Abstract
Natural products are a source for pesticide or drug discovery. In order to discover lead compounds with high fungicidal or herbicidal activity, new niacinamide derivatives derived from the natural product niacinamide, containing chiral flexible chains, were designed and synthesized. Their structures were confirmed by 1H NMR, 13C NMR and HRMS analysis. The fungicidal and herbicidal activities of these compounds were tested. The fungicidal activity results demonstrated that the compound (S)-2-(2-chloronicotinamido)propyl-2-methylbenzoate (3i) exhibited good fungicidal activity (92.3% inhibition) against the plant pathogen Botryosphaeria berengriana at 50 μg/mL and with an EC50 of 6.68 ± 0.72 μg/mL, which is the same as the positive control (fluxapyroxad). Compound 3i was not phytotoxic and could therefore be used as a fungicide on crops. Structure-activity relationships (SAR) were studied by molecular docking simulations with the succinate dehydrogenase of the fungal mitochondrial respiratory chain.
1. Introduction
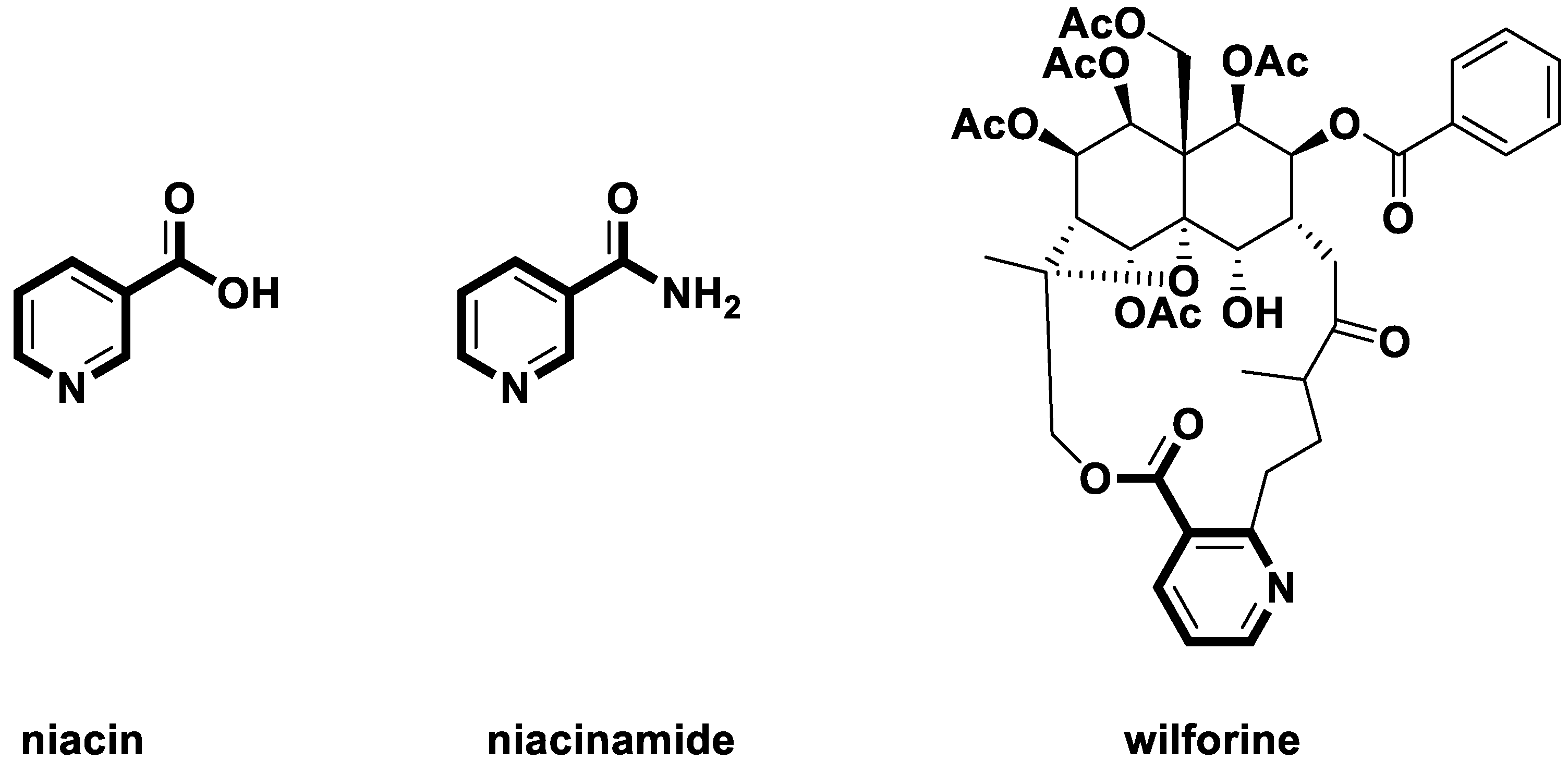
Natural products [1,2,3] have diverse structures, high biological activities, and novel mechanisms of action. Therefore, they are a rich source for pesticide discovery [4,5,6,7]. Nicotine is a class of pyridine type natural products which was first found in tobacco [8]. Nicotine has the effects of killing insects [9] and regulating human physiological activities [10]. Nicotine is also an important intermediate [11] in the synthesis of the natural product niacin and niacinamide (Figure 1), which are essential vitamins for humans. Nicotine acid derivatives can be found as many plant or microbial secondary metabolites. For example, wilforine (Figure 1) [12], which was found as a secondary metabolite in the Chinese traditional insecticidal plant thunder god vine (Tripterygium wilfordii), was used to treat rheumatoid arthritis, nephritis, lupus erythematosus, and thrombocytopenic purpura.
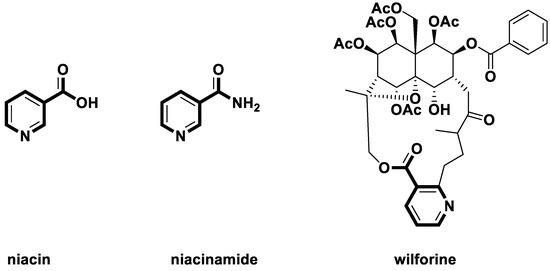
Figure 1.
Some natural niacin derivatives.
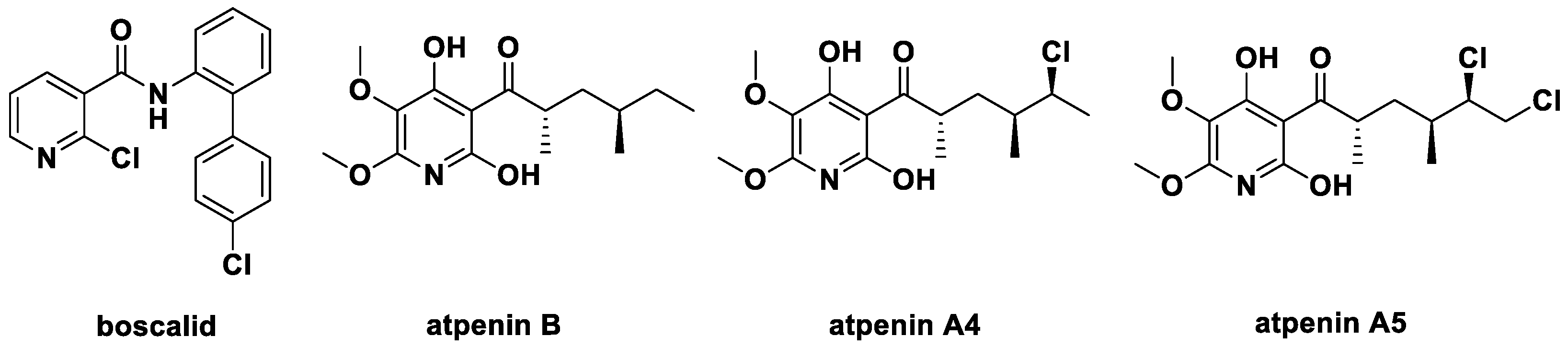
Furthermore, the pyridine structure is also a key skeleton in many agrochemicals [13,14,15]. They include the fungicide boscalid [16], the insecticide pyrifluquinazon [17], and the herbicide fluroxypyr [18]. The excellent succinate dehydrogenase inhibitor (SDHI) boscalid (2-chloro-N-(4′-chloro-[1,1′-biphenyl]-2-yl)nicotinamide, Figure 2) has high activity against plant pathogens Sclerotinia species., Zymospetoria species, and Colletotrichum species., and it has been widely used in agriculture. It became one of the important template molecules for the development of new fungicides [19,20,21] with a niacinamide skeleton (Figure 2). In 1990, Kumagai et al. [22] discovered three microbial secondary metabolites atpenin B, atpenin A4, atpenin A5 in Penicillium species, which contain pyridine ring, acyl group and a longer aliphatic chain with strong inhibitory activity against SDH from bovine heart.
