the human brain at 3.0 tesla. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 1999, 9, 531–538. [Google Scholar] [CrossRef]
- Ding, Y.M.; Zhou, C.C.; Zhao, Y.M.; Li, W.; Meng, S.Y. Construction of cRGD-iron oxide nanoparticles and its application in the tumor diagnosis by magnetic resonance imaging. Tumor 2010, 30, 277–282. [Google Scholar]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2020, 33, 1906539. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application. Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kokuryo, D.; Rafi, M.; Terada, Y.; Aoki, I.; Saga, T.; Takehiko, I.; Nishiyama, N.; Kataoka, K. Gd-DTPA-loaded polymer–metal complex micelles with high relaxivity for MR cancer imaging. Biomaterials 2013, 34, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.H.; Harkin, K.; Biller, D.; Rafi, M.; Terada, Y.; Aoki, I.; Saga, T.; Takehiko, I.; Nishiyama, N.; Kataoka, K. Investigation of suspected gadolinium neurotoxicity in a dog. Vet. Radiol. Ultrasound 2021, 62, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, S.; Divband, B.; Gharehaghaji, N. Magnetic resonance imaging property of doxorubicin-loaded gadolinium/13X zeolite/folic acid nanocomposite. J. Biomed. Phys. Eng. 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Liu, F.; Ma, L.; Liu, D.; Wang, Z. Nanoparticle-based systems for T 1-weighted magnetic resonance imaging contrast agents. Int. J. Mol. Sci. 2013, 14, 10591–10607. [Google Scholar] [CrossRef]
- Xie, Q.; Wen, T.; Yang, A.; Zhang, X.; Chen, B.; Meng, J.; Liu, J.; Gu, N.; Xu, H. A contrast examination of proinflammatory effects on kidney function for γ-Fe2O3 NP and gadolinium dimeglumine. Int. J. Nanomed. 2021, 16, 2271. [Google Scholar] [CrossRef]
- FDA. Development of Serious and Sometimes Fatal Nephrogenic Systemic Fibrosis/Nephrogenic Fibrosing Dermopathy. J. Radiol. Nurs. 2007, 26, 29–30. [Google Scholar] [CrossRef]
- Aime, S.; Cabella, C.; Colombatto, S.; Crich, S.G.; Gianolio, E.; Maggioni, F. Insights into the use of paramagnetic Gd (III) complexes in MR-molecular imaging investigations. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2002, 16, 394–406. [Google Scholar] [CrossRef]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2009, 30, 1259–1267. [Google Scholar] [CrossRef]
- Abdollah, M.R.; Carter, T.J.; Jones, C.; Kalber, T.L.; Rajkumar, V.; Tolner, B.; Gruettner, C.; Zaw-Thin, M.; Torres, J.B.; Ellis, M.; et al. Fucoidan prolongs the circulation time of dextran-coated iron oxide nanoparticles. ACS Nano 2018, 12, 1156–1169. [Google Scholar] [CrossRef]
- Janic, B.; Bhuiyan MP, I.; Ewing, J.R.; Ali, M.M. pH-Dependent Cellular Internalization of Paramagnetic Nanoparticle. ACS Sens. 2016, 1, 975–978. [Google Scholar] [CrossRef]
- Liu, W.; Deacon, J.; Yan, H.G.; Sun, B.; Liu, Y.F.; Hegan, D.; Li, Q.; Coman, D.; Parent, M.; Hyder, F.; et al. Tumor-targeted pH-low insertion peptide delivery of theranostic gadolinium nanoparticles for image-guided nanoparticle-enhanced radiation therapy. Transl. Oncol. 2020, 13, 100839. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Wu, A.G.; Chen, X.Y. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.H.; Jiang, H.; Lu, F.L.; Wang, H.W.; Zhang, X.M. Size and PEG Length-Controlled PEGylated Monocrystalline Superparamagnetic Iron Oxide Nanocomposite for MRI Contrast Agent. Int. J. Nanomed. 2021, 16, 201–211. [Google Scholar] [CrossRef]
- Khairnar, S.; More, N.; Mounika, C.; Kapusetti, G. Advances in Contrast Agents for Contrast-Enhanced Magnetic Resonance Imaging. J. Med. Imaging Radiat. Sci. 2019, 50, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liao, R.; Mahmood, A.A.; Xu, H.; Zhou, Q. pH-responsive pHLIP (pH low insertion peptide) nanoclusters of superparamagnetic iron oxide nanoparticles as a tumor-selective MRI contrast agent. Acta Biomater. 2017, 55, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.A.; Zhang, J.; Liao, R.; Pan, X.; Xu, D.; Xu, H.; Zhou, Q. Evaluation of non-targeting, C-or N-pH (low) insertion peptide modified superparamagnetic iron oxide nanoclusters for selective MRI of liver tumors and their potential toxicity in cirrhosis. RSC Adv. 2019, 9, 14051–14059. [Google Scholar] [CrossRef]
