Asiaticoside Increases Caspase-9 Activity in MCF-7 Cells and Inhibits TNF-α and IL-6 Expression in Nude Mouse Xenografts via the NF-κB Pathway
Abstract
1. Background
2. Results
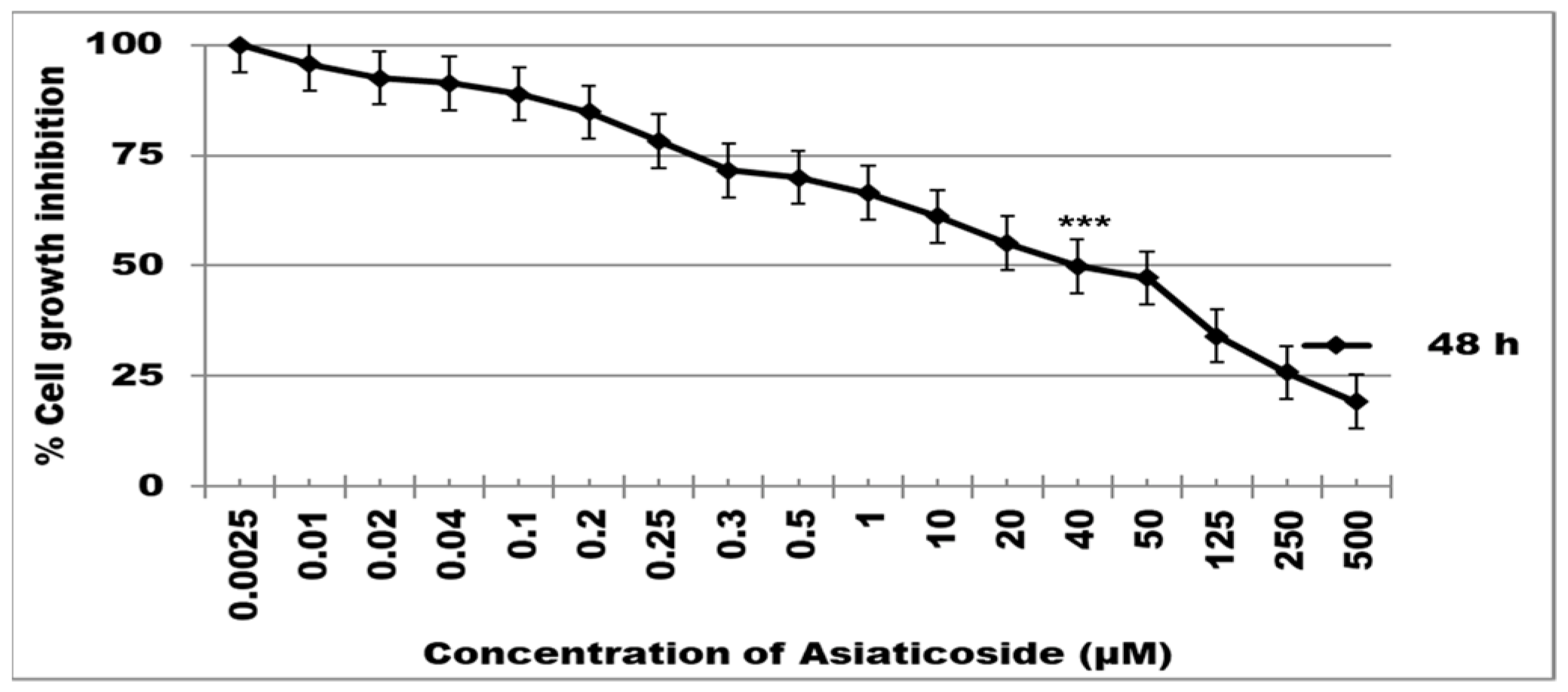
2.1. Cell Viability (MTT) Assay
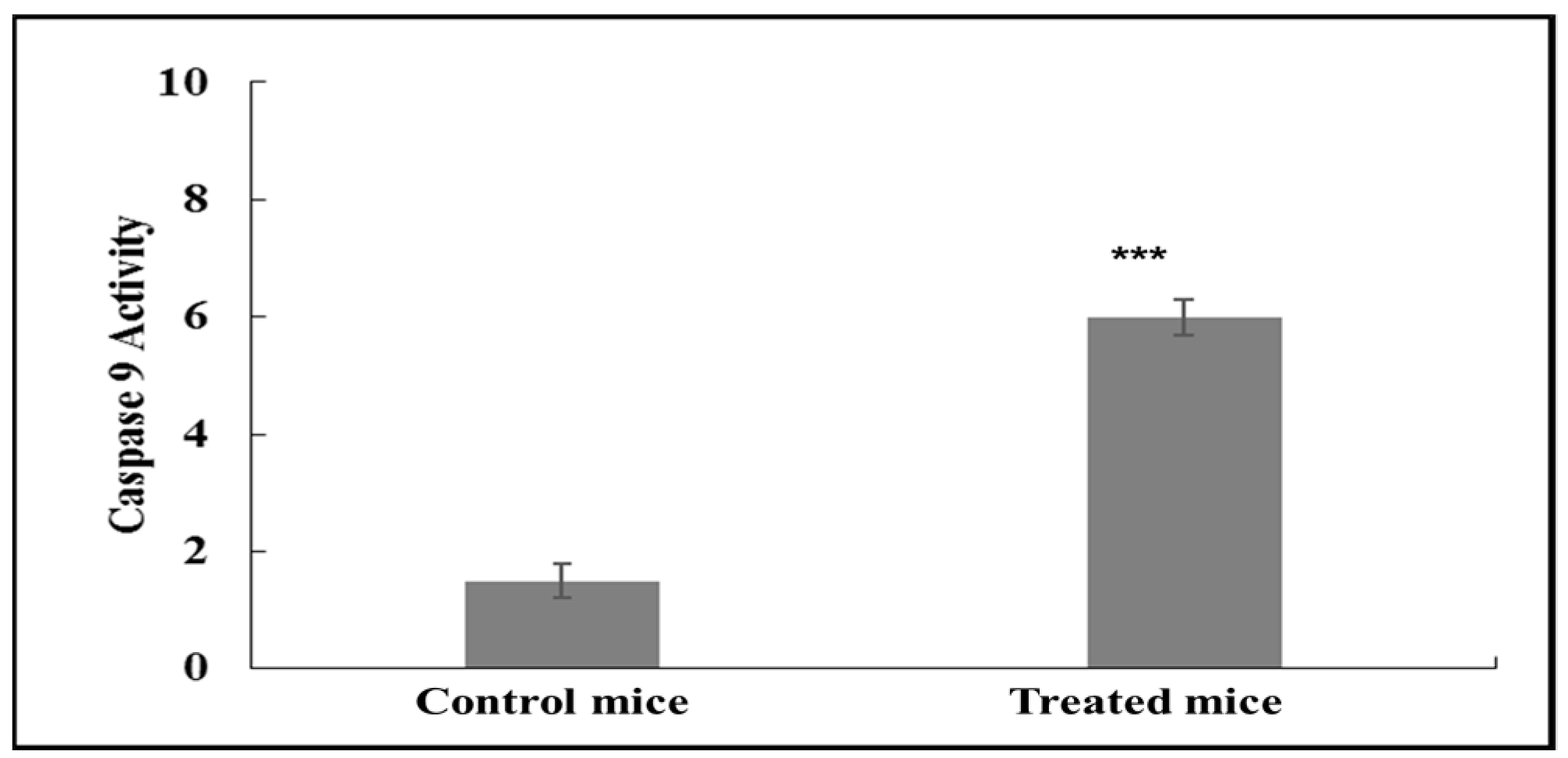
2.2. Asiaticoside Treatment Increases Caspase-9 Activity in MCF-7 Cells
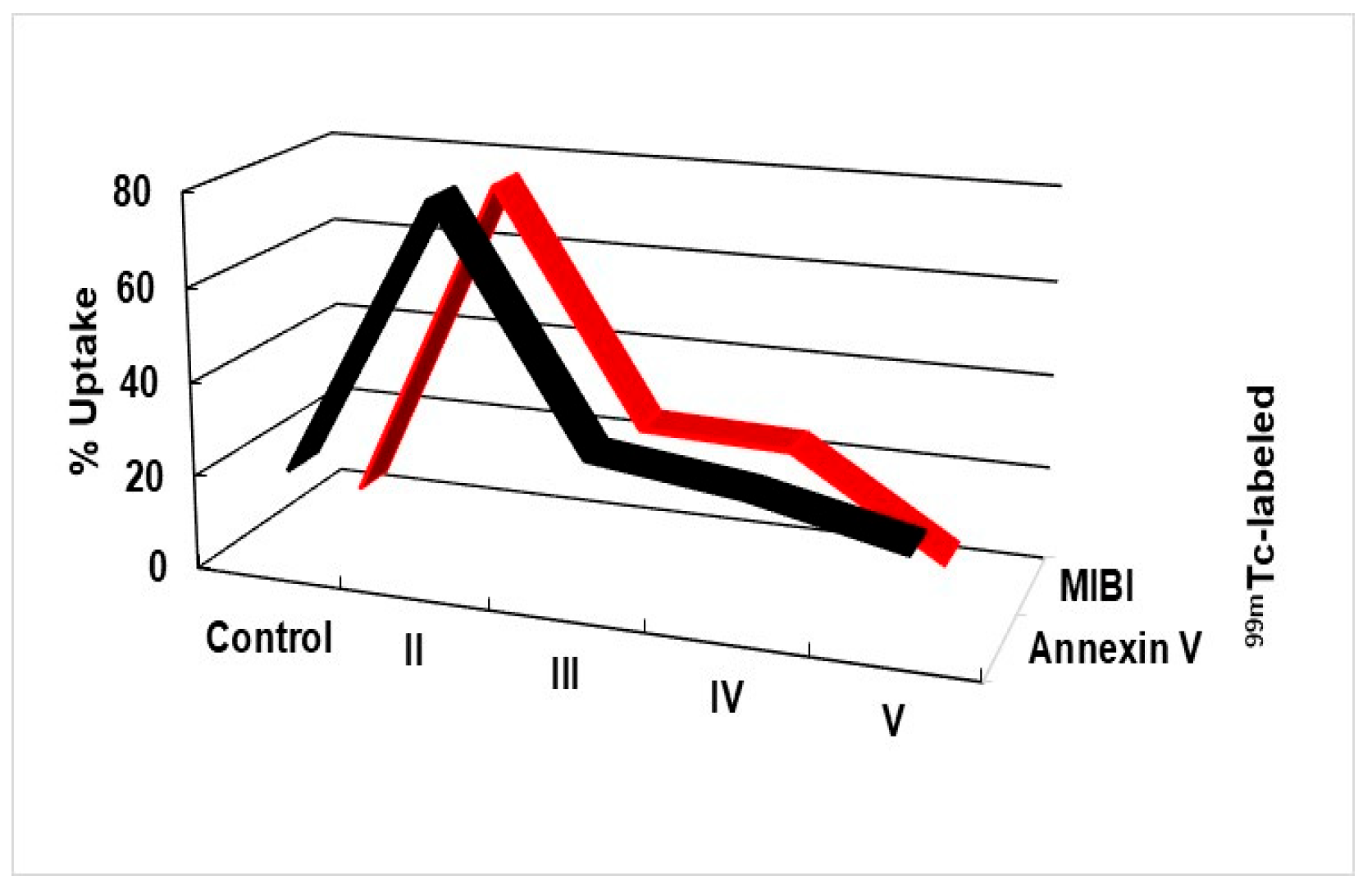
