Clinical Evaluation Based on a New Approach to Improve the Accuracy of 4β-Hydroxycholesterol Measurement as a Biomarker of CYP3A4 Activity
Abstract
1. Introduction
| A | B | C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Storage Conditions Temperature/Period | Human Plasma | Acidified Human Plasma | Human Serum | Storage Conditions Temperature/Period | Human Plasma | Storage Conditions Temperature/Period | Human Plasma | |||||
| K2-EDTA | Heparin Sodium | Heparin Lithium | 3.2% Sodium Citrate | K2-EDTA Containing Acetic Acid | Heparin Sodium Containing Acetic Acid | K2-EDTA | Heparin Sodium | K2-EDTA | ||||
| Before storage | 27.9 | 28.3 | 27.0 | 23.6 | 26.6 | 27.5 | 28.4 | Before storage | 32.5 | 30.0 | Before storage | 35.2 |
| −20 °C/1 month | 25.3 ± 0.6 | 26.5 ± 0.6 | 28.5 ± 1.8 | 22.6 ± 1.2 | 26.8 ± 1.3 | 25.9 ± 1.6 | 27.8 ± 2.2 | −20 °C/6 months | 30.3 ± 2.2 | 30.0 ± 1.6 | −70 °C/1 month | 35.3 ± 0.5 |
| (−9.3) | (−6.4) | (5.6) | (−4.2) | (0.8) | (−5.8) | (−2.1) | (−6.8) | (0.0) | (0.3) | |||
| −20 °C/2 months | 26.2 ± 2.4 | 25.2 ± 2.7 | 26.4 ± 0.8 | 22.2 ± 1.2 | 25.4 ± 2.0 | 25.9 ± 1.6 | 26.3 ± 0.9 | −20 °C/9 months | 37.4 ± 0.2 | 35.8 ± 0.9 | −70 °C/3 month | 33.4 ± 0.5 |
| (−6.1) | (−11.0) | (−2.2) | (−5.9) | (−4.5) | (−5.8) | (−7.4) | (* 15.1) | (* 19.3) | (−5.1) | |||
| −20 °C/6 months | 30.9 ± 1.2 | 29.2 ± 2.4 | 30.7 ± 1.4 | 24.9 ± 0.9 | 31.0 ± 0.7 | 41.2 ± 1.2 | 28.2 ± 3.7 | −20 °C/13 months | 45.7 ± 0.9 | 41.5 ± 0.5 | −70 °C/10 month | 35.7 ± 1.2 |
| (10.8) | (3.2) | (13.7) | (5.5) | (* 16.5) | (* 49.8) | (5.6) | (* 40.6) | (* 38.3) | (1.4) | |||
| −80 °C/1 month | 25.5 ± 1.0 | 26.1 ± 0.8 | 28.0 ± 0.2 | 24.2 ± 0.9 | 26.5 ± 1.0 | 26.5 ± 0.5 | 29.7 ± 1.0 | −80 °C/6 months | 29.5 ± 1.2 | 29.1 ± 1.1 | ||
| (−8.6) | (−7.8) | (3.7) | (2.5) | (−0.4) | (−3.6) | (4.6) | (−9.2) | (−3.0) | ||||
| −80 °C/2 months | 25.7 ± 1.4 | 26.0 ± 0.6 | 24.7 ± 0.8 | 22.3 ± 0.7 | 27.2 ± 0.5 | 26.2 ± 1.3 | 25.7 ± 1.7 | −80 °C/9 months | 32.0 ± 1.0 | 30.9 ± 1.3 | ||
| (−7.9) | (−8.1) | (−8.5) | (−5.5) | (2.3) | (−4.7) | (−9.5) | (−1.5) | (3.0) | ||||
| −80 °C/6 months | 29.7 ± 0.6 | 28.7 ± 1.2 | 28.3 ± 0.7 | 24.2 ± 0.8 | 28.7 ± 0.6 | 29.4 ± 0.3 | 29.0 ± 0.4 | −80 °C/13 months | 33.8 ± 1.4 | 33.1 ± 1.3 | ||
