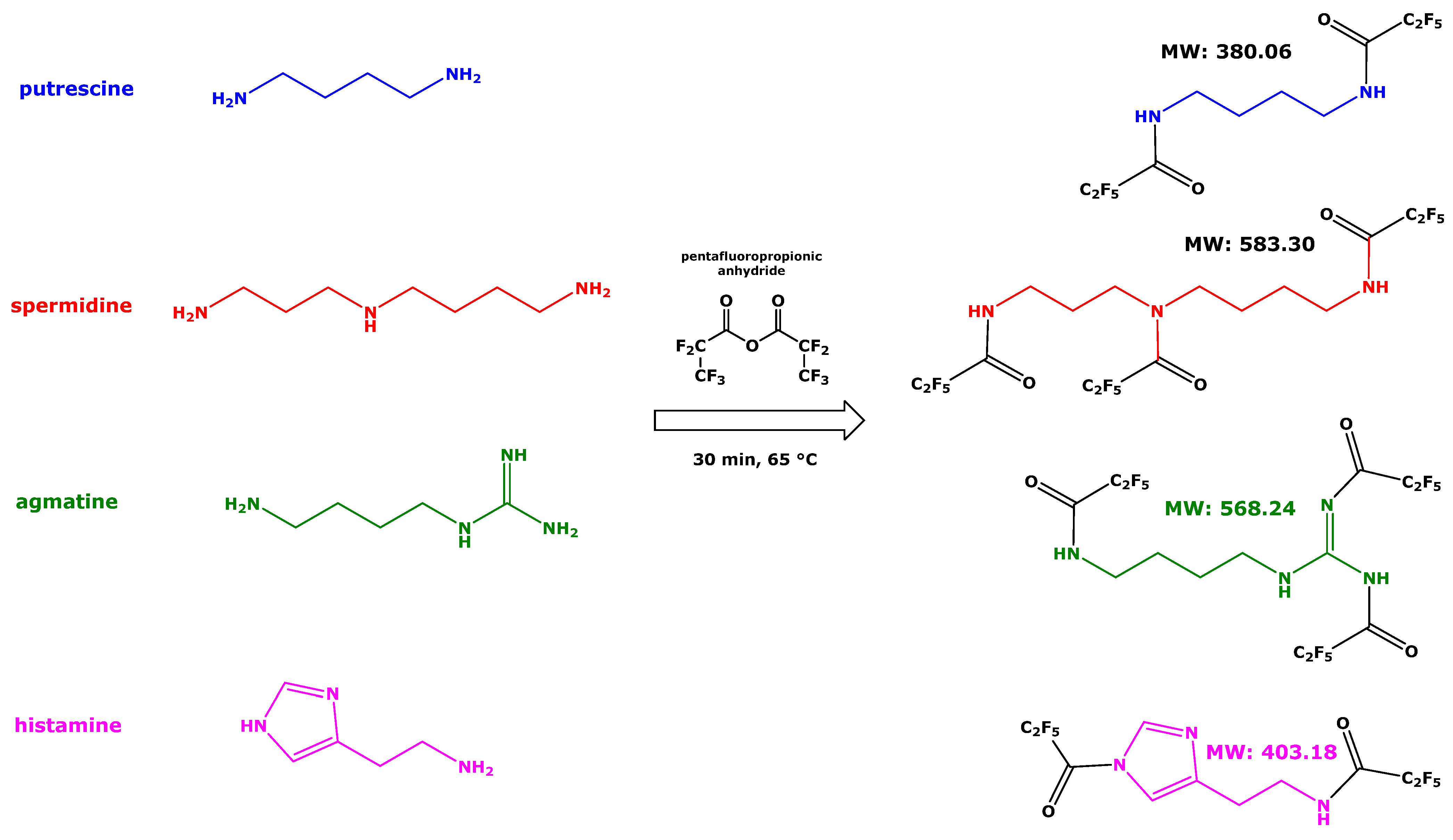
1. Introduction
Putrescine (PUT), spermidine (SPD), agmatine (AGM), histamine (HA) (
Scheme 1), and other amines, including cadaverine (CAD) and spermine (SPM), are widely distributed in nature and exert multiple biological functions [
1,
2]. They are biosynthesized by microorganisms, plants, animals, and humans [
3].
