Bioactive Clerodane Diterpenoids from the Leaves of Casearia coriacea Vent
Abstract
1. Introduction
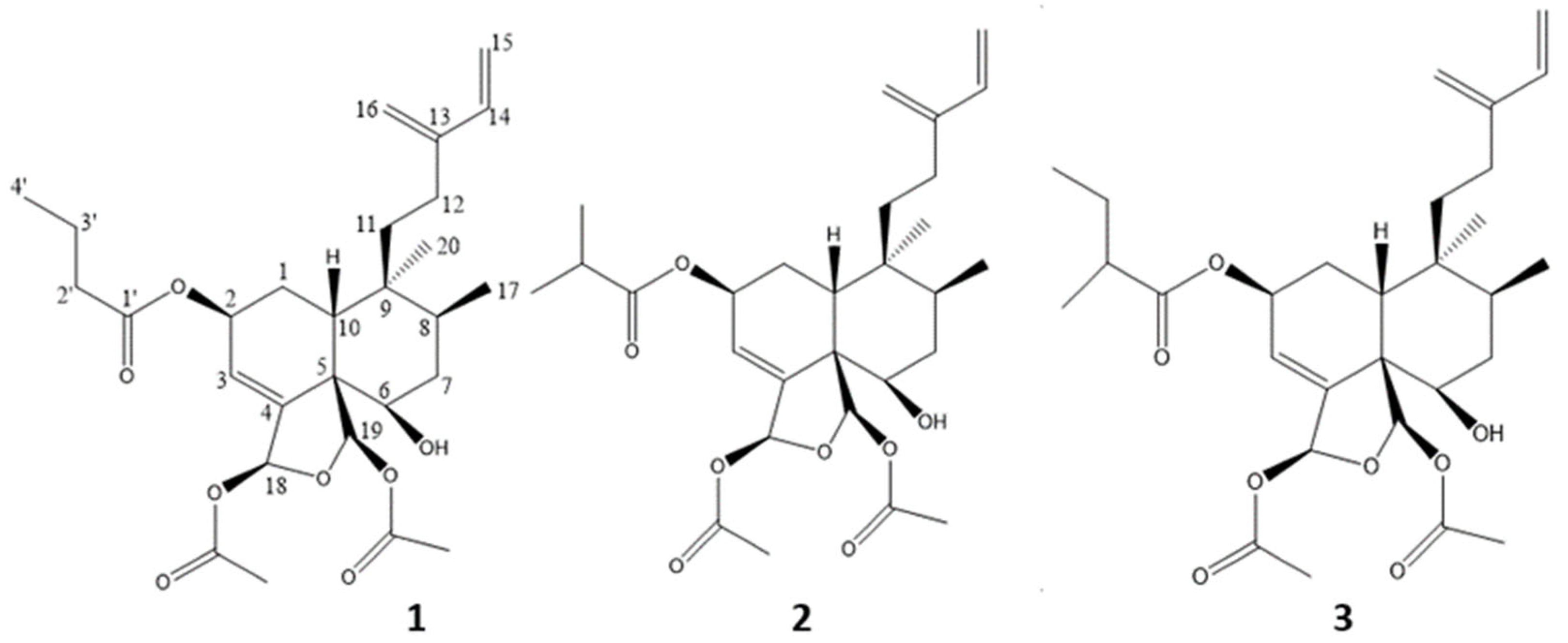
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. In Vitro Antiparasitic Activities
3.5. In Vitro Cytotoxic Activity
3.6. Zebrafish Embryos Acute Toxicity Test
3.7. Statistical Analysis
3.8. Illustrations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Malaria Report 2022. Available online: https://www.who.int/publications-detail-redirect/9789240064898 (accessed on 16 January 2023).
- WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk. Available online: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk (accessed on 16 January 2023).