| (6.5) | (1.4) | (4.8) | (2.5) | (7.9) | (6.9) | (2.1) | (4.0) | (10.3) | ||||
2. Results and Discussion
2.1. Stability of 4β-HC in Plasma/Serum Collected with Various Anticoagulants and Acid Solutions
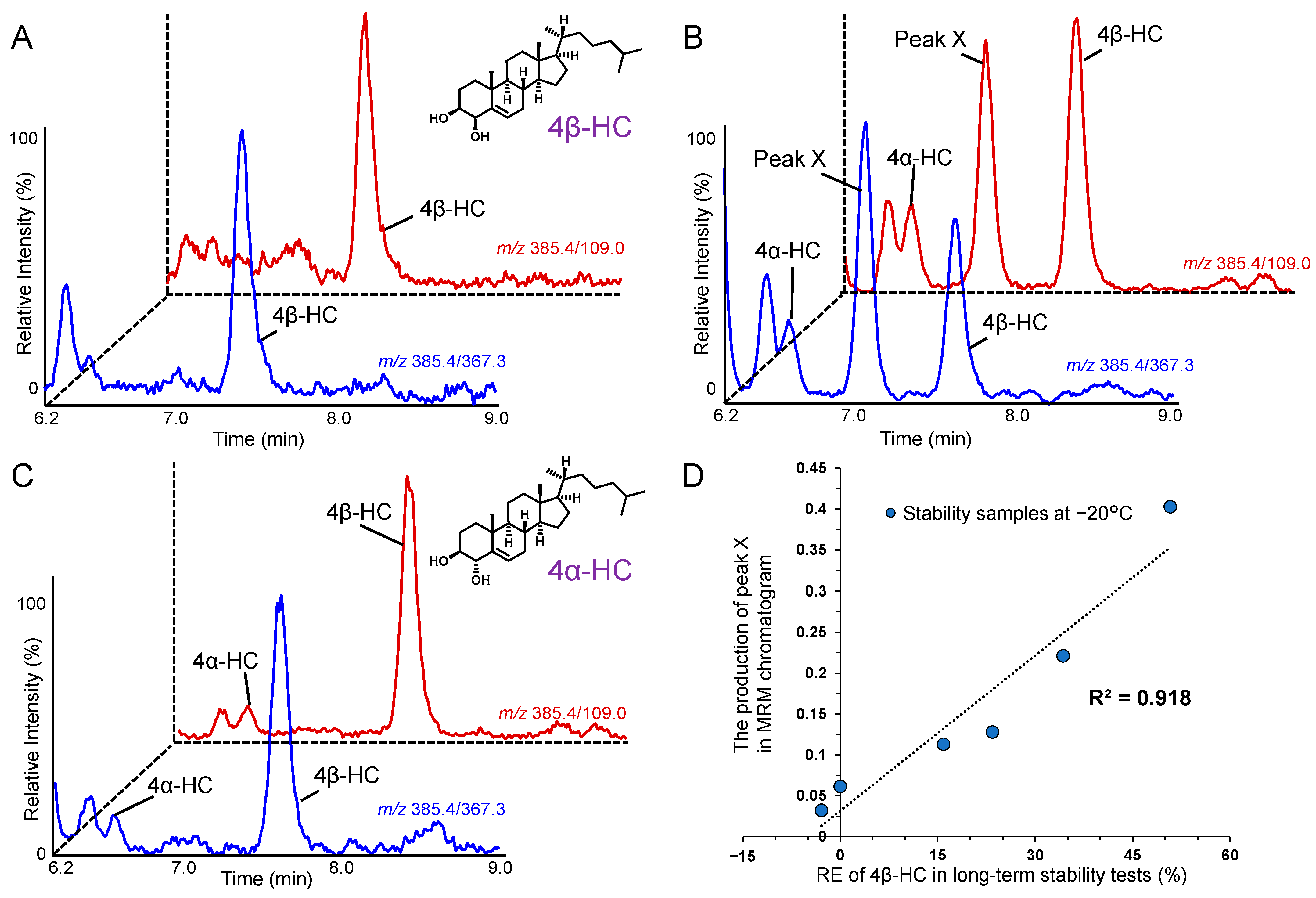
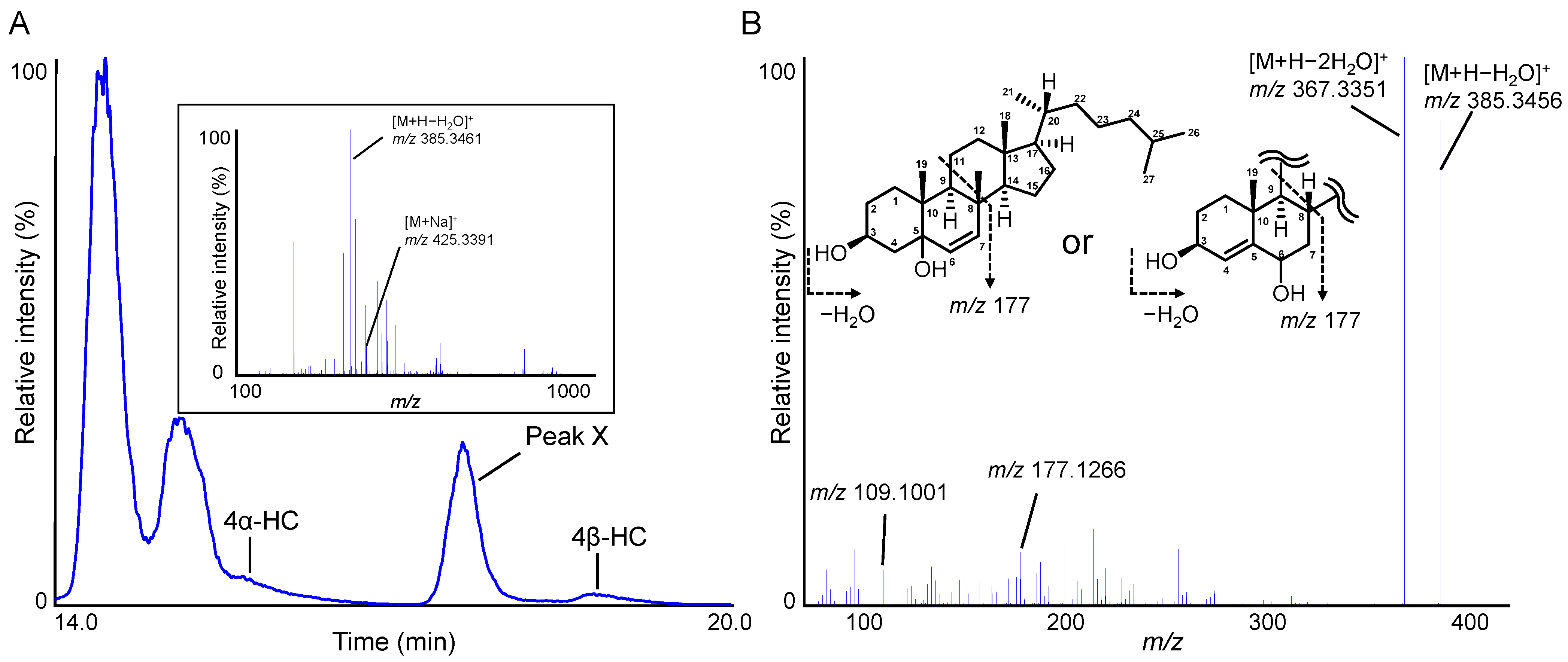
2.2. Indicators to Detect Defective Plasma That Contribute to Improving the Accuracy of 4β-HC Measurements in Plasma
2.3. DDI Studies to Evaluate Whether Weak Inducers of CYP3A4 (JTE-451 and JTE-761) Affect Plasma 4β-HC Levels and the AUC of Midazolam
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Investigation of Plasma Collection Methods, Storage Conditions, and Indicators of Defective Plasma Samples
3.3. Clinical DDI Studies of NCEs (JTE-451 and JTE-761)
3.4. LC-MS/MS Analysis of 4β-HC in Plasma and Serum
3.5. LC-HRMS Analysis of a Potential Indicator to Detect Defective Plasma
3.6. Other Measurements in Clinical DDI Studies
3.7. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bodin, K.; Andersson, U.; Rystedt, E.; Ellis, E.; Norlin, M.; Pikuleva, I.; Eggertsen, G.; Björkhem, I.; Diczfalusy, U. Metabolism of 4β-hydroxycholesterol in humans. J. Biol. Chem. 2002, 277, 31534–31540. [Google Scholar] [CrossRef]
- Bodin, K.; Bretillon, L.; Aden, Y.; Bertilsson, L.; Broomé, U.; Einarsson, C.; Diczfalusy, U. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterolin humans: Evidence for involvement of cytochrome P450 3A4. J. Biol. Chem. 2001, 276, 38685–38689. [Google Scholar] [CrossRef]
- Dutreix, C.; Lorenzo, S.; Wang, Y. Comparison of two endogenous biomarkers of CYP3A4 activity in a drug–drug interaction study between midostaurin and rifampicin. Eur. J. Clin. Pharmacol. 2014, 70, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Björkhem-Bergman, L.; Bäckström, T.; Nylén, H.; Rönquist-Nii, Y.; Bredberg, E.; Andersson, T.B.; Bertilsson, L.; Diczfalusy, U. Quinine compared to 4β-hydroxycholesterol and midazolam as markers for CYP3A induction by rifampicin. Drug Metab. Pharmacokinet. 