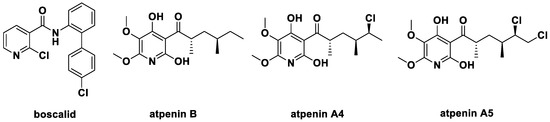
Figure 2.
The structure of boscalid and natural SDHI atpenins.
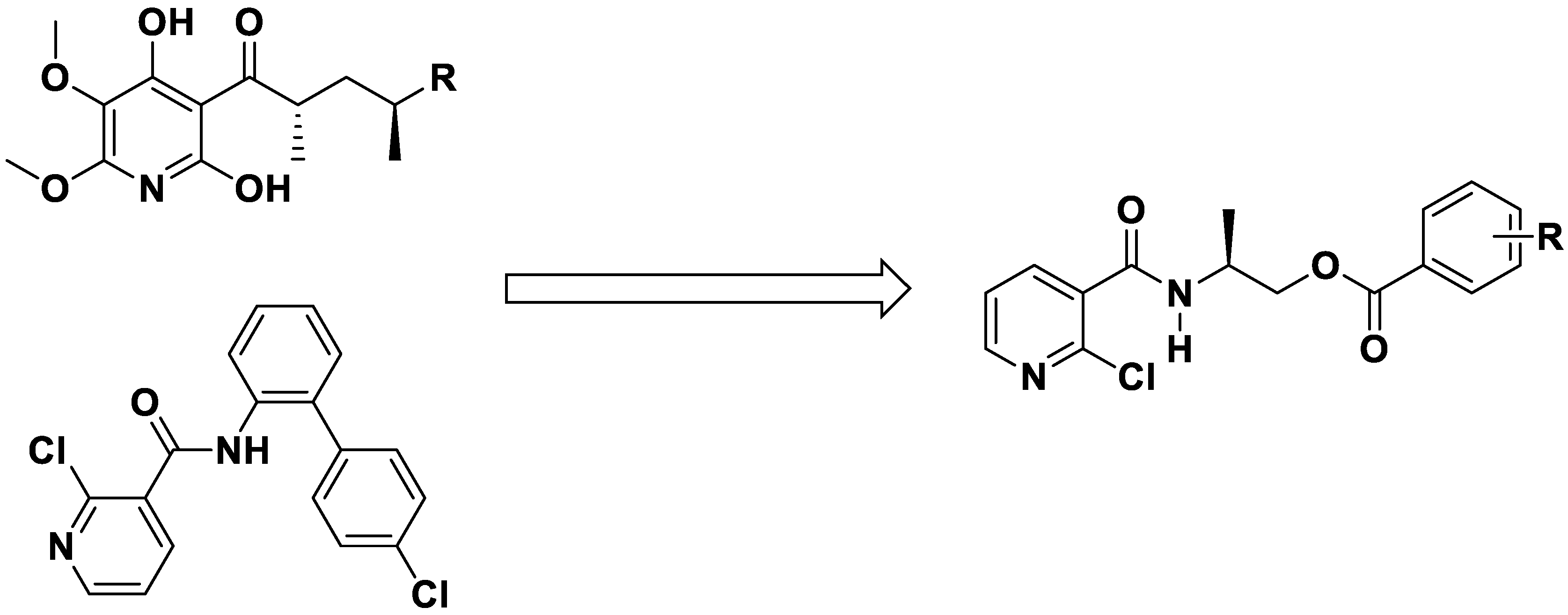
In our previous work, many bioactive heterocyclic compounds were designed and synthesized [23,24,25,26]. Of these, some pyridine derivatives exhibited good herbicidal, fungicidal, and plant growth regulation activity. In this paper, based on the structure of the natural product atpenin and the fungicide boscalid, a series of chiral niacinamide derivatives were designed. 2-Chloronicotinic acid was selected as a starting material and a carboxamide group and a flexible, chiral chain was introduced (Figure 3). Niacinamide derivatives were finally synthesized, and the structures were characterized by 1H NMR, 13C NMR and HRMS analysis. The fungicidal and herbicidal activity of chiral niacinamide compounds were evaluated, and the docking studies were also carried out to study the mode of action.
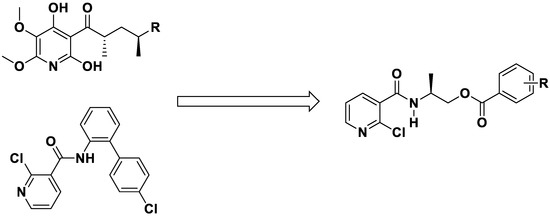
Figure 3.
The design strategy of title chiral niacinamide derivatives.
2. Results and Discussion
2.1. Synthesis and Spectra Analysis
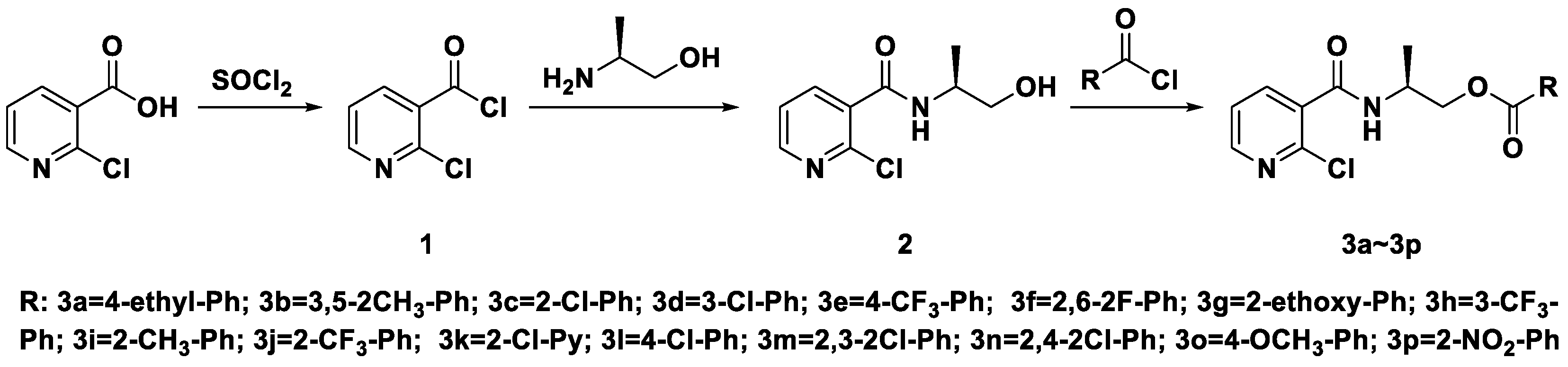
The synthetic route of (S)-2-(2-chloronicotinamido)propyl benzoates or nicotinate compounds is outlined in Scheme 1. In the process of synthesizing acyl chloride 1, SOCl2 is not only a reactant but also a solvent, so it needs to be excess. A tail gas absorption device should be added during the reaction to avoid environmental pollution. The reaction is judged by the state of the reaction liquid, which is changed from turbid to clear, the reaction can be stopped, then excess sulfoxide chloride was removed. In the synthesis of intermediate 2, the diluted acyl chloride 1 should be added slowly to a solution of (S)-2-aminopropan-1-ol, and the order cannot be changed. Furthermore, (S)-2-aminopropan-1-ol needs to be in excess (at least twice), which can improve the yield of intermediate 2. When the intermediate 2 is purified by column chromatography, the by-product ((S)-2-aminopropyl 2-chloronicotinate) is first obtained with eluent (ethyl acetate/petroleum ether = 1/1), and the intermediate 2 is directly given with pure ethyl acetate. For target product synthesis, following the addition of one equivalent of the respective substituted benzoyl chlorides (and nicotinyl chloride 3k), the reaction was stirred at room temperature and monitored by thin layer chromatography.
