Optimizing the Distillation of Greek Oregano—Do Process Parameters Affect Bioactive Aroma Constituents and In Vitro Antioxidant Activity?
Abstract
1. Introduction
2. Results and Discussion
2.1. Distillation Yields
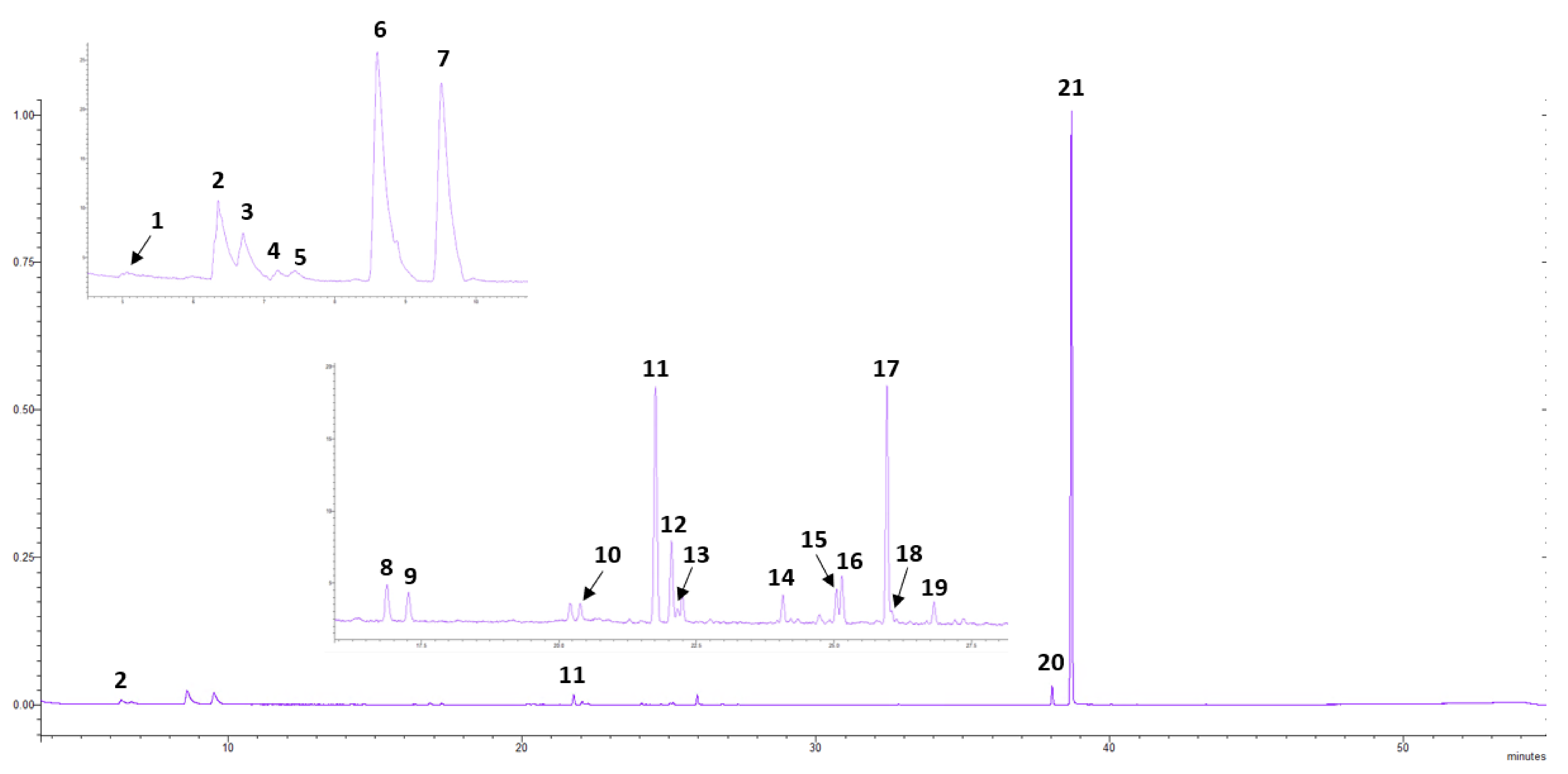
2.2. Headspace GC-MS Analysis
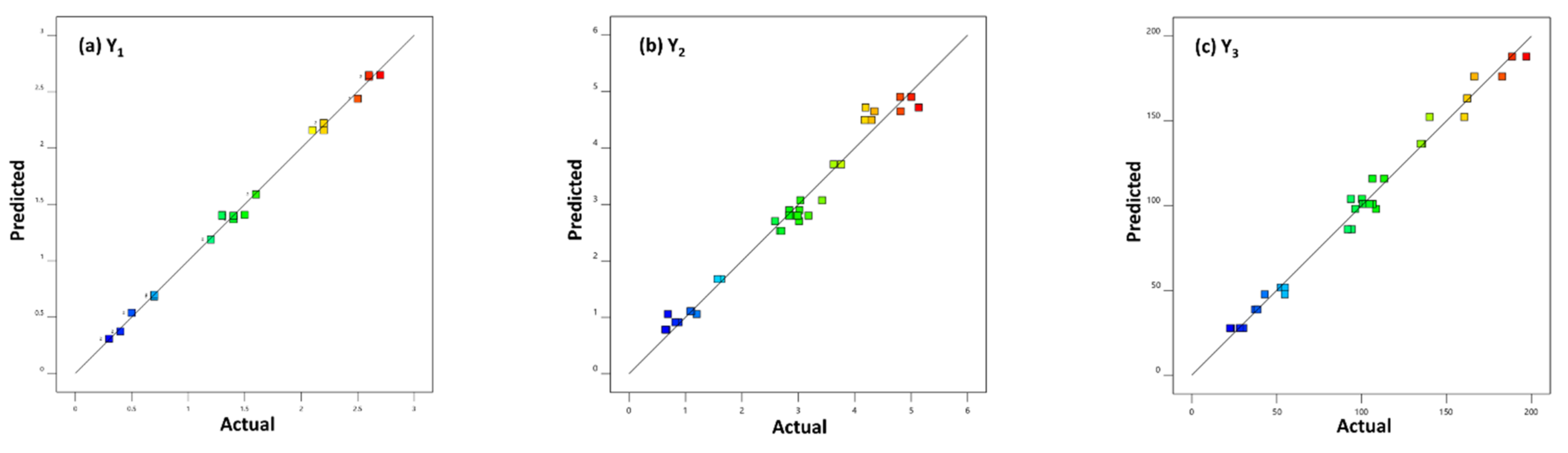
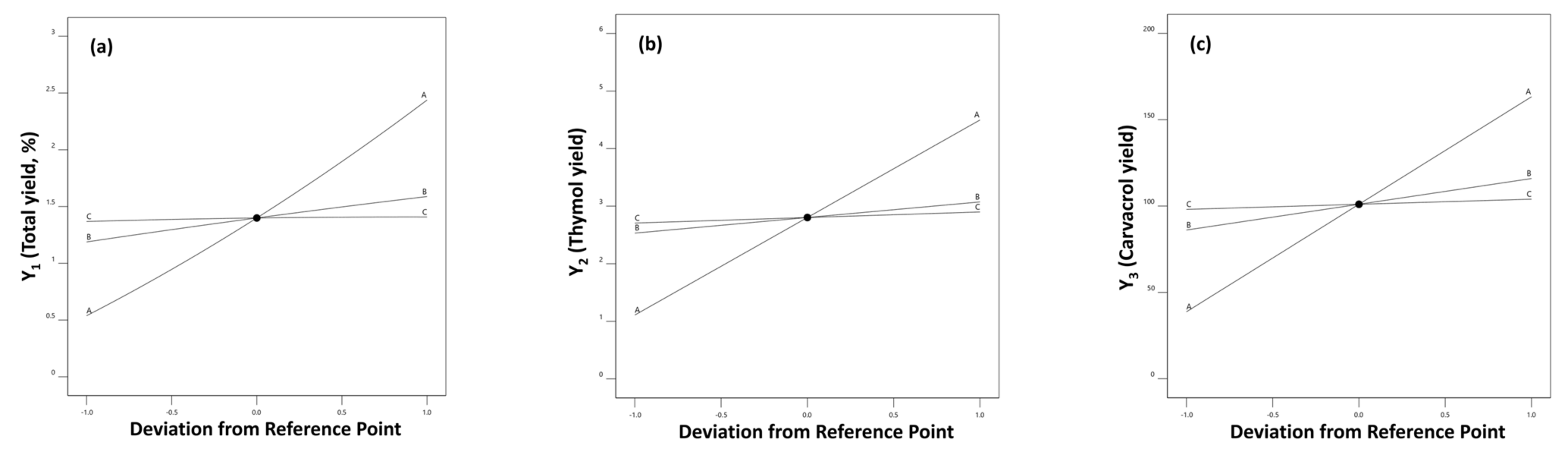
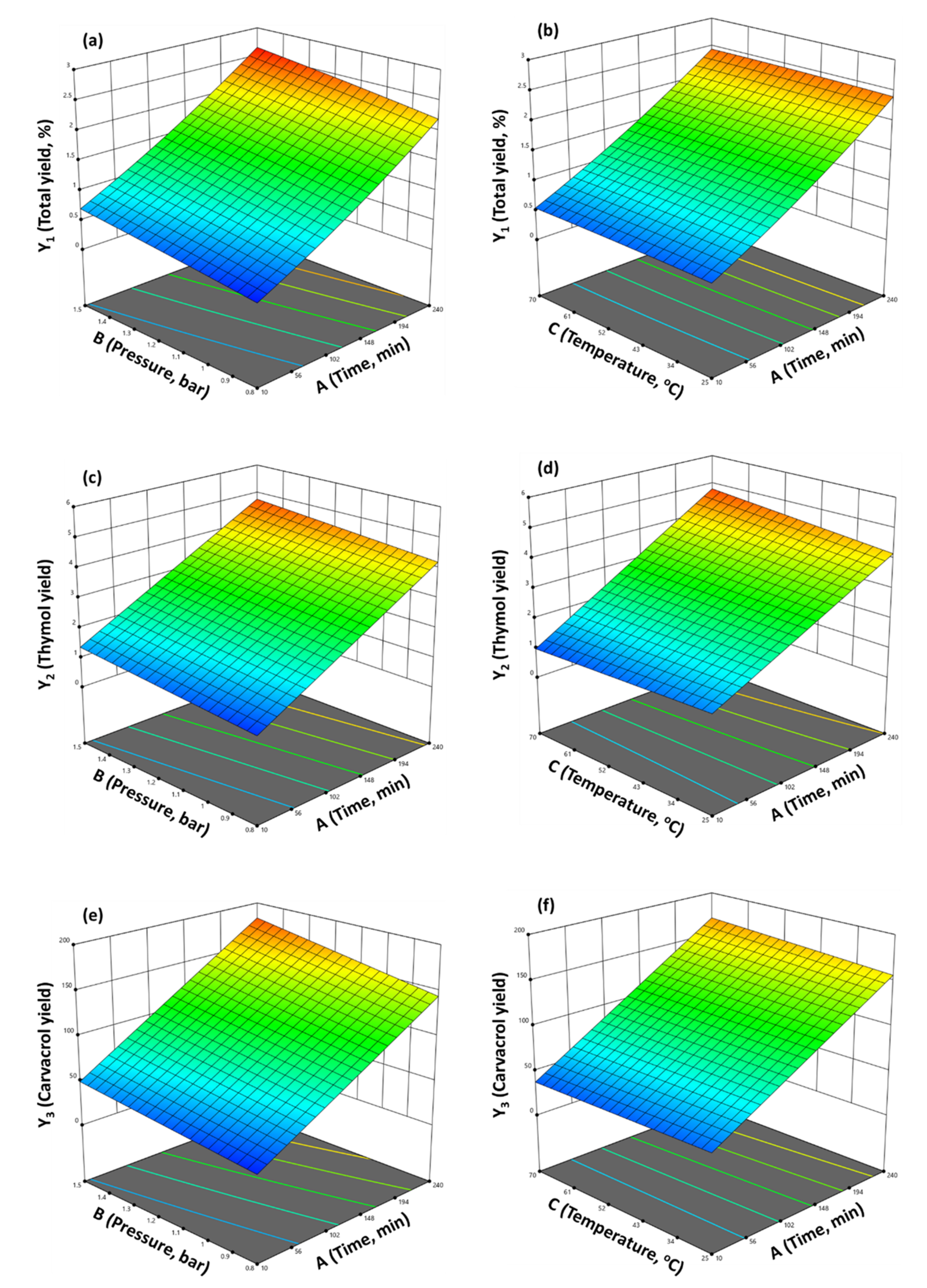
2.3. Experimental Design Results
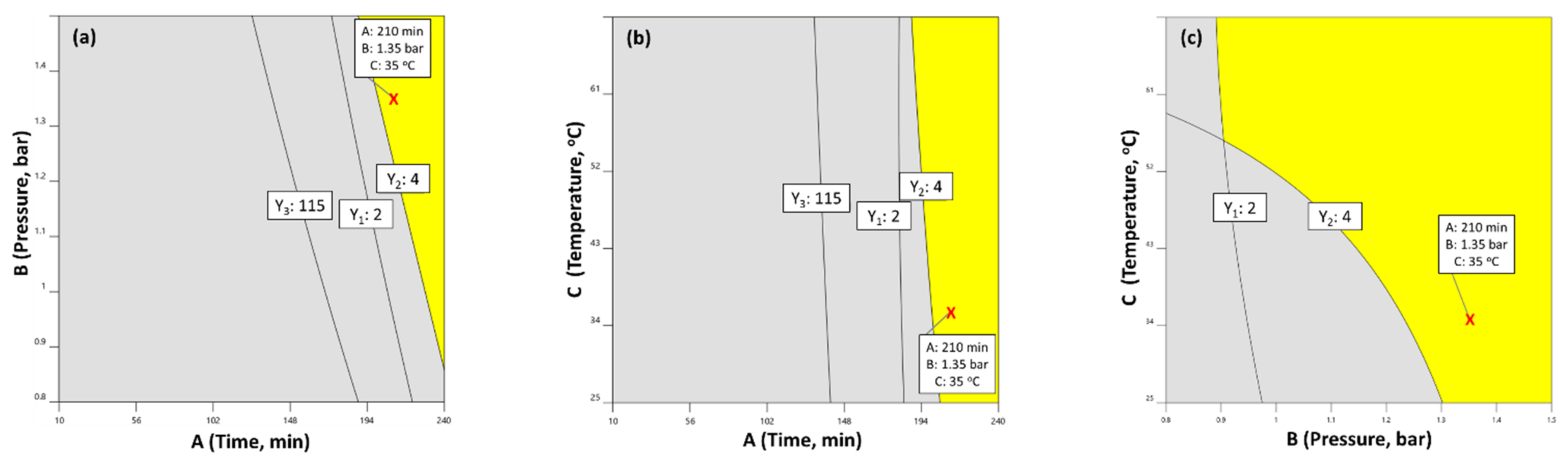
2.4. Distillation Parameters’ Optimization and Validation
2.5. Antioxidant Activity
3. Materials and Methods
3.1. Materials and Instruments
3.2. Experimental Design
3.3. Isolation of Essential Oils
3.4. Headspace Gas Chromatography—Mass Spectrometry Analysis
3.5. Data Processing and Analysis
3.6. Biological In Vitro Assays
3.7. Interaction with the Stable Radical 1,1-Diphenyl-picrylhydrazyl (DPPH)
3.8. Inhibition of Linoleic Acid Peroxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kintzios, S.E. Oregano: The Genera Origanum and Lippia. Medicinal and Aromatic Plants—Industrial Profiles; Kintzios, S.E., Ed.; Taylor and Francis: London, UK; New York, NY, USA, 2002; ISBN 978-0-415-36943-5. [Google Scholar]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Skoula, M.; Harborne, J.B. The Taxonomy and Chemistry of Origanum. In Oregano: The Genera Origanum and Lippia. Medicinal and Aromatic Plants—Industrial Profiles; Kintzios, S.E., Ed.; Taylor and Francis: New York, NY, USA, 2002; ISBN 978-0-415-36943-5. [Google Scholar]
- Kokkini, S. Taxonomy, Diversity and Distribution of Origanum Species. In Proceedings of the IPGRI International Workshop on Oregano, CIHEAM, Valenzano, Bari, Italy, 8–12 May 1996; pp. 122–132. [Google Scholar]
- Oliwier, G.W. The World Market of Oregano. In Proceedings of the IPGRI International Workshop on Oregano, CIHEAM, Valenzano, Bari, Italy, 8–12 May 1996; pp. 141–146. [Google Scholar]
