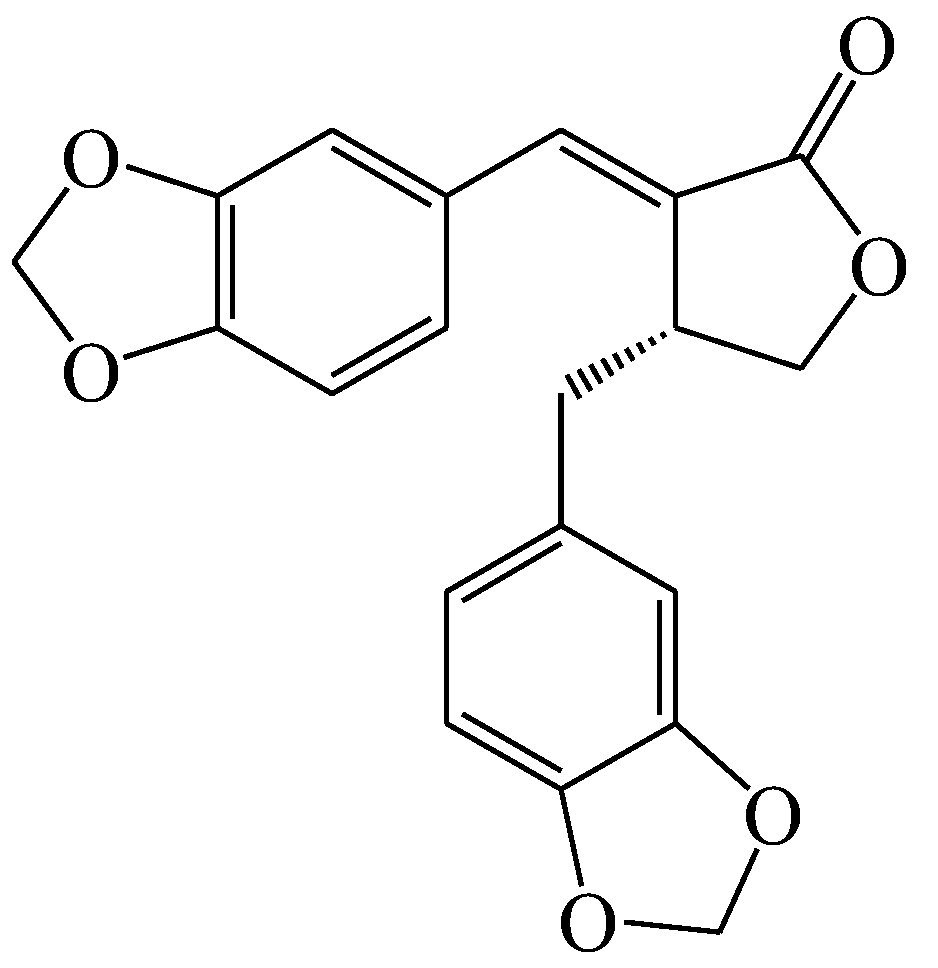
Neuroprotective Effects of Savinin on LPS-Induced Neuroinflammation In Vivo via Regulating MAPK/NF-κB Pathway and NLRP3 Inflammasome Activation
Abstract
1. Introduction
2. Results
2.1. Savinin Ameliorated LPS-Induced Histopathological Changes
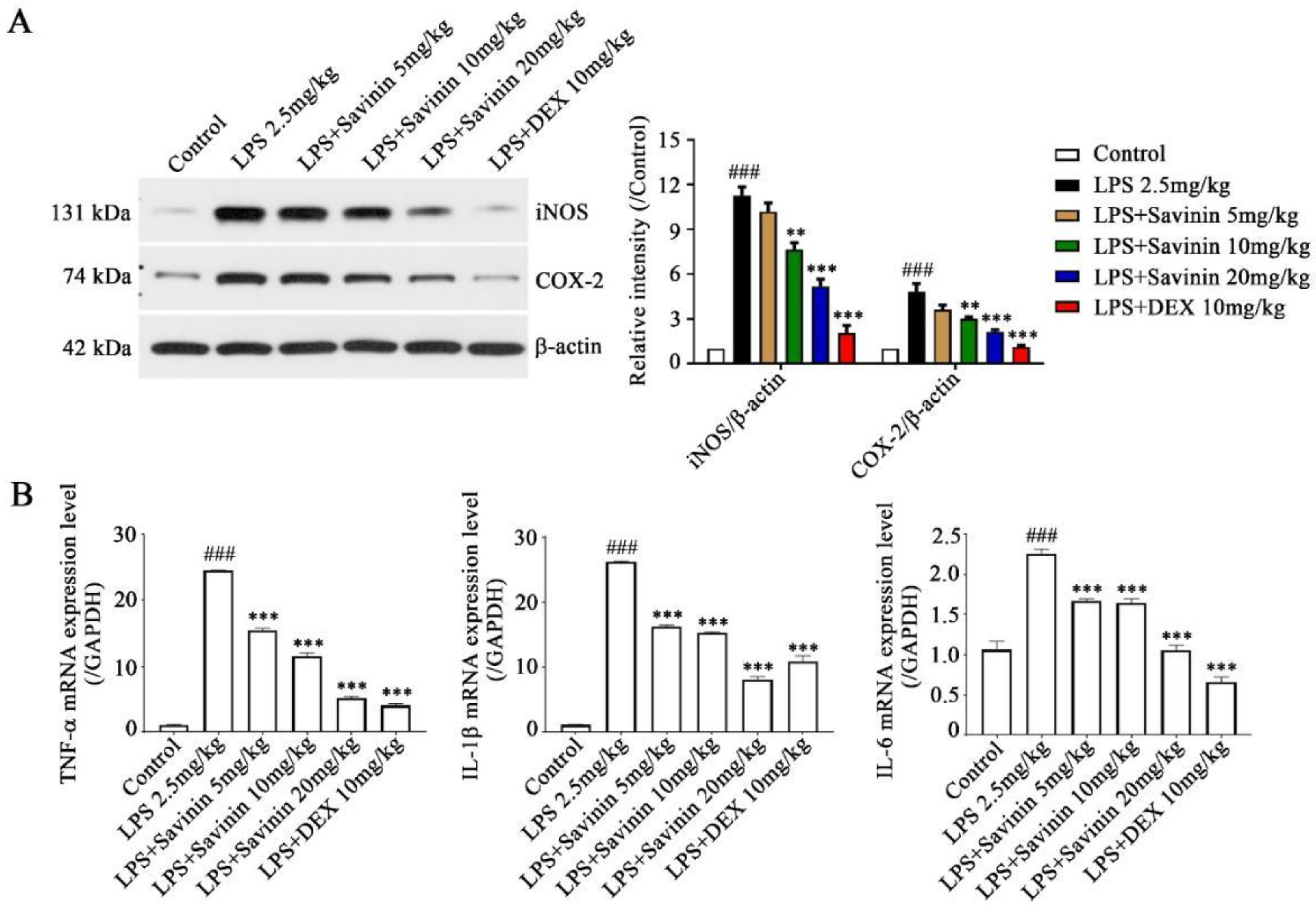
2.2. The Effect of Savinin on Expression of Inflammatory Factors in LPS-Treated Mice
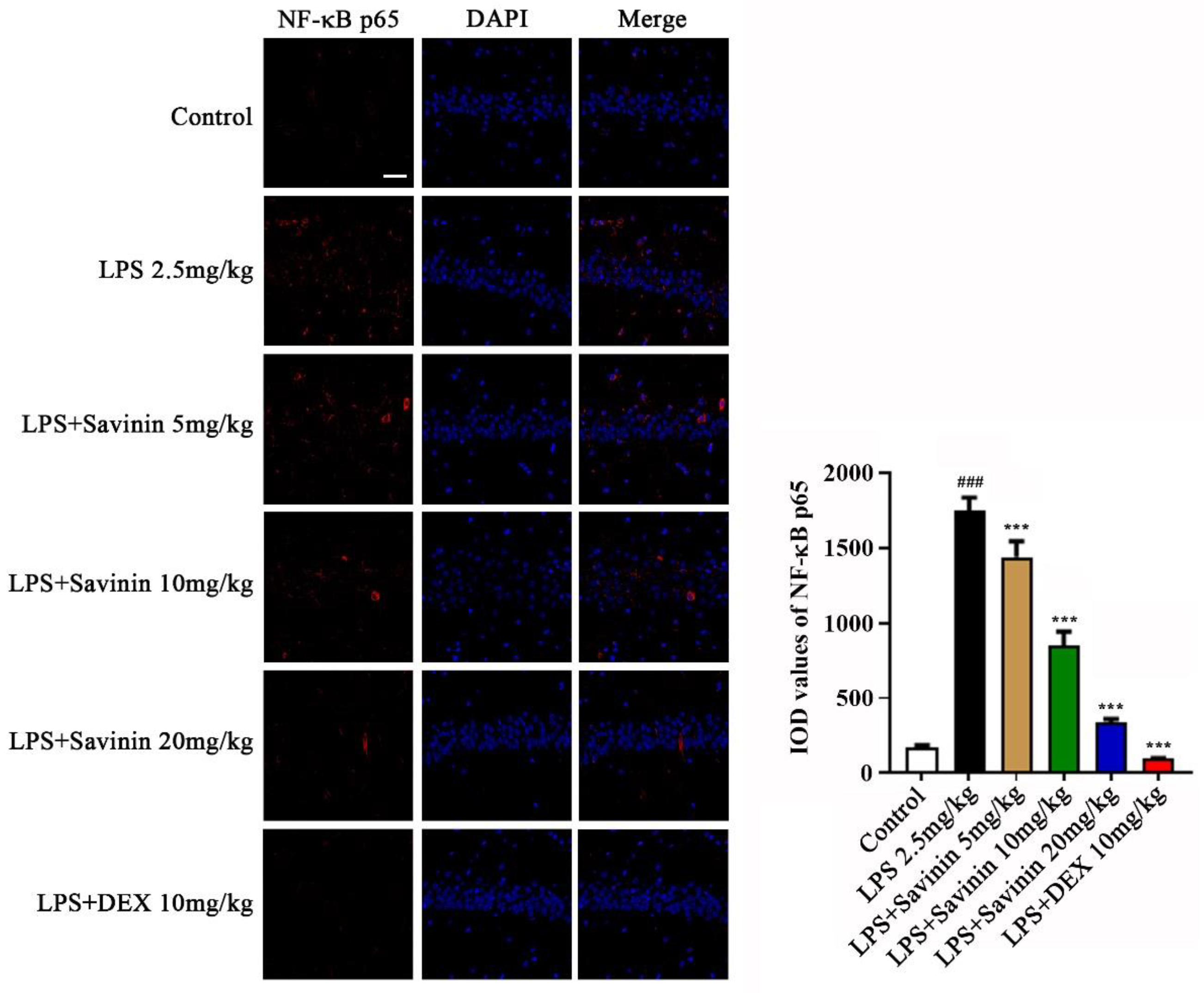
2.3. The Effect of Savinin on MAPK/NF-κB Signaling Pathway
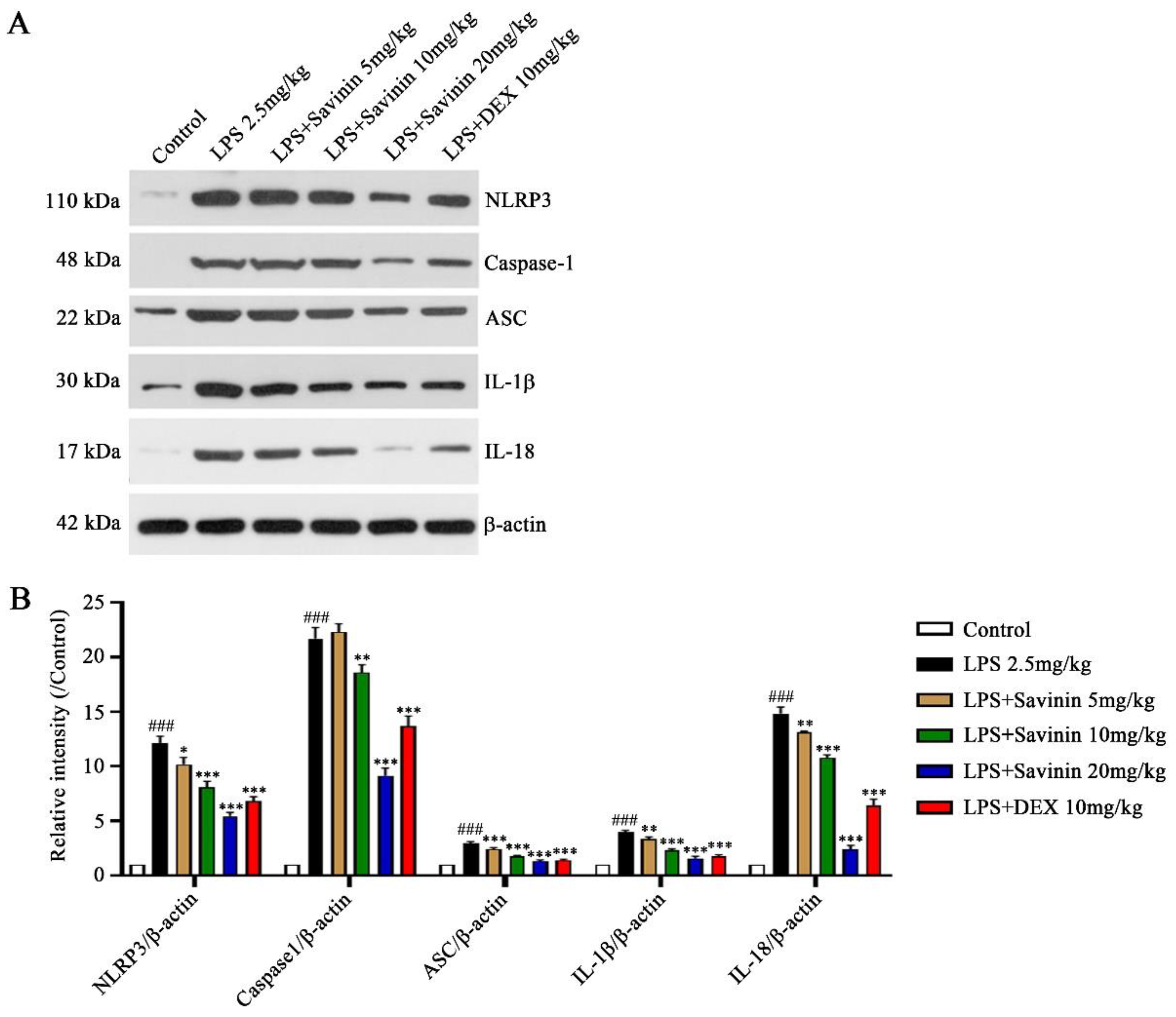
2.4. The Effect of Savinin on Formation of the NLRP3 Inflammasome Complex
2.5. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Experiments on Animals
4.3. Hematoxylin-Eosin (HE) Staining
4.4. Nitrite (NO) and Prostaglandin E2 (PGE2) Determination
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Western Blotting
4.8. Immunofluorescence (IF) Staining
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Chiu, C.C.; Liao, Y.E.; Yang, L.Y.; Wang, J.Y.; Tweedie, D.; Karnati, H.K.; Greig, N.H.; Wang, J.Y. Neuroinflammation in animal models of traumatic brain injury. J. Neurosci. Meth. 2016, 272, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Fakhri, S.; Gravandi, M.M.; Abdian, S.; Akkol, E.K.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective role of polydatin: Neuropharmacological mechanisms, molecular targets, therapeutic potentials, and clinical perspective. Molecules 2021, 26, 5985. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, G.; Bai, Y.; Tao, Y.; Wang, L.; Yang, L.; Wu, H.; Huang, F.; Shi, H.; Wu, X. Natural bear bile powder suppresses neuroinflammation in lipopolysaccharide-treated mice via regulating TGR5/AKT/NF-κB signaling pathway. J. Ethnopharmacol. 2022, 289, 115063. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, R.; Tong, Y.; Chen, P.; Shen, Y.; Miao, S.; Liu, X. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 140, 104814. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.J.; Park, S.K.; Park, J.H.; Jeong, H.R.; Lee, S.; Lee, H.; Seol, E.; Hoe, H.S. Donepezil regulates LPS and Aβ-stimulated neuroinflammation through MAPK/NLRP3 inflammasome/STAT3 signaling. Int. J. Mol. Sci. 2021, 22, 10637. [Google Scholar] [CrossRef]
- Zhu, H.; Jian, Z.; Zhong, Y.; Ye, Y.; Zhang, Y.; Hu, X.; Pu, B.; Gu, L.; Xiong, X. Janus kinase inhibition ameliorates ischemic stroke injury and neuroinflammation through reducing NLRP3 inflammasome activation via JAK2/STAT3 pathway inhibition. Front. Immunol. 2021, 12, 714943. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.; Zhan, F.; Luo, D.; Hua, F.; Xu, G. MicroRNA-138-5p regulates hippocampal neuroinflammation and cognitive impairment by NLRP3/Caspase-1 signaling pathway in rats. J. Inflamm. Res. 2021, 14, 1125–1143. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, C.; Siani, F.; Mus, L.; Ghezzi, C.; Cerri, S.; Pacchetti, B.; Bigogno, C.; Blandini, F. Neuroprotective effects of lignan 7-hydroxymatairesinol (HMR/lignan) in a rodent model of Parkinson’s disease. Nutrition 2020, 69, 110494. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Wu, S.; Wang, G.; Li, B.; Liu, B.; Lei, X. Pinoresinol diglucoside attenuates neuroinflammation, apoptosis and oxidative stress in a mice model with Alzheimer’s disease. Neuroreport 2021, 32, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Chinese Materia Medica of State Administration of Traditional Chinese Medicine. In Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999.
- Li, X.J.; Tang, S.Q.; Huang, H.; Luo, J.; Zhang, X.D.; Yook, C.S.; Whang, W.K.; Kim, Y.C.; Liu, X.Q. Acanthopanax henryi: Review of botany, phytochemistry and pharmacology. Molecules 2021, 26, 2215. [Google Scholar] [CrossRef]
- Li, X.J.; Kim, K.W.; Oh, H.; Liu, X.Q.; Kim, Y.C. Chemical constituents and an antineuroinflammatory lignan, savinin from the roots of Acanthopanax henryi. Evid.-Based Compl. Alt. 2019, 2019, 1856294. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, J.; Kim, P.S.; Yoo, E.S.; Baik, K.U.; Park, M.H. Savinin, a lignan from Pterocarpus santalinus inhibits tumor necrosis factor-alpha production and T cell proliferation. Biol. Pharm. Bull. 2001, 24, 167–171. [Google Scholar] [CrossRef]
- Lee, S.; Ban, H.S.; Kim, Y.P.; Kim, B.K.; Cho, S.H.; Ohuchi, K.; Shin, K.H. Lignans from Acanthopanax chiisanensis having an inhibitory activity on prostaglandin E2 production. Phytother. Res. 2005, 19, 103–106. [Google Scholar] [CrossRef]
