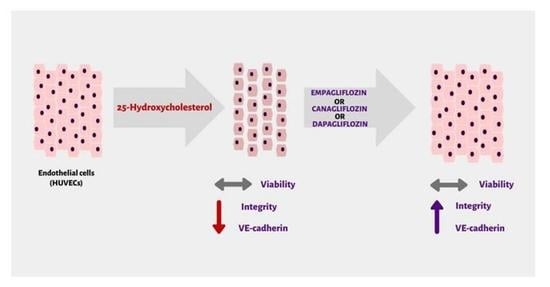
SGLT2 Inhibitors May Restore Endothelial Barrier Interrupted by 25-Hydroxycholesterol
Abstract
1. Introduction
2. Results
2.1. SGLT2 Inhibitors: Empagliflozin, Canagliflozin, and Dapagliflozin Significantly Increase Endothelial Integrity in Comparison to the Medium Control
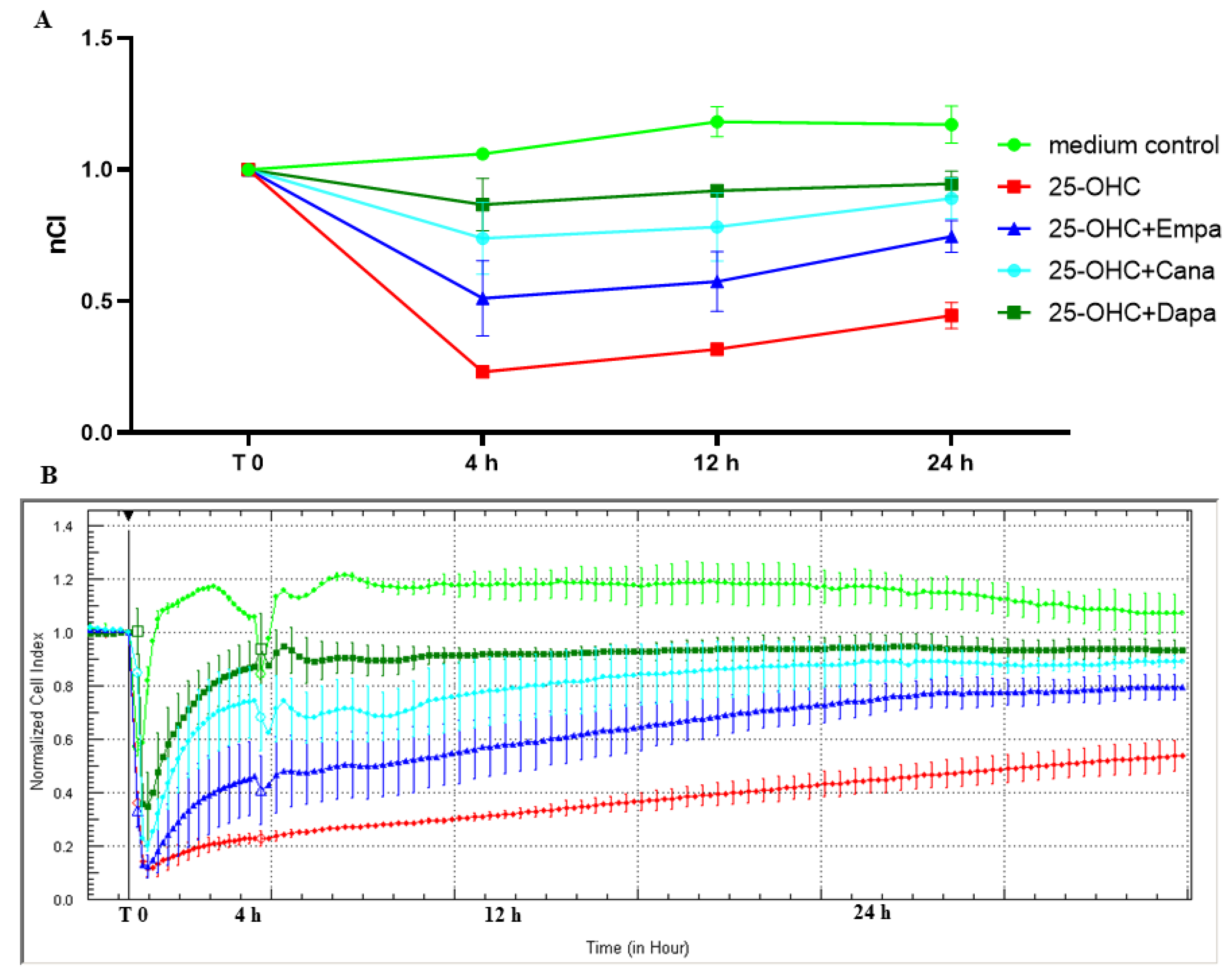
2.2. SGLT2 Inhibitors: Empagliflozin, Canagliflozin, and Dapagliflozin Improved Endothelial Cell Integrity Interrupted by 25-OHC
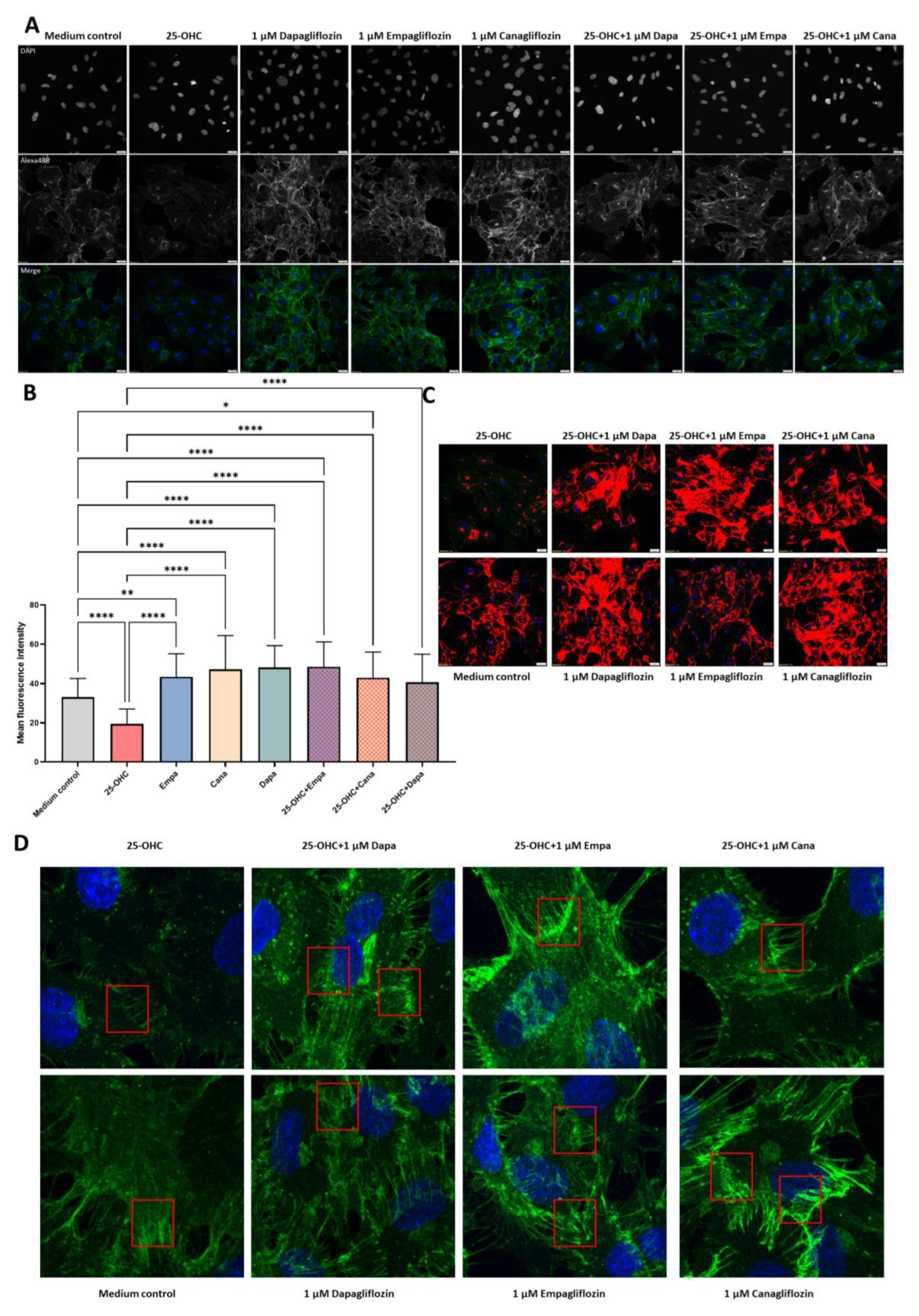
2.3. SGLT2 Inhibitors: Empagliflozin, Canagliflozin, and Dapagliflozin Completely Rescue VE-Cadherin Levels Decreased by 25-Hydroxycholesterol
3. Discussion
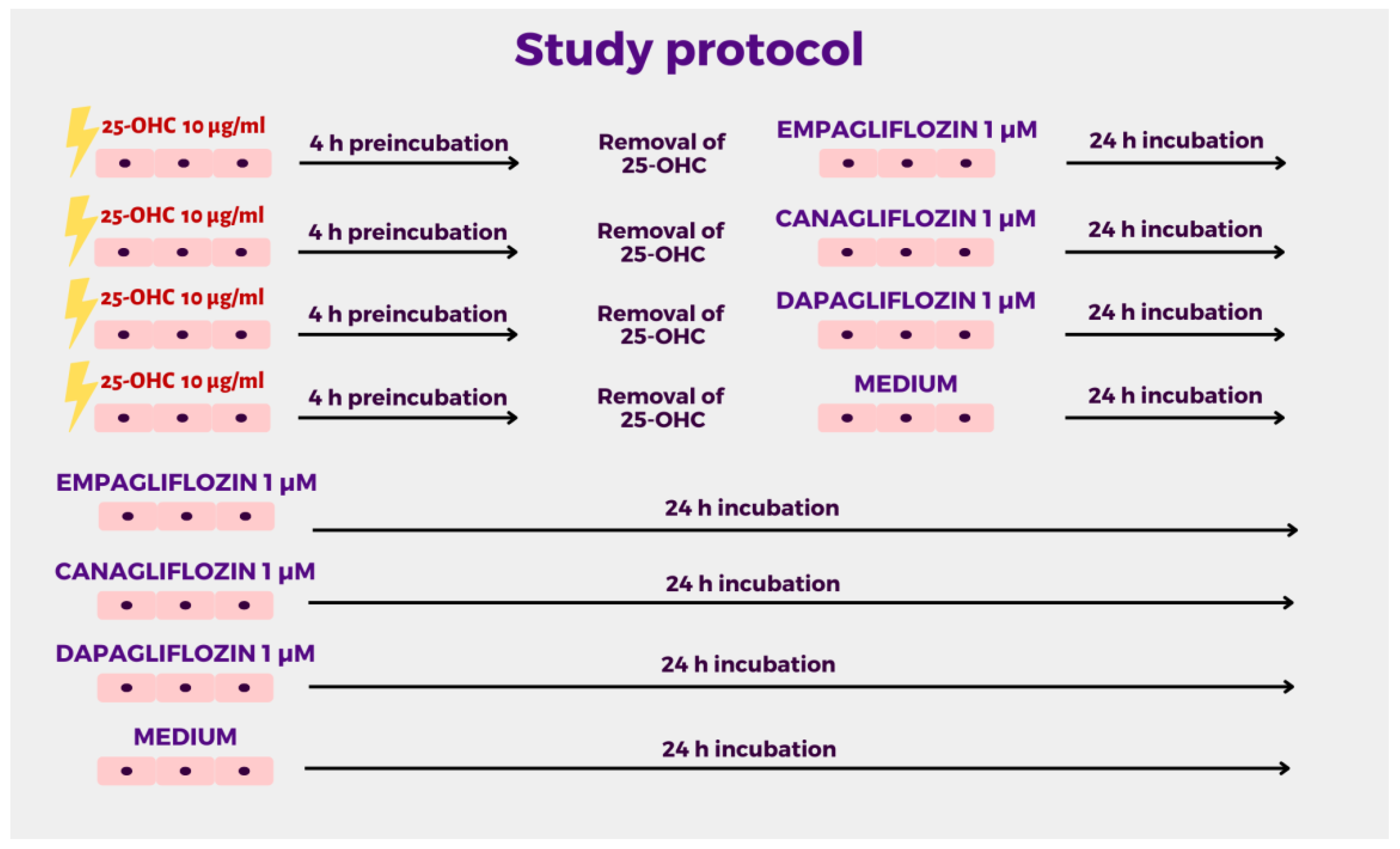
4. Materials and Methods
4.1. Chemicals
4.2. Cells
4.3. Cell Treatment
4.4. Cell Culture in the Real-Time Cell Electric Impedance Sensing System (RTCA-DP, xCELLigence)
4.5. Immunofluorescence and Confocal Microscopy
4.6. Statistical Analysis
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An Update on SGLT2 Inhibitors for the Treatment of Diabetes Mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. Z. Gefassmedizin 2016, 13, 17–18. [Google Scholar] [CrossRef]
- Li, N.; Lv, D.; Zhu, X.; Wei, P.; Gui, Y.; Liu, S.; Zhou, E.; Zheng, M.; Zhou, D.; Zhang, L. Effects of SGLT2 Inhibitors on Renal Outcomes in Patients with Chronic Kidney Disease: A Meta-Analysis. Front. Med. 2021, 8, 728089. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.L.; Pierce, K.N. New therapies for the treatment of heart failure with preserved ejection fraction. Am. J. Health Syst. Pharm. 