- Kokkini, S.; Vokou, D. Carvacrol-Rich Plants in Greece. Flavour Fragr. J. 1989, 4, 1–7. [Google Scholar] [CrossRef]
- Şahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H. Biological Activities of the Essential Oils and Methanol Extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia Region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Mertzanidis, D.; Nakas, A.; Assimopoulou, A.N.; Kokkini, S. Unravelling the Chemotaxonomic Identity of “White” and “Black” Oregano (Origanum vulgare) in Northern Greece. Planta Med. online ahead of print. [CrossRef] [PubMed]
- Franz, C.; Novak, J. Sources of Essential Oils. In Handbook of Essential Oils. Science, Technology, and Applications; Başer, K.H.C., Buchbauer, G., Eds.; Taylor & Francis Group: New York, NY, USA, 2010; ISBN 978-0-8153-7096-3. [Google Scholar]
- Lukas, B.; Schmiderer, C.; Novak, J. Phytochemical Diversity of Origanum vulgare L. subsp. vulgare (Lamiaceae) from Austria. Biochem. Syst. Ecol. 2013, 50, 106–113. [Google Scholar] [CrossRef]
- W’glarz, Z.; Osidska, E.; Geszprych, A.; Przybyb, J. Intraspecific Variability of Wild Marjoram (Origanum vulgare L.) Naturally Occurring in Poland. Rev. Bras. Pl. Med. 2006, 8, 23–26. [Google Scholar]
- Radušienė, J.; Ivanauskas, L.; Janulis, V.; Jakštas, V. Composition and Variability of Phenolic Compounds in Origanum vulgare from Lithuania. Biologija 2008, 54, 45–49. [Google Scholar] [CrossRef]
- Bonfanti, C.; Iannì, R.; Mazzaglia, A.; Lanza, C.M.; Napoli, E.M.; Ruberto, G. Emerging Cultivation of Oregano in Sicily: Sensory Evaluation of Plants and Chemical Composition of Essential Oils. Ind. Crops Prod. 2012, 35, 160–165. [Google Scholar] [CrossRef]
- Cattelan, M.G.; de Castilhos, M.B.M.; Niz da Silva, D.C.M.; Conti-Silva, A.C.; Hoffmann, F.L. Oregano Essential Oil: Effect on Sensory Acceptability. Nutr. Food Sci. 2015, 45, 574–582. [Google Scholar] [CrossRef]
