The Pleiotropic Role of Extracellular ATP in Myocardial Remodelling
Abstract
1. Introduction
2. Extracellular ATP Sources and Signalling
2.1. Cardiomyocytes
2.2. Cardiac Fibroblasts
2.3. Vascular Smooth Muscle Cells
2.4. Vascular Endothelial Cells
2.5. Circulating Red Blood Cells
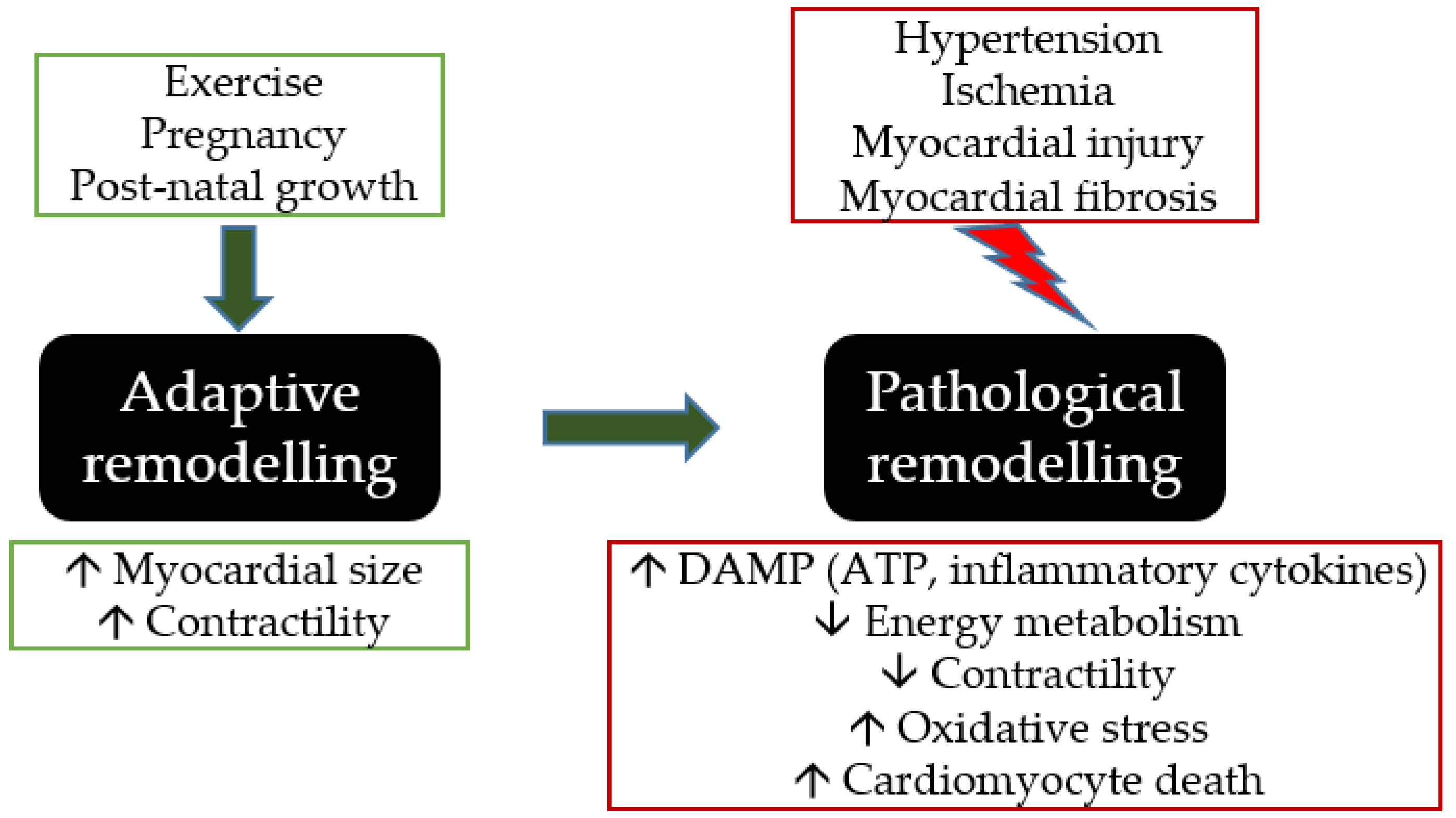
3. ATP in Cardiovascular Remodelling
3.1. Hypertension and Atherosclerosis
3.2. Ischemic Reperfusion (I/R) Injury
3.3. Angiogenesis
3.4. Myocardial Fibrosis
3.5. Hypertrophic and Atrophic Remodelling
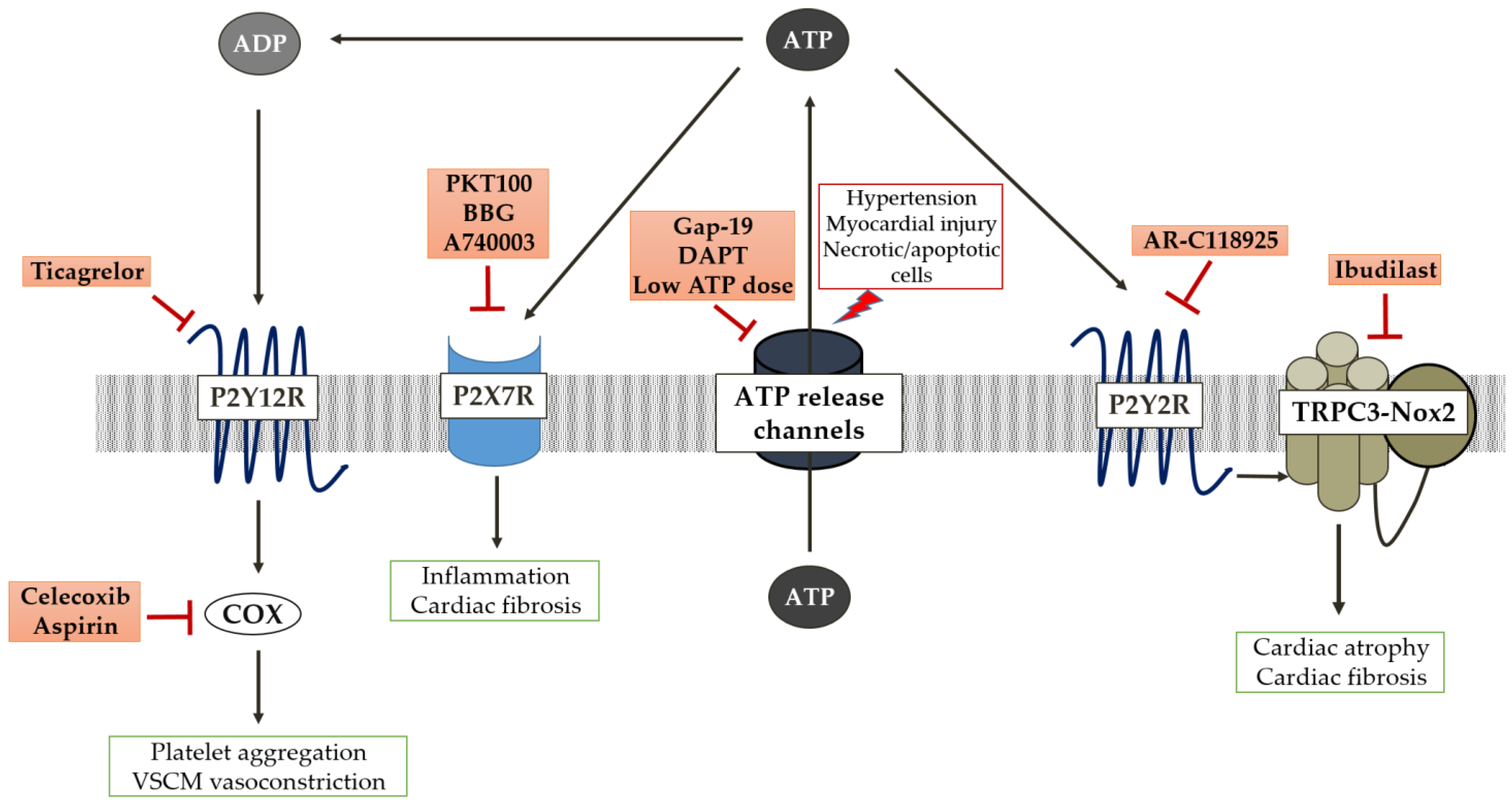
4. Therapeutic Insights of ATP Signalling in Cardiac Remodelling
4.1. Targeting ATP Release Channels
4.2. Targeting ATP-P2X Signalling Axis
4.3. Targeting ATP-P2Y2 and Downstream Effectors
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maillet, M.; van Berlo, J.H.; Molkentin, J.D. Molecular Basis of Physiological Heart Growth: Fundamental Concepts and New Players. Nat. Rev. Mol. Cell Biol. 2013, 14, 38–48. [Google Scholar] [CrossRef]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac Metabolism in Heart Failure: Implications beyond ATP Production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Gibb, A.A.; Hill, B.G. Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Bodin, P.; Burnstock, G. Purinergic Signalling: ATP Release. Neurochem. Res. 2001, 26, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H. Extracellular ATP and Other Nucleotides—Ubiquitous Triggers of Intercellular Messenger Release. Purinergic Signal. 2016, 12, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- Burnstock, G. A basis for distinguishing two types of purinergic receptor. In Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach; Straub, R.W., Bolis, L., Eds.; Raven Press: New York, NY, USA, 1978; pp. 107–118. [Google Scholar]
- Erlinge, D.; Burnstock, G. P2 Receptors in Cardiovascular Regulation and Disease. Purinergic Signal. 2008, 4, 1–20. [Google Scholar] [CrossRef]
- Taruno, A. ATP Release Channels. Int. J. Mol. Sci. 2018, 19, 808. [Google Scholar] [CrossRef]
- Lazarowski, E.R. Vesicular and Conductive Mechanisms of Nucleotide Release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Sesma, J.I.; Seminario-Vidal, L.; Kreda, S.M. Molecular Mechanisms of Purine and Pyrimidine Nucleotide Release. Adv. Pharmacol. 2011, 61, 221–261. [Google Scholar] [CrossRef]
- Myeong, J.; Ko, J.; Kwak, M.; Kim, J.; Woo, J.; Ha, K.; Hong, C.; Yang, D.; Kim, H.J.; Jeon, J.-H.; et al. Dual Action of the Galphaq-PLCbeta-PI(4,5)P2 Pathway on TRPC1/4 and TRPC1/5 Heterotetramers. Sci. Rep. 2018, 8, 12117. [Google Scholar] [CrossRef]
- Ralevic, V. P2X Receptors in the Cardiovascular System and Their Potential as Therapeutic Targets in Disease. Curr. Med. Chem. 2015, 22, 851–865. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Shestopalov, V.I.; Panchin, Y. Pannexins and Gap Junction Protein Diversity. Cell. Mol. Life Sci. 2008, 65, 376–394. [Google Scholar] [CrossRef]
- Smyth, J.W.; Shaw, R.M. Visualizing Cardiac Ion Channel Trafficking Pathways. Methods Enzymol. 2012, 505, 187–202. [Google Scholar] [CrossRef]
- Delmar, M.; Makita, N. Cardiac Connexins, Mutations and Arrhythmias. Curr. Opin. Cardiol. 2012, 27, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lan, Y.; Zhao, Y.; Zhang, Q.; Lin, T.; Lin, K.; Guo, J.; Yan, Y. Expression of Connexin 43 Protein in Cardiomyocytes of Heart Failure Mouse Model. Front. Cardiovasc. Med. 2022, 9, 1028558. [Google Scholar] [CrossRef] [PubMed]
- Warn-Cramer, B.J.; Cottrell, G.T.; Burt, J.M.; Lau, A.F. Regulation of Connexin-43 Gap Junctional Intercellular Communication by Mitogen-Activated Protein Kinase. J. Biol. Chem. 1998, 273, 9188–9196. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.F.; Hatch-Pigott, V.; Crow, D.S. Evidence That Heart Connexin43 Is a Phosphoprotein. J. Mol. Cell. Cardiol. 1991, 23, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Bowling, N.; Huang, X.; Sandusky, G.E.; Fouts, R.L.; Mintze, K.; Esterman, M.; Allen, P.D.; Maddi, R.; McCall, E.; Vlahos, C.J. Protein Kinase C-Alpha and -Epsilon Modulate Connexin-43 Phosphorylation in Human Heart. J. Mol. Cell. Cardiol. 2001, 33, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Keyse, S.M. Protein Phosphatases and the Regulation of Mitogen-Activated Protein Kinase Signalling. Curr. Opin. Cell Biol. 2000, 12, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Vessey, D.A.; Li, L.; Kelley, M. Pannexin-I/P2X 7 Purinergic Receptor Channels Mediate the Release of Cardioprotectants Induced by Ischemic Pre- and Postconditioning. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S.; Sasano, T.; Tateishi, Y.; Tamura, N.; Isobe, M.; Furukawa, T. Stretch of Atrial Myocytes Stimulates Recruitment of Macrophages via ATP Released through Gap-Junction Channels. J. Pharmacol. Sci. 2012, 120, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dahl, G. Pannexin1: A Multifunction and Multiconductance and/or Permeability Membrane Channel. Am. J. Physiol. Cell Physiol. 2018, 315, C290–C299. [Google Scholar] [CrossRef]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin Membrane Channels Are Mechanosensitive Conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef]
- Locovei, S.; Wang, J.; Dahl, G. Activation of Pannexin 1 Channels by ATP through P2Y Receptors and by Cytoplasmic Calcium. FEBS Lett. 2006, 580, 239–244. [Google Scholar] [CrossRef]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The Pannexin 1 Channel Activates the Inflammasome in Neurons and Astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef]
- Qiu, F.; Dahl, G. A Permeant Regulating Its Permeation Pore: Inhibition of Pannexin 1 Channels by ATP. Am. J. Physiol. Cell Physiol. 2009, 296, C250–C255. [Google Scholar] [CrossRef]
- Silverman, W.; Locovei, S.; Dahl, G. Probenecid, a Gout Remedy, Inhibits Pannexin 1 Channels. Am. J. Physiol. Cell Physiol. 2008, 295, C761–C767. [Google Scholar] [CrossRef]
- Mei, Q.; Liang, B.T. P2 Purinergic Receptor Activation Enhances Cardiac Contractility in Isolated Rat and Mouse Hearts. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H334–H341. [Google Scholar] [CrossRef]
- Shen, J.-B.; Pappano, A.J.; Liang, B.T. Extracellular ATP-Stimulated Current in Wild-Type and P2X4 Receptor Transgenic Mouse Ventricular Myocytes: Implications for a Cardiac Physiologic Role of P2X4 Receptors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 277–284. [Google Scholar] [CrossRef]
- Shen, J.-B.; Shutt, R.; Pappano, A.; Liang, B.T. Characterization and Mechanism of P2X Receptor-Mediated Increase in Cardiac Myocyte Contractility. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3056–H3062. [Google Scholar] [CrossRef] [PubMed]
- Musa, H.; Tellez, J.O.; Chandler, N.J.; Greener, I.D.; Maczewski, M.; Mackiewicz, U.; Beresewicz, A.; Molenaar, P.; Boyett, M.R.; Dobrzynski, H. P2 Purinergic Receptor MRNA in Rat and Human Sinoatrial Node and Other Heart Regions. Naunyn. Schmiedebergs. Arch. Pharmacol. 2009, 379, 541–549. [Google Scholar] [CrossRef]
- Hu, B.; Mei, Q.B.; Yao, X.J.; Smith, E.; Barry, W.H.; Liang, B.T. A Novel Contractile Phenotype with Cardiac Transgenic Expression of the Human P2X4 Receptor. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 2739–2741. [Google Scholar] [CrossRef]
- Hu, B.; Senkler, C.; Yang, A.; Soto, F.; Liang, B.T. P2X4 Receptor Is a Glycosylated Cardiac Receptor Mediating a Positive Inotropic Response to ATP. J. Biol. Chem. 2002, 277, 15752–15757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-Y.; Mamdani, M.; Qanud, K.; Shen, J.-B.; Pappano, A.J.; Kumar, T.S.; Jacobson, K.A.; Hintze, T.; Recchia, F.A.; Liang, B.T. Treatment of Heart Failure by a Methanocarba Derivative of Adenosine Monophosphate: Implication for a Role of Cardiac Purinergic P2X Receptors. J. Pharmacol. Exp. Ther. 2010, 333, 920–928. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.-M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G Protein-Coupled Nucleotide Receptors: From Molecular Mechanisms and Pathophysiology to Therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef]