2022, 79, 1424–1430. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Ou, Z.J.; Chen, J.; Dai, W.P.; Liu, X.; Yang, Y.K.; Li, Y.; Lin, Z.B.; Wang, T.T.; Wu, Y.Y.; Su, D.H.; et al. 25-Hydroxycholesterol impairs endothelial function and vasodilation by uncoupling and inhibiting endothelial nitric oxide synthase. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E781–E790. [Google Scholar] [CrossRef]
- Gorzelak-Pabiś, P.; Broncel, M.; Pawlos, A.; Wojdan, K.; Gajewski, A.; Cha, M. Dabigatran: Its protective effect against endothelial cell damage by oxysterol. Biomed. Pharmacother. 2022, 147, 112679. [Google Scholar] [CrossRef]
- Gory, S.; Vernet, M.; Laurent, M.; Dejana, E.; Dalmon, J.; Huber, P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood 1999, 93, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Gavard, J. Breaking the VE-cadherin bonds. FEBS Lett. 2009, 583, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: A paradigm shift in understanding their mechanism of action. Diabetes Care 2020, 43, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Osataphan, S.; Macchi, C.; Singhal, G.; Chimene-Weiss, J.; Sales, V.; Kozuka, C.; Dreyfuss, J.M.; Pan, H.; Tangcharoenpaisan, Y.; Morningstar, J.; et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 2019, 4, e123130. [Google Scholar] [CrossRef]
- Wicik, Z.; Nowak, A.; Jarosz-Popek, J.; Wolska, M.; Eyileten, C.; Siller-Matula, J.M.; von Lewinski, D.; Sourij, H.; Filipiak, K.J.; Postuła, M. Characterization of the SGLT2 Interaction Network and Its Regulation by SGLT2 Inhibitors: A Bioinformatic Analysis. Front. Pharmacol. 