2.3. Detection of Apoptosis Using 99mTc-Labeled Annexin V and MIBI
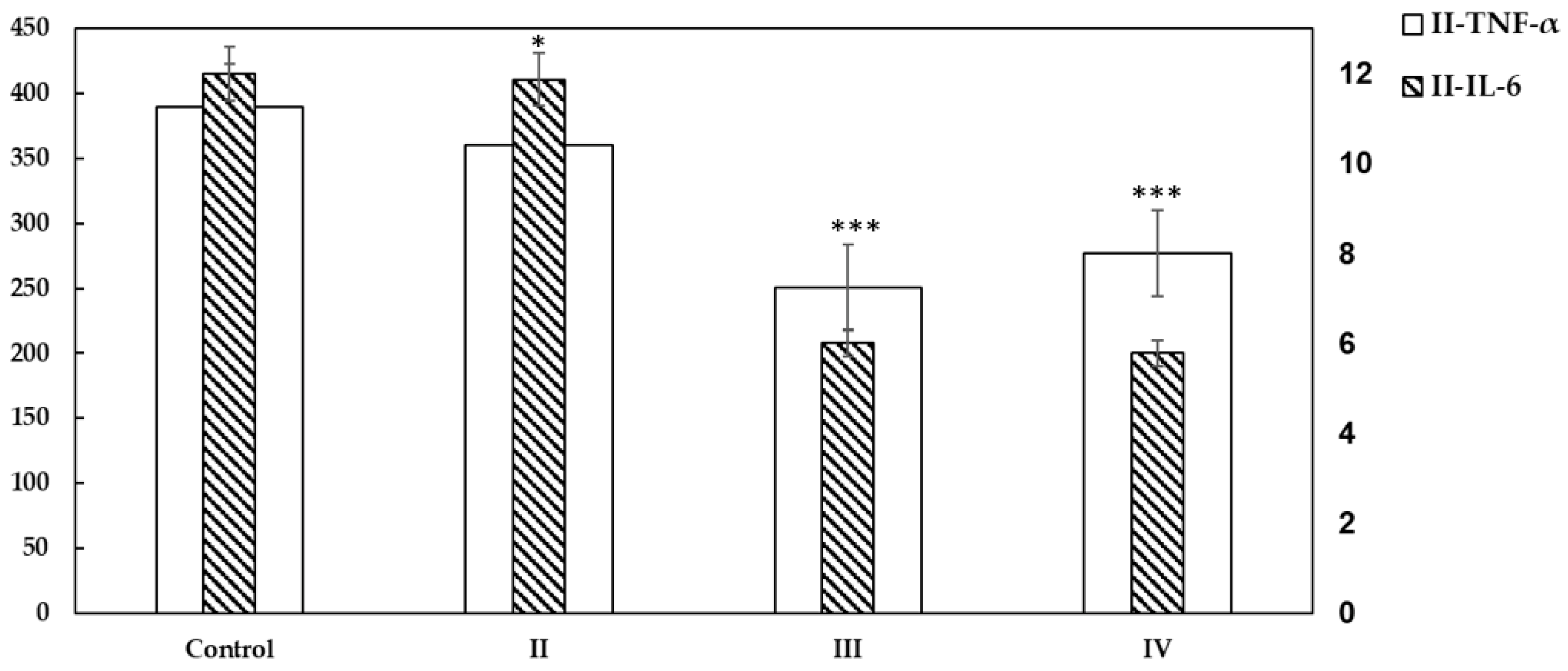
2.4. Cytokines Detection Using ELISA
2.5. Analyses of mRNA Expression
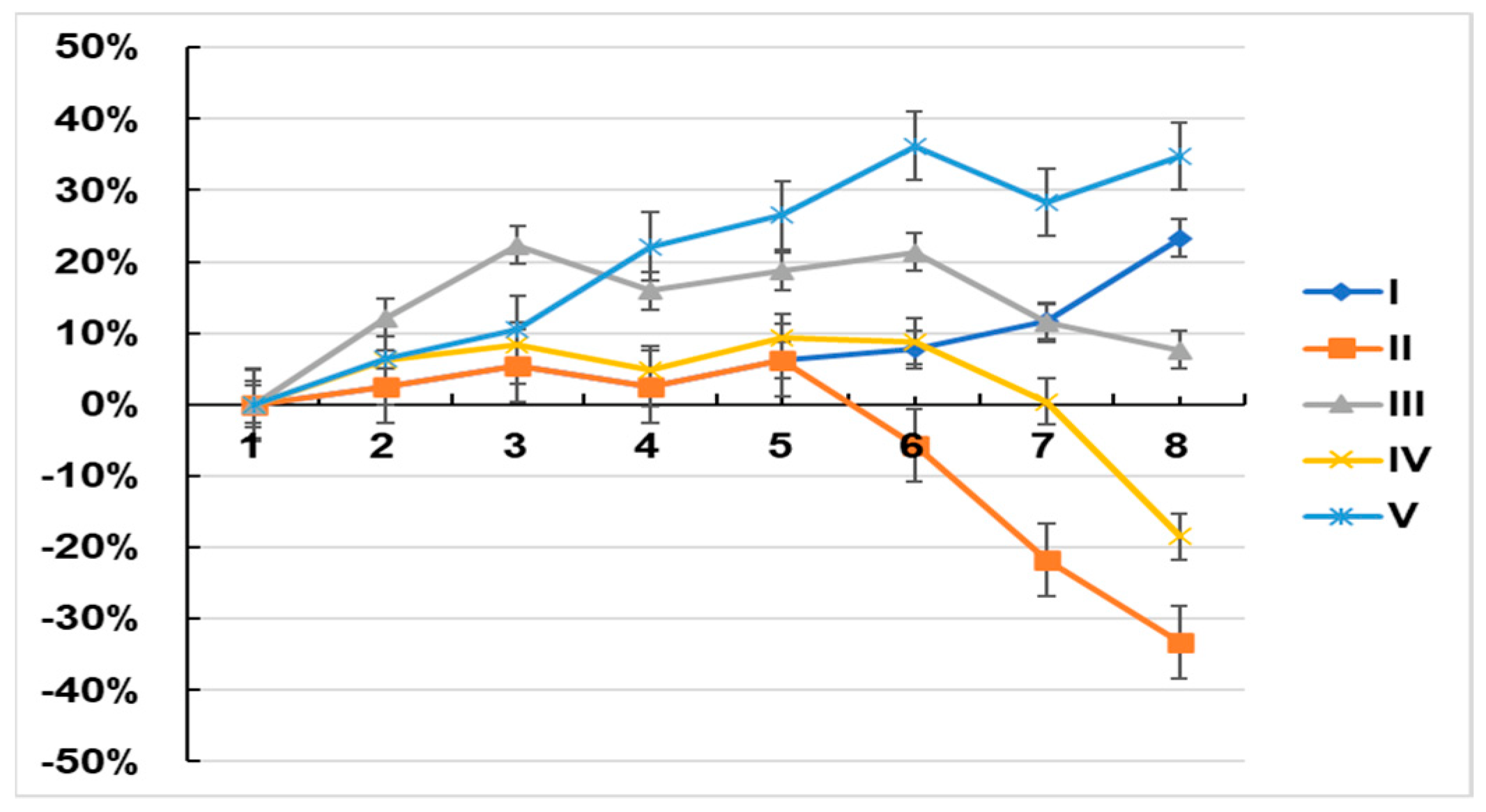
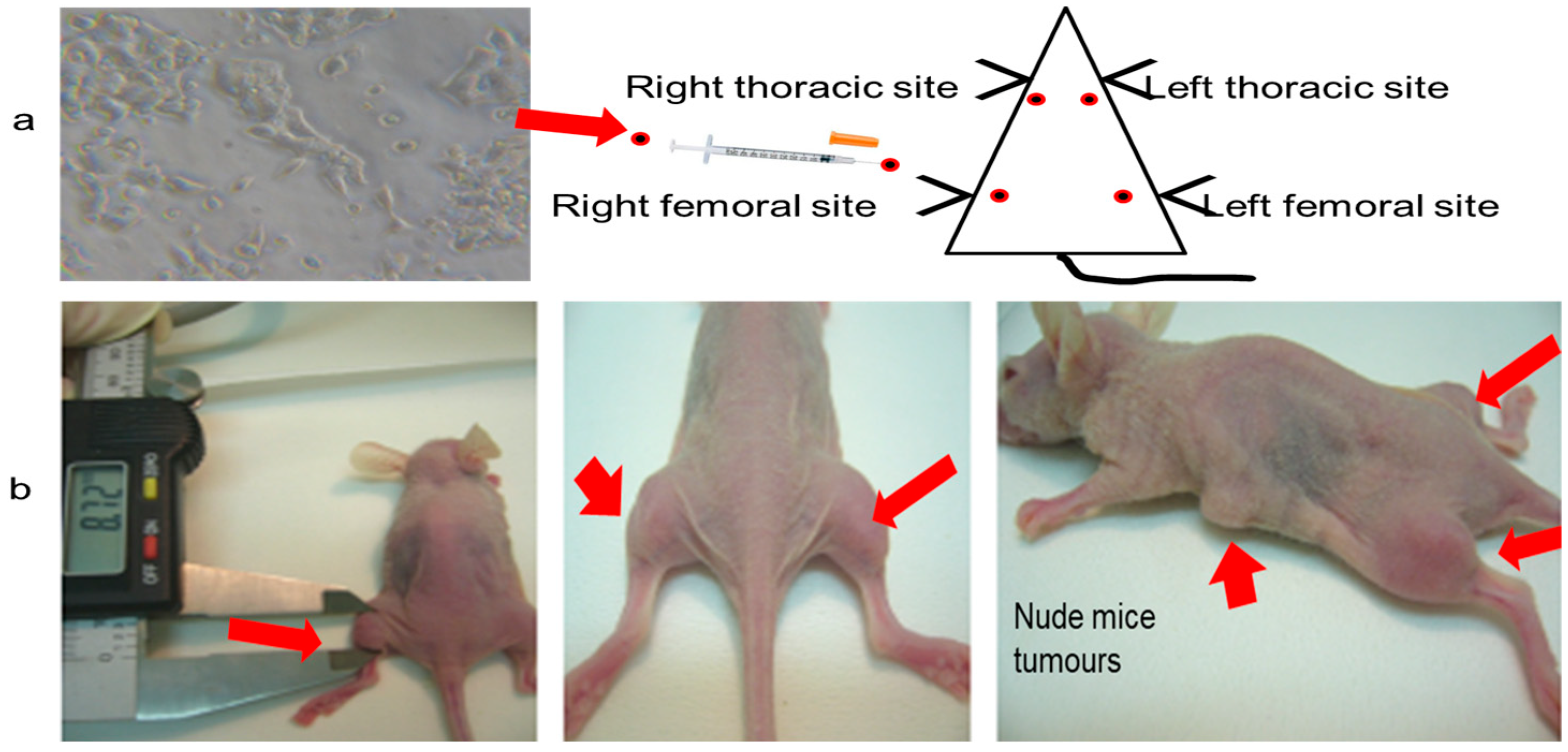
2.6. Tumor Formation and Growth in Xenograft Mice