Helicobacter pylori (Hp) was isolated from subjects infected with the bacterium produced by SPD and HA [
4]. Polyamines have therapeutic potential in many diseases, including cancer [
5]. Supplementation of SPD has been reported to exert beneficial effects on brain and heart and to extend lifespan [
6]. SPD’s autophagy and longevity and other potentially involved mechanisms and functions have been recently reviewed [
7,
8].
For the measurement of SPD, PUT, and AGM in human serum, we developed a gas chromatography-mass spectrometry (GC-MS) method [
9]. This approach includes a two-step extraction with
n-butanol from alkalinized serum and back to hydrochloric acid, one-step derivatization with pentafluoropropionic anhydride (PFPA) in ethyl acetate, extraction of the pentafluoropropionyl (PFP) derivatives with toluene and GC-MS analysis using a GC column temperature program starting at 70 °C. SPD and AGM form tri-PFP derivatives, i.e., SPD-(PFP)
3 and AGM-(PFP)
3, respectively; PUT forms a di-PFP derivative, i.e., PUT-(PFP)
2 [
9]. These observations suggest all amine groups of SPD, AGM, and PUT react with PFPA to form the corresponding PFP amides (
Scheme 1). In this method, we used a commercially available
13C
4-spermidine (
13C
4-SPD) as the internal standard for SPD, PUT, and AGM. By this GC-MS method, we faced difficulties in analyzing reliably HA as a PFP derivative. These included varying derivatization and extraction yields using different organic solvents, notably toluene. Others found by direct inlet probe MS analysis in the electron ionization (EI) mode that the derivatization of HA with PFPA in acetonitrile (30 min, 60 °C) forms a mono-PFP derivative [
10]. The derivatization of HA with PFPA in ethyl acetate-toluene-acetonitrile mixture (30 min, 50 °C) and its analysis by GC with flame ionization detection (FID) have been reported, but the structure of the derivative has not been reported [
11].
In previous work, we found the organic solvent, toluene, which is commonly used by our and other groups in GC-MS analyses of structurally related pentafluorobenzyl (PFB) derivatives of various endogenous analytes, is associated with considerable carryover effects for some analytes. This was especially the case for the PFB derivative of inorganic nitrate (i.e., PFB-ONO
2). This phenomenon occurred when we changed the single quadrupole GC-MS apparatus model DSQ by the single quadrupole GC-MS apparatus model ISQ from the same manufacturer (ThermoFisher) [
12]. We solved this problem by using ethyl acetate (EA) instead of toluene (TOL) for the extraction of PFB-ONO
2 from human plasma samples after derivatization of nitrate with PFB bromide [
12]. In the present work, we investigated the effects of these two solvents, as well as of two starting temperatures of the gas chromatographic (GC) column, i.e., 40 °C and 70 °C, on the GC-MS analysis of HA, SPD, PUT, and AGM using otherwise identical conditions.
We report here for the first time the derivatization of unlabelled HA (d0-HA) and deuterium-labelled HA (d4-HA) with PFPA, the structural characterization of the derivative and the quantitative GC-MS analysis of HA. Our present results show previously used derivatization and GC-MS conditions for SPD, PUT, and AGM are optimum for the simultaneous derivatization of HA, AGM, PUT, and SPD by GC-MS only when EA was used for the extraction of the PFP derivatives and when the starting GC column temperature was 40 °C. Commercially available 13C4-SPD and 2H4-HA were tested and found to be useful as internal standards for the analytes after standardization.