- Sinnis, P.; Fidock, D.A. The RTS,S vaccine—A chance to regain the upper hand against malaria? Cell 2022, 185, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, D.; Zongo, I.; Sagara, I.; Cairns, M.; Yerbanga, R.-S.; Diarra, M.; Nikièma, F.; Tapily, A.; Sompougdou, F.; Issiaka, D.; et al. Seasonal Malaria Vaccination with or without Seasonal Malaria Chemoprevention. N. Engl. J. Med. 2021, 385, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Rathod, G.K.; Jain, M.; Sharma, K.K.; Das, S.; Basak, A.; Jain, R. New structural classes of antimalarials. Eur. J. Med. Chem. 2022, 242, 114653. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Mamede, L.; Ledoux, A.; Jansen, O.; Frédérich, M. Natural Phenolic Compounds and Derivatives as Potential Antimalarial Agents. Planta Med. 2020, 86, 585–618. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, A.; St-Gelais, A.; Cieckiewicz, E.; Jansen, O.; Bordignon, A.; Illien, B.; Di Giovanni, N.; Marvilliers, A.; Hoareau, F.; Pendeville, H.; et al. Antimalarial Activities of Alkyl Cyclohexenone Derivatives Isolated from the Leaves of Poupartia borbonica. J. Nat. Prod. 2017, 80, 1750–1757. [Google Scholar] [CrossRef]
- Ledoux, A.; Mamede, L.; Palazzo, C.; Furst, T.; Jansen, O.; De Tullio, P.; Kagisha, V.; Pendeville, H.; Fillet, M.; Piel, G.; et al. Heparin-Coated Liposomes Improve Antiplasmodial Activity and Reduce the Toxicity of Poupartone B. Planta Med. Int. Open 2020, 7, e73–e80. [Google Scholar] [CrossRef]
- Ledoux, A.; Bériot, D.; Mamede, L.; Desdemoustier, P.; Detroz, F.; Jansen, O.; Frédérich, M.; Maquoi, E. Cytotoxicity of Poupartone B, an Alkyl Cyclohexenone Derivative from Poupartia borbonica, against Human Cancer Cell Lines. Planta Med. 2021, 87, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, A.; Frédérich, M.; Ledoux, A.; Campos, P.-E.; Clerc, P.; Hermann, T.; Quetin-Leclercq, J.; Cieckiewicz, E. In vitro antiplasmodial and cytotoxic activities of sesquiterpene lactones from Vernonia fimbrillifera Less. (Asteraceae). Nat. Prod. Res. 2018, 32, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, A.; Cao, M.; Jansen, O.; Mamede, L.; Campos, P.-E.; Payet, B.; Clerc, P.; Grondin, I.; Girard-Valenciennes, E.; Hermann, T.; et al. Antiplasmodial, anti-chikungunya virus and antioxidant activities of 64 endemic plants from the Mascarene Islands. Int. J. Antimicrob. Agents 2018, 52, 622–628. [Google Scholar] [CrossRef]
- Xia, L.; Guo, Q.; Tu, P.; Chai, X. The genus Casearia: A phytochemical and pharmacological overview. Phytochem. Rev. 2015, 14, 99–135. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Wang, C.-H.; Cheng, Y.-B.; Wang, L.-T.; Guh, J.-H.; Chien, C.-T.; Khalil, A.T. New Cytotoxic Clerodane Diterpenoids from the Leaves and Twigs of Casearia membranacea. J. Nat. Prod. 2004, 67, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Cheng, Y.-B.; Ahmed, A.F.; Lee, C.L.; Chen, S.-Y.; Chien, C.-T.; Kuo, Y.-H.; Tzeng, G.