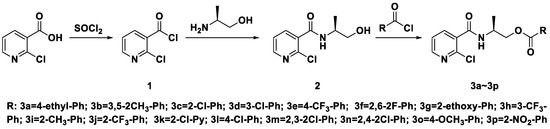
Scheme 1.
Synthetic route of the title compounds (S)-2-(2-chloronicotinamido)propyl benzoates or nicotinate compounds.
According to the 1H NMR spectra of compounds 3a~3p, it can be found that doublet peaks from the amide group can be found at about 6.4 ppm, with a coupling constant of 6.4 Hz. The protons of the pyridine ring and the benzene ring are located at 6–9 ppm, which are according to the aromatic ranges. The doublet peak of the chiral methyl group appearance at 1.29~1.43 ppm with coupling constant is 5.4 Hz; The CH group, which found as multiple peaks with shifts between 4–5 ppm. According to the high-resolution mass spectrometry, the difference between the measured value and the theoretical value is within 0.003.
2.2. Fungicidal Activity
Fungicidal activities of (S)-2-(2-chloronicotinamido)propyl benzoates or nicotinate compounds against Fusarium oxysporum, Cercospora arachidicola, Botryosphaeria berengriana, Alternaria solani, Gibberella zeae, Sclerotinia sclerotiorum, Botrytis cinerea, Rhizoctonia solani, Phytophthora infestans, Phytophthora capsica, and fluxapyroxad were evaluated at 50 μg/mL according to our previous work [27,28] (Table 1).

Table 1.
The fungicidal activity (% inhibition) of compounds 3a–3p and fluxapyroxad (FP) at 50 μg/mL on F. oxysporum (FO), C. arachidicola (CA), B. berengriana (BB), A. solani (AS), G. zeae (GZ), S. sclerotiorum (SS), B. cinerea (BC), R. solani (RS), P. infestans (PI) and P. capsici (PC). CK = control.
From Table 1, compounds 3a–3p showed good fungicidal activity (>60%) against B. berengriana, which is better than that of positive control fluxapyroxad (63.6%). Among them, compounds 3i, 3m, 3o, and 3p exhibited the best activity (>80%) against B. berengriana, while the fungicidal activity of compound 3i reached 92.3%, which was much higher than the control fluxapyroxad (63.6%). Against G. zeae, compounds 3g, 3h, 3j, 3k, 3m, 3n and 3p displayed good activities (about 70%), which were better than the positive control fluxapyroxad (28.6%). The other compounds also exhibited good activity (>30%) against G. zeae, except compounds 3c (21.9%) and 3d (25.0%). For A. solani, compounds 3j and 3p exhibited moderate activity (>60%), which was lower than that of l fluxapyroxad (88.9%). Compounds 3l and 3m exhibited good activity (>80%) against S. sclerotiorum, and most of the other compounds also possessed moderate activities (>50%), but all were lower than fluxapyroxad (96.4%). Against C. arachidicola, compounds 3g, 3k, 3l, 3m and 3n displayed moderate activities (>65%), but they were still lower than that of fluxapyroxad (100%). Compounds 3g, 3h, 3i and 3m displayed good activity (>57%) against B. cinerea, which is the same as the control (63.6%). Against F. oxysporum, P. infestans, P. capsici, R. solani, all compounds evaluated exhibited low activity.
Based on the preliminary fungicidal activity results of >80%, compounds 3i, 3l, 3m, 3o, and 3p were selected for further study. From Table 2, compounds 3i, 3m, 3o and 3p showed excellent fungicidal activity against B. berengriana with EC50 values of 6.68 ± 0.72, 15.14 ± 1.21, 11.98 ± 1.04 and 7.93 ± 1.05 μg mL−1, respectively, which are similar to fluxapyroxad (7.93 ± 1.05 μg mL−1). Among these compounds, compound 3i (6.68 ± 0.72 μg mL−1) exhibited the best activity, which is better than fluxapyroxad (7.93 ± 1.05 μg mL−1). For S. sclerotiorum, compounds 3l and 3m exhibited good activity with EC50 values of 12.69 ± 1.15 and 11.83 ± 1.03 μg mL−1, respectively, which were lower than that of fluxapyroxad (0.73 ± 0.11 μg mL−1).

Table 2.
The EC50 values against B. berengriana and S. sclerotiorum of select compounds.
2.3. Phytotoxicity
The phytotoxicity of (S)-2-(2-chloronicotinamido)propyl benzoates or nicotinate compounds are listed in Table 3. As shown in Table 3, most of the (S)-2-(2-chloronicotinamido)propyl benzoates or nicotinate compounds exhibited low phytotoxicity against lettuce (Lactuca sativa) and bentgrass (Agrostis stolonifera) at 1 mM, except compounds 3l and 3p. Compounds 3l and 3p possessed moderate phytotoxic (ranking 3) against Agrostis stolonifera at 1 mM. Compounds 3c, 3d, 3e, 3h, 3m, 3n possessed weak phytotoxicity (ranking 1~2) against Agrostis stolonifera at 1 mM. For the dicot lettuce, most of these compounds exhibited low weak phytotoxicity (ranking 1~2) against Agrostis stolonifera at 1 mM. The commercial herbicide aminotriazole was more active than any of the synthesized compounds on both lettuce and bentgrass.

Table 3.
Phytotoxicity against lettuce and bentgrass of title compounds at 1 mM.