2. Results
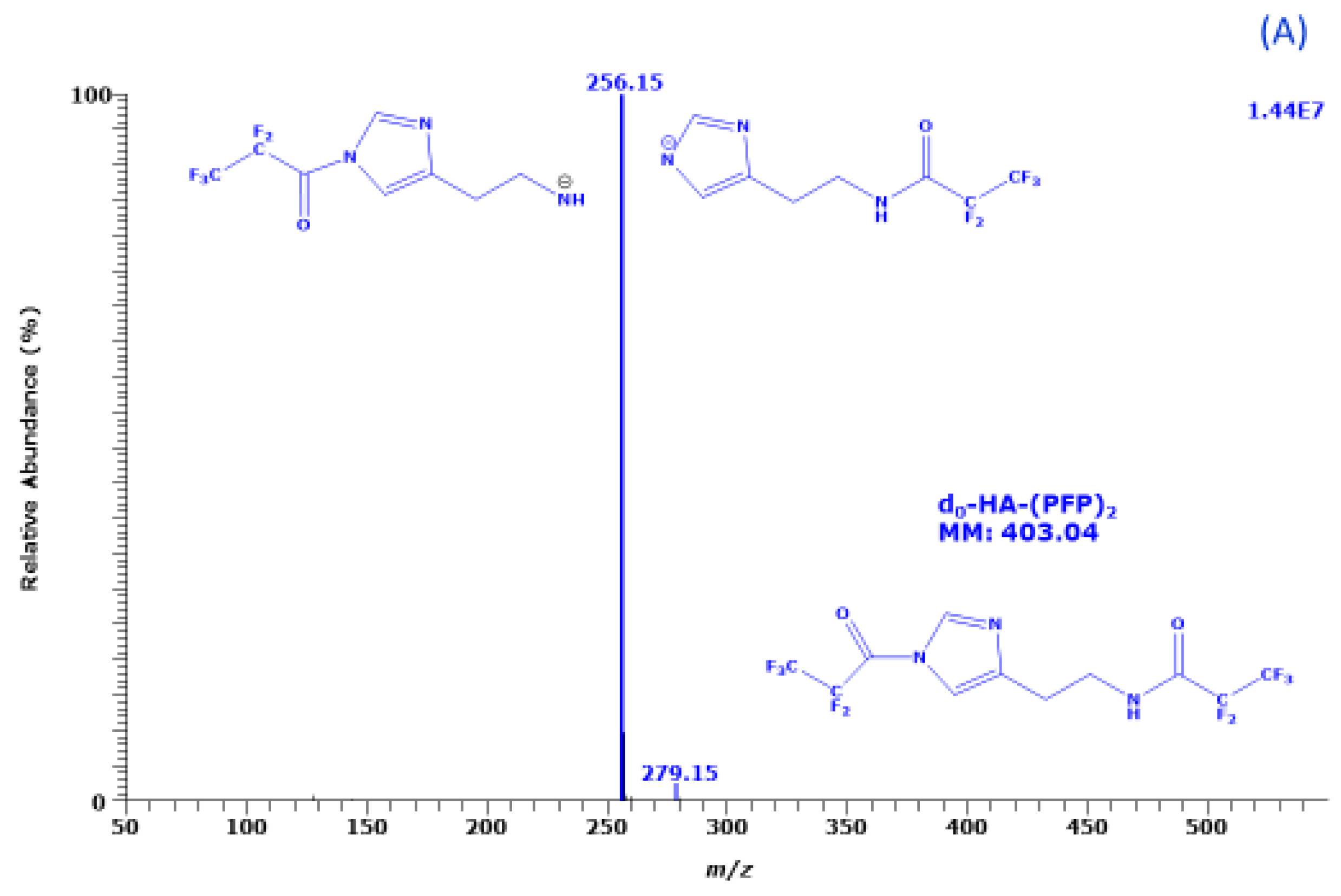
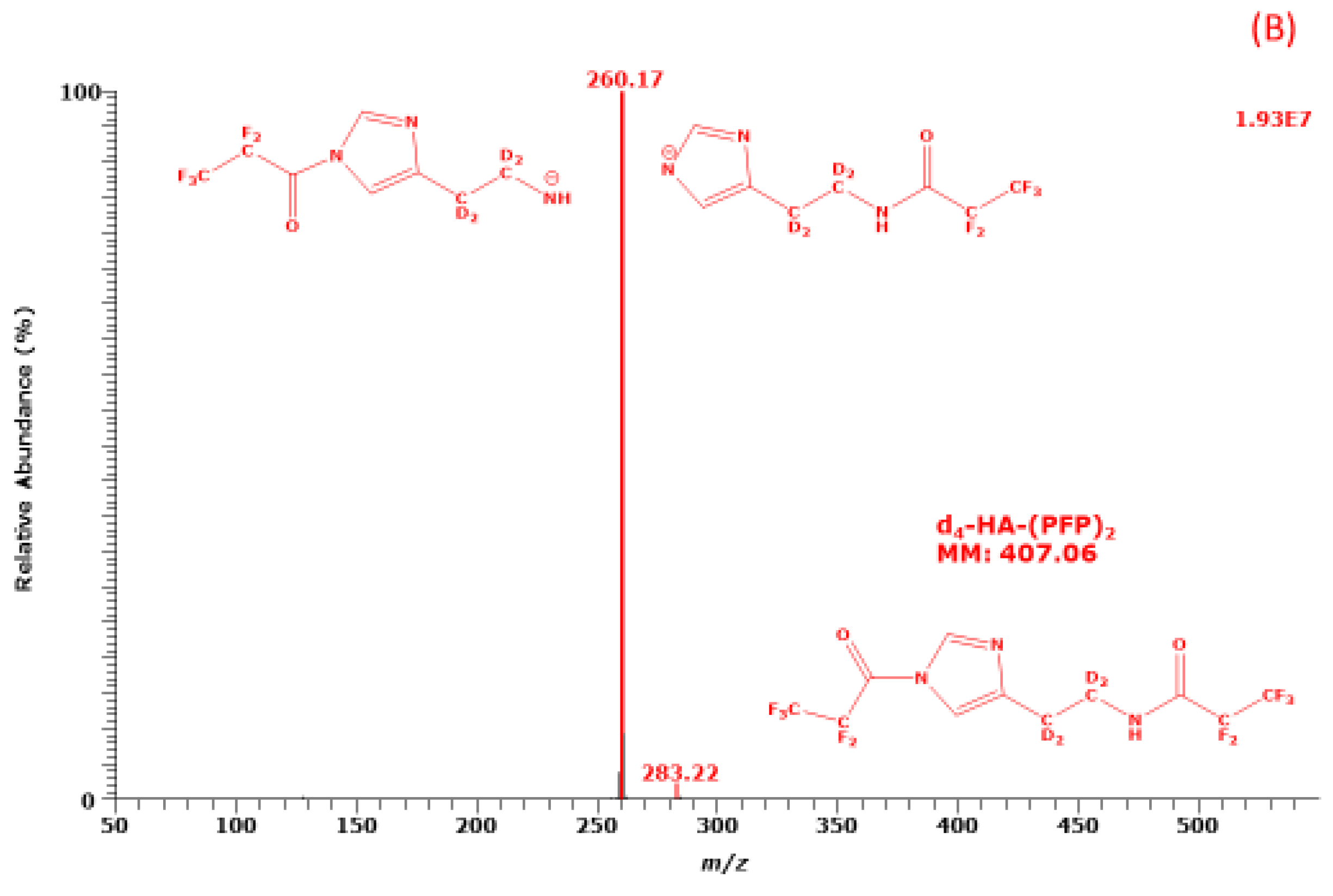
2.1. Derivatization of Histamine with PFPA and Structural Characterization of its PFP Derivative
The reaction of HA with PFPA resulted in a single intense GC peak in the relevant retention time window of 7 to 14 min. The GC-MS mass spectra of the PFP derivatives of unlabelled HA (d
0-HA) and
2H
4-HA (d
4-HA) from EA extracts are shown in
Figure 1.
The most intense ions in the GC-MS mass spectra of the PFP derivatives of d
0-HA and d
4-HA are
m/z 256 and
m/z 260, respectively. Much less intense ions in these mass spectra are
m/z 279 and
m/z 283, respectively. The difference of 4 Da in these two ion pairs and the shorter retention time of the d
4-HA derivative compared to the d
0-HA derivative are due to the presence of four deuterium atoms in the d
4-HA derivative. The occurrence of
m/z 279 and
m/z 283 in the GC-MS spectra suggests the derivatives each contain two PFP residues. Under the derivatization conditions (30 min, 65 °C) d
0-HA and d
4-HA react with PFPA form di-PFP derivatives, i.e., d
0-HA-(PFP)
2 and d
4-HA-(PFP)
2, respectively. The mass fragments,
m/z 279 and
m/z 283, are likely to result from the loss of a PFP residue (CF
3CF
2CO, 147 Da) from d
0-HA-(PFP)
2 (molecular mass, 403) and d
4-HA-(PFP)
2 (molecular mass, 407), respectively. The missing consecutive neutral losses of HF (20 Da) from
m/z 279 and
m/z 283 in the mass spectra to generate ions with
m/z 259 and
m/z 263, respectively, suggest the PFP residue (147 Da) of the imidazole ring of HA is lost during NICI [
9] (
Figure 1).