2014, 29, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Björkhem-Bergman, L.; Bäckström, T.; Nylén, H.; Rönquist-Nii, Y.; Bredberg, E.; Andersson, T.B.; Bertilsson, L.; Diczfalusy, U. Comparison of endogenous 4β-hydroxycholesterol with midazolam as markers for CYP3A4 induction by rifampicin. Drug Metab. Dispos. 2013, 41, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.J.; Hoffmann, M.; Tong, Z.; Wu, X.I.; Vallejo, M.; Melo, B.; Ye, Y.; Thomas, M.; Liu, Y.; Weiss, D.; et al. Use of 4β-hydroxycholesterol in animal and human plasma samples as a biomarker for CYP3A induction. Bioanalysis 2016, 8, 215–228. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Boulton, D.W.; Luo, W.L.; Rodrigues, A.D.; Yang, Z.; Goodenough, A.; Lee, M.; Jemal, M.; LaCreta, F. Validation of 4β-hydroxycholesterol and evaluation of other endogenous biomarkers for the assessment of CYP3A activity in healthy subjects. Br. J. Clin. Pharmacol. 2014, 78, 1122–1134. [Google Scholar] [CrossRef]
- Mao, J.; Martin, I.; McLeod, J.; Nolan, G.; van Horn, R.; Vourvahis, M.; Lin, Y.S. Perspective: 4β-hydroxycholesterol as an emerging endogenous biomarker of hepatic CYP3A. Drug Metab. Rev. 2017, 49, 18–34. [Google Scholar] [CrossRef]
- Dakappagari, N.; Zhang, H.; Stephen, L.; Amaravadi, L.; Khan, M.U. Recommendations for clinical biomarker specimen preservation and stability assessments. Bioanalysis 2017, 9, 643–653. [Google Scholar] [CrossRef]
- Jemal, M.; Ouyang, Z.; Xia, Y.Q. Systematic LC-MS/MS bioanalytical method development that incorporates plasma phospholipids risk avoidance, usage of incurred sample and well thought-out chromatography. Biomed. Chromatogr. 2010, 24, 2–19. [Google Scholar] [CrossRef]
- Hilhorst, M.; van Amsterdam, P.; Heinig, K.; Zwanziger, E.; Abbott, R. Stabilization of clinical samples collected for quantitative bioanalysis–a reflection from the European Bioanalysis Forum. Bioanalysis 2015, 7, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, A.K.; Onorato, J.M.; Ouyang, Z.; Chang, S.; Rodrigues, A.D.; Kasichayanula, S.; Huang, S.P.; Turley, W.; Burrell, R.; Bifano, M.; et al. Quantification of 4-beta-hydroxycholesterol in human plasma using automated sample preparation and LC-ESI-MS/MS analysis. Chem. Res. Toxicol. 2011, 24, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, Y.; Smith, L.; Edom, R.; Weng, N.; Mamidi, R.; Silva, J.; Evans, D.C.; Lim, H.K. LC–ESI-MS/MS quantification of 4β-hydroxycholesterol and cholesterol in plasma samples of limited volume. J. Pharm. Biomed. Anal. 2013, 85, 145–154. [Google Scholar] [CrossRef] [PubMed]