- von Kugelgen, I. Pharmacological Profiles of Cloned Mammalian P2Y-Receptor Subtypes. Pharmacol. Ther. 2006, 110, 415–432. [Google Scholar] [CrossRef]
- Strobaek, D.; Olesen, S.P.; Christophersen, P.; Dissing, S. P2-Purinoceptor-Mediated Formation of Inositol Phosphates and Intracellular Ca2+ Transients in Human Coronary Artery Smooth Muscle Cells. Br. J. Pharmacol. 1996, 118, 1645–1652. [Google Scholar] [CrossRef]
- Lutz, S.; Freichel-Blomquist, A.; Yang, Y.; Rümenapp, U.; Jakobs, K.H.; Schmidt, M.; Wieland, T. The Guanine Nucleotide Exchange Factor P63RhoGEF, a Specific Link between Gq/11-Coupled Receptor Signaling and RhoA. J. Biol. Chem. 2005, 280, 11134–11139. [Google Scholar] [CrossRef]
- Nishida, M.; Sato, Y.; Uemura, A.; Narita, Y.; Tozaki-Saitoh, H.; Nakaya, M.; Ide, T.; Suzuki, K.; Inoue, K.; Nagao, T.; et al. P2Y6 Receptor-Galpha12/13 Signalling in Cardiomyocytes Triggers Pressure Overload-Induced Cardiac Fibrosis. EMBO J. 2008, 27, 3104–3115. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left Ventricular Remodelling Post-Myocardial Infarction: Pathophysiology, Imaging, and Novel Therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Braun, O.O.; Lu, D.; Aroonsakool, N.; Insel, P.A. Uridine Triphosphate (UTP) Induces Profibrotic Responses in Cardiac Fibroblasts by Activation of P2Y2 Receptors. J. Mol. Cell. Cardiol. 2010, 49, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Soleymani, S.; Madakshire, R.; Insel, P.A. ATP Released from Cardiac Fibroblasts via Connexin Hemichannels Activates Profibrotic P2Y2 Receptors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 2580–2591. [Google Scholar] [CrossRef]
- Lu, D.; Insel, P.A. Hydrolysis of Extracellular ATP by Ectonucleoside Triphosphate Diphosphohydrolase (ENTPD) Establishes the Set Point for Fibrotic Activity of Cardiac Fibroblasts. J. Biol. Chem. 2013, 288, 19040–19049. [Google Scholar] [CrossRef]
- Chen, Y.; Epperson, S.; Makhsudova, L.; Ito, B.; Suarez, J.; Dillmann, W.; Villarreal, F. Functional Effects of Enhancing or Silencing Adenosine A2b Receptors in Cardiac Fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2478–H2486. [Google Scholar] [CrossRef]
- Epperson, S.A.; Brunton, L.L.; Ramirez-Sanchez, I.; Villarreal, F. Adenosine Receptors and Second Messenger Signaling Pathways in Rat Cardiac Fibroblasts. Am. J. Physiol. Cell Physiol. 2009, 296, C1171–C1177. [Google Scholar] [CrossRef]
- Phosri, S.; Arieyawong, A.; Bunrukchai, K.; Parichatikanond, W.; Nishimura, A.; Nishida, M.; Mangmool, S. Stimulation of Adenosine A2B Receptor Inhibits Endothelin-1-Induced Cardiac Fibroblast Proliferation and α-Smooth Muscle Actin Synthesis through the CAMP/Epac/PI3K/Akt-Signaling Pathway. Front. Pharmacol. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling and Vascular Cell Proliferation and Death. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 364–373. [Google Scholar] [CrossRef]
- Lohman, A.W.; Billaud, M.; Isakson, B.E. Mechanisms of ATP Release and Signalling in the Blood Vessel Wall. Cardiovasc. Res. 2012, 95, 269–280. [Google Scholar] [CrossRef]
- Billaud, M.; Lohman, A.W.; Straub, A.C.; Looft-Wilson, R.; Johnstone, S.R.; Araj, C.A.; Best, A.K.; Chekeni, F.B.; Ravichandran, K.S.; Penuela, S.; et al. Pannexin1 Regulates Alpha1-Adrenergic Receptor- Mediated Vasoconstriction. Circ. Res. 2011, 109, 80–85. [Google Scholar] [CrossRef]
- Buchet, R.; Tribes, C.; Rouaix, V.; Doumèche, B.; Fiore, M.; Wu, Y.; Magne, D.; Mebarek, S. Hydrolysis of Extracellular ATP by Vascular Smooth Muscle Cells Transdifferentiated into Chondrocytes Generates Pi but Not PPi. Int. J. Mol. Sci. 2021, 22, 2948. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimo, D.A.; Douglas, D.C.; Romani, A.M.; O’Neill, W.C.; Dubyak, G.R. Autocrine ATP Release Coupled to Extracellular Pyrophosphate Accumulation in Vascular Smooth Muscle Cells. Am. J. Physiol. Physiol. 2009, 296, C828–C839. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.H.; Prat, A.G.; Gerweck, L.; Seneveratne, T.; Arceci, R.J.; Kramer, R.; Guidotti, G.; Cantiello, H.F. The Multidrug Resistance (Mdr1) Gene Product Functions as an ATP Channel. Proc. Natl. Acad. Sci. USA 1993, 90, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Reisin, I.L.; Prat, A.G.; Abraham, E.H.; Amara, J.F.; Gregory, R.J.; Ausiello, D.A.; Cantiello, H.F. The Cystic Fibrosis Transmembrane Conductance Regulator Is a Dual ATP and Chloride Channel. J. Biol. Chem. 1994, 269, 20584–20591. [Google Scholar] [CrossRef] [PubMed]
- Robert, R.; Norez, C.; Becq, F. Disruption of CFTR Chloride Channel Alters Mechanical Properties and CAMP-Dependent Cl- Transport of Mouse Aortic Smooth Muscle Cells. J. Physiol. 2005, 568, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Vial, C.; Evans, R.J. P2X(1) Receptor-Deficient Mice Establish the Native P2X Receptor and a P2Y6-like Receptor in Arteries. Mol. Pharmacol. 2002, 62, 1438–1445. [Google Scholar] [CrossRef]
- Thyberg, J. Differentiated Properties and Proliferation of Arterial Smooth Muscle Cells in Culture. Int. Rev. Cytol. 1996, 169, 183–265. [Google Scholar] [CrossRef]
- Oketani, N.; Kakei, M.; Ichinari, K.; Okamura, M.; Miyamura, A.; Nakazaki, M.; Ito, S.; Tei, C. Regulation of K(ATP) Channels by P(2Y) Purinoceptors Coupled to PIP(2) Metabolism in Guinea Pig Ventricular Cells. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H757–H765. [Google Scholar] [CrossRef]
- Erlinge, D.; Hou, M.; Webb, T.E.; Barnard, E.A.; Moller, S. Phenotype Changes of the Vascular Smooth Muscle Cell Regulate P2 Receptor Expression as Measured by Quantitative RT-PCR. Biochem. Biophys. Res. Commun. 1998, 248, 864–870. [Google Scholar] [CrossRef]
- Hou, M.; Moller, S.; Edvinsson, L.; Erlinge, D. Cytokines Induce Upregulation of Vascular P2Y(2) Receptors and Increased Mitogenic Responses to UTP and ATP. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Malmsjo, M.; Hou, M.; Harden, T.K.; Pendergast, W.; Pantev, E.; Edvinsson, L.; Erlinge, D. Characterization of Contractile P2 Receptors in Human Coronary Arteries by Use of the Stable Pyrimidines Uridine 5’-O-Thiodiphosphate and Uridine 5’-O-3-Thiotriphosphate. J. Pharmacol. Exp. Ther. 2000, 293, 755–760. [Google Scholar] [PubMed]
- Wang, L.; Karlsson, L.; Moses, S.; Hultgardh-Nilsson, A.; Andersson, M.; Borna, C.; Gudbjartsson, T.; Jern, S.; Erlinge, D. P2 Receptor Expression Profiles in Human Vascular Smooth Muscle and Endothelial Cells. J. Cardiovasc. Pharmacol. 2002, 40, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, J.L.; Nachreiner, R.D.; Bhuller, A.S.; Condict, K.W.; Connors, B.A.; Herring, B.P.; Dalsing, M.C.; Unthank, J.L. Shear Level Influences Resistance Artery Remodeling: Wall Dimensions, Cell Density, and ENOS Expression. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1380–H1389. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Andersson, M.; Karlsson, L.; Watson, M.-A.; Cousens, D.J.; Jern, S.; Erlinge, D. Increased Mitogenic and Decreased Contractile P2 Receptors in Smooth Muscle Cells by Shear Stress in Human Vessels with Intact Endothelium. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1370–1376. [Google Scholar] [CrossRef]