- Terenina, M.B.; Misharina, T.A.; Krikunova, N.I.; Alinkina, E.S.; Fatkulina, L.D.; Vorob’yova, A.K. Oregano Essential Oil as an Inhibitor of Higher Fatty Acid Oxidation. Appl. Biochem. Microbiol. 2011, 47, 445–449. [Google Scholar] [CrossRef]
- Lagouri, V.; Blekas, G.; Tsimidou, M.; Kokkini, S.; Boskou, D. Composition and Antioxidant Activity of Essential Oils from Oregano Plants Grown Wild in Greece. Z Lebensm. Unters. Forch. 1993, 197, 20–23. [Google Scholar] [CrossRef]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and Cytotoxic Activities of Origanum Essential Oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; Badei, A.Z.M.A.; Hewedi, F.M.; El-Baroty, G.S.A. Antioxidant Activity of Some Spice Essential Oils on Linoleic Acid Oxidation in Aqueous Media. J. Am. Oil Chem. Soc. 1989, 66, 792–799. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and Antibacterial Capacities of Origanum vulgare L. Essential Oil from the Arid Andean Region of Chile and Its Chemical Characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; de Mejia, E.G. Bioactive Compounds from Culinary Herbs Inhibit a Molecular Target for Type 2 Diabetes Management, Dipeptidyl Peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic Profile, Antioxidant Activity and Enzyme Inhibitory Activities of Extracts from Aromatic Plants Used in Mediterranean Diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.; Picos-Salas, M.; Leyva-López, N.; Criollo-Mendoza, M.; Vazquez-Olivo, G.; Heredia, J. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef]
- Koldaş, S.; Demirtas, I.; Ozen, T.; Demirci, M.A.; Behçet, L. Phytochemical Screening, Anticancer and Antioxidant Activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a Plant of Traditional Usage. J. Sci. Food Agric. 2015, 95, 786–798. [Google Scholar] [CrossRef]