-L. Cytotoxic Clerodane Diterpenoids from Casearia membranacea. J. Nat. Prod. 2005, 68, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Cheng, Y.-B.; Chen, S.-Y.; Chien, C.-T.; Kuo, Y.-H.; Guh, J.-H.; Khalil, A.T.; Shen, Y.-C. New Bioactive Clerodane Diterpenoids from the Roots ofCasearia membranacea. CB 2008, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, J.; Shi, Z.; Zhang, Q.; Wang, H.; Li, D.; Song, Z.; Wang, C.; Jin, J.; Xu, J.; et al. Clerodane Diterpenoids Isolated from the Leaves of Casearia graveolens. J. Nat. Prod. 2020, 83, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.S.; Corley, D.G.; Carron, C.P.; Rowold, E.; Kilpatrick, B.F.; Durley, R.C. Four New Clerodane Diterpenes from the Leaves of Casearia guianensis Which Inhibit the Interaction of Leukocyte Function Antigen 1 with Intercellular Adhesion Molecule 1. J. Nat. Prod. 1997, 60, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.N.; da Silveira, M.R.; Danielski, L.G.; Florentino, D.; Petronilho, F.; Piovezan, A.P. Anti-inflammatory and antioxidant properties of hydroalcoholic crude extract from Casearia sylvestris Sw. (Salicaceae). J. Ethnopharmacol. 2013, 147, 612–617. [Google Scholar] [CrossRef]
- Bou, D.D.; Tempone, A.G.; Pinto, É.G.; Lago, J.H.G.; Sartorelli, P. Antiparasitic activity and effect of casearins isolated from Casearia sylvestris on Leishmania and Trypanosoma cruzi plasma membrane. Phytomedicine 2014, 21, 676–681. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.-H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef]
- De Mesquita, M.L.; Grellier, P.; Mambu, L.; de Paula, J.E.; Espindola, L.S. In vitro antiplasmodial activity of Brazilian Cerrado plants used as traditional remedies. J. Ethnopharmacol. 2007, 110, 165–170. [Google Scholar] [CrossRef]
- Simonsen, H.T.; Nordskjold, J.B.; Smitt, U.W.; Nyman, U.; Palpu, P.; Joshi, P.; Varughese, G. In vitro screening of Indian medicinal plants for antiplasmodial activity. J. Ethnopharmacol. 2001, 74, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Kanarsa, T.; Buayairaksa, M. New Bioactive Clerodane Diterpenoids from the Bark of Casearia grewiifolia. J. Nat. Prod. 2005, 68, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Truong, N.B.; Doan, H.T.M.; Litaudon, M.; Retailleau, P.; Do, T.T.; Nguyen, H.V.; Chau, M.V.; Pham, C.V. Cytotoxic Clerodane Diterpenoids from the Leaves of Casearia grewiifolia. J. Nat. Prod. 2015, 78, 2726–2730. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Júnior, G.M.; Dutra, L.A.; Ferreira, P.M.P.; De Moraes, M.O.; Costa Lotufo, L.V.; Pessoa, C.D.Ó.; Torres, R.B.; Boralle, N.; Bolzani, V.D.S.; Cavalheiro, A.J. Cytotoxic clerodane diterpenes from casearia rupestris. J. Nat. Prod. 2011, 74, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Oda, F.B.; Crevelin, E.J.; Crotti, A.E.M.; Orlando, A.B.; de Medeiros, A.I.; Nogueira, F.A.R.; dos Santos, A.G. Acidic and hepatic derivatives of bioactive clerodane diterpenes casearins J and O. Fitoterapia 2019, 137, 104197. [Google Scholar] [CrossRef]