- Nissanka, A.P.; Karunaratne, V.; Bandara, B.M.; Kumar, V.; Nakanishi, T.; Nishi, M.; Inada, A.; Tillekeratne, L.M.; Wijesundara, D.S.; Gunatilaka, A.A. Antimicrobial alkaloids from Zanthoxylum tetraspermum and caudatum. Phytochemistry 2001, 56, 857–861. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, H.H.; Piao, X.L.; Kim, J.S.; Kang, S.S.; Shin, K.H. Anti-estrogenic activity of lignans from Acanthopanax chiisanensis root. Arch. Pharm. Res. 2005, 28, 186–189. [Google Scholar] [CrossRef]
- Verma, S.; Twilley, D.; Esmear, T.; Oosthuizen, C.B.; Reid, A.M.; Nel, M.; Lall, N. Anti-SARS-CoV natural products with the potential to inhibit SARS-CoV-2 (COVID-19). Front. Pharmacol. 2020, 11, 561334. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, L.; Tizziani, T.; Souza, G.B.; Moreira, M.A.; Neto, J.; Dos Santos, C.; de Carvalho, M.G.; Dalmarco, E.M.; Turqueti, L.B.; Scotti, M.T.; et al. Natural products with tandem anti-inflammatory, immunomodulatory and anti-SARS-CoV/2 effects: A drug discovery perspective against SARS-CoV-2. Curr. Med. Chem. 2022, 29, 2530–2564. [Google Scholar] [CrossRef] [PubMed]
- Swargiary, A.; Mahmud, S.; Saleh, M.A. Screening of phytochemicals as potent inhibitor of 3-chymotrypsin and papain-like proteases of SARS-CoV2: An in silico approach to combat COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Masdeu, J.C.; Pascual, B.; Fujita, M. Imaging neuroinflammation in neurodegenerative disorders. J. Nucl. Med. 2022, 63 (Suppl. S1), 45S–52S. [Google Scholar] [CrossRef]
- Tatu, L.; Vuillier, F. Structure and vascularization of the human hippocampus. Front. Neurol. Neurosci. 2014, 34, 18–25. [Google Scholar]
- Weerasinghe-Mudiyanselage, P.D.E.; Ang, M.J.; Kang, S.; Kim, J.S.; Moon, C. Structural plasticity of the hippocampus in neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 3349. [Google Scholar] [CrossRef]