Under the same derivatization, extraction, and GC-MS conditions, the mass spectrum of the PUT derivative contained the most intense ion at
m/z 340, suggesting formation of PUT-(PFP)
2. The most intense ion in the mass spectrum of AGM derivative was
m/z 528 due to AGM-(PFP)
3. The most intense ions in the GC-MS spectra of the derivatives of SPD (
13C
0-SPD) and
13C
4-SPD were
m/z 361 and
m/z 365, respectively, suggesting formation of SPD-(PFP)
3 (molecular mass, 583).
13C
0-SPD-(PFP)
3 and
13C
4-SPD-(PFP)
3 eluted virtually at the same time. The difference of 4 Da is due to the presence of four
13C atoms in the
13C
4-SPD derivative. These observations confirm previous results for SPD, PUT and AGM [
9].
2.2. Linearity and Standardization of 2H4-Histamine
Aqueous solutions of d0-HA and d4-HA were used for the standardization of the commercially available d4-HA. Varying amounts of d0-HA (0, 70, 280, 420, 700 pmol) were spiked with a fixed amount of d4-HA (nominal amount, 300 pmol). After solvent evaporation to dryness by a stream of nitrogen gas, PFPA derivatization (30 min, 65 °C) was carried out, derivatives were extracted with EA, and GC-MS analysis in the SIM mode (m/z 256 and m/z 260) was performed. Linear regression analysis between the PAR of m/z 256 to m/z 260 (y) against the amount of d0-HA (x) resulted in the regression equation, y = 0.026 + 0.0011 × x (r2 = 0.9977, p < 0.0001). The reciprocal slope of the regression equation corresponds to the amount of d4-HA used in this experiment and is calculated to be 906 pmol. In quantitative analyses, the corrected concentration of the internal standard d4-HA in its stock solution was used. The PAR of m/z 256 to m/z 260 in the d4-HA sample was determined to be 0.022, indicating an isotopic purity of about 98% 2H, which is closed to the declared isotopic purity.
2.3. Effects of Ethyl Acetate on the GC-MS Analysis of the Amines as PFP Derivatives
EA extracts of derivatized mixtures of amines were analyzed by GC-MS. The retention time (and the coefficient of variation CV, %) of the PUT derivative was 7.942 min (0.05%). The retention time of the AGM derivative was 9.26 min (0.00%). The retention times of the PFP derivatives of d0-HA and d4-HA were 10.93 min (0.08%) and 10.90 min (0.07%), respectively. The retention times of the PFP derivatives of 13C0-SPD and 13C4-SPD were 11.42 min (0.04%) and 11.42 min (0.00%), respectively.
Plotting the PAR (y) of the peak areas of the PFP derivatives of the amines to the peak areas of 13C4-SPD against the amount of the derivatized amines (x) resulted in the following regression equations using EA and OTP40: y = 0.66 + 0.023 x (r2 = 0.9925, p < 0.0001) for PUT; y = −0.27 + 0.029 × x (r2 = 0.9968, p < 0.0001) for AGM; y = 0.03 + 0.0027 × x (r2 = 0.9893, p < 0.0001) for SPD; and y = −0.01 + 0.00074 × x (r2 = 0.8949, p = 0.0043) for HA.
Plotting the PAR (y) of the peak area of the PFP derivatives of the amines to the peak area of d4-HA against the amount of the derivatized amines (x) resulted in the following linear regression equations using EA and OTP40: y = 0.27 + 0.067 × x (r2 = 0.9863, p = 0.0007) for PUT; y = −1.65 + 0.079 × x (r2 = 0.9737, p = 0.0018) for AGM; y = 0.18 + 0.0007 × x (r2 = 0.771, p = 0.020) for SPD; and y = −0.27 + 0.029 × x (r2 = 0.9968, p < 0.0001) for HA.
These results suggest PUT, AGM, SPD, and HA can be quantified simultaneously by GC-MS as their PFP derivatives in EA extracts using either 13C4-SPD or d4-HA as internal standards. Yet, the highest linearity is observed when 13C4-SPD was used as the internal standard for SPD and d4-HA as the internal standard for HA.