2.6.1. TNF-α and IL-6 Expression Is Reduced and Tumour Necrosis Is Increased in Xenograft Tumors from Mice Treated with Asiaticoside
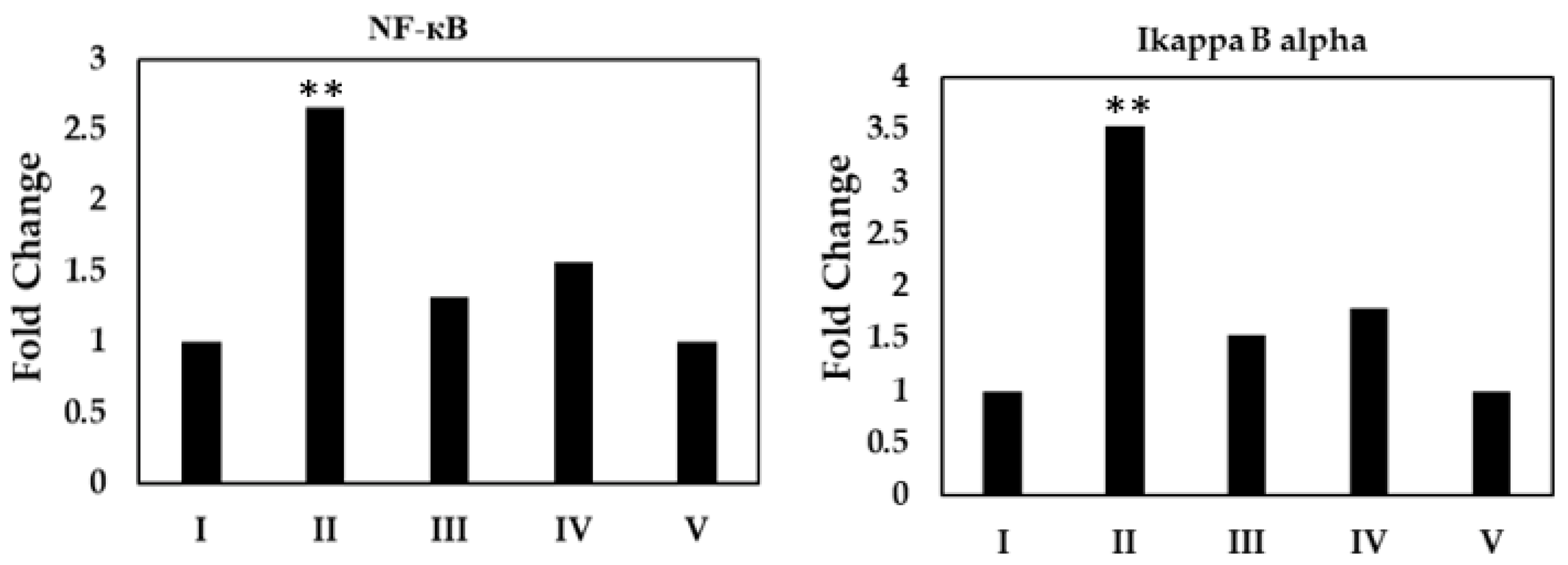
2.6.2. Activation of NF-kB Assay
3. Methods and Materials
3.1. Preparation of Asiaticoside
3.2. Cell Culture
3.3. In Vitro Cell Experiments
3.4. Fluorometric Assay Assessing Caspase-9 Activation and Apoptosis
3.5. Cytokines Detection Using Enzyme-Linked Immunosorbent Assay (ELISA)