- Đurović, S.; Micić, D.; Pezo, L.; Radić, D.; Bazarnova, J.G.; Smyatskaya, Y.A.; Blagojević, S. The Effect of Various Extraction Techniques on the Quality of Sage (Salvia officinalis L.) Essential Oil, Expressed by Chemical Composition, Thermal Properties and Biological Activity. Food Chem. X 2022, 13, 100213. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Karakaya, S.; El, S.N.; Karagözlü, N.; Şahin, S. Antioxidant and Antimicrobial Activities of Essential Oils Obtained from Oregano (Origanum vulgare ssp. hirtum) by Using Different Extraction Methods. J. Med. Food 2011, 14, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.T.; Liao, P.; Crocoll, C.; Boachon, B.; Förster, C.; Leidecker, F.; Wiese, N.; Zhao, D.; Wood, J.C.; Buell, C.R.; et al. The Biosynthesis of Thymol, Carvacrol, and Thymohydroquinone in Lamiaceae Proceeds via Cytochrome P450s and a Short-Chain Dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2110092118. [Google Scholar] [CrossRef] [PubMed]
- Mitraka, G.-C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability 2021, 13, 8818. [Google Scholar] [CrossRef]
- Anthony, K.P.; Deolu-Sobogun, S.A.; Saleh, M.A. Comprehensive Assessment of Antioxidant Activity of Essential Oils. J. Food Sci. 2012, 77, C839–C843. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The Yield, Chemical Composition, and Antioxidant Activities of Essential Oils from Different Plant Parts of the Wild and Cultivated Oregano (Origanum vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Yang, D.-P.; Tang, G.-Y. Multipotent Antioxidants: From Screening to Design. Drug Discov. Today 2006, 11, 749–754. [Google Scholar] [CrossRef]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative Stress in Alzheimer’s Disease: Are We Connecting the Dots? Miniperspective. J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef]
- Górnicki, A.; Gutsze, A. In Vivo and in Vitro Influence of Etretinate on Erythrocyte Membrane Fluidity. Eur. J. Pharmacol. 2001, 423, 127–134. [Google Scholar] [CrossRef]
- Guthrie, W.F. NIST/SEMATECH e-Handbook of Statistical Methods (NIST Handbook 151). Available online: http://www.itl.nist.gov/div898/handbook/ (accessed on 14 March 2020).