2022, 13, ehac544-2689. [Google Scholar] [CrossRef]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front. Physiol. 2021, 12, 1589. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Guo, X.; Zeng, Z.; Wu, J.; Liu, Y.; He, J.; Wang, R.; Huang, Q.; Chen, Z. Sirt1 protects endothelial cells against LPS-induced barrier dysfunction. Oxid. Med. Cell. Longev. 2017, 2017, 4082102. [Google Scholar] [CrossRef]
- Dioum, E.M.; Chen, R.; Alexander, M.S.; Zhang, Q.; Hogg, R.T.; Gerard, R.D.; Garcia, J.A. Regulation of Hypoxia-Inducible Factor 2α Signaling by the Stress-Responsive Deacetylase Sirtuin 1. Science 2009, 324, 1289–1293. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, W.; Tu, A.B.; Pasupneti, S.; Shuffle, E.; Dahms, P.; Zhang, P.; Cai, H.; Dinh, T.T.; Liu, B.; et al. Endothelial Hypoxia-Inducible Factor-2 Is Required for the Maintenance of Airway Microvasculature. Circulation 2019, 139, 502–517. [Google Scholar] [CrossRef]
- Le Bras, A.; Lionneton, F.; Mattot, V.; Lelièvre, E.; Caetano, B.; Spruyt, N.; Soncin, F. HIF-2α specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene 2007, 26, 7480–7489. [Google Scholar] [CrossRef]
- Wei, R.; Wang, W.; Pan, Q.; Guo, L. Effects of SGLT-2 Inhibitors on Vascular Endothelial Function and Arterial Stiffness in Subjects with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 826604. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, E.; Broncel, M.; Bukowska, B.; Gorzelak-Pabiś, P. The protective effect of dabigatran and rivaroxaban on DNA oxidative changes in a model of vascular endothelial damage with oxidized cholesterol. Int. J. Mol. Sci. 2020, 21, 1953. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, M.G.; Dejana, E.; Giampietro, C. Vascular endothelial (VE)-cadherin, endothelial adherens junctions, and vascular disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029322. [Google Scholar] [CrossRef]
- Guo, M.; Breslin, J.W.; Wu, M.H.; Gottardi, C.J.; Yuan, S.Y. VE-cadherin and β-catenin binding dynamics during histamine-induced endothelial hyperpermeability. Am. J. Physiol.-Cell Physiol. 2008, 294, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Lin, J.; Pang, Z.; Zhang, H.; Wang, Y.; Ma, L.; Zhang, H.; Zhang, X.; Chen, M.; Zhang, X.; et al. Aberrant Cholesterol Metabolism and Wnt/β-Catenin Signaling Coalesce via Frizzled5 in Supporting Cancer Growth. Adv. Sci. 2022, 9, 2200750. [Google Scholar] [CrossRef]
- Cai, C.; Wu, F.; Zhuang, B.; Ou, Q.; Peng, X.; Shi, N.; Peng, L.; Li, Z.; Wang, J.; Cai, S.; et al. Empagliflozin activates Wnt/β-catenin to stimulate FUNDC1-dependent mitochondrial quality surveillance against type-3 cardiorenal syndrome. Mol. Metab. 2022, 64, 101553. [Google Scholar] [CrossRef]
- Woźniak, E.; Broncel, M.; Bukowska, B.; Gorzelak-Pabiś, P. The protective effect of empagliflozin on DNA oxidative changes in a model of vascular endothelial damage with oxidized cholesterol. Atherosclerosis 2022, 355, 8–9. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, P.X.; Guo, X.H.; Huang, Q.B. Role of moesin, Src, and ROS in advanced glycation end product-induced vascular endothelial dysfunction. Microcirculation 2017, 24, e12358. [Google Scholar] [CrossRef]