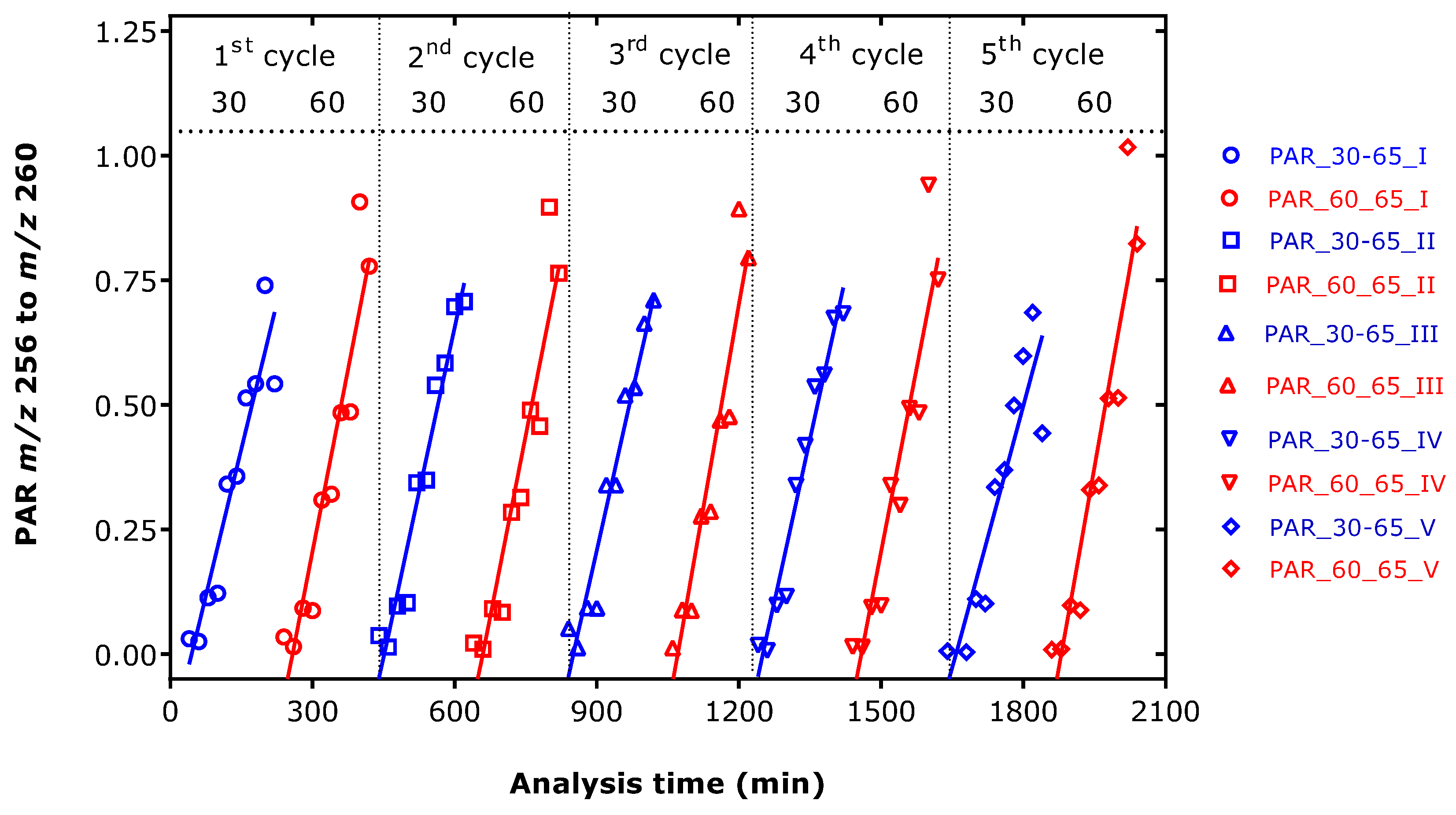
2.4. Effect of Derivatization Time and Long-Term Stability of the Histamine PFP Derivative
Standard curves of d
0-HA and d
4-HA were prepared as described above, yet for two derivatization times, i.e., 30 min and 60 min, in duplicate for each concentration. The PFPA derivatization temperature of 65 °C and a reaction time of 30 min were found sufficient for the GC-MS analysis of many amino acids and amines [
7,
9,
12]. Higher derivatization temperatures were not tested in the present study. The samples were placed in the autosampler (at about 19 °C), and the EA extracts were analyzed consecutively in five cycles, beginning from the lower to the higher d
0-HA amounts. Samples were injected every 20 min. The results of this experiment are illustrated in
Figure 2 and indicate close similarity except of the highest amount d
0-HA that was derivatized for 60 min. The mean CV values for all analyses were 91% for 0 pmol d
0-HA, 10.7% for 70 pmol d
0-HA, 9.7% for 280 pmol d
0-HA, 7.3% for 420 pmol d
0-HA, and 13.5% for 700 pmol d
0-HA. Linear regression analysis between the PAR of
m/z 256 to
m/z 260 (
y) and the amount of d
0-HA (
x, pmol) resulted in the following regression equations (
r2 range, 0.88 to 0.96,
p < 0.0001):
y = −0.2 + 0.0039 ×
x;
y = −1.3 + 0.0049 ×
x;
y = −2 + 0.0044 ×
x;
y = −3 + 0.0048 ×
x;
y = −3.60 + 0.0042 ×
x;
y = −6 + 0.0054 ×
x;
y = −6 + 0.0054 ×
x;
y = −5 + 0.0043 ×
x;
y = −7 + 0.0049 ×
x;
y = −6 + 0.0035 ×
x; and
y = −10 + 0.0054 ×
x, respectively (
Figure 2).