2.4. Molecular Docking Studies
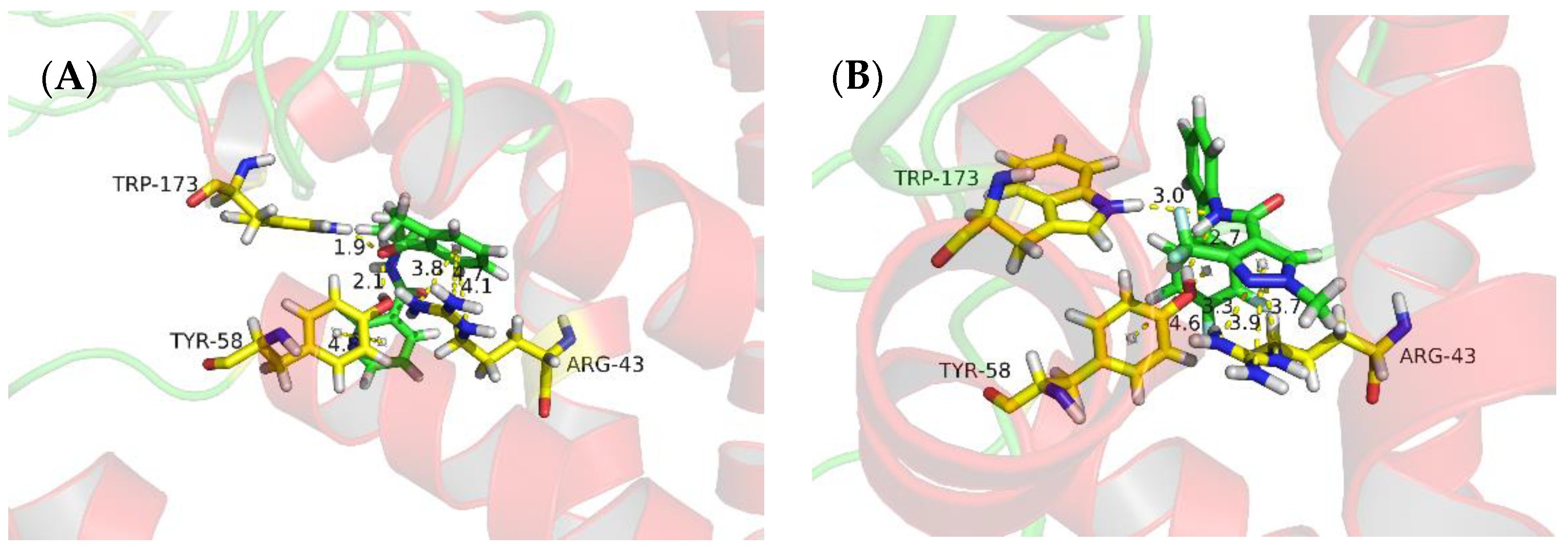
The niacinamide compounds in this study were designed from reported SDH inhibitors. The fungicidal activity of 3i is good, so we conducted molecular modeling analysis of binding of this compound with SDH. The molecular docking was done and results are shown in Table 4 and Figure 4. From Table 4, the docking scores of compound 3i and the positive control, fluxapyroxad, are similar. As shown in Figure 4A, there are two hydrogen bonds between Trp173 or Tyr58 and the O atom of CONH group with the distances of 1.9 Å and 2.1 Å, respectively. On the other hand, there were three π-cation interactions between Arg43 and the phenyl ring with the distances of 3.8 Å, 4.1 Å, 4.7 Å. Furthermore, there was a π-π interaction between the pyridine ring and Tyr58 with a distance of 4.8 Å. As for the positive control fluxapyroxad (Figure 4B), it had a different molecular interaction mode with compound 3i, although it also has two hydrogen bonds, three π-cation interactions and a π-π interaction with the same amino acid residue. The π–cation interactions were between the pyrazole ring and the Arg43, and the hydrogen bonds exist between the NH and Trp173 or Tyr58. This may be the reason of different fungicidal activities between 3i and fluxapyroxad.

Table 4.
The docking results of compounds 3i and fluxapyroxad with SDH.
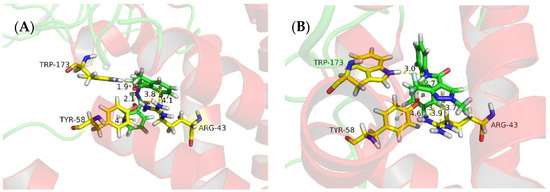
Figure 4.
Molecular docking result of 3i (A) and fluxapyroxad (B) with SDH.
3. Materials and Methods
3.1. Instruments
Melting points were determined using an X-4 apparatus and uncorrected. 1H NMR and 13C NMR spectra were measured on a Bruker AC-P500 and AC-P400 instrument using TMS as an internal standard and CDCl3 as the solvent. HR-ESI-MS was tested using an Agilent 1100 HPLC-JEOL AccuTOF instrument. All reagents were of analytical grade or freshly prepared before use.
3.2. Synthesis
3.2.1. Synthesis of Intermediate 2
The synthetic route is shown in Scheme 1. 2-Chloronicotinic acid (3.4 g, 22 mmol) and SOCl2 (10 mL) were heated at reflux for 3h, until the reaction solution becomes clear, then reflux for another 30min, evaporated SOCl2 to obtain yellow liquid 1 without purification. Then, the solution of 2-chloronicotinoyl chloride (14.1 g, 0.08mol) in THF (10 mL) was added in a dropwise fashion to the mixture of (S)-2-aminopropan-1-ol (7.5g, 0.1 mol) and triethylamine (8 mL) in THF (20 mL), the mixture was stirred for 4 h. The solvent was removed, and pure product of intermediate 2 was given as a white solid by column chromatography. White solid, m.p.62–64 °C, 1H NMR (CDCl3, 500 MHz), δ: 1.25 (d, J = 5.5 Hz, 3H, CH3), 3.35 (t, J = 4.5 Hz, 1H, OH), 3.57–3.62 (m, 1H, CH2), 3.69–3.73 (m, 1H, CH2), 4.18–4.23 (m, 1H, CH), 6.93 (d, J = 6.1 Hz, 1H, NH), 7.27–7.29 (m, 1H, Py), 7.92–7.94 (m, 1H, Py), 8.37–8.39 (m, 1H, Py).
3.2.2. Synthesis of Target Compounds 3
The synthetic route is shown in Scheme 1. To a solution of intermediate 2 (0.3 g, 1.4 mmol) and triethylamine (2 mL) in THF (10 mL), substituted benzoyl chloride (1.5 mmol) was added dropwise. The mixture was stirred at room temperature for 3h. The THF was removed, and the target compounds 3a–3p were given by column chromatography. The structure of the pure title product was confirmed by 1 H NMR, 13C NMR and HRMS. The spectra are given as supporting information.