- Li, X.; Römer, G.; Kerindongo, R.P.; Hermanides, J.; Albrecht, M.; Hollmann, M.W.; Zuurbier, C.J.; Preckel, B.; Weber, N.C. Sodium glucose co-transporter 2 inhibitors ameliorate endothelium barrier dysfunction induced by cyclic stretch through inhibition of reactive oxygen species. Int. J. Mol. Sci. 2021, 22, 6044. [Google Scholar] [CrossRef]
- Uthman, L.; Homayr, A.; Juni, R.P.; Spin, E.L.; Kerindongo, R.; Boomsma, M.; Hollmanna Benedikt Preckel, M.W.; Koolwijk, P.; Van Hinsbergh, V.W.M.; Zuurbier, C.J.; et al. Empagliflozin and dapagliflozin reduce ROS generation and restore no bioavailability in tumor necrosis factor α-stimulated human coronary arterial endothelial cells. Cell. Physiol. Biochem. 2019, 53, 865–886. [Google Scholar] [CrossRef]
- Sidibé, A.; Imhof, B.A. VE-cadherin phosphorylation decides: Vascular permeability or diapedesis. Nat. Immunol. 2014, 15, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Wessel, F.; Winderlich, M.; Holm, M.; Frye, M.; Rivera-Galdos, R.; Vockel, M.; Linnepe, R.; Ipe, U.; Stadtmann, A.; Zarbock, A.; et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat. Immunol. 2014, 15, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.J.; Boyd, D.; Katwan, O.J.; Strembitska, A.; Almabrouk, T.A.; Kennedy, S.; Palmer, T.M.; Salt, I.P. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci. Rep. 2018, 8, 5276. [Google Scholar] [CrossRef] [PubMed]
- Behnammanesh, G.; Durante, Z.E.; Peyton, K.J.; Martinez-Lemus, L.A.; Brown, S.M.; Bender, S.B.; Durante, W. Canagliflozin inhibits human endothelial cell proliferation and tube formation. Front. Pharmacol. 2019, 10, 362. [Google Scholar] [CrossRef] [PubMed]