- Ju, I.G.; Huh, E.; Kim, N.; Lee, S.; Choi, J.G.; Hong, J.; Oh, M.S. Artemisiae Iwayomogii Herba inhibits lipopolysaccharide-induced neuroinflammation by regulating NF-κB and MAPK signaling pathways. Phytomedicine 2021, 84, 153501. [Google Scholar] [CrossRef]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef]
- Milner, M.T.; Maddugoda, M.; Götz, J.; Burgener, S.S.; Schroder, K. The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer’s disease. Curr. Opin. Immunol. 2021, 68, 116–124. [Google Scholar] [CrossRef]
- O’Brien, W.T.; Pham, L.; Symons, G.F.; Monif, M.; Shultz, S.R.; McDonald, S.J. The NLRP3 inflammasome in traumatic brain injury: Potential as a biomarker and therapeutic target. J. Neuroinflamm. 2020, 17, 104. [Google Scholar] [CrossRef]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The role of the inflammasome in neurodegenerative diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Xie, G.; Wei, M.; Wang, P.; Chen, L. The protective effect of Epimedii Folium and Curculiginis Rhizoma on Alzheimer’s disease by the inhibitions of NF-κB/MAPK pathway and NLRP3 inflammasome. Oncotarget 2017, 8, 43709–43720. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Chen, L.; Chen, L.; Tan, A.; Xie, G.; Long, Q.; Ning, F.; Lan, Z.; Wang, P. Epimedii Folium and Curculiginis Rhizoma ameliorate lipopolysaccharides-induced cognitive impairment by regulating the TREM2 signaling pathway. J. Ethnopharmacol. 2022, 284, 114766. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Xu, M.; Li, T.; Fan, K.; Yan, T.; Xiao, F.; Bi, K.; Jia, Y. Nootkatone, a neuroprotective agent from Alpiniae Oxyphyllae Fructus, improves cognitive impairment in lipopolysaccharide-induced mouse model of Alzheimer’s disease. Int. Immunopharmacol. 2018, 62, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yan, T.; Gong, G.; Wu, B.; He, B.; Du, Y.; Xiao, F.; Jia, Y. Purification, structural characterization, and cognitive improvement activity of a polysaccharides from Schisandra chinensis. Int. J. Biol. Macromol. 2020, 163, 497–507. [Google Scholar] [CrossRef]
- Dong, D.; Xu, Z.; Zhong, W.; Peng, S. Parallelization of molecular docking: A review. Curr. Top. Med. Chem. 2018, 18, 1015–1028. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular docking: Shifting paradigms in drug discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]






| Receptors | Binding Affinity (kcal/mol) | Binding Residues |
|---|---|---|
| MAPK13 | −7.1 | T92 (Thr92), K6 (Lys6), F9 (Phe9), P22 (Pro22), T24 (Thr24), R46 (Arg46), P351 (Pro351), D89 (Asp89), V90 (Val90), F91 (Phe91), I346 (Ile346) |
| NF-κB | −6.7 | H142 (His142), L174 (Leu174), T164 (Thr164), V72 (Val72), E101 (Glu101), R73 (Arg73), Q162 (Gln162), N138 (Asn138), N139 (Asn139) |
| NLRP3 | −9.1 | Y381 (Tyr381), R167 (Arg167), I151 (Ile151), I234 (Ile234), T233 (Thr233), G231 (Gly231), H522 (His522), I521 (Ile521), P412 (Pro412), L413 (Leu413), W416 (Trp416) |
| Gene | Sequence (5’-3’) |
|---|---|
| GAPDH | Forward: TCAACGGCACAGTCAAGG Reverse: TGAGCCCTTCCACGATG |