- Hogarth, D.K.; Sandbo, N.; Taurin, S.; Kolenko, V.; Miano, J.M.; Dulin, N.O. Dual Role of PKA in Phenotypic Modulation of Vascular Smooth Muscle Cells by Extracellular ATP. Am. J. Physiol. Cell Physiol. 2004, 287, C449–C456. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.C.S.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Lamas, S.; Marsden, P.A.; Li, G.K.; Tempst, P.; Michel, T. Endothelial Nitric Oxide Synthase: Molecular Cloning and Characterization of a Distinct Constitutive Enzyme Isoform. Proc. Natl. Acad. Sci. USA 1992, 89, 6348–6352. [Google Scholar] [CrossRef]
- McAdam, B.F.; Catella-Lawson, F.; Mardini, I.A.; Kapoor, S.; Lawson, J.A.; FitzGerald, G.A. Systemic Biosynthesis of Prostacyclin by Cyclooxygenase (COX)-2: The Human Pharmacology of a Selective Inhibitor of COX-2. Proc. Natl. Acad. Sci. USA 1999, 96, 272–277. [Google Scholar] [CrossRef]
- Davenport, A.P.; Kuc, R.E.; Maguire, J.J.; Harland, S.P. ETA Receptors Predominate in the Human Vasculature and Mediate Constriction. J. Cardiovasc. Pharmacol. 1995, 26 (Suppl. 3), S265–S267. [Google Scholar] [CrossRef]
- Mantovani, A.; Bussolino, F.; Introna, M. Cytokine Regulation of Endothelial Cell Function: From Molecular Level to the Bedside. Immunol. Today 1997, 18, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Webster, N.R. Physiology of the Endothelium. Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Korenaga, R.; Kamiya, A.; Ando, J. Fluid Shear Stress Activates Ca(2+) Influx into Human Endothelial Cells via P2X4 Purinoceptors. Circ. Res. 2000, 87, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Korenaga, R.; Kamiya, A.; Qi, Z.; Sokabe, M.; Ando, J. P2X(4) Receptors Mediate ATP-Induced Calcium Influx in Human Vascular Endothelial Cells. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H285–H292. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Furuya, K.; Nakamura, M.; Kobatake, E.; Sokabe, M.; Ando, J. Visualization of Flow-Induced ATP Release and Triggering of Ca2+ Waves at Caveolae in Vascular Endothelial Cells. J. Cell Sci. 2011, 124, 3477–3483. [Google Scholar] [CrossRef] [PubMed]
- Hisadome, K.; Koyama, T.; Kimura, C.; Droogmans, G.; Ito, Y.; Oike, M. Volume-Regulated Anion Channels Serve as an Auto/Paracrine Nucleotide Release Pathway in Aortic Endothelial Cells. J. Gen. Physiol. 2002, 119, 511–520. [Google Scholar] [CrossRef]
- Mugisho, O.O.; Green, C.R.; Kho, D.T.; Zhang, J.; Graham, E.S.; Acosta, M.L.; Rupenthal, I.D. The Inflammasome Pathway Is Amplified and Perpetuated in an Autocrine Manner through Connexin43 Hemichannel Mediated ATP Release. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 385–393. [Google Scholar] [CrossRef]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial Cation Channel PIEZO1 Controls Blood Pressure by Mediating Flow-Induced ATP Release. J. Clin. Invest. 2016, 126, 4527–4536. [Google Scholar] [CrossRef]
- Schwiebert, E.M.; Zsembery, A. Extracellular ATP as a Signaling Molecule for Epithelial Cells. Biochim. Biophys. Acta Biomembr. 2003, 1615, 7–32. [Google Scholar] [CrossRef]
- Boo, Y.C.; Sorescu, G.; Boyd, N.; Shiojima, I.; Walsh, K.; Du, J.; Jo, H. Shear Stress Stimulates Phosphorylation of Endothelial Nitric-Oxide Synthase at Ser1179 by Akt-Independent Mechanisms: Role of Protein Kinase A. J. Biol. Chem. 2002, 277, 3388–3396. [Google Scholar] [CrossRef]
- Bae, S.W.; Kim, H.S.; Cha, Y.N.; Park, Y.S.; Jo, S.A.; Jo, I. Rapid Increase in Endothelial Nitric Oxide Production by Bradykinin Is Mediated by Protein Kinase A Signaling Pathway. Biochem. Biophys. Res. Commun. 2003, 306, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Grygorczyk, R.; Orlov, S.N. Effects of Hypoxia on Erythrocyte Membrane Properties—Implications for Intravascular Hemolysis and Purinergic Control of Blood Flow. Front. Physiol. 2017, 8, 1110. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, J.E.; Jayaraman, A.; Ristenpart, W.D. Centrifugation-Induced Release of ATP from Red Blood Cells. PLoS ONE 2018, 13, e0203270. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Forsyth, A.M.; Stone, H.A. Red Blood Cell Dynamics: From Cell Deformation to ATP Release. Integr. Biol. (Camb) 2011, 3, 972–981. [Google Scholar] [CrossRef]
- Melhorn, M.I.; Brodsky, A.S.; Estanislau, J.; Khoory, J.A.; Illigens, B.; Hamachi, I.; Kurishita, Y.; Fraser, A.D.; Nicholson-Weller, A.; Dolmatova, E.; et al. CR1-Mediated ATP Release by Human Red Blood Cells Promotes CR1 Clustering and Modulates the Immune Transfer Process*. J. Biol. Chem. 2013, 288, 31139–31153. [Google Scholar] [CrossRef]
- Bergfeld, G.R.; Forrester, T. Release of ATP from Human Erythrocytes in Response to a Brief Period of Hypoxia and Hypercapnia. Cardiovasc. Res. 1992, 26, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Sprague, R.S.; Ellsworth, M.L.; Stephenson, A.H.; Kleinhenz, M.E.; Lonigro, A.J. Deformation-Induced ATP Release from Red Blood Cells Requires CFTR Activity. Am. J. Physiol. 1998, 275, H1726–H1732. [Google Scholar] [CrossRef]
- Sprague, R.S.; Ellsworth, M.L.; Stephenson, A.H.; Lonigro, A.J. Participation of CAMP in a Signal-Transduction Pathway Relating Erythrocyte Deformation to ATP Release. Am. J. Physiol. Cell Physiol. 2001, 281, C1158–C1164. [Google Scholar] [CrossRef]
- Ellsworth, M.L.; Ellis, C.G.; Sprague, R.S. Role of Erythrocyte-Released ATP in the Regulation of Microvascular Oxygen Supply in Skeletal Muscle. Acta Physiol. 2016, 216, 265–276. [Google Scholar] [CrossRef]
- Sridharan, M.; Adderley, S.P.; Bowles, E.A.; Egan, T.M.; Stephenson, A.H.; Ellsworth, M.L.; Sprague, R.S. Pannexin 1 Is the Conduit for Low Oxygen Tension-Induced ATP Release from Human Erythrocytes. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1146–H1152. [Google Scholar] [CrossRef]
- Forsyth, A.M.; Wan, J.; Owrutsky, P.D.; Abkarian, M.; Stone, H.A. Multiscale Approach to Link Red Blood Cell Dynamics, Shear Viscosity, and ATP Release. Proc. Natl. Acad. Sci. USA 2011, 108, 10986–10991. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, N.; Leal Denis, M.F.; Pignataros, O.P.; Kobatake, E.; Lazarowski, E.R.; Schwarzbaum, P.J. Homeostasis of Extracellular ATP in Human Erythrocytes. J. Biol. Chem. 2011, 286, 38397–38407. [Google Scholar] [CrossRef]
- Marginedas-Freixa, I.; Alvarez, C.L.; Moras, M.; Leal Denis, M.F.; Hattab, C.; Halle, F.; Bihel, F.; Mouro-Chanteloup, I.; Lefevre, S.D.; Le Van Kim, C.; et al. Human Erythrocytes Release ATP by a Novel Pathway Involving VDAC Oligomerization Independent of Pannexin-1. Sci. Rep. 2018, 8, 11384. [Google Scholar] [CrossRef] [PubMed]
- Skals, M.; Leipziger, J.; Praetorius, H.A. Haemolysis Induced by α-Toxin from Staphylococcus Aureus Requires P2X Receptor Activation. Pflügers Arch. Eur. J. Physiol. 2011, 462, 669. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Olivecrona, G.; Gotberg, M.; Olsson, M.L.; Winzell, M.S.; Erlinge, D. ADP Acting on P2Y13 Receptors Is a Negative Feedback Pathway for ATP Release from Human Red Blood Cells. Circ. Res. 2005, 96, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, S.K.; Skals, M.; Leipziger, J.; Praetorius, H.A. P2X Receptor-Dependent Erythrocyte Damage by α-Hemolysin from Escherichia Coli Triggers Phagocytosis by THP-1 Cells. Toxins 2013, 5, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Hejl, J.L.; Skals, M.; Leipziger, J.; Praetorius, H.A. P2X Receptor Stimulation Amplifies Complement-Induced Haemolysis. Pflügers Arch. Eur. J. Physiol. 2013, 465, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Tanneur, V.; Duranton, C.; Brand, V.B.; Sandu, C.D.; Akkaya, C.; Kasinathan, R.S.; Gachet, C.; Sluyter, R.; Barden, J.A.; Wiley, J.S.; et al. Purinoceptors Are Involved in the Induction of an Osmolyte Permeability in Malaria-Infected and Oxidized Human Erythrocytes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 133–135. [Google Scholar] [CrossRef]