3.6. Real-Time (RT)-PCR Analysis of Gene Expression in MCF-7 Cells
3.7. Detection of Apoptosis Using 99mTc-Labeled Annexin V and MIBI
3.8. Experimental Animals and Ethics Statement
3.9. The General Care of the Animals
3.10. Animal Experiments
3.11. Experimental Protocol
3.12. Real-Time (RT)-PCR Analysis of Gene Expression in Tumours
3.13. Western Blot Analysis
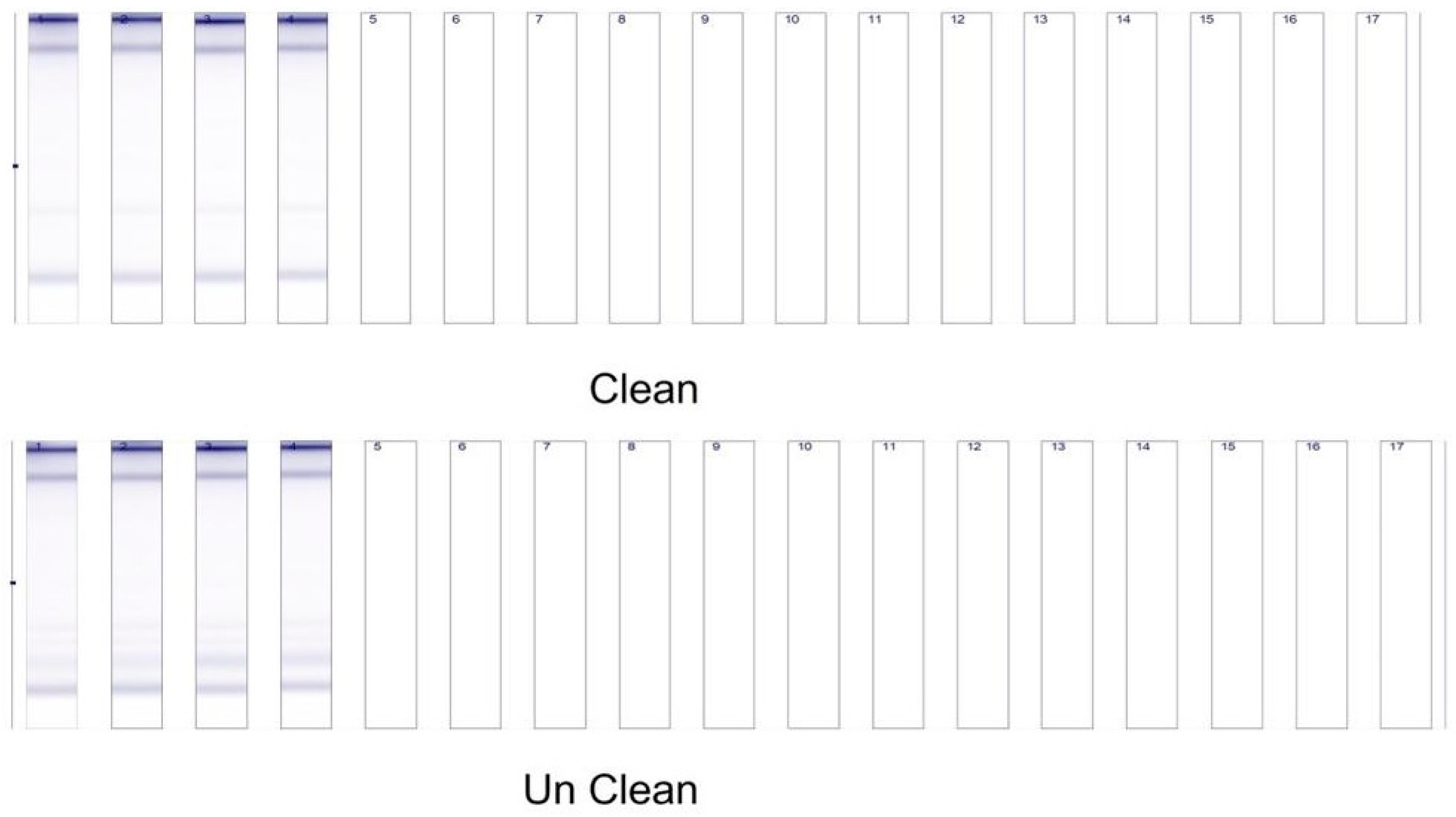
3.14. Ribonucleic Acid (RNA) Purification and Microarrays
3.15. Statistical Analysis
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization-Breast Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer#:~:text=Reducing%20global%20breast%20cancer%20mortality,and%20comprehensive%20breast%20cancer%20management (accessed on 6 January 2023).