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2013; ISBN 978-1-118-14692-7. [Google Scholar]
- Virgiliou, C.; Zisi, C.; Kontogiannopoulos, K.N.; Nakas, A.; Iakovakis, A.; Varsamis, V.; Gika, H.G.; Assimopoulou, A.N. Headspace Gas Chromatography-Mass Spectrometry in the Analysis of Lavender’s Essential Oil: Optimization by Response Surface Methodology. J. Chromatogr. B 2021, 1179, 122852. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, Wiley Series in Probability and Statistics, 4th ed.; Wiley: Hoboken, NJ, USA, 2016; ISBN 978-1-118-91601-8. [Google Scholar]
- Liargkova, T.; Hadjipavlou-Litina, D.J.; Koukoulitsa, C.; Voulgari, E.; Avgoustakis, C. Simple Chalcones and Bis -Chalcones Ethers as Possible Pleiotropic Agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Antioxidant and Anti-Inflammatory Activity of Aryl-Acetic and Hydroxamic Acids as Novel Lipoxygenase Inhibitors. MC 2006, 2, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and Pharmacochemical Evaluation of Novel Aryl-Acetic Acid Inhibitors of Lipoxygenase, Antioxidants, and Anti-Inflammatory Agents. Bioorg. Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef] [PubMed]
| Factors | Responses | |||||
|---|---|---|---|---|---|---|
| Sample | A Time (min) | B Pressure (bar) | C Temperature (°C) | Y1 Total Yield (% w/w) | Y2 Thymol Yield (‱) | Y3 Carvacrol Yield (‱) |
| 30 | 10 | 0.80 | 25.0 | 0.3 | 0.66 | 22.99 |
| 3 | 10 | 0.80 | 25.0 | 0.3 | 0.64 | 22.50 |
| 19 | 240 | 0.80 | 25.0 | 2.1 | 3.76 | 135.55 |
| 11 | 240 | 0.80 | 25.0 | 2.2 | 3.63 | 134.85 |
| 26 | 10 | 1.50 | 25.0 | 0.7 | 1.63 | 52.34 |
| 20 | 10 | 1.50 | 25.0 | 0.7 | 1.57 | 54.81 |
| 24 | 240 | 1.50 | 25.0 | 2.6 | 4.81 | 182.76 |
| 6 | 240 | 1.50 | 25.0 | 2.6 | 4.35 | 166.40 |
| 27 | 10 | 0.80 | 70.0 | 0.4 | 0.88 | 30.28 |
| 10 | 10 | 0.80 | 70.0 | 0.4 | 0.82 | 28.24 |
| 9 | 240 | 0.80 | 70.0 | 2.2 | 5.13 | 140.03 |
| 17 | 240 | 0.80 | 70.0 | 2.2 | 4.19 | 160.49 |
| 32 | 10 | 1.50 | 70.0 | 0.7 | 0.69 | 54.75 |
| 31 | 10 | 1.50 | 70.0 | 0.7 | 1.20 | 42.91 |
| 12 | 240 | 1.50 | 70.0 | 2.7 | 4.81 | 188.59 |
| 22 | 240 | 1.50 | 70.0 | 2.6 | 5.00 | 196.97 |
| 4 | 10 | 1.15 | 47.5 | 0.5 | 1.10 | 37.29 |
| 18 | 10 | 1.15 | 47.5 | 0.5 | 1.08 | 38.67 |
| 1 | 240 | 1.15 | 47.5 | 2.5 | 4.30 | 162.26 |
| 23 | 240 | 1.15 | 47.5 | 2.5 | 4.18 | 162.17 |
| 16 | 125 | 0.80 | 47.5 | 1.2 | 2.68 | 94.19 |
| 5 | 125 | 0.80 | 47.5 | 1.2 | 2.70 | 91.79 |
| 13 | 125 | 1.50 | 47.5 | 1.6 | 3.42 | 113.26 |
| 2 | 125 | 1.50 | 47.5 | 1.6 | 3.04 | 106.37 |
| 15 | 125 | 1.15 | 25.0 | 1.4 | 3.01 | 108.46 |
| 28 | 125 | 1.15 | 25.0 | 1.4 | 2.59 | 96.26 |
| 25 | 125 | 1.15 | 70.0 | 1.3 | 2.84 | 93.65 |
| 21 | 125 | 1.15 | 70.0 | 1.5 | 3.02 | 100.24 |
| 8 | 125 | 1.15 | 47.5 | 1.4 | 2.84 | 100.72 |
| 7 | 125 | 1.15 | 47.5 | 1.4 | 3.18 | 106.45 |
| 29 | 125 | 1.15 | 47.5 | 1.4 | 2.96 | 102.28 |
| 14 | 125 | 1.15 | 47.5 | 1.3 | 2.99 | 104.69 |
| Compound | RT (min) | RI Polar (NIST) ** | Sample | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 3 | 19 | 11 | 26 | 20 | 24 | 6 | 27 | 10 | 9 | 17 | 32 | 31 | 12 | 22 | |||
| β-Pinene | 5.09 | 1112 ± 7 | 0.00% | 0.00% | 0.17% | 0.19% | 0.00% | 0.00% | 0.13% | 0.16% | 0.00% | 0.00% | 0.22% | 0.17% | 0.09% | 0.15% | 0.24% | 0.18% |
| β-Myrcene | 6.36 | 1161 ± 7 | 1.65% | 1.78% | 3.61% | 4.15% | 2.08% | 1.62% | 3.13% | 4.16% | 1.70% | 2.14% | 3.93% | 2.77% | 2.11% | 3.43% | 3.39% | 2.66% |
| α-Terpinene | 6.71 | 1180 ± 8 | 1.41% | 1.45% | 2.19% | 2.54% | 1.55% | 1.12% | 1.90% | 2.37% | 1.37% | 1.84% | 2.40% | 1.68% | 1.49% | 2.45% | 1.97% | 1.53% |
| Limonene | 7.19 | 1200 ± 7 | 0.14% | 0.15% | 0.22% | 0.32% | 0.15% | 0.14% | 0.16% | 0.14% | 0.12% | 0.17% | 0.32% | 0.20% | 0.20% | 0.34% | 0.20% | 0.15% |
| β-Phellandrene | 7.45 | 1211 ± 7 | 0.12% | 0.14% | 0.25% | 0.39% | 0.16% | 0.09% | 0.26% | 0.36% | 0.11% | 0.16% | 0.37% | 0.20% | 0.20% | 0.34% | 0.23% | 0.19% |