- Kirk, K.; Lehane, A.M. Membrane Transport in the Malaria Parasite and Its Host Erythrocyte. Biochem. J. 2014, 457, 1–18. [Google Scholar] [CrossRef]
- Sprague, R.S.; Ellsworth, M.L. Erythrocyte-Derived ATP and Perfusion Distribution: Role of Intracellular and Intercellular Communication. Microcirculation 2012, 19, 430–439. [Google Scholar] [CrossRef]
- Olearczyk, J.J.; Ellsworth, M.L.; Stephenson, A.H.; Lonigro, A.J.; Sprague, R.S. Nitric Oxide Inhibits ATP Release from Erythrocytes. J. Pharmacol. Exp. Ther. 2004, 309, 1079–1084. [Google Scholar] [CrossRef]
- Ning, B.; Chen, Y.; Waqar, A.B.; Yan, H.; Shiomi, M.; Zhang, J.; Chen, Y.E.; Wang, Y.; Itabe, H.; Liang, J.; et al. Hypertension Enhances Advanced Atherosclerosis and Induces Cardiac Death in Watanabe Heritable Hyperlipidemic Rabbits. Am. J. Pathol. 2018, 188, 2936–2947. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and Microvascular Endothelial Dysfunction in Diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Burnstock, G.; Ralevic, V. Purinergic Signaling and Blood Vessels in Health and Disease. Pharmacol. Rev. 2014, 66, 102–192. [Google Scholar] [CrossRef]
- Shatarat, A.; Dunn, W.R.; Ralevic, V. Raised Tone Reveals ATP as a Sympathetic Neurotransmitter in the Porcine Mesenteric Arterial Bed. Purinergic Signal. 2014, 10, 639–649. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, T.; Shi, P. Plasma ATP Increase Is a Biomarker of Hypertension and Triggers Low-Grade Inflammation through P2X7 Receptor. FASEB J. 2019, 33, 692.2. [Google Scholar] [CrossRef]
- Zhao, T.V.; Li, Y.; Liu, X.; Xia, S.; Shi, P.; Li, L.; Chen, Z.; Yin, C.; Eriguchi, M.; Chen, Y.; et al. ATP Release Drives Heightened Immune Responses Associated with Hypertension. Sci. Immunol. 2019, 4, eaau6426. [Google Scholar] [CrossRef] [PubMed]
- Stachon, P.; Geis, S.; Peikert, A.; Heidenreich, A.; Michel, N.A.; Ünal, F.; Hoppe, N.; Dufner, B.; Schulte, L.; Marchini, T.; et al. Extracellular ATP Induces Vascular Inflammation and Atherosclerosis via Purinergic Receptor Y2 in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Bracey, N.A.; Beck, P.L.; Muruve, D.A.; Hirota, S.A.; Guo, J.; Jabagi, H.; Wright, J.R.J.; Macdonald, J.A.; Lees-Miller, J.P.; Roach, D.; et al. The Nlrp3 Inflammasome Promotes Myocardial Dysfunction in Structural Cardiomyopathy through Interleukin-1β. Exp. Physiol. 2013, 98, 462–472. [Google Scholar] [CrossRef]
- Stachon, P.; Heidenreich, A.; Merz, J.; Hilgendorf, I.; Wolf, D.; Willecke, F.; von Garlen, S.; Albrecht, P.; Härdtner, C.; Ehrat, N.; et al. P2X(7) Deficiency Blocks Lesional Inflammasome Activity and Ameliorates Atherosclerosis in Mice. Circulation 2017, 135, 2524–2533. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-Activated Gasdermin D Causes Pyroptosis by Forming Membrane Pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Goonetilleke, L.; Ralevic, V.; Dunn, W.R. Influence of Pressure on Adenosine Triphosphate Function as a Sympathetic Neurotransmitter in Small Mesenteric Arteries from the Spontaneously Hypertensive Rat. J. Hypertens. 2013, 31, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Mark, A.; Esler, M. The Sympathetic Nervous System Alterations in Human Hypertension. Circ. Res. 2015, 116, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Paton, J.F.R. The Sympathetic Nervous System and Blood Pressure in Humans: Implications for Hypertension. J. Hum. Hypertens. 2012, 26, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, S.; Hoggatt, A.; Tang, H.; Hacker, T.A.; Obukhov, A.G.; Herring, P.B.; Seye, C.I. Endothelial Cell-Specific Deletion of P2Y2 Receptor Promotes Plaque Stability in Atherosclerosis-Susceptible ApoE-Null Mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sokabe, T.; Matsumoto, T.; Yoshimura, K.; Shibata, M.; Ohura, N.; Fukuda, T.; Sato, T.; Sekine, K.; Kato, S.; et al. Impaired Flow-Dependent Control of Vascular Tone and Remodeling in P2X4-Deficient Mice. Nat. Med. 2006, 12, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Buvinic, S.; Briones, R.; Huidobro-Toro, J.P. P2Y(1) and P2Y(2) Receptors Are Coupled to the NO/CGMP Pathway to Vasodilate the Rat Arterial Mesenteric Bed. Br. J. Pharmacol. 2002, 136, 847–856. [Google Scholar] [CrossRef]
- Raqeeb, A.; Sheng, J.; Ao, N.; Braun, A.P. Purinergic P2Y2 Receptors Mediate Rapid Ca(2+) Mobilization, Membrane Hyperpolarization and Nitric Oxide Production in Human Vascular Endothelial Cells. Cell Calcium 2011, 49, 240–248. [Google Scholar] [CrossRef]
- Seye, C.I.; Yu, N.; Jain, R.; Kong, Q.; Minor, T.; Newton, J.; Erb, L.; Gonzalez, F.A.; Weisman, G.A. The P2Y2 Nucleotide Receptor Mediates UTP-Induced Vascular Cell Adhesion Molecule-1 Expression in Coronary Artery Endothelial Cells. J. Biol. Chem. 2003, 278, 24960–24965. [Google Scholar] [CrossRef]
- Cao, G.; Yang, G.; Timme, T.L.; Saika, T.; Truong, L.D.; Satoh, T.; Goltsov, A.; Park, S.H.; Men, T.; Kusaka, N.; et al. Disruption of the Caveolin-1 Gene Impairs Renal Calcium Reabsorption and Leads to Hypercalciuria and Urolithiasis. Am. J. Pathol. 2003, 162, 1241–1248. [Google Scholar] [CrossRef]
- Burnstock, G. Control of Vascular Tone by Purines and Pyrimidines. Br. J. Pharmacol. 2010, 161, 527–529. [Google Scholar] [CrossRef]
- Inscho, E.W.; Cook, A.K.; Clarke, A.; Zhang, S.; Guan, Z. P2X1 Receptor-Mediated Vasoconstriction of Afferent Arterioles in Angiotensin II-Infused Hypertensive Rats Fed a High-Salt Diet. Hypertension 2011, 57, 780–787. [Google Scholar] [CrossRef]
- Govindan, S.; Taylor, E.J.A.; Taylor, C.W. Ca(2+) Signalling by P2Y Receptors in Cultured Rat Aortic Smooth Muscle Cells. Br. J. Pharmacol. 2010, 160, 1953–1962. [Google Scholar] [CrossRef]
- Malmsjö, M.; Edvinsson, L.; Erlinge, D. P2X Receptors Counteract the Vasodilatory Effects of Endothelium Derived Hyperpolarising Factor. Eur. J. Pharmacol. 2000, 390, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Harhun, M.I.; Povstyan, O.V.; Albert, A.P.; Nichols, C.M. ATP-Evoked Sustained Vasoconstrictions Mediated by Heteromeric P2X1/4 Receptors in Cerebral Arteries. Stroke 2014, 45, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Erlinge, D. Extracellular ATP: A Growth Factor for Vascular Smooth Muscle Cells. Gen. Pharmacol. 1998, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Sunggip, C.; Tozaki-Saitoh, H.; Shimauchi, T.; Numaga-Tomita, T.; Hirano, K.; Ide, T.; Boeynaems, J.-M.; Kurose, H.; Tsuda, M.; et al. Purinergic P2Y6 Receptors Heterodimerize with Angiotensin AT1 Receptors to Promote Angiotensin II-Induced Hypertension. Sci. Signal. 2016, 9, ra7. [Google Scholar] [CrossRef] [PubMed]
- Chaulet, H.; Desgranges, C.; Renault, M.A.; Dupuch, F.; Ezan, G.; Peiretti, F.; Loirand, G.; Pacaud, P.; Gadeau, A.P. Extracellular Nucleotides Induce Arterial Smooth Muscle Cell Migration via Osteopontin. Circ. Res. 2001, 89, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Agca, Y.; Qian, S.; Agca, C.; Seye, C.I. Direct Evidence for P2Y2 Receptor Involvement in Vascular Response to Injury. J. Vasc. Res. 2016, 53, 163–171. [Google Scholar] [CrossRef]
- Guieu, R.; Brignole, M.; Deharo, J.C.; Deharo, P.; Mottola, G.; Groppelli, A.; Paganelli, F.; Ruf, J. Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases? Int. J. Mol. Sci. 2021, 22, 7584. [Google Scholar] [CrossRef]
- Gaudry, M.; Vairo, D.; Marlinge, M.; Gaubert, M.; Guiol, C.; Mottola, G.; Gariboldi, V.; Deharo, P.; Sadrin, S.; Maixent, J.M.; et al. Adenosine and Its Receptors: An Expected Tool for the Diagnosis and Treatment of Coronary Artery and Ischemic Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5321. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Sabirov, R.Z.; Uramoto, H.; Okada, Y. Role of ATP-Conductive Anion Channel in ATP Release from Neonatal Rat Cardiomyocytes in Ischaemic or Hypoxic Conditions. J. Physiol. 2004, 559, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yang, X.-J.; Jiang, T.-B.; Chen, Y. Ischemia Triggered ATP Release through Pannexin-1 Channel by Myocardial Cells Activates Sympathetic Fibers. Microvasc. Res. 2016, 104, 32–37. [Google Scholar] [CrossRef]