- Pershina, A.G.; Brikunova, O.Y.; Demin, A.M.; Abakumov, M.A.; Vaneev, A.N.; Naumenko, V.A.; Erofeev, A.S.; Gorelkin, P.V.; Nizamov, T.R.; Muslimov, A.R. Variation in tumor pH affects pH-triggered delivery of peptide-modified magnetic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102317. [Google Scholar] [CrossRef]
- Al-Baradie, R.S. Nanobodies as versatile tools: A focus on targeted tumor therapy, tumor imaging and diagnostics. Hum. Antibodies 2020, 28, 259–272. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yu, M.M.; Wang, Z.G. Inhibition of MDA-MB-231 cell proliferation by pHLIP(Var7)-P1AP andSPECT imaging of MDA-MB-231 breast cancer-bearing nude miceusing 125I-pHLIP(Var7)-P1AP. Nukl.-Nucl. 2021, 60, 240–248. [Google Scholar]
- Wei, X.; Zhao, H.; Huang, G.; Liu, J.; He, W.; Huang, Q. ES-MION-Based Dual-Modality PET/MRI Probes for Acidic Tumor Microenvironment Imaging. ACS Omega 2022, 7, 3442–3451. [Google Scholar] [CrossRef]
- Demoin, D.W.; Wyatt, L.C.; Edwards, K.J.; Abdel-Atti, D.; Sarparanta, M.; Pourat, J.; Longo, V.A.; Carlin, S.D.; Engelman, D.M.; Andreev, O.A.; et al. PET Imaging of Extracellular pH in Tumors with (64)Cu- and (18)F-Labeled pHLIP Peptides: A Structure-Activity Optimization Study. Bioconjugate Chem. 2016, 27, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.S.; Steinberg, I.; Vermesh, O.; Berg, N.S.V.D.; Rosenthal, E.L.; van Dam, G.M.; Ntziachristos, V.; Gambhir, S.S.; Hernot, S.; Rogalla, S. Emerging intraoperative imaging modalities to improve surgical precision. Mol. Imaging Biol. 2018, 20, 705–715. [Google Scholar] [CrossRef]
- Shibutani, H.; Fujii, K.; Kawakami, R.; Imanaka, T.; Kawai, K.; Tsujimoto, S.; Matsumura, K.; Otagaki, M.; Morishita, S.; Hashimoto, K.; et al. Tangential signal dropout artefact in optical frequency domain imaging. Eurointervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2021, 17, e326–e331. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-photon small-molecule fluorescence-based agents for sensing, imaging, and therapy within biological systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.I.; Center, I.A. Research Progress and Application of Modern Microimaging Technology. China Med. Devices 2018, 33, 107–110. [Google Scholar]
- Behzadi, P.; Ranjbar, R. DNA microarray technology and bioinformatic web services. Acta Microbiol. Immunol. Hung. 2019, 66, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, C.W.; Khanal, A.; Zeiderman, M.; Khanal, B.R.; Burton, N.C.; McMasters, K.M.; Vickers, S.M.; Grizzle, W.E.; McNally, L.R. Targeting Acidity in Pancreatic Adenocarcinoma: Multispectral Optoacoustic Tomography Detects pH-Low Insertion Peptide Probes In VivoMSOT Detects pHLIP Probes. Clin. Cancer Res. 2015, 21, 4576–4585. [Google Scholar] [CrossRef]
- Golijanin, J.; Amin, A.; Moshnikova, A.; Brito, J.M.; Tran, T.Y.; Adochite, R.-C.; Andreev, G.O.; Crawford, T.; Engelman, D.M.; Andreev, O.A. Targeted imaging of urothelium carcinoma in human bladders by an ICG pHLIP peptide ex vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 11829–11834. [Google Scholar] [CrossRef]
- Brito, J.; Golijanin, B.; Kott, O.; Moshnikova, A.; Mueller-Leonhard, C.; Gershman, B.; Andreev, O.A.; Reshetnyak, Y.K.; Amin, A.; Golijanin, D. Ex-vivo Imaging of Upper Tract Urothelial Carcinoma Using Novel pH Low Insertion Peptide (Variant 3), a Molecular Imaging Probe. Urology 2020, 139, 134–140. [Google Scholar] [CrossRef]
- Adochite, R.-C.; Moshnikova, A.; Golijanin, J.; Andreev, O.A.; Katenka, N.V.; Reshetnyak, Y.K. Comparative study of tumor targeting and biodistribution of pH (low) insertion peptides (pHLIP® peptides) conjugated with different fluorescent dyes. Mol. Imaging Biol. 2016, 18, 686–696. [Google Scholar] [CrossRef]
- Mitrou, A.; Feng, X.; Khan, A.; Yaroslavsky, A.N. Feasibility of dual-contrast fluorescence imaging of pathological breast tissues. J. Biophotonics 2021, 14, e202100007. [Google Scholar] [CrossRef] [PubMed]