- Aimaiti, S.; Suzuki, A.; Saito, Y.; Fukuyoshi, S.; Goto, M.; Miyake, K.; Newman, D.J.; O’Keefe, B.R.; Lee, K.-H.; Nakagawa-Goto, K. Corymbulosins I–W, Cytotoxic Clerodane Diterpenes from the Bark of Laetia corymbulosa. J. Org. Chem. 2018, 83, 951–963. [Google Scholar] [CrossRef]
- Gibbons, S.; Gray, A.I.; Waterman, P.G. Clerodane diterpenes from the bark of Casearia tremula. Phytochemistry 1996, 41, 565–570. [Google Scholar] [CrossRef]
- Sai Prakash, C.V.; Hoch, J.M.; Kingston, D.G.I. Structure and Stereochemistry of New Cytotoxic Clerodane Diterpenoids from the Bark of Casearia l ucida from the Madagascar Rainforest. J. Nat. Prod. 2002, 65, 100–107. [Google Scholar] [CrossRef]
- Viira, B.; Gendron, T.; Lanfranchi, D.A.; Cojean, S.; Horvath, D.; Marcou, G.; Varnek, A.; Maes, L.; Maran, U.; Loiseau, P.; et al. In Silico Mining for Antimalarial Structure-Activity Knowledge and Discovery of Novel Antimalarial Curcuminoids. Molecules 2016, 21, 853. [Google Scholar] [CrossRef]
- Gan, F.-F.; Kaminska, K.K.; Yang, H.; Liew, C.-Y.; Leow, P.-C.; So, C.-L.; Tu, L.N.L.; Roy, A.; Yap, C.-W.; Kang, T.-S.; et al. Identification of Michael Acceptor-Centric Pharmacophores with Substituents That Yield Strong Thioredoxin Reductase Inhibitory Character Correlated to Antiproliferative Activity. Antioxid. Redox Signal. 2013, 19, 1149–1165. [Google Scholar] [CrossRef]
- Acquaviva, R.; Malfa, G.A.; Loizzo, M.R.; Xiao, J.; Bianchi, S.; Tundis, R. Advances on Natural Abietane, Labdane and Clerodane Diterpenes as Anti-Cancer Agents: Sources and Mechanisms of Action. Molecules 2022, 27, 4791. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Risinger, A.L.; Petersen, C.L.; Grkovic, T.; O’Keefe, B.R.; Mooberry, S.L.; Cichewicz, R.H. Anacolosins A-F and Corymbulosins X and Y, Clerodane Diterpenes from Anacolosa clarkii Exhibiting Cytotoxicity toward Pediatric Cancer Cell Lines. J. Nat. Prod. 2019, 82, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Shuo, Y.; Zhang, C.; Yang, X.; Liu, F.; Zhang, Q.; Li, A.; Ma, J.; Lee, D.; Ohizumi, Y.; Guo, Y. Clerodane diterpenoids from Casearia kurzii and their cytotoxic activities. J. Nat. Med. 2019, 73, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Chapter 25. Zebrafish Model for Safety and Toxicity Testing of Nutraceuticals|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/B9780128021477000255?token=2D6D32DC8137B7B4B20BDA9F7F366074B12258665FA0A2E5CA3FB3BE0B5C2FC449B1B7F16BF1B49BE9775985535EFCEA&originRegion=eu-west-1&originCreation=20230106072706 (accessed on 6 January 2023).
- Ali, S.; van Mil, H.G.J.; Richardson, M.K. Large-Scale Assessment of the Zebrafish Embryo as a Possible Predictive Model in Toxicity Testing. PLoS ONE 2011, 6, e21076. [Google Scholar] [CrossRef] [PubMed]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Catteau, L.; Schioppa, L.; Beaufay, C.; Girardi, C.; Hérent, M.-F.; Frédérich, M.; Quetin-Leclercq, J. Antiprotozoal activities of Triterpenic Acids and Ester Derivatives Isolated from the Leaves of Vitellaria paradoxa. Planta Med. 2021, 87, 860–867. [Google Scholar] [CrossRef]