- Cinar, E.; Zhou, S.; DeCourcey, J.; Wang, Y.; Waugh, R.E.; Wan, J. Piezo1 Regulates Mechanotransductive Release of ATP from Human RBCs. Proc. Natl. Acad. Sci. USA 2015, 112, 11783–11788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Charles, E.J.; Zhao, Y.; Narahari, A.K.; Baderdinni, P.K.; Good, M.E.; Lorenz, U.M.; Kron, I.L.; Bayliss, D.A.; Ravichandran, K.S.; et al. Pannexin-1 Channels on Endothelial Cells Mediate Vascular Inflammation during Lung Ischemia-Reperfusion Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L301–L312. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T.; Mager, A.; Kuper, N.; Karcher, C.; Weissmuller, T.; Boengler, K.; Schulz, R.; Robson, S.C.; Colgan, S.P. ATP Release from Activated Neutrophils Occurs via Connexin 43 and Modulates Adenosine-Dependent Endothelial Cell Function. Circ. Res. 2006, 99, 1100–1108. [Google Scholar] [CrossRef]
- Dolmatova, E.; Spagnol, G.; Boassa, D.; Baum, J.R.; Keith, K.; Ambrosi, C.; Kontaridis, M.I.; Sorgen, P.L.; Sosinsky, G.E.; Duffy, H.S. Cardiomyocyte ATP Release through Pannexin 1 Aids in Early Fibroblast Activation. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1208–H1218. [Google Scholar] [CrossRef]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.M.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular Danger Signals Guide Neutrophils to Sites of Sterile Inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef]
- Riegel, A.-K.; Faigle, M.; Zug, S.; Rosenberger, P.; Robaye, B.; Boeynaems, J.-M.; Idzko, M.; Eltzschig, H.K. Selective Induction of Endothelial P2Y6 Nucleotide Receptor Promotes Vascular Inflammation. Blood 2011, 117, 2548–2555. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Macmanus, C.F.; Colgan, S.P. Neutrophils as Sources of Extracellular Nucleotides: Functional Consequences at the Vascular Interface. Trends Cardiovasc. Med. 2008, 18, 103–107. [Google Scholar] [CrossRef]
- Hou, M.; Malmsjo, M.; Moller, S.; Pantev, E.; Bergdahl, A.; Zhao, X.H.; Sun, X.Y.; Hedner, T.; Edvinsson, L.; Erlinge, D. Increase in Cardiac P2X1-and P2Y2-Receptor MRNA Levels in Congestive Heart Failure. Life Sci. 1999, 65, 1195–1206. [Google Scholar] [CrossRef]
- Hochhauser, E.; Cohen, R.; Waldman, M.; Maksin, A.; Isak, A.; Aravot, D.; Jayasekara, P.S.; Muller, C.E.; Jacobson, K.A.; Shainberg, A. P2Y2 Receptor Agonist with Enhanced Stability Protects the Heart from Ischemic Damage in Vitro and in Vivo. Purinergic Signal. 2013, 9, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, S.B.; Skovsted, G.F.; Berchtold, L.A.; Radziwon-Balicka, A.; Dreisig, K.; Edvinsson, L.; Sheykhzade, M.; Haanes, K.A. Role of Pannexin and Adenosine Triphosphate (ATP) Following Myocardial Ischemia/Reperfusion. Scand. Cardiovasc. J. 2018, 52, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Shainberg, A.; Hochhauser, E.; Cheporko, Y.; Tobar, A.; Birk, E.; Pinhas, L.; Leipziger, J.; Don, J.; Porat, E. UTP Reduces Infarct Size and Improves Mice Heart Function after Myocardial Infarct via P2Y2 Receptor. Biochem. Pharmacol. 2011, 82, 1126–1133. [Google Scholar] [CrossRef]
- Walkowski, B.; Kleibert, M.; Majka, M.; Wojciechowska, M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells 2022, 11, 1553. [Google Scholar] [CrossRef]
- Vessey, D.A.; Li, L.; Kelley, M. P2X7 Receptor Agonists Pre- and Postcondition the Heart against Ischemia-Reperfusion Injury by Opening Pannexin-1/P2X(7) Channels. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H881–H887. [Google Scholar] [CrossRef]
- Matsuura, H.; Kojima, A.; Fukushima, Y.; Xie, Y.; Mi, X.; Sabirov, R.Z.; Okada, Y. Positive Inotropic Effects of ATP Released via the Maxi-Anion Channel in Langendorff-Perfused Mouse Hearts Subjected to Ischemia-Reperfusion. Front. Cell Dev. Biol. 2021, 9, 597997. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Role of G-Protein-Coupled Adenosine Receptors in Downregulation of Inflammation and Protection from Tissue Damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef]
- Thompson, L.F.; Eltzschig, H.K.; Ibla, J.C.; Van De Wiele, C.J.; Resta, R.; Morote-Garcia, J.C.; Colgan, S.P. Crucial Role for Ecto-5’-Nucleotidase (CD73) in Vascular Leakage during Hypoxia. J. Exp. Med. 2004, 200, 1395–1405. [Google Scholar] [CrossRef]
- Eckle, T.; Faigle, M.; Grenz, A.; Laucher, S.; Thompson, L.F.; Eltzschig, H.K. A2B Adenosine Receptor Dampens Hypoxia-Induced Vascular Leak. Blood 2008, 111, 2024–2035. [Google Scholar] [CrossRef]
- Eckle, T.; Krahn, T.; Grenz, A.; Kohler, D.; Mittelbronn, M.; Ledent, C.; Jacobson, M.A.; Osswald, H.; Thompson, L.F.; Unertl, K.; et al. Cardioprotection by Ecto-5’-Nucleotidase (CD73) and A2B Adenosine Receptors. Circulation 2007, 115, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Kohler, D.; Eckle, T.; Faigle, M.; Grenz, A.; Mittelbronn, M.; Laucher, S.; Hart, M.L.; Robson, S.C.; Muller, C.E.; Eltzschig, H.K. CD39/Ectonucleoside Triphosphate Diphosphohydrolase 1 Provides Myocardial Protection during Cardiac Ischemia/Reperfusion Injury. Circulation 2007, 116, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Djerada, Z.; Feliu, C.; Richard, V.; Millart, H. Current Knowledge on the Role of P2Y Receptors in Cardioprotection against Ischemia-Reperfusion. Pharmacol. Res. 2017, 118, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Banfi, C.; Burbiel, J.C.; Luo, H.; Tremoli, E.; Abbracchio, M.P. Cardiomyocyte Death Induced by Ischaemic/Hypoxic Stress Is Differentially Affected by Distinct Purinergic P2 Receptors. J. Cell. Mol. Med. 2012, 16, 1074–1084. [Google Scholar] [CrossRef]
- Hemanthakumar, K.A.; Kivelä, R. Angiogenesis and Angiocrines Regulating Heart Growth. Vasc. Biol. (Bristol, England) 2020, 2, R93–R104. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Akazawa, H.; Naito, A.T.; Komuro, I. Angiogenesis and Cardiac Hypertrophy. Circ. Res. 2014, 114, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Shiojima, I.; Sato, K.; Izumiya, Y.; Schiekofer, S.; Ito, M.; Liao, R.; Colucci, W.S.; Walsh, K. Disruption of Coordinated Cardiac Hypertrophy and Angiogenesis Contributes to the Transition to Heart Failure. J. Clin. Invest. 2005, 115, 2108–2118. [Google Scholar] [CrossRef]
- Phung, T.L.; Ziv, K.; Dabydeen, D.; Eyiah-Mensah, G.; Riveros, M.; Perruzzi, C.; Sun, J.; Monahan-Earley, R.A.; Shiojima, I.; Nagy, J.A.; et al. Pathological Angiogenesis Is Induced by Sustained Akt Signaling and Inhibited by Rapamycin. Cancer Cell 2006, 10, 159–170. [Google Scholar] [CrossRef]
- Narmoneva, D.A.; Vukmirovic, R.; Davis, M.E.; Kamm, R.D.; Lee, R.T. Endothelial Cells Promote Cardiac Myocyte Survival and Spatial Reorganization: Implications for Cardiac Regeneration. Circulation 2004, 110, 962–968. [Google Scholar] [CrossRef]
- Luxán, G.; Dimmeler, S. The Vasculature: A Therapeutic Target in Heart Failure? Cardiovasc. Res. 2022, 118, 53–64. [Google Scholar] [CrossRef]
- Seye, C.I.; Yu, N.; Gonzalez, F.A.; Erb, L.; Weisman, G.A. The P2Y2 Nucleotide Receptor Mediates Vascular Cell Adhesion Molecule-1 Expression through Interaction with VEGF Receptor-2 (KDR/Flk-1). J. Biol. Chem. 2004, 279, 35679–35686. [Google Scholar] [CrossRef]