(S)-2-(2-chloronicotinamido)propyl 4-ethylbenzoate (3a)
White solid, yield 43.4%, m.p. 77–79 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.26 (t, J = 6.0 Hz, 3H, CH3), 1.40 (d, J = 5.7Hz, 3H, CH3), 2.69–2.73 (m, 2H, CH2), 4.43–4.45 (m, 2H, CH2), 4.62–4.66 (m, 1H, CH), 6.77 (d, J = 6.4 Hz, 1H, NH), 7.27 (d, J = 6.5 Hz, 2H, Ph), 7.30–7.33 (m, 1H, Py), 7.96 (d, J = 6.5 Hz, 2H, Ph), 8.01–8.03 (m, 1H, Py), 8.43–8.44 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ: 15.18, 17.21, 28.95, 45.64, 66.87, 122.72, 127.01, 128.01(2C, Ph), 129.78(2C, Ph), 131.28, 139.53, 147.06, 150.26, 150.89, 164.27, 166.63; HRMS (ESI) for C18H19ClN2O3 m/z: Calculated, 347.1157, Found, 347.1153 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 3,5-dimethylbenzoate (3b)
White solid, yield 57.4%, m.p. 100–103 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.41 (d, J = 5.6 Hz, 3H, CH3), 2.37 (s, 6H, CH3), 4.41–4.46 (m, 2H, CH2), 4.61–4.66 (m, 1H, CH), 6.79 (d, J = 6.3 Hz, 1H, NH), 7.21 (s, 1H, Ph), 7.32–7.34 (m, 1H, Py), 7.66 (s, 2H, Ph), 8.06–8.07 (m, 1H, Py), 8.44–8.46 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.28, 21.15, 45.73, 66.86, 73.53, 122.78, 127.35(2C, Ph), 129.42, 131.14, 134.99, 138.18(2C, Ph), 139.79, 147.86, 150.99, 164.13, 166.91; HRMS (ESI) for C18H19ClN2O3 m/z: Calculated, 347.1157, Found, 347.1155 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2-chlorobenzoate (3c)
White solid, yield 57.6%, m.p. 77–79 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.42 (d, J = 5.5 Hz, 3H, CH3), 4.45–4.47 (m, 2H, CH2), 4.64–4.68 (m, 1H, CH), 6.75 (d, J = 6.4 Hz, 1H, NH), 7.30–7.32 (m, 1H, Py), 7.32–7.35 (m, 1H, Ph), 7.42–7.46 (m, 2H, Ph), 7.86–7.87 (m, 1H, Ph), 8.01–8.03 (m, 1H, Py), 8.42–8.44 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.35, 45.31, 67.67, 122.70, 126.75, 129.51, 131.14, 131.27, 131.69, 132.93, 133.57, 139.45, 147.06, 150.93, 164.29, 165.67; HRMS (ESI) for C16H14Cl2N2O3 m/z: Calculated, 353.0454, Found, 353.0456 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 3-chlorobenzoate (3d)
White solid, yield 47.9%, m.p. 126–128 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.42 (d, J = 5.4 Hz, 3H, CH3), 4.44–4.48 (m, 2H, CH2), 4.63–4.67 (m, 1H, CH), 6.71 (d, J = 6.4 Hz, 1H, NH), 7.32–7.35 (m, 1H, Py), 7.39–7.42 (m, 1H, Ph), 7.55–7.57 (m, 1H, Ph), 7.93–7.95 (m, 1H, Ph), 8.01–8.02 (m, 1H, Ph), 8.03–8.04 (m, 1H, Py), 8.44–8.45 (m, 1H, Py); 13C NMR (CDCl3, 125 MHz) δ: 17.19, 45.50, 67.43, 122.81, 127.79, 129.71, 129.86, 131.14, 131.35, 133.34, 134.66, 139.68, 147.01, 151.03, 164.28, 165.33; HRMS (ESI) for C16H14Cl2N2O3 m/z: Calculated, 353.0454, Found, 353.0460 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 4-(trifluoromethyl)benzoate (3e)
White solid, yield 54.6%, m.p. 104–107 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.41 (d, J = 5.4 Hz, 3H, CH3), 4.45–4.49 (m, 2H, CH2), 4.64–4.69 (m, 1H, CH), 6.71 (d, J = 6.4 Hz, 1H, NH), 7.31–7.33 (m, 1H, Py), 7.71 (d, J = 6.6 Hz, 2H, Ph), 8.00–8.02 (m, 1H, Py), 8.16 (d, J = 6.5 Hz, 2H, Ph), 8.42–8.44 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.17, 45.44, 67.59, 122.82(2C, Ph), 124.85(CF3, J = 272Hz), 125.48(q, J = 4Hz), 130.06(2C, Ph), 131.05, 132.81, 134.57, 139.68, 146.93, 151.07, 164.26, 165.31; HRMS (ESI) for C17H14ClF3N2O3 m/z: Calculated, 387.0718, Found, 387.0719 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2,6-difluorobenzoate (3f)
White solid, yield 67.3%, m.p. 61–63 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.41 (d, J = 5.6 Hz, 3H, CH3), 4.48–4.52 (m, 2H, CH2), 4.62–4.66 (m, 1H, CH), 6.69 (d, J = 6.4 Hz, 1H, NH), 6.97 (t, J = 6.6 Hz, 2H, Ph), 7.32–7.35 (m, 1H, Py), 7.42–7.47 (m, 1H, Ph), 8.05–8.07 (m, 1H, Py), 8.44–8.45 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.19, 45.14, 67.85, 109.99, 112.06(d, J = 3Hz), 112.29(d, J = 3Hz), 122.72, 131.10, 133.35(t, J = 10.1Hz), 139.64, 147.12, 150.98, 162.07, 164.15; HRMS (ESI) for C16H13ClF2N2O3 m/z: Calculated, 355.0656, Found, 355.0660 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2-ethoxybenzoate (3g)
Waxy solid, yield 55.6%; 1H NMR (CDCl3, 500 MHz), δ: 1.36 (t, J = 5.6 Hz, 3H, CH3), 1.41 (d, J = 5.4 Hz, 3H, CH3), 4.06–4.10 (m, 2H, CH2), 4.40–4.45 (m, 2H, CH2), 4.59–4.64 (m, 1H, CH), 6.78 (d, J = 6.4 Hz, 1H, NH), 6.94–6.98 (m, 2H, Ph), 7.29–7.31 (m, 1H, Py), 7.44–7.47 (m, 2H, Ph), 7.79–7.81 (m, 2H, Ph), 7.99–8.01 (m, 1H, Py), 8.42–8.43 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ: 14.76, 17.42, 45.47, 64.31, 66.95, 112.97, 119.67, 120.10, 122.65, 131.46, 131.82, 133.77, 139.33, 147.15, 150.84, 158.43, 164.23, 166.57; HRMS (ESI) for C18H19ClN2O4 m/z: Calculated, 385.0926, Found, 385.0922 [M + Na]+.