- van de Merbel, N.C.; Bronsema, K.J.; van Hout, M.W.; Nilsson, R.; Sillén, H. A validated liquid chromatography–tandem mass spectrometry method for the quantitative determination of 4β-hydroxycholesterol in human plasma. J. Pharm. Biomed. Anal. 2011, 55, 1089–1095. [Google Scholar] [CrossRef]
- Hasan, M.; Siegmund, W.; Oswald, S. Rapid LC–MS/MS method for the determination of 4-hydroxycholesterol/cholesterol ratio in serum as endogenous biomarker for CYP3A activity in human and foals. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1033, 193–199. [Google Scholar] [CrossRef]
- Diczfalusy, U.; Nylén, H.; Elander, P.; Bertilsson, L. 4β-hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br. J. Clin. Pharmacol. 2011, 71, 183–189. [Google Scholar] [CrossRef]
- Suzuki, Y.; Oda, A.; Negami, J.; Toyama, D.; Tanaka, R.; Ono, H.; Ando, T.; Shin, T.; Mimata, H.; Itoh, H.; et al. Sensitive UHPLC-MS/MS quantification method for 4β-and 4α-hydroxycholesterol in plasma for accurate CYP3A phenotyping. J. Lipid. Res. 2022, 63, 100184. [Google Scholar] [CrossRef]
- Japan Tobacco Inc. Available online: https://www.jt.com/investors/results/S_information/pharmaceuticals/index.html (accessed on 11 December 2022).
- Fuhr, U.; Jetter, A.; Kirchheiner, J. Appropriate phenotyping procedures for drug metabolizing enzymes and transporters in humans and their simultaneous use in the “cocktail” approach. Clin. Pharmacol. Ther. 2007, 81, 270–283. [Google Scholar] [CrossRef]
- Aubry, A.F.; Dean, B.; Diczfalusy, U.; Goodenough, A.; Iffland, A.; McLeod, J.; Weng, N.; Yang, Z. Recommendations on the development of a bioanalytical assay for 4β-hydroxycholesterol, an emerging endogenous biomarker of CYP3A activity. AAPS J. 2016, 18, 1056–1066. [Google Scholar] [CrossRef]