- Węgłowska, E.; Koziołkiewicz, M.; Kamińska, D.; Grobelski, B.; Pawełczak, D.; Kołodziejczyk, M.; Bielecki, S.; Gendaszewska-Darmach, E. Extracellular Nucleotides Affect the Proangiogenic Behavior of Fibroblasts, Keratinocytes, and Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 238. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, P.; Eccles, S.A.; Yaqoob, M.M. Coupling between the TRPC3 Ion Channel and the NCX1 Transporter Contributed to VEGF-Induced ERK1/2 Activation and Angiogenesis in Human Primary Endothelial Cells. Cell. Signal. 2017, 37, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cubbon, R.M.; Wilson, L.A.; Amer, M.S.; McKeown, L.; Hou, B.; Majeed, Y.; Tumova, S.; Seymour, V.A.L.; Taylor, H.; et al. Orai1 and CRAC Channel Dependence of VEGF-Activated Ca2+ Entry and Endothelial Tube Formation. Circ. Res. 2011, 108, 1190–1198. [Google Scholar] [CrossRef]
- Gerasimovskaya, E.V.; Woodward, H.N.; Tucker, D.A.; Stenmark, K.R. Extracellular ATP Is a Pro-Angiogenic Factor for Pulmonary Artery Vasa Vasorum Endothelial Cells. Angiogenesis 2008, 11, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Mühleder, S.; Fuchs, C.; Basílio, J.; Szwarc, D.; Pill, K.; Labuda, K.; Slezak, P.; Siehs, C.; Pröll, J.; Priglinger, E.; et al. Purinergic P2Y(2) Receptors Modulate Endothelial Sprouting. Cell. Mol. Life Sci. 2020, 77, 885–901. [Google Scholar] [CrossRef]
- Gast, R.E.; König, S.; Rose, K.; Ferenz, K.B.; Krieglstein, J. Binding of ATP to Vascular Endothelial Growth Factor Isoform VEGF-A165 Is Essential for Inducing Proliferation of Human Umbilical Vein Endothelial Cells. BMC Biochem. 2011, 12, 28. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Yu, Y.-Q.; Yang, H.; Yang, H.; Huo, Y.-F.; Huang, Y.; Tian, X.-X.; Fang, W.-G. Extracellular ATP Promotes Angiogenesis and Adhesion of TNBC Cells to Endothelial Cells via Upregulation of CTGF. Cancer Sci. 2022, 113, 2457–2471. [Google Scholar] [CrossRef]
- Rumjahn, S.M.; Yokdang, N.; Baldwin, K.A.; Thai, J.; Buxton, I.L.O. Purinergic Regulation of Vascular Endothelial Growth Factor Signaling in Angiogenesis. Br. J. Cancer 2009, 100, 1465–1470. [Google Scholar] [CrossRef]
- Hill, L.M.; Gavala, M.L.; Lenertz, L.Y.; Bertics, P.J. Extracellular ATP May Contribute to Tissue Repair by Rapidly Stimulating Purinergic Receptor X7-Dependent Vascular Endothelial Growth Factor Release from Primary Human Monocytes. J. Immunol. 2010, 185, 3028–3034. [Google Scholar] [CrossRef]
- Gidlof, O.; Sathanoori, R.; Magistri, M.; Faghihi, M.A.; Wahlestedt, C.; Olde, B.; Erlinge, D. Extracellular Uridine Triphosphate and Adenosine Triphosphate Attenuate Endothelial Inflammation through MiR-22-Mediated ICAM-1 Inhibition. J. Vasc. Res. 2015, 52, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gjorgjieva, M.; Ay, A.-S.; Correia de Sousa, M.; Delangre, E.; Dolicka, D.; Sobolewski, C.; Maeder, C.; Fournier, M.; Sempoux, C.; Foti, M. MiR-22 Deficiency Fosters Hepatocellular Carcinoma Development in Fatty Liver. Cells 2022, 11, 2860. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac Fibrosis: Cell Biological Mechanisms, Molecular Pathways and Therapeutic Opportunities. Mol. Aspects Med. 2019, 65, 70–99. [Google Scholar] [CrossRef] [PubMed]
- Bazhutina, A.; Balakina-Vikulova, N.A.; Kursanov, A.; Solovyova, O.; Panfilov, A.; Katsnelson, L.B. Mathematical Modelling of the Mechano-Electric Coupling in the Human Cardiomyocyte Electrically Connected with Fibroblasts. Prog. Biophys. Mol. Biol. 2021, 159, 46–57. [Google Scholar] [CrossRef]
- Brocklehurst, P.; Zhang, H.; Ye, J. Effects of Fibroblast on Electromechanical Dynamics of Human Atrial Tissue-Insights from a 2D Discrete Element Model. Front. Physiol. 2022, 13, 938497. [Google Scholar] [CrossRef]
- Baum, J.; Duffy, H.S. Fibroblasts and Myofibroblasts: What Are We Talking About? J. Cardiovasc. Pharmacol. 2011, 57, 376–379. [Google Scholar] [CrossRef]
- Erb, L.; Liao, Z.; Seye, C.I.; Weisman, G.A. P2 Receptors: Intracellular Signaling. Pflugers Arch. 2006, 452, 552–562. [Google Scholar] [CrossRef]
- Kitajima, N.; Watanabe, K.; Morimoto, S.; Sato, Y.; Kiyonaka, S.; Hoshijima, M.; Ikeda, Y.; Nakaya, M.; Ide, T.; Mori, Y.; et al. TRPC3-Mediated Ca2+ Influx Contributes to Rac1-Mediated Production of Reactive Oxygen Species in MLP-Deficient Mouse Hearts. Biochem. Biophys. Res. Commun. 2011, 409, 108–113. [Google Scholar] [CrossRef]
- Kitajima, N.; Numaga-tomita, T.; Watanabe, M.; Kuroda, T.; Nishimura, A.; Miyano, K.; Yasuda, S.; Kuwahara, K.; Sato, Y.; Ide, T.; et al. TRPC3 Positively Regulates Reactive Oxygen Species Driving Maladaptive Cardiac Remodeling. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Numaga-Tomita, T.; Kitajima, N.; Kuroda, T.; Nishimura, A.; Miyano, K.; Yasuda, S.; Kuwahara, K.; Sato, Y.; Ide, T.; Birnbaumer, L.; et al. TRPC3-GEF-H1 Axis Mediates Pressure Overload-Induced Cardiac Fibrosis. Sci. Rep. 2016, 6, 39383. [Google Scholar] [CrossRef]
- Lassegue, B.; San Martin, A.; Griendling, K.K. Biochemistry, Physiology, and Pathophysiology of NADPH Oxidases in the Cardiovascular System. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef]
- Looi, Y.H.; Grieve, D.J.; Siva, A.; Walker, S.J.; Anilkumar, N.; Cave, A.C.; Marber, M.; Monaghan, M.J.; Shah, A.M. Involvement of Nox2 NADPH Oxidase in Adverse Cardiac Remodeling after Myocardial Infarction. Hypertension 2008, 51, 319–325. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Walker, S.J.; Dworakowski, R.; Lakatta, E.G.; Shah, A.M. Involvement of NADPH Oxidase in Age-Associated Cardiac Remodeling. J. Mol. Cell. Cardiol. 2010, 48, 765–772. [Google Scholar] [CrossRef]
- Noubade, R.; Wong, K.; Ota, N.; Rutz, S.; Eidenschenk, C.; Valdez, P.A.; Ding, J.; Peng, I.; Sebrell, A.; Caplazi, P.; et al. NRROS Negatively Regulates Reactive Oxygen Species during Host Defence and Autoimmunity. Nature 2014, 509, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Shimauchi, T.; Numaga-Tomita, T.; Ito, T.; Nishimura, A.; Matsukane, R.; Oda, S.; Hoka, S.; Ide, T.; Koitabashi, N.; Uchida, K.; et al. TRPC3-Nox2 Complex Mediates Doxorubicin-Induced Myocardial Atrophy. JCI Insight 2017, 2, e93358. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Armillei, M.K.; Yu, A.S.; Liang, B.T.; Runnels, L.W.; Yue, L. Ca(2+) Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases. J. Cardiovasc. Dev. Dis. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Park, H.; Minami, T. Calcineurin-Nuclear Factor for Activated T Cells (NFAT) Signaling in Pathophysiology of Wound Healing. Inflamm. Regen. 2021, 41, 26. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Kurahara, L.-H.; Hiraishi, K. TRP Channels in Cardiac and Intestinal Fibrosis. Semin. Cell Dev. Biol. 2019, 94, 40–49. [Google Scholar] [CrossRef]
- Harada, M.; Luo, X.; Qi, X.Y.; Tadevosyan, A.; Maguy, A.; Ordog, B.; Ledoux, J.; Kato, T.; Naud, P.; Voigt, N.; et al. Transient Receptor Potential Canonical-3 Channel-Dependent Fibroblast Regulation in Atrial Fibrillation. Circulation 2012, 126, 2051–2064. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, G.; Quan, Y.; Li, J.; Wang, X.; Wu, W.; Li, M.; Liu, X. Inhibition of P2X7 Purinergic Receptor Ameliorates Cardiac Fibrosis by Suppressing NLRP3/IL-1β Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 7956274. [Google Scholar] [CrossRef]
- Tian, G.; Zhou, J.; Quan, Y.; Kong, Q.; Wu, W.; Liu, X. P2Y1 Receptor Agonist Attenuates Cardiac Fibroblasts Activation Triggered by TGF-Β1. Front. Pharmacol. 2021, 12, 627773. [Google Scholar] [CrossRef]
- Franke, H.; Sauer, C.; Rudolph, C.; Krügel, U.; Hengstler, J.G.; Illes, P. P2 Receptor-Mediated Stimulation of the PI3-K/Akt-Pathway in Vivo. Glia 2009, 57, 1031–1045. [Google Scholar] [CrossRef]
- D’Andrea, A.; La Gerche, A.; Golia, E.; Teske, A.J.; Bossone, E.; Russo, M.G.; Calabro, R.; Baggish, A.L. Right Heart Structural and Functional Remodeling in Athletes. Echocardiography 2015, 32 (Suppl. 1), S11–S22. [Google Scholar] [CrossRef]