| γ-Terpinene | 8.61 | 1246 ± 9 | 8.19% | 9.24% | 10.61% | 11.43% | 7.72% | 7.01% | 8.95% | 11.00% | 9.07% | 11.68% | 11.50% | 8.24% | 7.48% | 13.42% | 8.77% | 6.68% |
| p-Cymene | 9.53 | 1272 ± 8 | 3.87% | 3.86% | 8.48% | 12.04% | 4.69% | 3.82% | 8.28% | 10.57% | 3.75% | 4.74% | 9.71% | 6.92% | 5.76% | 8.16% | 8.93% | 6.73% |
| 1-Octen-3-ol | 16.89 | 1450 ± 7 | 0.21% | 0.31% | 0.49% | 0.55% | 0.32% | 0.30% | 0.47% | 0.57% | 0.30% | 0.35% | 0.54% | 0.42% | 0.23% | 0.55% | 0.41% | 0.33% |
| 4-Thujanol | 17.27 | 1465 ± 9 | 0.10% | 0.14% | 0.37% | 0.36% | 0.20% | 0.15% | 0.39% | 0.48% | 0.13% | 0.14% | 0.41% | 0.32% | 0.12% | 0.27% | 0.36% | 0.30% |
| Linalool | 20.40 | 1547 ± 7 | 0.07% | 0.10% | 0.13% | 0.09% | 0.10% | 0.08% | 0.10% | 0.12% | 0.09% | 0.09% | 0.11% | 0.10% | 0.05% | 0.13% | 0.09% | 0.08% |
| β-Caryophyllene | 21.77 | 1595 ± 16 | 1.67% | 1.76% | 2.65% | 1.85% | 2.00% | 1.54% | 1.51% | 1.73% | 1.69% | 1.95% | 1.74% | 1.47% | 1.14% | 2.49% | 1.47% | 1.34% |
| Terpinen-4-ol | 22.05 | 1602 ± 9 | 0.52% | 0.55% | 0.76% | 0.71% | 0.61% | 0.49% | 0.56% | 0.64% | 0.53% | 0.62% | 0.61% | 0.51% | 0.33% | 0.82% | 0.52% | 0.46% |
| Dihydrocarvone | 22.16 | 1624 ± 21 | 0.07% | 0.09% | 0.11% | 0.09% | 0.08% | 0.08% | 0.07% | 0.08% | 0.09% | 0.09% | 0.07% | 0.07% | 0.06% | 0.15% | 0.07% | 0.07% |
| α-Caryophyllene | 24.08 | 1667 ± 14 | 0.18% | 0.17% | 0.23% | 0.13% | 0.21% | 0.13% | 0.12% | 0.12% | 0.17% | 0.16% | 0.12% | 0.11% | 0.08% | 0.19% | 0.09% | 0.10% |
| α-Terpineol | 25.05 | 1697 ± 10 | 0.12% | 0.11% | 0.24% | 0.13% | 0.18% | 0.11% | 0.15% | 0.13% | 0.12% | 0.13% | 0.14% | 0.14% | 0.06% | 0.26% | 0.15% | 0.16% |
| endo-Borneol | 25.15 | 1702 ± 15 | 0.25% | 0.29% | 0.45% | 0.35% | 0.32% | 0.27% | 0.34% | 0.39% | 0.27% | 0.33% | 0.37% | 0.31% | 0.15% | 0.40% | 0.34% | 0.31% |
| β-Bisabolene | 25.97 | 1727 ± 11 | 2.02% | 2.07% | 2.05% | 1.21% | 1.96% | 1.97% | 0.94% | 0.87% | 2.03% | 2.10% | 1.01% | 1.12% | 0.87% | 2.63% | 0.77% | 0.71% |
| Carvone | 26.06 | 1740 ± 12 | 0.04% | 0.06% | 0.10% | 0.04% | 0.06% | 0.07% | 0.08% | 0.08% | 0.05% | 0.05% | 0.05% | 0.06% | 0.02% | 0.10% | 0.08% | 0.08% |
| δ-Cadinene | 26.83 | 1758 ± 13 | 0.20% | 0.20% | 0.16% | 0.09% | 0.19% | 0.18% | 0.05% | 0.05% | 0.18% | 0.18% | 0.07% | 0.08% | 0.08% | 0.22% | 0.04% | 0.04% |
| Thymol | 38.04 | 2189 ± 9 | 2.20% | 2.14% | 1.79% | 1.65% | 2.33% | 2.24% | 1.85% | 1.67% | 2.19% | 2.05% | 2.33% | 1.91% | 0.98% | 1.71% | 1.78% | 1.92% |
| Carvacrol | 38.69 | 2236 ± 10 | 76.63% | 74.98% | 64.55% | 61.30% | 74.77% | 78.30% | 70.29% | 64.00% | 75.69% | 70.60% | 63.65% | 72.95% | 78.22% | 61.30% | 69.85% | 75.76% |
| Compound | RT (min) | RI Polar (NIST) ** | Sample | |||||||||||||||
| 4 | 18 | 1 | 23 | 16 | 5 | 13 | 2 | 15 | 28 | 25 | 21 | 8 | 7 | 29 | 14 | |||
| β-Pinene | 5.09 | 1112 ± 7 | 0.00% | 0.00% | 0.22% | 0.23% | 0.08% | 0.09% | 0.21% | 0.19% | 0.15% | 0.18% | 0.17% | 0.24% | 0.20% | 0.17% | 0.16% | 0.13% |
| β-Myrcene | 6.36 | 1161 ± 7 | 1.88% | 1.81% | 3.88% | 3.77% | 1.66% | 1.97% | 2.91% | 3.34% | 2.23% | 3.23% | 2.56% | 3.39% | 2.83% | 2.16% | 2.46% | 1.67% |
| α-Terpinene | 6.71 | 1180 ± 8 | 1.31% | 1.22% | 2.02% | 1.96% | 0.99% | 1.13% | 1.48% | 2.08% | 1.32% | 1.91% | 1.62% | 2.01% | 1.57% | 1.26% | 1.37% | 0.94% |
| Limonene | 7.19 | 1200 ± 7 | 0.11% | 0.11% | 0.34% | 0.26% | 0.13% | 0.10% | 0.16% | 0.24% | 0.19% | 0.23% | 0.18% | 0.21% | 0.29% | 0.20% | 0.20% | 0.16% |
| β-Phellandrene | 7.45 | 1211 ± 7 | 0.10% | 0.10% | 0.32% | 0.34% | 0.13% | 0.10% | 0.19% | 0.27% | 0.21% | 0.29% | 0.20% | 0.26% | 0.29% | 0.22% | 0.23% | 0.17% |
| γ-Terpinene | 8.61 | 1246 ± 9 | 8.51% | 7.40% | 11.07% | 11.19% | 6.52% | 7.37% | 6.70% | 10.82% | 7.28% | 10.03% | 8.82% | 10.53% | 6.94% | 7.32% | 8.32% | 5.25% |