(S)-2-(2-chloronicotinamido)propyl 3-(trifluoromethyl)benzoate (3h)
White solid, yield 48.6%, m.p. 112–114 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.41 (d, J = 5.6 Hz, 3H, CH3), 4.44–4.51 (m, 2H, CH2), 4.62–4.68 (m, 1H, CH), 6.74 (d, J = 6.4 Hz, 1H, NH), 7.31–7.33 (m, 1H, Py), 7.60 (t, J = 6.2 Hz, 1H, Ph), 7.83 (d, J = 6.2 Hz, 1H, Ph), 8.01–8.03 (m, 1H, Py), 8.23 (d, J = 6.3 Hz, 1H, Ph), 8.30 (s, 1H, Ph), 8.42–8.44 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ: 17.15, 45.46, 67.57, 122.80, 124.88(CF3, J = 270Hz), 126.52(q, J = 4Hz), 129.22, 129.79, 130.48, 131.04, 131.33, 132.85, 139.71, 146.94, 151.05, 164.27, 165.20; HRMS (ESI) for C17H14ClF3N2O3 m/z: Calculated, 387.0718, Found, 387.0732 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2-methylbenzoate (3i)
White solid, yield 41.7%, m.p.92–94 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.42 (d, J = 5.4 Hz, 3H, CH3), 2.61 (s, 3H, CH3), 4.41–4.47 (m, 2H, CH2), 4.62–4.68 (m, 1H, CH), 6.71 (d, J = 6.2 Hz, 1H, NH), 7.25–7.28 (m, 2H, Ph), 7.32–7.35 (m, 1H, Py), 7.41–7.44 (m, 1H, Ph), 7.93–7.95 (m, 1H, Ph), 8.04–8.06 (m, 1H, Py), 8.45–8.46 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.36, 21.86, 45.62, 66.83, 122.76, 125.81, 130.60, 131.13, 131.83, 132.36, 138.06, 139.70, 140.47, 147.03, 151.00, 164.16, 167.36; HRMS (ESI) for C17H17ClN2O3 m/z: Calculated, 333.1000, Found, 333.1009 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2-(trifluoromethyl)benzoate (3j)
Oil, yield 57.9%; 1H NMR (CDCl3, 500 MHz), δ: 1.31 (d, J = 5.4 Hz, 3H, CH3), 4.35–4.42 (m, 2H, CH2), 4.50–4.57 (m, 1H, CH), 6.85 (d, J = 6.5 Hz, 1H, NH), 7.20–7.23 (m, 1H, Py), 7.56–7.59 (m, 2H, Ph), 7.68–7.70 (m, 1H, Ph), 7.75–7.79 (m, 1H, Ph), 7.84–7.86 (m, 1H, Py), 8.31–8.32 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ: 17.09, 45.20, 68.22, 109.98, 121.98, 122.70, 124.70(CF3, J = 271Hz), 126.65(q, J = 5Hz), 130.50, 131.22, 131.52, 131.93, 139.39, 147.07, 150.92, 164.34, 166.74; HRMS (ESI) for C17H14ClF3N2O3 m/z: Calculated, 387.0718, Found, 387.0720 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2-chloronicotinate (3k)
White solid, yield 65.1%, m.p.106–108 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.43 (d, J = 5.4 Hz, 3H, CH3), 4.46–4.52 (m, 2H, CH2), 4.64–4.70 (m, 1H, CH), 6.67 (d, J = 6.5 Hz, 1H, NH), 7.32–7.37 (m, 2H, Py), 8.02–8.04 (m, 1H, Py), 8.22–8.23 (m, 1H, Py), 8.43–8.45 (m, 1H, Py), 8.52–8.53 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.30, 45.22, 68.23, 122.26, 122.78, 126.43, 131.05, 139.57, 140.69, 146.98, 149.84, 151.06, 152.17, 164.29, 164.50; HRMS (ESI) for C15H13Cl2N3O3 m/z: Calculated, 354.0407, Found, 354.0409 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 4-chlorobenzoate (3l)
White solid, yield 52.8%, m.p. 89–91 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.31 (d, J = 5.4 Hz, 3H, CH3), 4.45–4.51 (m, 2H, CH2), 4.62–4.69 (m, 1H, CH), 6.68 (d, J = 6.5 Hz, 1H, NH), 7.27–7.30 (m, 2H, Ph), 7.32–7.34 (m, 1H, Py), 7.60–7.62 (m, 1H, Ph), 7.69–7.71 (m, 1H, Ph), 8.02–8.04 (m, 1H, Py), 8.44–8.45 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.32, 45.28, 68.00, 122.77, 127.28, 129.29(2C, Ph), 131.08, 132.32, 133.46(2C, Ph), 139.59, 147.02, 151.04, 164.24, 165.34;HRMS (ESI) for C16H14Cl2N2O3 m/z: Calculated, 353.0454, Found, 353.0450 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2,3-dichlorobenzoate (3m)
White solid, yield 47.9%, m.p. 89–91 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.42 (d, J = 5.4 Hz, 3H, CH3), 4.45–4.51 (m, 2H, CH2), 4.62–4.69 (m, 1H, CH), 6.66 (d, J = 6.5 Hz, 1H, NH), 7.27–7.30 (m, 1H, Ph), 7.32–7.35 (m, 1H, Py), 7.61–7.63 (m, 1H, Ph), 7.69–7.71 (m, 1H, Ph), 8.03–8.05 (m, 1H, Py), 8.44–8.46 (m, 1H, Py); 13C NMR (CDCl3, 125 MHz) δ: 17.32, 45.29, 68.00, 122.77, 127.30, 129.31, 131.13, 131.53, 132.34, 133.47, 134.69, 139.56, 147.04, 151.04, 164.27, 165.35; HRMS (ESI) for C16H13Cl3N2O3 m/z: Calculated, 387.0065, Found, 387.0071 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2,4-dichlorobenzoate (3n)
White solid, yield 48.3%, m.p. 116–118 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.42 (d, J = 5.6 Hz, 3H, CH3), 4.44–4.49 (m, 2H, CH2), 4.63–4.70 (m, 1H, CH), 6.66 (d, J = 6.5 Hz, 1H, NH), 7.32–7.36 (m, 2H, Ph, Py), 7.49 (s, 1H, Ph), 7.86 (d, J = 6.7 Hz, 1H, Ph), 8.04–8.06 (m, 1H, Py), 8.45–8.46 (m, 1H, Py); 13C NMR (CDCl3, 125 MHz) δ: 17.36, 45.29, 67.89, 122.79, 127.23, 127.76, 131.11, 131.13, 132.84, 134.82, 138.81, 139.65, 147.04, 151.06, 164.23, 164.78; HRMS (ESI) for C16H13Cl3N2O3 m/z: Calculated, 387.0065, Found, 387.0070 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 4-methoxybenzoate (3o)
White solid, yield 67.6%, m.p. 108–110 °C; 1H NMR (CDCl3, 500 MHz), δ: 1.41 (d, J = 5.4 Hz, 3H, CH3), 4.39–4.46 (m, 2H, CH2), 4.60–4.67 (m, 1H, CH), 6.73 (d, J = 6.4 Hz, 1H, NH), 6.93 (d, J = 6.6 Hz, 2H, Ph), 7.32–7.34 (m, 1H, Py), 8.01 (d, J = 6.7 Hz, 2H, Ph), 8.02–8.04 (m, 1H, Py), 8.44–8.45 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.22, 45.70, 55.45, 66.78, 113.74(2C, Ph), 121.91, 122.74, 131.26, 131.72(2C, Ph), 139.59, 147.05, 150.93, 163.62, 164.24, 166.32; HRMS (ESI) for C17H17ClN2O4 m/z: Calculated, 349.0950, Found, 349.0961 [M + H]+.
(S)-2-(2-chloronicotinamido)propyl 2-nitrobenzoate (3p)
oil, yield 63.6%; 1H NMR (CDCl3, 500 MHz), δ: 1.29 (d, J = 5.4 Hz, 3H, CH3), 4.28–4.31 (m, H, CH), 4.44–4.53 (m, 2H, CH2), 6.78 (d, J = 6.4 Hz, 1H, NH), 7.22–7.24 (m, 1H, Py), 7.60–7.66 (m, 2H, Ph), 7.75–7.77 (m, 1H, Ph), 7.79–7.81 (m, 1H, Ph), 7.85–7.88 (m, 1H, Py), 8.31–8.33 (m, 1H, Py); 13C NMR (CDCl3, 101 MHz) δ:17.01, 45.16, 68.51, 122.62, 123.70, 126.62, 130.36, 131.41, 132.26, 132.91, 139.14, 139.17, 147.24, 150.81, 164.56, 165.00; HRMS (ESI) for C16H14ClN3O5 m/z: Calculated, 364.0695, Found, 364.0700 [M + H]+.
3.3. Fungicide Bioassays
The fungicidal activity of title chiral niacinamide derivatives 3a~3p were tested in vitro against F. oxysporum (FO), C. arachidicola (CA), B.berengriana (BB), A. solani (AS), G. zeae (GZ), S. sclerotiorum (SS), B. cinerea (BC), R. solani (RS), P. infestans (PI) and P. capsici (PC), and their relative percent inhibition (%) was determined using the mycelium growth rate method according to the previous work [27,28]. Fluxapyroxad was used as the positive control. Each compound was dissolved in DMSO with 1% tween to prepare the 10 g/L stock solution. The ten fungal species were inoculated into a Petri dish containing 50 μg/mL stock solution and incubated in a 25 °C biochemical incubator in darkness. The solvent DMSO with 1% tween was used as a blank assay. The fungicidal effect was determined 3 days later. Each treatment (species and compound) was repeated three times. The inhibition of compounds compared to the blank assay was calculated via the following equation:
where CK is the average diameter of mycelia in the blank test and AI is the average diameter of mycelia in the presence of those compounds. The EC50 values were also calculated.