| TNF-α | Forward: ATCCGCGACGTGGAACTG Reverse: ACCGCCTGGAGTTCTGGAA |
| IL-1β | Forward: GAGCACCTTCTmCCTTCATCTT Reverse: TCACACACCAGCAGGTT |
| IL-6 | Forward: TCCGGAGAGGAGACTTCACA Reverse: TTGCCATTGCACAACTCTTTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Li, C.; Suo, Z.; Xu, Y.; Wei, K.; Zhao, L.; Huang, H.; Liu, X.; Liu, D.; Li, X. Neuroprotective Effects of Savinin on LPS-Induced Neuroinflammation In Vivo via Regulating MAPK/NF-κB Pathway and NLRP3 Inflammasome Activation. Molecules 2023, 28, 1575. https://doi.org/10.3390/molecules28041575
Tang S, Li C, Suo Z, Xu Y, Wei K, Zhao L, Huang H, Liu X, Liu D, Li X. Neuroprotective Effects of Savinin on LPS-Induced Neuroinflammation In Vivo via Regulating MAPK/NF-κB Pathway and NLRP3 Inflammasome Activation. Molecules. 2023; 28(4):1575. https://doi.org/10.3390/molecules28041575
Chicago/Turabian StyleTang, Siqi, Chunying Li, Zongwu Suo, Yi Xu, Kaixin Wei, Lei Zhao, Hao Huang, Xiangqian Liu, Dongxu Liu, and Xiaojun Li. 2023. "Neuroprotective Effects of Savinin on LPS-Induced Neuroinflammation In Vivo via Regulating MAPK/NF-κB Pathway and NLRP3 Inflammasome Activation" Molecules 28, no. 4: 1575. https://doi.org/10.3390/molecules28041575
APA StyleTang, S., Li, C., Suo, Z., Xu, Y., Wei, K., Zhao, L., Huang, H., Liu, X., Liu, D., & Li, X. (2023). Neuroprotective Effects of Savinin on LPS-Induced Neuroinflammation In Vivo via Regulating MAPK/NF-κB Pathway and NLRP3 Inflammasome Activation. Molecules, 28(4), 1575. https://doi.org/10.3390/molecules28041575