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast cancer: Presentation, investigation and management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Ahmad, A. Breast Cancer Statistics: Recent Trends. Adv. Exp. Med. Biol. 2019, 1152, 1–7. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef]
- Al-Saeedi, F.J. Mangiferin protect oxidative stress against deoxynivalenol induced damages through Nrf2 signalling pathways in endothelial cells. Clin. Exp. Pharmacol. Physiol. 2021, 48, 389–400. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Katoh, M. Human FOX gene family (Review). Int. J. Oncol. 2004, 25, 1495–1500. [Google Scholar] [CrossRef]
- Al-Saeedi, F.J. Study of the cytotoxicity of asiaticoside on rats and tumour cells. BMC Cancer 2014, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Hanapi, N.A.; Mohamad Arshad, A.S.; Abdullah, J.M.; Tengku Muhammad, T.S.; Yusof, S.R. Blood-Brain Barrier Permeability of Asiaticoside, Madecassoside and Asiatic Acid in Porcine Brain Endothelial Cell Model. J. Pharm. Sci. 2021, 110, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Yingchun, L.; Huihan, W.; Rong, Z.; Guojun, Z.; Ying, Y.; Zhuogang, L. Antitumor Activity of Asiaticoside against Multiple Myeloma Drug-Resistant Cancer Cells Is Mediated by Autophagy Induction, Activation of Effector Caspases, and Inhibition of Cell Migration, Invasion, and STAT-3 Signaling Pathway. Med. Sci. Monit. 2019, 25, 1355–1361. [Google Scholar] [CrossRef]
- Kwon, K.J.; Bae, S.; Kim, K.; An, I.S.; Ahn, K.J.; An, S.; Cha, H.J. Asiaticoside, a component of Centella asiatica, inhibits melanogenesis in B16F10 mouse melanoma. Mol. Med. Rep. 2014, 10, 503–507. [Google Scholar] [CrossRef]
- Garanti, T.; Stasik, A.; Burrow, A.J.; Alhnan, M.A.; Wan, K.W. Anti-glioma activity and the mechanism of cellular uptake of asiatic acid-loaded solid lipid nanoparticles. Int. J. Pharm. 2016, 500, 305–315. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, J.; Wang, J.; Wang, C.; Zhang, Y.; Zhao, L.; Li, J.; Feng, X. Asiaticoside Antagonizes Proliferation and Chemotherapeutic Drug Resistance in Hepatocellular Carcinoma (HCC) Cells. Med. Sci. Monit. 2020, 26, e924435. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jia, Z.; Li, X.; Hu, Z.; Yu, X.; Xia, J. Asiaticoside hampers epithelial-mesenchymal transition by promoting PPARG expression and suppressing P2RX7-mediated TGF-β/Smad signaling in triple-negative breast cancer. Phytother. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeedi, F.J.; Bitar, M.; Pariyani, S. Effect of asiaticoside on 99mTc-tetrofosmin and 99mTc-sestamibi uptake in MCF-7 cells. J. Nucl. Med. Technol. 2011, 39, 279–283. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Cartwright, T.; Perkins, N.D.; Wilson, L.W. NFKB1: A suppressor of inflammation, ageing and cancer. FEBS J. 2016, 283, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, T.M.; Devonshire, A.S.; Ellison, S.L.; Foy, C.A. Evaluation of a novel approach for the measurement of RNA quality. BMC Res. Notes 2010, 3, 89. [Google Scholar] [CrossRef]
- Anukunwithaya, T.; Tantisira, M.H.; Tantisira, B.; Khemawoot, P. Pharmacokinetics of a Standardized Extract of Centella asiatica ECa 233 in Rats. Planta Med. 2017, 83, 710–717. [Google Scholar] [CrossRef]
- Tan, S.C.; Bhattamisra, S.K.; Chellappan, D.K.; Candasamy, M. Actions and Therapeutic Potential of Madecassoside and Other Major Constituents of Centella asiatica: A Review. Appl. Sci. 2021, 11, 8475. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, Y.Y.; Chang, J.Y.; Choi, H.G.; Lim, S.J.; Lee, M.K. Development of a novel dosage form for intramuscular injection of titrated extract of Centella asiatica in a mixed micellar system. Int. J. Pharm. 2001, 220, 141–147. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.; Oliveira, P.A.; Pinho-Oliveira, J.; Teixeira-Guedes, C.; Soares-Maia, R.; da Costa, R.G.; Colaço, B.; Pires, M.J.; Colaço, J.; Ferreira, R.; et al. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Anim. 2013, 42, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P.; Liu, Y.; Zheng, X.; Li, Q. Caspase-9: Structure, mechanisms and clinical application. Oncotarget 2017, 8, 23996–24008. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, D.; Tsutsumi, K.; Oyake, D.; Ohta, T.; Nishikawa, H.; Koizuka, I. Inhibition of caspase-9 activity and Apaf-1 expression in cisplatin-resistant head and neck squamous cell carcinoma cells. Auris Nasus Larynx 2003, 30, S85–S88. [Google Scholar] [CrossRef] [PubMed]