Inhibition (%) = (CK − AI)/CK × 100%
3.4. Herbicide Bioassays
The bioassay method of Dayan et al. [29] was used. Briefly, seeds of lettuce (Latuca sativa—cv. Iceberg A Crisphead from Burpee Seeds of Warminster, PA, USA) and bentrass (Agrostis stolonifera—Penncross variety obtained from Turf-Seed, Inc. of Hubbard, OR, USA) were surface sterilized with a 0.5% to 1% (v/v) sodium hypochlorite solution for approximately 10 min, rinsed with deionized water and dried in a sterile environment. A filter paper disk (Whatman Grade 1, 1.5 cm) was placed in each well of a 24-well plate. The control wells contained 200 μL of deionized water. The control + solvent well contained 180 μL of water and 20 μL of the solvent. All sample wells contained 180 μL of water and 20 μL of the appropriate dilution of the sample. Water was pipetted into the well before the sample or solvent. Test samples were dissolved in acetone and the final concentration of acetone in the wells was 10%. For the bioassay five lettuce seeds or 20 mg of bentgrass seeds were placed in each well before sealing the plate with Parafilm. The plates were incubated for seven days in a Percival Scientific (Perry, IA, USA) CU-36L5 incubator under continuous light conditions at 26 °C and 120 μmol∙s−1∙m−2 average photosynthetically active radiation (PAR). A qualitative estimate of phytotoxicity was made by assigning a rating of 0 for no effect (sample well plants looked identical to the control plus solvent well plants; seeds had germinated and resulting seedlings had grown normally), 1 less than 20% inhibited germination, 2 for about 20–40% germination inhibition, 3 for about 40–60% germination inhibition, 4 for more than 60% germination inhibition, and 5 for no germination of the seeds. Each experiment was repeated.
3.5. Molecular Docking
Modeled protein succinate dehydrogenase (SDH) and compound 3i and positive control fluxapyroxad were prepared using Discovery Studio 3.5 software according to our previous work [30,31]. All docking calculations were performed using the Discovery Studio 3.5 software package and energy minimization was carried out by CHARMm [32] force field using the ligand partial charge method CFF (Consistent Force Field). Minimization was carried out until an energy gradient of 0.01 was reached. The CDOCKER was used for docking of the two compounds. The X-ray crystal structure of succinate dehydrogenase (SDH, 2FBW [33]) was downloaded from the protein data bank, which was used and refined with CHARMm in DS 3.5 at physiological pH. Consequently, compound 3i and the positive control fluxapyroxad were docked into the active site; ten conformations of each compound were obtained through CDOCKER based on the docking score after energy minimization using the smart minimizer method, which begins with the steepest descent method, followed by the conjugate gradient method. For each final pose, the CHARMm energy and the interaction energy are calculated. The poses are sorted by CHARMm energy, and the tentop scoring poses are retained [34]. The binding modes were validated by interactions between the candidate molecules and active site residues.
4. Conclusions
In conclusion, a series of (S)-2-(2-chloronicotinamido)propyl benzoate compounds was synthesized using 2-chloronicotinic acid as starting material. These compounds were introduced with a flexible, chiral chain. The fungicidal and herbicidal activity of the synthesized compounds against ten fungi and two plant species were tested, and some of the compounds showed good fungicidal activity against B. berengriana and S. sclerotiorum, and low phytotoxic against the monocot and dicot, allowing them to be used in plant fungal protection programs. Molecular binding studies indicate that the most active compound 3i is a probably a SDH inhibitor. These structures can be further optimized for discovery of new fungicides.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28010047/s1, Figures S1–S47: Spectroscopy of target compounds.
Author Contributions
Methodology, Z.-C.W. and Q.W.; formal analysis, Z.-C.W. and Q.W.; investigation, Z.-C.W. and Q.W.; data curation, Z.-C.W. and Q.W.; writing—original draft preparation, Z.-C.W.; writing—review and editing, X.-H.L., S.O.D., C.L.C., J.B.-H., L.-J.M., L.H., J.-Q.W., C.-X.T., N.-B.S. and Y.-X.L.; supervision, X.-H.L. and S.O.D.; funding acquisition, X.-H.L., L.H. and S.O.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by Zhejiang Provincial Natural Science Foundation of China (No. LTGN23C140002, LY19C140002, LY19B020009) and the Chemical Company for Research (KYY-HX-20210140, KYY-HX-20190720); the Open Fund of Key Laboratory of Study, Discovery of Small Targeted Molecules of Hunan Province (2022CG02); the Institute for Frontiers and Interdisciplinary Sciences, Zhejiang University of Technology (2022JCY04); Partial funding was provided by the USDA Non-Assistance Cooperative Agreement 58-6060-1-001 grant to the University of Mississippi.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Wang, D.L.; Li, M.; Yuan, C.X.; Fang, Y.L.; Zhang, Z.J. Guaiacol as a natural melanin biosynthesis inhibitor to control northern corn leaf blight. Pest Manag. Sci. 2022, 78, 4557–4568. [Google Scholar] [CrossRef]
- Cardenas, D.M.; Bajsa-Hirschel, J.; Cantrell, C.L.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macias, F.A. Evaluation of the phytotoxic and antifungal activity of C-17-sesquiterpenoids as potential biopesticides. Pest Manag. Sci. 2022, 78, 4240–4251. [Google Scholar] [CrossRef]
- Barilli, E.; Agudo, F.J.; Masi, M.; Nocera, P.; Evidente, A.; Rubiales, D. Anthraquinones and their analogues as potential biocontrol agents of rust and powdery mildew diseases of field crops. Pest Manag. Sci. 2022, 78, 3489–3497. [Google Scholar] [CrossRef]
- Che, Z.P.; Guo, X.L.; Li, Y.H.; Zhang, S.; Zhu, L.N.; He, J.X.; Sun, D.; Guo, Y.H.; Liu, Y.B.; Wei, R.X.; et al. Synthesis of paeonol ester derivatives and their insecticidal, nematicidal, and anti-oomycete activities. Pest Manag. Sci. 2022, 78, 3442–3455. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.W.; Zhang, L.; Liu, S.T.; Zhang, J.R.; Zhou, X.; Wang, P.Y.; Yang, S. Discovery of novel rost-4-ene derivatives as potential plant activators for preventing phytopathogenic bacterial infection: Design, synthesis and biological studies. Pest Manag. Sci. 2022, 78, 3404–3415. [Google Scholar] [CrossRef]
- Velasco-Azorsa, R.; Zeferino-Diaz, R.; Alvarado-Rodriguez, J.G.; Lopez-Ruiz, H.; Rojas-Lima, S.; Flores-Castro, K.; Prado-Vera, I.C.; Alatorre-Rosas, R.; Tut-Pech, F.; Carrillo-Benitez, M.G.; et al. Nematicidal activity of furanoeremophilenes against Meloidogyne incognita and Nacobbus aberrans. Pest Manag. Sci. 2022, 78, 2571–2580. [Google Scholar] [CrossRef]