- The ICH M10 Guideline on Bioanalytical Method Validation and Study Sample Analysis (24 May 2022). Available online: https://database.ich.org/sites/default/files/M10_Guideline_Step4_2022_0524.pdf (accessed on 11 December 2022).
- van de Merbel, N.; Savoie, N.; Yadav, M.; Ohtsu, Y.; White, J.; Riccio, M.F.; Dong, K.; de Vries, R.; Diancin, J. Stability: Recommendation for best practices and harmonization from the Global Bioanalysis Consortium Harmonization Team. AAPS J. 2014, 16, 392–399. [Google Scholar] [CrossRef]
- Redrup, M.J.; Igarashi, H.; Schaefgen, J.; Lin, J.; Geisler, L.; Ben M’Barek, M.; Ramachandran, S.; Cardoso, T.; Hillewaert, V. Sample management: Recommendation for best practices and harmonization from the global bioanalysis consortium harmonization team. AAPS J. 2016, 18, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, Z.A.; Pratt, D.A. Cholesterol autoxidation revisited: Debunking the dogma associated with the most vilified of lipids. J. Am. Chem. Soc. 2016, 138, 6932–6935. [Google Scholar] [CrossRef]
- Zielinski, Z.A.; Pratt, D.A. H-atom abstraction vs addition: Accounting for the diverse product distribution in the autoxidation of cholesterol and its esters. J. Am. Chem. Soc. 2019, 141, 3037–3051. [Google Scholar] [CrossRef]
- Helberg, J.; Pratt, D.A. Autoxidation vs. antioxidants—The fight for forever. Chem. Soc. Rev. 2021, 50, 7343–7358. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Q.; Gosset, J.R.; Fahmi, O.A.; Lin, Z.; Chabot, J.R.; Terra, S.G.; Le, V.; Chidsey, K.; Nouri, P.; Kim, A.; et al. Examination of the human cytochrome P450 3A4 induction potential of PF-06282999, an irreversible myeloperoxidase inactivator: Integration of preclinical, in silico, and biomarker methodologies in the prediction of the clinical outcome. Drug Metab. Dispos. 2017, 45, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rodrigues, A.D. Does the long plasma half-life of 4β-hydroxycholesterol impact its utility as a cytochrome P450 3A (CYP3A) metric? J. Clin. Pharmacol. 2010, 50, 1330–1338. [Google Scholar] [CrossRef]
- The FDA Guideline on Clinical Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry (January 2020). Available online: https://www.fda.gov/media/134581/download (accessed on 11 December 2022).