- Chehade, H.; Fox, A.; Mor, G.G.; Alvero, A.B. Determination of Caspase Activation by Western Blot. Methods Mol. Biol. 2021, 2255, 1–12. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Mueller, T.; Voigt, W.; Simon, H.; Fruehauf, A.; Bulankin, A.; Grothey, A.; Schmoll, H.J. Failure of activation of caspase-9 induces a higher threshold for apoptosis and cisplatin resistance in testicular cancer. Cancer Res 2003, 63, 513–521. [Google Scholar]
- Zhou, X.; Ke, C.; Lv, Y.; Ren, C.; Lin, T.; Dong, F.; Mi, Y. Asiaticoside suppresses cell proliferation by inhibiting the NF-κB signaling pathway in colorectal cancer. Int. J. Mol. Med. 2020, 46, 1525–1537. [Google Scholar] [CrossRef]
- He, Y.; Peng, X.; Zheng, L.; Tang, Y.; Li, J.; Huang, X. Asiaticoside inhibits epithelial-mesenchymal transition and stem cell-like properties of pancreatic cancer PANC-1 cells by blocking the activation of p65 and p38MAPK. J. Gastrointest. Oncol. 2021, 12, 196–206. [Google Scholar] [CrossRef]
- Sh Ahmed, A.; Taher, M.; Mandal, U.K.; Jaffri, J.M.; Susanti, D.; Mahmood, S.; Zakaria, Z.A. Pharmacological properties of Centella asiatica hydrogel in accelerating wound healing in rabbits. BMC Complement. Altern. Med. 2019, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S. Pharmacological Effects of Centella asiatica on Skin Diseases: Evidence and Possible Mechanisms. Evid.-Based Complement. Alternat. Med. 2021, 2021, 5462633. [Google Scholar] [CrossRef]
- Jing, L.; Haitao, W.; Qiong, W.; Fu, Z.; Nan, Z.; Xuezheng, Z. Anti inflammatory effect of asiaticoside on human umbilical vein endothelial cells induced by ox-LDL. Cytotechnology 2018, 70, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kurebayashi, J.; Kurosumi, M.; Kunisue, H.; Otsuki, T.; Tanaka, K.; Sonoo, H. Combined effects of docetaxel and fluoropyrimidines on tumor growth and expression of interleukin-6 and thymidine phosphorylase in breast cancer xenografts. Cancer Chemother. Pharmacol. 2001, 48, 283–288. [Google Scholar] [CrossRef]
- Jiang, X.P.; Yang, D.C.; Elliott, R.L.; Head, J.F. Down-regulation of expression of interleukin-6 and its receptor results in growth inhibition of MCF-7 breast cancer cells. Anticancer Res. 2011, 31, 2899–2906. [Google Scholar]
- Sasser, A.K.; Sullivan, N.J.; Studebaker, A.W.; Hendey, L.F.; Axel, A.E.; Hall, B.M. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007, 21, 3763–3770. [Google Scholar] [CrossRef]
- Kurebayashi, J. Regulation of interleukin-6 secretion from breast cancer cells and its clinical implications. Breast Cancer 2000, 7, 124–129. [Google Scholar] [CrossRef]
- Cai, Z.; Bettaieb, A.; Mahdani, N.E.; Legrès, L.G.; Stancou, R.; Masliah, J.; Chouaib, S. Alteration of the sphingomyelin/ceramide pathway is associated with resistance of human breast carcinoma MCF7 cells to tumor necrosis factor-alpha-mediated cytotoxicity. J. Biol. Chem. 1997, 272, 6918–6926. [Google Scholar] [CrossRef] [PubMed]
- Leek, R.; Landers, R.; Fox, S.; Ng, F.; Harris, A.; Lewis, C. Association of tumour necrosis factor alpha and its receptors with thymidine phosphorylase expression in invasive breast carcinoma. Br. J. Cancer 1998, 77, 2246–2251. [Google Scholar] [CrossRef]
- Miles, D.; Happerfield, L.; Naylor, M.; Bobrow, L.; Rubens, R.; Balkwwill, F. Expression of tumour necrosis factor (TNFα) and its receptors in benign and malignant breast tissue. Int. J. Cancer 1994, 56, 777–782. [Google Scholar] [CrossRef]
- Sheen-Chen, S.M.; Chen, W.J.; Eng, H.L.; Chou, F.F. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res. Treat. 1997, 43, 211–215. [Google Scholar] [CrossRef]
- Chukiatsiri, S.; Siriwong, S.; Thumanu, K. Pupae protein extracts exert anticancer effects by downregulating the expression of IL-6, IL-1β and TNF-α through biomolecular changes in human breast cancer cells. Biomed Pharmacother. 2020, 128, 110278. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Veiby, O.P.; Read, M.A. Chemoresistance: Impact of nuclear factor (NF)-kappaB inhibition by small interfering RNA. Commentary re J. Guo et al. Enhanced chemosensitivity to irinotecan by RNA interference-mediated down-regulation of the NF-kappaB p65 subunit. Clin Cancer Res 2004;10:3333-3341. Clin. Cancer Res. 2004, 10, 3262–3264. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Kim, H.; Oh, W.K.; Yu, K.A.; Choe, Y.K.; Ahn, J.S.; Kim, D.S.; Kim, S.H.; Dinarello, C.A.; Kim, K.; et al. Tetramethoxy hydroxyflavone p7F downregulates inflammatory mediators via the inhibition of nuclear factor kappaB. Ann. N. Y. Acad. Sci. 2004, 1030, 555–568. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef]