| p-Cymene | 9.53 | 1272 ± 8 | 4.25% | 4.75% | 9.99% | 10.12% | 4.74% | 4.72% | 9.79% | 9.07% | 5.50% | 8.45% | 6.73% | 7.84% | 8.22% | 5.48% | 6.43% | 4.58% |
| 1-Octen-3-ol | 16.89 | 1450 ± 7 | 0.27% | 0.28% | 0.53% | 0.50% | 0.30% | 0.27% | 0.29% | 0.44% | 0.21% | 0.46% | 0.38% | 0.36% | 0.45% | 0.26% | 0.40% | 0.23% |
| 4-Thujanol | 17.27 | 1465 ± 9 | 0.14% | 0.15% | 0.37% | 0.44% | 0.19% | 0.15% | 0.24% | 0.33% | 0.15% | 0.34% | 0.25% | 0.22% | 0.29% | 0.17% | 0.26% | 0.17% |
| Linalool | 20.40 | 1547 ± 7 | 0.10% | 0.08% | 0.12% | 0.11% | 0.07% | 0.08% | 0.09% | 0.10% | 0.05% | 0.09% | 0.09% | 0.09% | 0.11% | 0.07% | 0.10% | 0.07% |
| β-Caryophyllene | 21.77 | 1595 ± 16 | 2.13% | 1.37% | 1.76% | 1.77% | 1.39% | 1.80% | 1.81% | 1.76% | 1.02% | 1.50% | 1.67% | 2.34% | 1.75% | 1.62% | 1.76% | 1.32% |
| Terpinen-4-ol | 22.05 | 1602 ± 9 | 0.58% | 0.49% | 0.59% | 0.62% | 0.51% | 0.50% | 0.64% | 0.62% | 0.37% | 0.54% | 0.56% | 0.51% | 0.60% | 0.42% | 0.54% | 0.43% |
| Dihydrocarvone | 22.16 | 1624 ± 21 | 0.09% | 0.09% | 0.09% | 0.09% | 0.07% | 0.08% | 0.10% | 0.10% | 0.06% | 0.09% | 0.09% | 0.10% | 0.10% | 0.07% | 0.08% | 0.06% |
| α-Caryophyllene | 24.08 | 1667 ± 14 | 0.22% | 0.13% | 0.12% | 0.13% | 0.12% | 0.17% | 0.14% | 0.13% | 0.07% | 0.09% | 0.13% | 0.22% | 0.16% | 0.13% | 0.15% | 0.12% |
| α-Terpineol | 25.05 | 1697 ± 10 | 0.15% | 0.15% | 0.14% | 0.15% | 0.16% | 0.15% | 0.16% | 0.16% | 0.15% | 0.14% | 0.18% | 0.18% | 0.16% | 0.15% | 0.15% | 0.15% |
| endo-Borneol | 25.15 | 1702 ± 15 | 0.28% | 0.27% | 0.34% | 0.39% | 0.30% | 0.26% | 0.24% | 0.34% | 0.28% | 0.37% | 0.36% | 0.32% | 0.30% | 0.21% | 0.29% | 0.23% |
| β-Bisabolene | 25.97 | 1727 ± 11 | 2.48% | 1.56% | 1.01% | 0.93% | 1.42% | 1.84% | 1.41% | 1.14% | 0.83% | 1.03% | 1.32% | 1.78% | 1.26% | 1.38% | 1.48% | 1.12% |
| Carvone | 26.06 | 1740 ± 12 | 0.06% | 0.06% | 0.08% | 0.07% | 0.06% | 0.07% | 0.06% | 0.06% | 0.04% | 0.06% | 0.06% | 0.08% | 0.06% | 0.05% | 0.05% | 0.04% |
| δ-Cadinene | 26.83 | 1758 ± 13 | 0.22% | 0.16% | 0.07% | 0.07% | 0.15% | 0.16% | 0.11% | 0.09% | 0.06% | 0.06% | 0.10% | 0.16% | 0.10% | 0.10% | 0.12% | 0.11% |
| Thymol | 38.04 | 2189 ± 9 | 2.21% | 2.17% | 1.72% | 1.67% | 2.23% | 2.25% | 2.14% | 1.90% | 2.15% | 1.85% | 2.18% | 2.01% | 2.03% | 2.27% | 2.12% | 2.30% |
| Carvacrol | 38.69 | 2236 ± 10 | 74.57% | 77.34% | 64.91% | 64.87% | 78.49% | 76.49% | 70.79% | 66.48% | 77.47% | 68.76% | 72.04% | 66.82% | 71.95% | 76.04% | 73.05% | 80.53% |
| Responses * | ||||||
|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | ||||
| Factors ** | F-Value | p-Value *** | F-Value | p-Value *** | F-Value | p-Value *** |
| A | 6762.13 | <0.0001 | 982.38 | <0.0001 | 1832.17 | <0.0001 |
| B | 299.71 | <0.0001 | 25.12 | <0.0001 | 105.08 | <0.0001 |
| C | - | - | - | - | - | - |
| AB | - | - | - | - | 5.86 | 0.0231 |
| AC | - | - | 13.11 | 0.0013 | 5.86 | 0.0231 |
| BC | - | - | 9.64 | 0.0047 | - | - |
| A2 | 15.57 | 0.0007 | - | - | - | - |
| R2 | 0.9969 | 0.9764 | 0.9874 | |||
| Sample * | % Inhibition of Lipid Peroxidation (± SD) | % Interaction with DPPH–RA% (Reducing Activity) 20 min (± SD) | % Interaction with DPPH–RA% (Reducing Activity) 60 min (± SD) | Carvacrol (%) ** | Thymol (%) ** |
|---|---|---|---|---|---|
| 30 | 80 ± 1.1 | 52.7 ± 1.2 | 77.2 ± 2.3 | 76.63 | 2.20 |
| 3 | 49.7 ± 0.9 | 64.4 ± 2.7 | 74.98 | 2.14 | |
| 19 | 49.3 ± 0.8 | 64.9 ± 1.8 | 64.55 | 1.79 | |
| 11 | 48.3 ± 1.3 | 63.2 ± 2.1 | 61.30 | 1.65 | |
| 26 | 54.5 ± 0.8 | 66.0 ± 2.4 | 74.77 | 2.33 | |
| 20 | 48.9 ± 075 | 58.2 ± 0.7 | 78.30 | 2.24 | |
| 24 | 33 ± 0.03 | 61.0 ± 1.3 | 80.0 ± 2.9 | 70.29 | 1.85 |
| 6 | 58.0 ± 0.5 | 69.0 ± 1.1 | 64.00 | 1.67 | |
| 27 | 3 ± 0.001 | 56.0 ± 1.3 | 70.0 ± 1.9 | 75.69 | 2.19 |
| 10 | 57.0 ± 1.1 | 68.0 ± 0.7 | 70.60 | 2.05 | |
| 9 | 49.0 ± 0.4 | 62.0 ± 0.4 | 63.65 | 2.33 | |
| 17 | 51.8 ± 03 | 69.0 ± 0.2 | 72.95 | 1.91 | |
| 32 | 15 ± 0.01 | 61.4 ± 1.7 | 61.0 ± 1.0 | 78.22 | 0.98 |