| Extracting Solvent | Yield (%m/m) | IC50 (µg/mL) ± SD (n = 3) |
|---|---|---|
| EtOH | 4.19 | 0.70 ± 0.12 |
| MeOH | 4.03 | 1.89 ± 0.05 |
| CH2Cl2 | 4.32 | 0.62 ± 0.07 |
| Sample | P. falciparum 3D7 IC50 (n = 3) (µg/mL) (µM) | Leishmania IC50 (n = 3) (µg/mL) (µM) | Trypanosoma IC50 (n = 3) (µg/mL) (µM) | Hemolysis (%) |
|---|---|---|---|---|
| DCM extract | 0.62 ± 0.07 - | NT | NT | <1% |
| Caseamembrin T (1) | 0.25 ± 0.10 0.49 ± 0.19 | 26.05 ± 0.64 51.69 ± 1.27 | 3.08 ± 0.33 6.11 ± 0.65 | <1% |
| Corymbulosin I (2) | 0.40 ± 0.13 0.79 ± 0.26 | 10.27 ± 0.23 20.38 ± 0.46 | 3.00 ± 0.60 5.95 ± 1.19 | <1% |
| Isocaseamembrin E (3) | 0.51 ± 0.13 0.98 ± 0.25 | 26.04 ± 0.95 50.27 ± 1.83 | 3.03 ± 0.49 5.85 ± 0.95 | <1% |
| Artemisinin | 0.004 ± 0.001 0.014 ± 0.003 | - | - | - |
| Triton 20% | - | - | - | 100% |
| Pentamidine | - | 0.02 ± 0.00 0.06 ± 0.00 | - | |
| Suramine | - | 0.05 ± 0.008 0.04 ± 0.008 | - |
| Sample |
MDA-MB-231 IC50 (n = 3) (µg/mL) (µM) |
PANC-1 IC50 (n = 3) (µg/mL) (µM) |
A549 IC50 (n = 3) (µg/mL) (µM) |
|---|---|---|---|
| DCM extract | 0.89 ± 0.15 - | 0.59 ± 0.04 - | 0.95 ± 0.12 - |
| Caseamembrin T (1) | 1.34 ± 0.01 2.66 ± 0.02 | 0.74 ± 0.08 1.47 ± 0.16 | 1.62 ± 0.18 3.21 ± 0.36 |
| Corymbulosin I (2) | 0.47 ± 0.02 0.93 ± 0.04 | 0.31 ± 0.10 0.62 ± 0.19 | 0.75 ± 0.07 1.49 ± 0.14 |
| Isocaseamembrin E (3) | 1.05 ± 0.25 2.03 ± 0.48 | 0.61 ± 0.08 1.18 ± 0.15 | 1.50 ± 0.08 2.89 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledoux, A.; Hamann, C.; Bonnet, O.; Jullien, K.; Quetin-Leclercq, J.; Tchinda, A.; Smadja, J.; Gauvin-Bialecki, A.; Maquoi, E.; Frédérich, M. Bioactive Clerodane Diterpenoids from the Leaves of Casearia coriacea Vent. Molecules 2023, 28, 1197. https://doi.org/10.3390/molecules28031197
Ledoux A, Hamann C, Bonnet O, Jullien K, Quetin-Leclercq J, Tchinda A, Smadja J, Gauvin-Bialecki A, Maquoi E, Frédérich M. Bioactive Clerodane Diterpenoids from the Leaves of Casearia coriacea Vent. Molecules. 2023; 28(3):1197. https://doi.org/10.3390/molecules28031197
Chicago/Turabian StyleLedoux, Allison, Carla Hamann, Olivier Bonnet, Kateline Jullien, Joëlle Quetin-Leclercq, Alembert Tchinda, Jacqueline Smadja, Anne Gauvin-Bialecki, Erik Maquoi, and Michel Frédérich. 2023. "Bioactive Clerodane Diterpenoids from the Leaves of Casearia coriacea Vent" Molecules 28, no. 3: 1197. https://doi.org/10.3390/molecules28031197
APA StyleLedoux, A., Hamann, C., Bonnet, O., Jullien, K., Quetin-Leclercq, J., Tchinda, A., Smadja, J., Gauvin-Bialecki, A., Maquoi, E., & Frédérich, M. (2023). Bioactive Clerodane Diterpenoids from the Leaves of Casearia coriacea Vent. Molecules, 28(3), 1197. https://doi.org/10.3390/molecules28031197