- Harvey, P.A.; Leinwand, L.A. Cardiac Atrophy and Remodeling. Cell. Mol. Pathobiol. Cardiovasc. Dis. 2014, 3, 37–50. [Google Scholar] [CrossRef]
- Musolino, V.; Palus, S.; Tschirner, A.; Drescher, C.; Gliozzi, M.; Carresi, C.; Vitale, C.; Muscoli, C.; Doehner, W.; von Haehling, S.; et al. Megestrol Acetate Improves Cardiac Function in a Model of Cancer Cachexia-Induced Cardiomyopathy by Autophagic Modulation. J. Cachexia. Sarcopenia Muscle 2016, 7, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Weeks, K.L.; Gao, X.; Du, X.-J.; Boey, E.J.H.; Matsumoto, A.; Bernardo, B.C.; Kiriazis, H.; Cemerlang, N.; Tan, J.W.; Tham, Y.K.; et al. Phosphoinositide 3-Kinase P110alpha Is a Master Regulator of Exercise-Induced Cardioprotection and PI3K Gene Therapy Rescues Cardiac Dysfunction. Circ. Heart Fail. 2012, 5, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Onohara, N.; Nishida, M.; Inoue, R.; Kobayashi, H.; Sumimoto, H.; Sato, Y.; Mori, Y.; Nagao, T.; Kurose, H. TRPC3 and TRPC6 Are Essential for Angiotensin II-Induced Cardiac Hypertrophy. EMBO J. 2006, 25, 5305–5316. [Google Scholar] [CrossRef]
- Wettschureck, N.; Rutten, H.; Zywietz, A.; Gehring, D.; Wilkie, T.M.; Chen, J.; Chien, K.R.; Offermanns, S. Absence of Pressure Overload Induced Myocardial Hypertrophy after Conditional Inactivation of Galphaq/Galpha11 in Cardiomyocytes. Nat. Med. 2001, 7, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Post, G.R.; Goldstein, D.; Thuerauf, D.J.; Glembotski, C.C.; Brown, J.H. Dissociation of P44 and P42 Mitogen-Activated Protein Kinase Activation from Receptor-Induced Hypertrophy in Neonatal Rat Ventricular Myocytes. J. Biol. Chem. 1996, 271, 8452–8457. [Google Scholar] [CrossRef]
- Zheng, J.S.; Boluyt, M.O.; O’Neill, L.; Crow, M.T.; Lakatta, E.G. Extracellular ATP Induces Immediate-Early Gene Expression but Not Cellular Hypertrophy in Neonatal Cardiac Myocytes. Circ. Res. 1994, 74, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Sunggip, C.; Shimoda, K.; Oda, S.; Tanaka, T.; Giles, W.R.; Nishiyama, K.; Mangmool, S.; Nishimura, A.; Numaga-Tomita, T.; Nishida, M. TRPC5-ENOS Axis Negatively Regulates ATP-Induced Cardiomyocyte Hypertrophy. Front. Pharmacol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Yang, T.; Shen, J.; Yang, R.; Redden, J.; Dodge-Kafka, K.; Grady, J.; Jacobson, K.A.; Liang, B.T. Novel Protective Role of Endogenous Cardiac Myocyte P2X4 Receptors in Heart Failure. Circ. Hear. Fail. 2014, 7, 510–518. [Google Scholar] [CrossRef]
- Nakayama, H.; Wilkin, B.J.; Bodi, I.; Molkentin, J.D. Calcineurin-Dependent Cardiomyopathy Is Activated by TRPC in the Adult Mouse Heart. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Sudi, S.B.; Tanaka, T.; Oda, S.; Nishiyama, K.; Nishimura, A.; Sunggip, C.; Mangmool, S.; Numaga-Tomita, T.; Nishida, M. TRPC3-Nox2 Axis Mediates Nutritional Deficiency-Induced Cardiomyocyte Atrophy. Sci. Rep. 2019, 9, 9785. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Gibson, S.B. Starvation-Induced Autophagy Is Regulated by Mitochondrial Reactive Oxygen Species Leading to AMPK Activation. Cell. Signal. 2013, 25, 50–65. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, H.-X.; Guo, S.-B.; Yang, H.; Zeng, X.-J.; Fang, Q.; Tang, C.-S.; Du, J.; Li, H.-H. Transcriptional Effects of E3 Ligase Atrogin-1/MAFbx on Apoptosis, Hypertrophy and Inflammation in Neonatal Rat Cardiomyocytes. PLoS ONE 2013, 8, e53831. [Google Scholar] [CrossRef]
- Ohman, J.; Kudira, R.; Albinsson, S.; Olde, B.; Erlinge, D. Ticagrelor Induces Adenosine Triphosphate Release from Human Red Blood Cells. Biochem. Biophys. Res. Commun. 2012, 418, 754–758. [Google Scholar] [CrossRef]
- van Giezen, J.J.J.; Sidaway, J.; Glaves, P.; Kirk, I.; Björkman, J.-A. Ticagrelor Inhibits Adenosine Uptake in Vitro and Enhances Adenosine-Mediated Hyperemia Responses in a Canine Model. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 164–172. [Google Scholar] [CrossRef]
- Nylander, S.; Femia, E.A.; Scavone, M.; Berntsson, P.; Asztély, A.-K.; Nelander, K.; Löfgren, L.; Nilsson, R.G.; Cattaneo, M. Ticagrelor Inhibits Human Platelet Aggregation via Adenosine in Addition to P2Y12 Antagonism. J. Thromb. Haemost. 2013, 11, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease Developed in Collaboration with EACTS. Eur. J. cardio-thoracic Surg. Off. J. Eur. Assoc. Cardio-thoracic Surg. 2018, 53, 34–78. [Google Scholar] [CrossRef]
- Grzesk, G.; Kozinski, M.; Navarese, E.P.; Krzyzanowski, M.; Grzesk, E.; Kubica, A.; Siller-Matula, J.M.; Castriota, F.; Kubica, J. Ticagrelor, but Not Clopidogrel and Prasugrel, Prevents ADP-Induced Vascular Smooth Muscle Cell Contraction: A Placebo-Controlled Study in Rats. Thromb. Res. 2012, 130, 65–69. [Google Scholar] [CrossRef]
- Thomas, M.R.; Storey, R.F. Impact of Aspirin Dosing on the Effects of P2Y12 Inhibition in Patients with Acute Coronary Syndromes. J. Cardiovasc. Transl. Res. 2014, 7, 19–28. [Google Scholar] [CrossRef]
- Grosser, T.; Fries, S.; FitzGerald, G.A. Biological Basis for the Cardiovascular Consequences of COX-2 Inhibition: Therapeutic Challenges and Opportunities. J. Clin. Invest. 2006, 116, 4–15. [Google Scholar] [CrossRef]
- Cattaneo, M.; Lecchi, A. Inhibition of the Platelet P2Y12 Receptor for Adenosine Diphosphate Potentiates the Antiplatelet Effect of Prostacyclin. J. Thromb. Haemost. 2007, 5, 577–582. [Google Scholar] [CrossRef]
- Grześk, G.; Kozinski, M.; Tantry, U.S.; Wicinski, M.; Fabiszak, T.; Navarese, E.P.; Grzesk, E.; Jeong, Y.-H.; Gurbel, P.A.; Kubica, J. High-Dose, but Not Low-Dose, Aspirin Impairs Anticontractile Effect of Ticagrelor Following ADP Stimulation in Rat Tail Artery Smooth Muscle Cells. Biomed Res. Int. 2013, 2013, 928271. [Google Scholar] [CrossRef]
- Kunugi, S.; Iwabuchi, S.; Matsuyama, D.; Okajima, T.; Kawahara, K. Negative-Feedback Regulation of ATP Release: ATP Release from Cardiomyocytes Is Strictly Regulated during Ischemia. Biochem. Biophys. Res. Commun. 2011, 416, 409–415. [Google Scholar] [CrossRef]
- Crassous, P.-A.; Shu, P.; Huang, C.; Gordan, R.; Brouckaert, P.; Lampe, P.D.; Xie, L.-H.; Beuve, A. Newly Identified NO-Sensor Guanylyl Cyclase/Connexin 43 Association Is Involved in Cardiac Electrical Function. J. Am. Heart Assoc. 2017, 6, e006397. [Google Scholar] [CrossRef]
- Rong, B.; Xie, F.; Sun, T.; Hao, L.; Lin, M.-J.; Zhong, J.-Q. Nitric Oxide, PKC-ε, and Connexin43 Are Crucial for Ischemic Preconditioning-Induced Chemical Gap Junction Uncoupling. Oncotarget 2016, 7, 69243–69255. [Google Scholar] [CrossRef]
- Pogoda, K.; Kameritsch, P.; Retamal, M.A.; Vega, J.L. Regulation of Gap Junction Channels and Hemichannels by Phosphorylation and Redox Changes: A Revision. BMC Cell Biol. 2016, 17 (Suppl. 1), 11. [Google Scholar] [CrossRef]
- Wang, N.; De Vuyst, E.; Ponsaerts, R.; Boengler, K.; Palacios-Prado, N.; Wauman, J.; Lai, C.P.; De Bock, M.; Decrock, E.; Bol, M.; et al. Selective Inhibition of Cx43 Hemichannels by Gap19 and Its Impact on Myocardial Ischemia/Reperfusion Injury. Basic Res. Cardiol. 2013, 108, 309. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Zhang, L.; Chen, B.; Yang, L.; Li, X.; Li, Y.; Yu, H. Inhibition of Connexin 43 Hemichannels Alleviates Cerebral Ischemia/Reperfusion Injury via the TLR4 Signaling Pathway. Front. Cell. Neurosci. 2018, 12, 372. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Huang, S.; Chen, W.; Mao, H.; Ouyang, X.; Chen, L.; Li, L. Apelin-13/APJ Induces Cardiomyocyte Hypertrophy by Activating the Pannexin-1/P2X7 Axis and FAM134B-Dependent Reticulophagy. J. Cell. Physiol. 2022, 237, 2230–2248. [Google Scholar] [CrossRef]