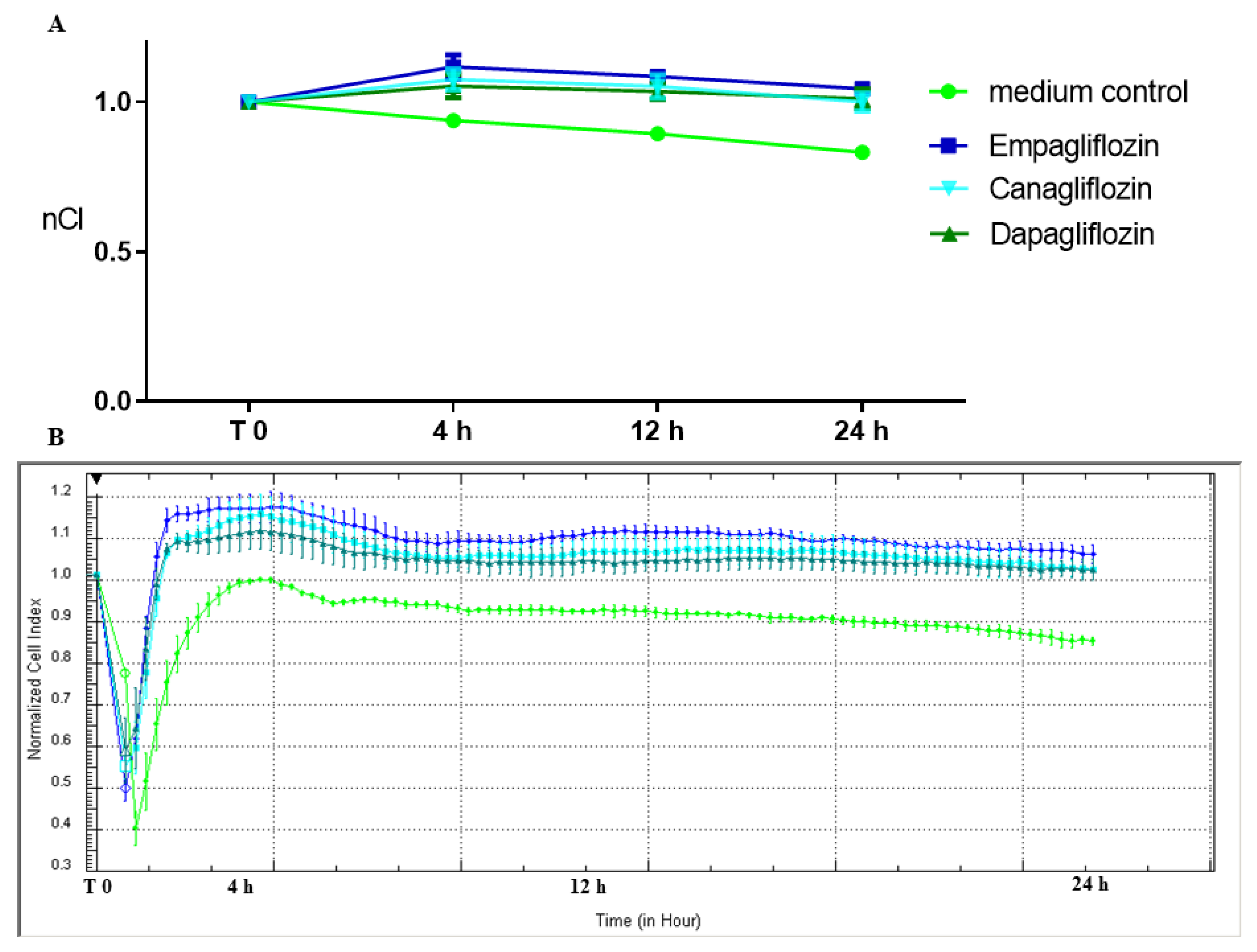
| nCI | T:0 | T:4 h | T:12 h | T:24 h | |
|---|---|---|---|---|---|
| mean ± SD | medium control | 1 ± 0 | 0.94 ± 0.01 | 0.89 ± 0.01 | 0.83 ± 0.01 |
| Empa 1 µM | 1 ± 0 | 1.12 ± 0.04 | 1.08 ± 0.01 | 1.04 ± 0.01 | |
| Cana 1 µM | 1 ± 0 | 1.07 ± 0.04 | 1.05 ± 0.04 | 1.00 ± 0.03 | |
| Dapa 1 µM | 1 ± 0 | 1.05 ± 0.04 | 1.03 ± 0.03 | 1.01 ± 0.03 | |
| Adjusted p value * | sp vs. Empa | >0.9999 | <0.0001 | <0.0001 | <0.0001 |
| sp vs. Dapa | >0.9999 | <0.0001 | <0.0001 | <0.0001 | |
| sp vs. Cana | >0.9999 | <0.0001 | <0.0001 | <0.0001 | |
| Empa vs. Dapa | >0.9999 | 0.0133 | 0.0698 | 0.3398 | |
| Empa vs. Cana | >0.9999 | 0.1580 | 0.3407 | 0.1356 | |
| Dapa vs. Cana | >0.9999 | 0.6874 | 0.8257 | 0.9502 |
| nCI | T:0 | T:4 h | T:12 h | T:24 h | |
|---|---|---|---|---|---|
| mean ± SD | Medium control | 1 ± 0 | 1.06 ± 0.01 | 1.18 ± 0.06 | 1.17 ± 0.07 |
| 25-OHC | 1 ± 0 | 0.23 ± 0.02 | 0.32 ± 0.02 | 0.45 ± 0.05 | |
| 25-OHC+Empa 1 | 1 ± 0 | 0.51 ± 0.14 | 0.57 ± 0.11 | 0.75 ± 0.06 | |
| 25-OHC+Cana 1 | 1 ± 0 | 0.74 ± 0.14 | 0.78 ± 0.13 | 0.89 ± 0.08 | |
| 25-OHC+Dapa 1 | 1 ± 0 | 0.87 ± 0.1 | 0.92 ± 0.02 | 0.95 ± 0.05 | |
| Adjusted p value * | Medium control vs. 25-OHC | >0.9999 | <0.0001 | <0.0001 | <0.0001 |
| Medium control vs. 25-OHC + Empa 1 | >0.9999 | <0.0007 | <0.0001 | <0.0001 | |
| 25-OHC vs. 25-OHC + Empa 1 | >0.9999 | 0.0020 | 0.0019 | 0.0005 | |
| Medium control vs. 25-OHC + Cana 1 | >0.9999 | <0.0009 | <0.0001 | 0.0028 | |
| 25-OHC vs. 25-OHC + Cana 1 | >0.9999 | <0.0001 | <0.0001 | <0.0001 | |