- Hallaj, T.; Azizi, N.; Amjadi, M. A dual-mode colorimetric and fluorometric nanosensor for detection of uric acid based on N, P co-doped carbon dots and in-situ formation of Au/Ag core-shell nanoparticles. Microchem. J. 2021, 162, 105865. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Farag, M.A.; Yehia, A.M. A gold–carbon dots nanoprobe for dual mode detection of ketamine HCl in soda drinks. New J. Chem. 2020, 44, 7058–7064. [Google Scholar] [CrossRef]
- Liao, G.; Wang, L.; Yu, W. Application of novel targeted molecular imaging probes in the early diagnosis of upper urinary tract epithelial carcinoma. Oncol. Lett. 2018, 16, 6349–6354. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.J.; Wang, N.; Zhang, X.M.; Sun, T.Y.; Zhu, Q.; Kong, W.; Wang, F.; Zhu, G.Y. An upconversion nanoplatform with extracellular pH-driven tumor-targeting ability for improved photodynamic therapy. Nanoscale 2018, 10, 4432–4441. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhao, X.; Chen, L.J.; Yang, C.X.; Yan, X.P. pH-Driven Targeting Nanoprobe with Dual-Responsive Drug Release for Persistent Luminescence Imaging and Chemotherapy of Tumor. Anal. Chem. 2020, 92, 1179–1188. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Pei, Y.; Wei, M.-Y. Newly-engineered materials for bio-imaging technology: A focus on the hybrid system of ultrasound and fluorescence. Front. Bioeng. Biotechnol. 2019, 7, 88. [Google Scholar] [CrossRef]
- Xu, S.; Shi, X.; Chu, C.; Liu, G. A TME-activated in situ nanogenerator for magnetic resonance/fluorescence/photoacoustic imaging. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–156. [Google Scholar]
- Lin, L.; Wang LH, V. The emerging role of photoacoustic imaging in clinical oncology. Nat. Rev. Clin. Oncol. 2022, 19, 365–384. [Google Scholar] [CrossRef]
- Reshetnyak, Y.K. Imaging Tumor Acidity: pH-Low Insertion Peptide Probe for Optoacoustic Tomography. Clin. Cancer Res. 2015, 21, 4502–4504. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Teng, Z.; Tian, W.; Luo, S.; Kong, X.; Su, X.; Tang, Y.; Wang, S.; Lu, G. pH-Dependent Transmembrane Activity of Peptide-Functionalized Gold Nanostars for Computed Tomography/Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Memorial Sloan Kettering Cancer Center. Study of the Imaging Agent 18F-Var3 in Patients with Breast Cancer. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04054986 (accessed on 17 May 2022).
- Jin Zh Tsuji Ab Degardin, M.; Dumy, P.; Boturyn, D.; Higashi, T. Multiplexed Imaging Reveals the Spatial Relationship of the Extracellular Acidity-Targeting pHLIP with Necrosis, Hypoxia, and the Integrin-Targeting cRGD Peptide. Cells 2022, 11, 3499. [Google Scholar]
- Gillies Rj Verduzco, D.; Gatenby, R.A. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer 2012, 12, 487–493. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Macholl, S.; Morrison, M.S.; Iveson, P.; Arbo, B.E.; Andreev, O.A.; Reshetnyak, Y.K.; Engelman, D.M.; Johannesen, E. In Vivo pH Imaging with Tc-99m-Phlip. Mol. Imaging Biol. 2012, 14, 725–734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-C.; Wang, Z.-X.; Pan, J.-Y.; Wang, L.-Q.; Dai, X.-Y.; Wu, K.-F.; Ye, X.-W.; Xu, X.-L. Recent Advances in Imaging Agents Anchored with pH (Low) Insertion Peptides for Cancer Theranostics. Molecules 2023, 28, 2175. https://doi.org/10.3390/molecules28052175
Liu Y-C, Wang Z-X, Pan J-Y, Wang L-Q, Dai X-Y, Wu K-F, Ye X-W, Xu X-L. Recent Advances in Imaging Agents Anchored with pH (Low) Insertion Peptides for Cancer Theranostics. Molecules. 2023; 28(5):2175. https://doi.org/10.3390/molecules28052175
Chicago/Turabian StyleLiu, Yu-Cheng, Zhi-Xian Wang, Jing-Yi Pan, Ling-Qi Wang, Xin-Yi Dai, Ke-Fei Wu, Xue-Wei Ye, and Xiao-Ling Xu. 2023. "Recent Advances in Imaging Agents Anchored with pH (Low) Insertion Peptides for Cancer Theranostics" Molecules 28, no. 5: 2175. https://doi.org/10.3390/molecules28052175
APA StyleLiu, Y.-C., Wang, Z.-X., Pan, J.-Y., Wang, L.-Q., Dai, X.-Y., Wu, K.-F., Ye, X.-W., & Xu, X.-L. (2023). Recent Advances in Imaging Agents Anchored with pH (Low) Insertion Peptides for Cancer Theranostics. Molecules, 28(5), 2175. https://doi.org/10.3390/molecules28052175