- Qiu, J.; Yu, L.; Zhang, X.; Wu, Q.; Wang, D.; Wang, X.; Xia, C.; Feng, H. Asiaticoside attenuates lipopolysaccharide-induced acute lung injury via down-regulation of NF-κB signaling pathway. Int. Immunopharmacol. 2015, 26, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zaman, F.Y.; Orchard, S.G.; Haydon, A.; Zalcberg, J.R. Non-aspirin non-steroidal anti-inflammatory drugs in colorectal cancer: A review of clinical studies. Br. J. Cancer 2022, 127, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Salaheldin, T.A.; Adhami, V.M.; Fujioka, K.; Mukhtar, H.; Mousa, S.A. Photochemoprevention of ultraviolet Beam Radiation-induced DNA damage in keratinocytes by topical delivery of nanoformulated Epigallocatechin-3-gallate. Nanomedicine 2022, 44, 102580. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, M.; Sarwat, M.; Siddique, H.R. Apigenin in Cancer Prevention and Therapy: A Systematic Review and Meta-Analysis of Animal Models. Crit. Rev. Oncol. Hematol. 2022, 176, 103751. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, C.; Balsano, R.; Corianò, M.; Pellegrino, B.; Saba, G.; Bardanzellu, F.; Denaro, N.; Ramundo, M.; Toma, I.; Fusaro, A.; et al. Long-Term Effects of Breast Cancer Therapy and Care: Calm after the Storm? J. Clin. Med. 2022, 11, 7239. [Google Scholar] [CrossRef] [PubMed]

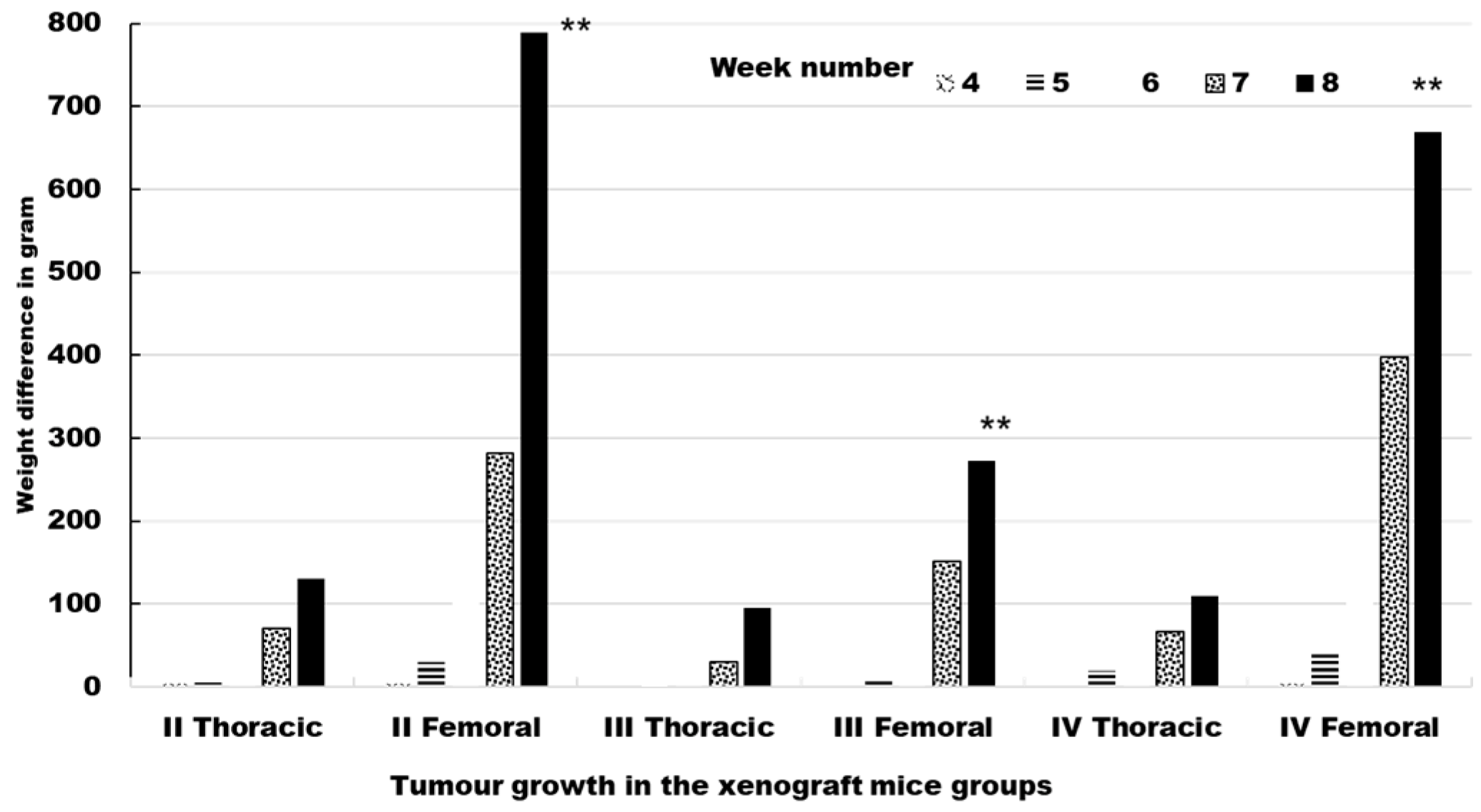
| Time (Week) | |||||
|---|---|---|---|---|---|
| Tumour Site | 4 | 5 | 6 | 7 | 8 |
| II Thoracic | 3.66 ± 0.5 | 5.34 ± 0.3 | 36.12 ± 0.08 | 70.04 ± 0.4 | 130.32 ± 0.7 |
| II Femoral | 3.78 ± 0.6 | 29.42 ± 0.4 | 159.58 ± 0.6 | 278.98 ± 0.9 | 787.9 ± 0.8 |
| III Thoracic | 0 ± 0.0 | 2.08 ± 0.03 | 9.08 ± 0.07 | 30.04 ± 0.3 | 94.98 ± 0.6 |
| III Femoral | 0.84 ± 0.03 | 8.98 ± 0.2 | 15.97 ± 0.05 | 150.98 ± 0.7 | 273.09 ± 0.9 |
| IV Thoracic | 0 ± 0.0 | 18.95 ± 0.3 | 45.89 ± 0.7 | 65.98 ± 0.8 | 109.04 ± 0.8 |
| IV Femoral | 3.89 ± 0.4 | 42.97 ± 0.5 | 189.96 ± 0.8 | 396.94 ± 0.9 | 670.02 ± 0.9 |
| Group | I | II | III | IV | V |
|---|---|---|---|---|---|
| TNF-α (pg/mg protein) | 124.34 ± 5.21 | 297.31 ± 7.81 *** | 167.33 ± 2.68 ** | 176.95 ± 4.38 * | 112.36 ± 5.31 |
| IL-6 (pg/mg protein) | 152 ± 12.67 | 375 ± 19.35 *** | 210.52 ± 4.89 ** | 255.34 ± 3.67 * | 143.57 ± 4.2 |
| Time (Week) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| I | Saline | Saline | Saline | Saline | Saline | Saline | Saline | Saline |
| II | Saline | Saline | MCF-7 cell injection | Saline | Saline | Saline | Saline | Saline |
| III | Asiaticoside | Asiaticoside | MCF-7 cell injection | Asiaticoside | Asiaticoside | Asiaticoside | Asiaticoside | Asiaticoside |
| IV | Saline | Saline | MCF-7 cell injection | Saline | Saline | Asiaticoside | Asiaticoside | Asiaticoside |
| V | Asiaticoside | Asiaticoside | Asiaticoside | Asiaticoside | Asiaticoside | Asiaticoside | Asiaticoside | Asiaticoside |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saeedi, F.J. Asiaticoside Increases Caspase-9 Activity in MCF-7 Cells and Inhibits TNF-α and IL-6 Expression in Nude Mouse Xenografts via the NF-κB Pathway. Molecules 2023, 28, 2101. https://doi.org/10.3390/molecules28052101
Al-Saeedi FJ. Asiaticoside Increases Caspase-9 Activity in MCF-7 Cells and Inhibits TNF-α and IL-6 Expression in Nude Mouse Xenografts via the NF-κB Pathway. Molecules. 2023; 28(5):2101. https://doi.org/10.3390/molecules28052101
Chicago/Turabian StyleAl-Saeedi, Fatma J. 2023. "Asiaticoside Increases Caspase-9 Activity in MCF-7 Cells and Inhibits TNF-α and IL-6 Expression in Nude Mouse Xenografts via the NF-κB Pathway" Molecules 28, no. 5: 2101. https://doi.org/10.3390/molecules28052101
APA StyleAl-Saeedi, F. J. (2023). Asiaticoside Increases Caspase-9 Activity in MCF-7 Cells and Inhibits TNF-α and IL-6 Expression in Nude Mouse Xenografts via the NF-κB Pathway. Molecules, 28(5), 2101. https://doi.org/10.3390/molecules28052101