- Sparks, T.C.; Duke, S.O. Structural simplification of natural products as a lead generation approach in crop protection compound discovery. J. Agric. Food Chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef]
- Posselt, W.; Reimann, L. Chemische untersuchungen des tabaks und darstellung eines eigenthümlich wirksamen prinzips dieser pflanze. Geigers Mag. Pharmac. 1828, 6, 138–161. [Google Scholar]
- Casanova, H.; Ortiz, C.; Pelaez, C.; Vallejo, A.; Moreno, M.E.; Acevedo, M. Insecticide formulations based on nicotine oleate stabilized by sodium caseinate. J. Agric. Food Chem. 2002, 50, 6389–6394. [Google Scholar] [CrossRef]
- Celie, P.H.N.; van Rossum-Fikkert, S.E.; van Dijk, W.J.; Brejc, K.; Smit, A.B.; Sixma, T.K. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 2004, 41, 907–914. [Google Scholar] [CrossRef]
- Carneiro, C.; Nardone, B.; Kiguradze, T.; Posligua, A.; West, D.P.; Rani, M. Frequency of nonmelanoma skin cancer recurrence in cancer patients receiving niacinamide or niacin: A retrospective case-control study. J. Am. Acad. Dermatol. 2016, 74, AB198. [Google Scholar]
- Ma, S.J.; Ma, T.; Ren, M.R.; Li, H.; Ma, Z.Q. Insecticidal action of the botanical insecticide wilforine on Mythimna separata (Walker) related with the changes of ryanodine receptor expression. Ecotoxicol. Environ. Saf. 2021, 213, 112025. [Google Scholar] [CrossRef] [PubMed]
- Zakharychev, V.V.; Kuzenkov, A.V.; Martsynkevich, A.M. Good pyridine hunting: A biomimic compound, a modifier and a unique pharmacophore in agrochemicals. Chem. Heterocycl. Compod. 2020, 56, 1491–1516. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Nakamura, T.; Kimura, H.; Nakayama, H. Synthesis and application of trifluoromethylpyridines as a key structural motif in active agrochemical and pharmaceutical ingredients. J. Pestic. Sci. 2021, 46, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Burriss, A.; Edmunds, A.J.F.; Emery, D.; Hall, R.G.; Jacob, O.; Schaetzer, J. The importance of trifluoromethyl pyridines in crop protection. Pest Manag. Sci. 2018, 74, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Eicken, K.; Goetz, N.; Harreus, A.; Ammermann, E.; Lorenz, G.; Rang, H. Anilide Derivatives and Their Use to Combat Botrytis. EP Patent 0,545,099, 7 November 1992. [Google Scholar]
- Maienfisch, P. Selective feeding blockers: Pymetrozine, flonicamid, and pyrifluquinazon. In Modern Crop Protection Compounds; Jeschke, P., Witschel, M., Krämer, W., Schirmer, U., Eds.; Wiley-VCH: Weinheim, Germany, 2019; Volume 3, pp. 1501–1526. [Google Scholar] [CrossRef]
- McGregor, S.D. Herbicidal Use of Aminohalopyridyloxy Acids and Derivatives Thereof. U.S. Patent 4,110,104, 23 December 1976. [Google Scholar]
- Wen, F.; Zhang, H.; Yu, Z.Y.; Jin, H.; Yang, Q.A.; Hou, T.P. Design, synthesis and antifungal/insecticidal evaluation of novel nicotinamide derivatives. Pestic. Biochem. Physiol. 2010, 98, 248–253. [Google Scholar] [CrossRef]
- Shi, Y.H.; Zhang, S.; Wan, F.X.; Sun, C.X.; Jiang, L. Synthesis, fungicidal activity and molecular docking study of novel N-[2-((substitutedphenyl)amino)pyridin-3-yl]-pyrimidine-4-carboxamides. Chin. J. Org. Chem. 2020, 40, 1948–1954. [Google Scholar] [CrossRef]
- Zhang, P.P.; Wang, Q.; Min, L.J.; Wu, H.K.; Weng, J.Q.; Tan, C.X.; Zhang, Y.G.; Liu, X.H. Synthesis, crystal structure, fungicidal activity and molecular docking of nicotinic acyl urea derivatives. J. Mol. Struct. 2020, 1205, 127485. [Google Scholar] [CrossRef]
- Kumagai, H.; Nishida, H.; Imamura, N.; Tomoda, H.; Omura, S. The structures of atpenins A4, A5 and B, new antifungal antibiotics produced by Penicillium sp. J. Antibiot. 1990, 43, 1553–1558. [Google Scholar] [CrossRef]
- Liu, X.H.; Wen, Y.H.; Cheng, L.; Xu, T.M.; Wu, N.J. Design, synthesis, pesticidal activities of pyrimidin-4-amine derivatives bearing a 5-(trifluoromethyl)-1,2,4-oxadiazole moiety. J. Agric. Food Chem. 2021, 69, 6968–6980. [Google Scholar] [CrossRef]
- Yu, C.S.; Wang, Q.; Bajsa-Hirschel, J.; Cantrell, C.; Duke, S.O.; Liu, X.H. Synthesis, crystal structure, herbicidal activity and SAR study of novel N-(arylmethoxy)-2-chloronicotinamides derived from nicotinic acid. J. Agric. Food Chem. 2021, 69, 6423–6430. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Cai, P.P.; Cheng, L.; Zhong, L.K.; Tan, C.X.; Shen, Z.H.; Han, L.; Xu, T.M.; Liu, X.H. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manag. Sci. 2020, 76, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.H.; Cheng, L.; Xu, T.M.; Liu, X.H.; Wu, N.J. Synthesis, insecticidal activities and DFT study of pyrimidin-4-amine derivatives containing the 1,2,4-oxadiazole motif. Front. Chem. Sci. Eng. 2022, 16, 1090–1100. [Google Scholar] [CrossRef]
- Min, L.J.; Wang, H.; Bajsa-Hirschel, J.; Yu, C.S.; Wang, B.; Yao, M.M.; Han, L.; Cantrell, C.L.; Duke, S.O.; Sun, N.B.; et al. Novel dioxolane ring compounds for the management of phytopathogen diseases as ergosterol biosynthesis inhibitors: Synthesis, biological activities and molecular docking. J. Agric. Food Chem. 2022, 70, 4303–4315. [Google Scholar] [CrossRef]
- Liu, X.H.; Yu, W.; Min, L.J.; Wedge, D.E.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Cantrell, C.L.; Bajsa-Hischel, J.; Hua, X.W.; et al. Synthesis and pesticidal activities of new quinoxalines. J. Agric. Food Chem. 2020, 68, 7324–7332. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Min, L.J.; Shen, Z.H.; Bajsa-Hirschel, J.; Cantrell, C.L.; Han, L.; Hua, X.W.; Liu, X.H.; Duke, S.O. Synthesis, crystal structure, herbicidal activity and mode of action of new cyclopropane-1,1-dicarboxylic acid analogues. Pestic. Biochem. Physiol. 2022, 188, 105228. [Google Scholar] [CrossRef]
- Bin, W.; Chen, W.T.; Min, L.J.; Han, L.; Sun, N.B.; Liu, X.H. Synthesis, structure, and antifungal activities of 3-(difluoromethyl)-pyrazole-4-carboxylic oxime ester derivatives. Heteroat. Chem. 2022, 2022, 6078017. [Google Scholar] [CrossRef]
- Rarey, M.; Kramer, B.; Lengauer, T.; Klebe, G. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996, 261, 470–489. [Google Scholar] [CrossRef]
- Huang, L.S.; Sun, G.; Cobessi, D.; Wang, A.C.; Shen, J.T.; Tung, E.Y.; Anderson, V.E.; Berry, E.A. 3-Nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 2006, 281, 5965–5972. [Google Scholar] [CrossRef]
- Ji, J.; Lao, K.; Hu, J.; Pang, T.; Jiang, Z.; Yuan, H.; Miao, J.; Chen, X.; Ning, S.; Xiang, H.; et al. Discovery of novel aromatase inhibitors using a homogeneous time-resolved fluorescence assay. Acta Pharmacol. Sin. 2014, 35, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).