- Umezawa, H.; Mizuno, S.; Yamazaki, H.; Nitta, K. Inhibition of DNA-dependent RNA synthesis by rifamycins. J. Antibiot. 1968, 21, 234–236. [Google Scholar] [CrossRef]
- Sugimoto, H.; Kakehi, M.; Satomi, Y.; Kamiguchi, H.; Jinno, F. Method development for the determination of 24S-hydroxycholesterol in human plasma without derivatization by high-performance liquid chromatography with tandem mass spectrometry in atmospheric pressure chemical ionization mode. J. Sep. Sci. 2015, 38, 3516–3524. [Google Scholar] [CrossRef]
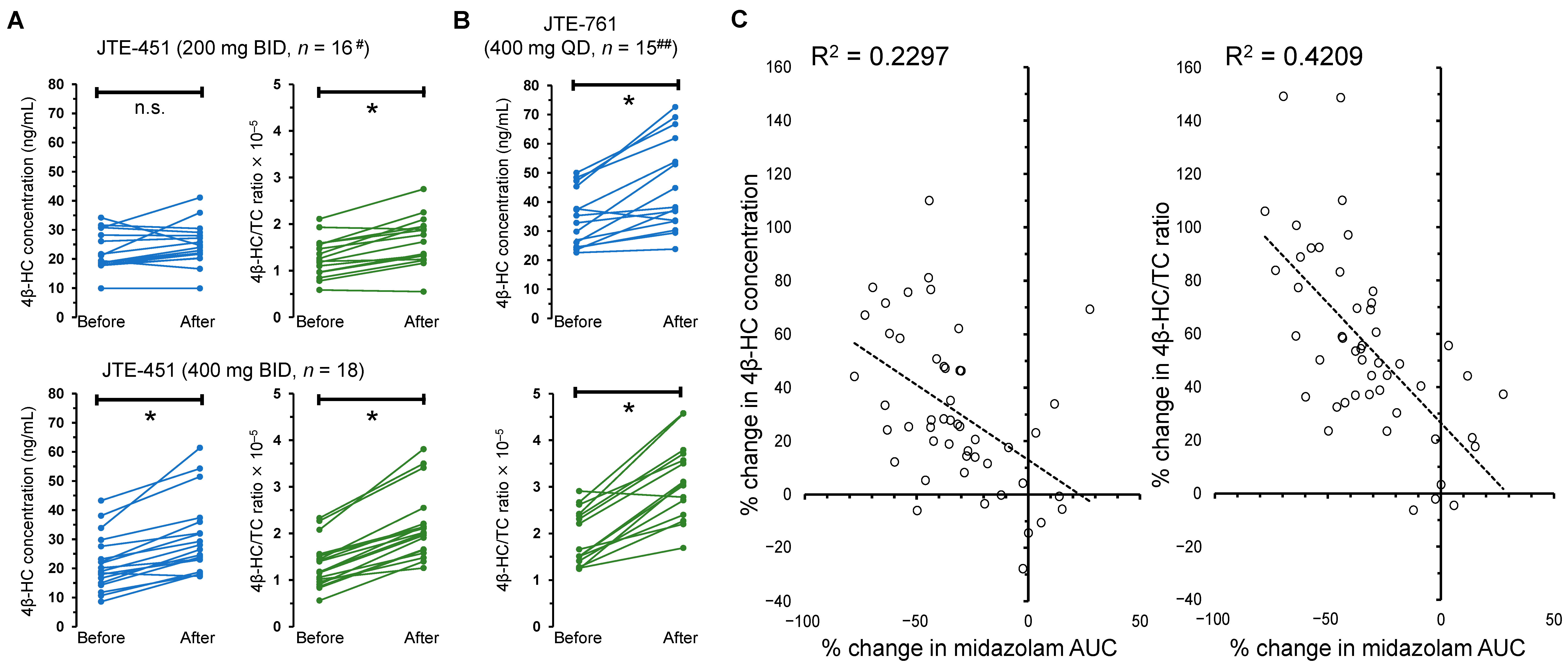
| Parameter | JTE-451 200 mg BID (n = 16 #) | JTE-451 400 mg BID (n = 18) | JTE-761 400 mg QD (n = 15 ##) | |
|---|---|---|---|---|
| 4β-HC concentration (ng/mL) | Before administration | 22.7 ± 6.7 | 21.8 ± 9.5 | 34.1 ± 9.8 |
| After administration | 25.0 ± 7.3 | 30.8 ± 13.0 | 45.6 ± 16.0 | |
| % change (before vs. after) | 11.8 ± 22.4 | 46.1 ± 28.9 | 33.6 ± 25.7 | |
| Significance (before vs. after) | p = 0.059 | p < 0.01 | p < 0.01 | |
| TC concentration (mg/dL) | Before administration | 177.9 ± 35.0 | 163.5 ± 27.0 | 181.9 ± 38.3 |
| After administration | 157.6 ± 35.1 | 141.7 ± 26.5 | 145.0 ± 27.3 | |
| % change (before vs. after) | −13.3 ± 13.1 | −13.4 ± 6.9 | −19.7 ± 7.7 | |
| 4β-HC/TC ratio (×105) | Before administration | 1.28 ± 0.42 | 1.32 ± 0.50 | 1.93 ± 0.59 |
| After administration | 1.65 ± 0.53 | 2.16 ± 0.73 | 3.13 ± 0.84 | |
| % change (before vs. after) | 29.3 ± 19.4 | 68.5 ± 30.6 | 68.1 ± 37.0 | |
| Significance (before vs. after) | p < 0.01 | p < 0.01 | p < 0.01 | |