| 31 | 62.4 ± 2.2 | 73.0 ± 1.7 | 61.30 | 1.71 | |
| 12 | 58.3 ± 2.0 | 69.0 ± 0.6 | 69.85 | 1.78 | |
| 22 | 58.6 ± 1.1 | 68.0 ± 1.6 | 75.76 | 1.92 | |
| 4 | 53.4 ± 0.5 | 66.0 ± 1.3 | 74.57 | 2.21 | |
| 18 | 48.2 ± 0.3 | 61.0 ± 0.4 | 77.34 | 2.17 | |
| 1 | 47.5 ± 0.2 | 61.0 ± 0.7 | 64.91 | 1.72 | |
| 23 | 50.4 ± 0.9 | 66.0 ± 1.6 | 64.87 | 1.67 | |
| 16 | 56.2 ± 1,0 | 67.0 ± 1.4 | 78.49 | 2.23 | |
| 5 | 45.0 ± 0.4 | 62.0 ± 2.1 | 76.49 | 2.25 | |
| 13 | 39.1 ± 0.6 | 51.0 ± 1.1 | 70.79 | 2.14 | |
| 2 | 45.7 ± 0.4 | 65.0 ± 0.9 | 66.48 | 1.90 | |
| 15 | 45.5 ± 0.9 | 65.0 ± 1.0 | 77.47 | 2.15 | |
| 28 | 47.4 ± 1.3 | 65.0 ± 1.4 | 68.76 | 1.85 | |
| 25 | 30.0 ± 0.8 | 45.0 ± 0.7 | 72.04 | 2.18 | |
| 21 | 47.4 ± 1.4 | 65.0 ± 1.1 | 66.82 | 2.01 | |
| 8 | 49.6 ± 1.6 | 65.0 ± 0.7 | 71.95 | 2.03 | |
| 7 | 53.4 ± 2.0 | 69.0 ± 1.6 | 76.04 | 2.27 | |
| 29 | 46.1 ± 0.7 | 63.0 ± 1.2 | 73.05 | 2.12 | |
| 14 | 49.3 ± 0.3 | 68.0 ± 2.4 | 80.53 | 2.30 | |
| NDGA | 88.0 ± 2.3 | 93.0 ± 3.2 | |||
| Trolox | 92 ± 1.9 |
| Samples | RA% 20 Min | RA% 60 Min | Mean Values of % Carvacrol | Time (Min) | Pressure (Bar) | Temperature (°C) |
|---|---|---|---|---|---|---|
| 8/7/29/14 | 49.5 | 66.3 | 75.4 | 125 | 1.15 | 47.5 |
| 15/28 | 46.5 | 65.0 | 73.0 | 125 | 1.15 | 25.0 |
| 25/21 | 37.2 | 55.0 | 69.4 | 125 | 1.15 | 70.0 |
| 2/13 | 42.4 | 58.0 | 68.6 | 125 | 1.50 | 47.5 |
| 1/23 | 49.0 | 63.5 | 64.9 | 240 | 1.15 | 47.5 |
| 11/19 | 48.8 | 64.1 | 63.0 | 240 | 0.80 | 25.0 |
| Samples | RA% 20 Min | RA% 60 Min | Mean Values of % Carvacrol | Time (Min) | Pressure (Bar) | Temperature (°C) |
|---|---|---|---|---|---|---|
| 16/5 | 51.0 | 64.5 | 77.5 | 125 | 0.80 | 47.5 |
| 26/20 | 51.7 | 62.0 | 76.6 | 10 | 1.50 | 25.0 |
| 4/18 | 51.0 | 63.5 | 76.0 | 10 | 1.15 | 47.5 |
| 30/3 | 51.2 | 70.8 | 75.8 | 10 | 0.80 | 25.0 |
| 9/17 | 50.4 | 65.5 | 68.3 | 240 | 0.80 | 70.0 |
| Samples | RA% 20 Min | RA% 60 Min | MeanValues of % Carvacrol | Time (Min) | Pressure (Bar) | Temperature (°C) |
| 27/10 | 56.5 | 69.0 | 74.6 | 10 | 0.80 | 70.0 |
| 12/22 | 58.5 | 68.5 | 72.8 | 240 | 1.50 | 70.0 |
| 32/31 | 62.2 | 67.0 | 69.8 | 10 | 1.50 | 70.0 |
| 24/6 | 59.5 | 74.5 | 67.0 | 240 | 1.50 | 25.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakas, A.; Giannarelli, G.; Fotopoulos, I.; Chainoglou, E.; Peperidou, A.; Kontogiannopoulos, K.N.; Tsiaprazi-Stamou, A.; Varsamis, V.; Gika, H.; Hadjipavlou-Litina, D.; et al. Optimizing the Distillation of Greek Oregano—Do Process Parameters Affect Bioactive Aroma Constituents and In Vitro Antioxidant Activity? Molecules 2023, 28, 971. https://doi.org/10.3390/molecules28030971
Nakas A, Giannarelli G, Fotopoulos I, Chainoglou E, Peperidou A, Kontogiannopoulos KN, Tsiaprazi-Stamou A, Varsamis V, Gika H, Hadjipavlou-Litina D, et al. Optimizing the Distillation of Greek Oregano—Do Process Parameters Affect Bioactive Aroma Constituents and In Vitro Antioxidant Activity? Molecules. 2023; 28(3):971. https://doi.org/10.3390/molecules28030971
Chicago/Turabian StyleNakas, Alexandros, Georgia Giannarelli, Ioannis Fotopoulos, Eirini Chainoglou, Aikaterini Peperidou, Konstantinos N. Kontogiannopoulos, Artemis Tsiaprazi-Stamou, Vasilios Varsamis, Helen Gika, Dimitra Hadjipavlou-Litina, and et al. 2023. "Optimizing the Distillation of Greek Oregano—Do Process Parameters Affect Bioactive Aroma Constituents and In Vitro Antioxidant Activity?" Molecules 28, no. 3: 971. https://doi.org/10.3390/molecules28030971
APA StyleNakas, A., Giannarelli, G., Fotopoulos, I., Chainoglou, E., Peperidou, A., Kontogiannopoulos, K. N., Tsiaprazi-Stamou, A., Varsamis, V., Gika, H., Hadjipavlou-Litina, D., & Assimopoulou, A. N. (2023). Optimizing the Distillation of Greek Oregano—Do Process Parameters Affect Bioactive Aroma Constituents and In Vitro Antioxidant Activity? Molecules, 28(3), 971. https://doi.org/10.3390/molecules28030971
