- Liu, J.; Prell, T.; Stubendorff, B.; Keiner, S.; Ringer, T.; Gunkel, A.; Tadic, V.; Goldhammer, N.; Malci, A.; Witte, O.W.; et al. Down-Regulation of Purinergic P2X7 Receptor Expression and Intracellular Calcium Dysregulation in Peripheral Blood Mononuclear Cells of Patients with Amyotrophic Lateral Sclerosis. Neurosci. Lett. 2016, 630, 77–83. [Google Scholar] [CrossRef]
- Stock, T.C.; Bloom, B.J.; Wei, N.; Ishaq, S.; Park, W.; Wang, X.; Gupta, P.; Mebus, C.A. Efficacy and Safety of CE-224,535, an Antagonist of P2X7 Receptor, in Treatment of Patients with Rheumatoid Arthritis Inadequately Controlled by Methotrexate. J. Rheumatol. 2012, 39, 720–727. [Google Scholar] [CrossRef]
- Keystone, E.C.; Wang, M.M.; Layton, M.; Hollis, S.; McInnes, I.B. Clinical Evaluation of the Efficacy of the P2X7 Purinergic Receptor Antagonist AZD9056 on the Signs and Symptoms of Rheumatoid Arthritis in Patients with Active Disease despite Treatment with Methotrexate or Sulphasalazine. Ann. Rheum. Dis. 2012, 71, 1630–1635. [Google Scholar] [CrossRef]
- Hansen, T.; Karimi Galougahi, K.; Besnier, M.; Genetzakis, E.; Tsang, M.; Finemore, M.; O’Brien-Brown, J.; Di Bartolo, B.A.; Kassiou, M.; Bubb, K.J.; et al. The Novel P2X7 Receptor Antagonist PKT100 Improves Cardiac Function and Survival in Pulmonary Hypertension by Direct Targeting of the Right Ventricle. Am. J. Physiol. Circ. Physiol. 2020, 319, H183–H191. [Google Scholar] [CrossRef]
- Yang, R.; Beqiri, D.; Shen, J.-B.; Redden, J.M.; Dodge-Kafka, K.; Jacobson, K.A.; Liang, B.T. P2X4 Receptor-ENOS Signaling Pathway in Cardiac Myocytes as a Novel Protective Mechanism in Heart Failure. Comput. Struct. Biotechnol. J. 2015, 13, 1–7. [Google Scholar] [CrossRef]
- Jiang, L.; Bardini, M.; Keogh, A.; dos Remedios, C.G.; Burnstock, G. P2X1 Receptors Are Closely Associated with Connexin 43 in Human Ventricular Myocardium. Int. J. Cardiol. 2005, 98, 291–297. [Google Scholar] [CrossRef]
- Bennetts, F.M.; Mobbs, J.I.; Ventura, S.; Thal, D.M. The P2X1 Receptor as a Therapeutic Target. Purinergic Signal. 2022, 18, 421–433. [Google Scholar] [CrossRef]
- Kindon, N.; Davis, A.; Dougall, I.; Dixon, J.; Johnson, T.; Walters, I.; Thom, S.; McKechnie, K.; Meghani, P.; Stocks, M.J. From UTP to AR-C118925, the Discovery of a Potent Non Nucleotide Antagonist of the P2Y2 Receptor. Bioorg. Med. Chem. Lett. 2017, 27, 4849. [Google Scholar] [CrossRef]
- Rafehi, M.; Burbiel, J.C.; Attah, I.Y.; Abdelrahman, A.; Müller, C.E. Synthesis, Characterization, and in Vitro Evaluation of the Selective P2Y(2) Receptor Antagonist AR-C118925. Purinergic Signal. 2017, 13, 89–103. [Google Scholar] [CrossRef]
- Golan, O.; Issan, Y.; Isak, A.; Leipziger, J.; Robaye, B.; Shainberg, A. Extracellular Nucleotide Derivatives Protect Cardiomyocytes against Hypoxic Stress. Biochem. Pharmacol. 2011, 81, 1219–1227. [Google Scholar] [CrossRef]
- Certal, M.; Vinhas, A.; Pinheiro, A.R.; Ferreirinha, F.; Barros-Barbosa, A.R.; Silva, I.; Costa, M.A.; Correia-de-Sa, P. Calcium Signaling and the Novel Anti-Proliferative Effect of the UTP-Sensitive P2Y11 Receptor in Rat Cardiac Myofibroblasts. Cell Calcium 2015, 58, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Numaga-Tomita, T.; Fujimoto, Y.; Tanaka, T.; Toyama, C.; Nishimura, A.; Yamashita, T.; Matsunaga, N.; Koyanagi, S.; Azuma, Y.-T.; et al. Ibudilast Attenuates Doxorubicin-Induced Cytotoxicity by Suppressing Formation of TRPC3 Channel and NADPH Oxidase 2 Protein Complexes. Br. J. Pharmacol. 2019, 176, 3723–3738. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Nishiyama, K.; Man Lee, J.; Ibuki, Y.; Imai, Y.; Noda, T.; Kamiya, N.; Kusakabe, T.; Kanda, Y.; Nishida, M. TRPC3-Nox2 Protein Complex Formation Increases the Risk of SARS-CoV-2 Spike Protein-Induced Cardiomyocyte Dysfunction through ACE2 Upregulation. Int. J. Mol. Sci. 2023, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Freichel, M.; Berlin, M.; Schurger, A.; Mathar, I.; Bacmeister, L.; Medert, R.; Frede, W.; Marx, A.; Segin, S.; Londono, J.E.C. TRP Channels in the Heart; Emir, T.L.R., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 149–185. ISBN 9781315152837. [Google Scholar]
- Dastidar, S.G.; Rajagopal, D.; Ray, A. Therapeutic Benefit of PDE4 Inhibitors in Inflammatory Diseases. Curr. Opin. Investig. Drugs 2007, 8, 364–372. [Google Scholar] [PubMed]
- Kato, Y.; Nishiyama, K.; Nishimura, A.; Noda, T.; Okabe, K.; Kusakabe, T.; Kanda, Y.; Nishida, M. Drug Repurposing for the Treatment of COVID-19. J. Pharmacol. Sci. 2022, 149, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Szadujkis-Szadurska, K.; Matusiak, G.; Malinowski, B.; Gajdus, M.; Wiciński, M.; Szadujkis-Szadurski, L. Influence of Celecoxib on the Vasodilating Properties of Human Mesenteric Arteries Constricted with Endothelin-1. Biomed. Rep. 2014, 2, 412–418. [Google Scholar] [CrossRef] [PubMed]
| ATP Sources | ATP-Released Channels | Autocrine/Paracrine Signalling of Extracellular ATP |
|---|---|---|
| Cardiomyocytes | Connexin-43 Pannexin 1 Pannexin 2 Pannexin-1/P2X7 complex | P2X4 –basal cardiac contractility P2Y/Gq—PLC—↑ Ca2+—contraction P2Y11/Gs—cAMP—↑ Ca2+—contraction and relaxation |
| CF | Connexin-43 Connexin-45 | P2Y2—α-SMA/TGF-β/PAI-1—sufficient scar formation and inflammatory response |
| VSCM | Connexin-43 Pannexin-1 ABC transporters | P2X1—basal vascular contractility |
| EC | Cav-1 VRAC Connexin hemichannels | P2X4—↑ Ca2+—NO—vasodilation P2Y/Gq—PLC—↑ Ca2+—NO—vasodilation |
| RBC | CR-1 CTFR Pannexin-1 VDAC | P2Y1—↑ osmolyte permeability—absorption of sufficient nutrient P2X1 and P2X7—PS exposure—hemolysis |
| Pathological Condition | Protective Signalling | Pathological Signalling |
|---|---|---|
| Hypertension and Atherosclerosis | ATP-P2X4-NO-Vasodilation ATP→Adenosine-A2A-NO-Vasodilation | ATP-P2X7-NLRP3-Pro-inflammatory cytokines ATP-P2X1-Vasocontriction ATP-P2X1/P2X4-Vasocontriction ATP-P2Y2-VSMC migration/proliferation/hypertrophic vascular remodelling |
| Ischemic/ Reperfusion Injury | ATP/P2Y2-AKT, ERK-Cardiomyocyte survival ATP-Pannexin1/P2X7-Cardioprotectant (SP1, adenosine) ATP→Adenosine-A2B-HIF1α-protection against hypoxia | ATP-P2X7-NLRP3-Pro-inflammatory cytokines/fibroblast differentiation ATP-P2Y6-Vascular inflammation |
| Angiogenesis | ATP/P2Y2-AKT, ERK, mTOR-VEGF-Angiogenesis ATP/VEGF165-Angiogenesis ATP/P2Y2-miR-22-ICAM-1 downregulation-tumourgenesis suppression | ATP/P2Y2-CTGF, VEGF, VCAM-1-TNBC progression and metastasis ATP/P2X7-VEGF-Angiogenesis |
| Myocardial fibrosis | ATP/P2Y1-reduce ERK-Impaired production of pro-fibrotic factors | ATP-P2X7-NLRP3-Pro-inflammatory cytokines/fibroblast differentiation ATP-P2Y2-TRPC3-replacement fibrosis ATP-P2Y2-MAPK-pro-fibrotic factors |
| Myocardial hypertrophy and atrophy | ATP/P2Y2-TRPC5/eNOS-inhibit cardiomyocyte hypertrophy ATP-P2X4-eNOS-inhibit cardiomyocyte hypertrophy | ATP-P2Y2-TRPC3/Nox2-cardiomyocyte atrophy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudi, S.; Thomas, F.M.; Daud, S.K.; Ag Daud, D.M.; Sunggip, C. The Pleiotropic Role of Extracellular ATP in Myocardial Remodelling. Molecules 2023, 28, 2102. https://doi.org/10.3390/molecules28052102
Sudi S, Thomas FM, Daud SK, Ag Daud DM, Sunggip C. The Pleiotropic Role of Extracellular ATP in Myocardial Remodelling. Molecules. 2023; 28(5):2102. https://doi.org/10.3390/molecules28052102
Chicago/Turabian StyleSudi, Suhaini, Fiona Macniesia Thomas, Siti Kadzirah Daud, Dayang Maryama Ag Daud, and Caroline Sunggip. 2023. "The Pleiotropic Role of Extracellular ATP in Myocardial Remodelling" Molecules 28, no. 5: 2102. https://doi.org/10.3390/molecules28052102
APA StyleSudi, S., Thomas, F. M., Daud, S. K., Ag Daud, D. M., & Sunggip, C. (2023). The Pleiotropic Role of Extracellular ATP in Myocardial Remodelling. Molecules, 28(5), 2102. https://doi.org/10.3390/molecules28052102