| Midazolam AUC (hr × ng/mL) | Before administration | 39.2 ± 11.5 | 39.7 ± 14.9 | 40.8 ± 16.8 |
| After administration | 36.3 ± 10.5 | 22.8 ± 7.9 | 18.9 ± 7.8 | |
| % change (before vs. after) | −7.0 ± 18.8 | −40.3 ± 11.7 | −49.8 ± 20.9 | |
| CYP3A4 induction category | Very weak | Weak | Weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taya, Y.; Mizunaga, M.; Nakao, S.; Jutanom, M.; Shimizu, N.; Nomura, Y.; Nakagawa, K. Clinical Evaluation Based on a New Approach to Improve the Accuracy of 4β-Hydroxycholesterol Measurement as a Biomarker of CYP3A4 Activity. Molecules 2023, 28, 1576. https://doi.org/10.3390/molecules28041576
Taya Y, Mizunaga M, Nakao S, Jutanom M, Shimizu N, Nomura Y, Nakagawa K. Clinical Evaluation Based on a New Approach to Improve the Accuracy of 4β-Hydroxycholesterol Measurement as a Biomarker of CYP3A4 Activity. Molecules. 2023; 28(4):1576. https://doi.org/10.3390/molecules28041576
Chicago/Turabian StyleTaya, Yuki, Mari Mizunaga, Shunsuke Nakao, Mirinthorn Jutanom, Naoki Shimizu, Yukihiro Nomura, and Kiyotaka Nakagawa. 2023. "Clinical Evaluation Based on a New Approach to Improve the Accuracy of 4β-Hydroxycholesterol Measurement as a Biomarker of CYP3A4 Activity" Molecules 28, no. 4: 1576. https://doi.org/10.3390/molecules28041576
APA StyleTaya, Y., Mizunaga, M., Nakao, S., Jutanom, M., Shimizu, N., Nomura, Y., & Nakagawa, K. (2023). Clinical Evaluation Based on a New Approach to Improve the Accuracy of 4β-Hydroxycholesterol Measurement as a Biomarker of CYP3A4 Activity. Molecules, 28(4), 1576. https://doi.org/10.3390/molecules28041576