| Medium control vs. 25-OHC + Dapa 1 | >0.9999 | 0.0007 | <0.0001 | 0.0001 | |
| 25-OHC vs. 25-OHC + Dapa 1 | >0.9999 | <0.0001 | <0.0001 | <0.0001 |
| Compounds | Concentration | Cell Viability [%] | ANOVA I |
|---|---|---|---|
| Control | 0 µg/mL | 98.9 ± 0.8 | - |
| 25-hydroxycholesterol | 10 µg/mL | 97.3 ± 1.6 | p > 0.05 |
| Empagliflozin | 1 µM | 98.4 ± 0.8 | p > 0.05 |
| Dapagliflozin | 1 µM | 98.1 ± 1.3 | p > 0.05 |
| Canagliflozin | 1 µM | 98.5 ± 1.1 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlos, A.; Broncel, M.; Woźniak, E.; Markiewicz, Ł.; Piastowska-Ciesielska, A.; Gorzelak-Pabiś, P. SGLT2 Inhibitors May Restore Endothelial Barrier Interrupted by 25-Hydroxycholesterol. Molecules 2023, 28, 1112. https://doi.org/10.3390/molecules28031112
Pawlos A, Broncel M, Woźniak E, Markiewicz Ł, Piastowska-Ciesielska A, Gorzelak-Pabiś P. SGLT2 Inhibitors May Restore Endothelial Barrier Interrupted by 25-Hydroxycholesterol. Molecules. 2023; 28(3):1112. https://doi.org/10.3390/molecules28031112
Chicago/Turabian StylePawlos, Agnieszka, Marlena Broncel, Ewelina Woźniak, Łukasz Markiewicz, Agnieszka Piastowska-Ciesielska, and Paulina Gorzelak-Pabiś. 2023. "SGLT2 Inhibitors May Restore Endothelial Barrier Interrupted by 25-Hydroxycholesterol" Molecules 28, no. 3: 1112. https://doi.org/10.3390/molecules28031112
APA StylePawlos, A., Broncel, M., Woźniak, E., Markiewicz, Ł., Piastowska-Ciesielska, A., & Gorzelak-Pabiś, P. (2023). SGLT2 Inhibitors May Restore Endothelial Barrier Interrupted by 25-Hydroxycholesterol. Molecules, 28(3), 1112. https://doi.org/10.3390/molecules28031112