2.5. Effects of Ethyl Acetate and Toluene and of the Starting GC Column Temperature on the Amine Analysis by GC-MS as PFP Derivatives
Typical GC-MS chromatograms from analyses of the PFP derivatives in EA and TOL extracts using OTP40 are shown in
Figure 3. The PFP derivatives of PUT, AGM, SPD, and
13C
4-SPD had almost identical retention times in EA and TOL extracts. The PFP derivatives of d
0-HA and d
4-HA were found in larger abundance in the EA extracts. On a molar basis, the PFP derivatives of PUT and AGM had similar peak areas in both extracts. The PFP derivatives of SPD and
13C
4-SPD had similar peak areas in both extracts as well, but they were considerably smaller compared to those of PUT and AGM. The peak areas of the PFP derivatives of d
0-HA and d
4-HA were small in EA and not present in TOL extracts (
Figure 3). The main results are reported in
Table 1 for the individual amines and include retention time (
tR) and peak area (PA) of the derivatives, linearity between PA (
y) and amine amount
(x), and signal-to-noise (
S/N) values.
Table 1 also reports the peak area ratio (PAR) of the PFP derivatives of
13C
0-SPD-(PFP)
3 and
13C
4-SPD-(PFP)
3. The detailed results of this experiment for PUT, AGM, and SPD are presented in the
Supplementary Material (Table S1) to this work.
The results of
Table 1 suggest PUT, AGM, and SPD can be analyzed with comparable sensitivity using EA or TOL for extraction and OTP40 or OTP70 for GC separation. The mean of the slope of the regression equation of the PAR of
13C
0-SPD/
13C
4-SPD is 0.002755 (CV, 4.7%). The reciprocal of the mean value of the slope corresponds to 363 pmol
13C
4-SPD (nominal amount, 300 pmol for standardized
13C
4-SPD).
The results of these experiments with the pure amines were confirmed after extraction of the above-mentioned amines from aqueous buffer of pH 7.4 using a standard two-step
n-butanol/HCl extraction [
9], derivatization with PFPA (30 min, 65 °C), extraction of the derivatives with EA, and GC-MS analysis using OTP40, as described in this work (
Table 2). d
0-HA and d
4-HA were not detectable by GC-MS when TOL was used for the extraction of the native amines and of the PFP derivatives. d
4-HA and
13C
4-SPD were used at constant amounts. The PA values of the PFP derivatives of d
4-HA and
13C
4-SPD in the EA extracts varied by 5.9% and 18.3%, respectively.
2.6. Limits of Detection
The peaks shown in
Figure 3 were obtained by injecting 1670 fmol of each amine assuming complete derivatization and extraction. The
S/N values obtained from these analyses are summarized in
Table 1. The
S/N values of PUT were 1087:1 in EA and 685:1 in TOL. The
S/N values of AGM were 4696:1 in EA and 2573:1 in TOL. The
S/N values of SPD were 480:1 in EA and 228:1 in TOL. The
S/N values of
13C
4-SPD were 398:1 in EA and 237:1 in TOL.
The lowest
S/N values were observed for
2H
0-HA (4:1) and
2H
4-HA (9:1) using EA and OTP40. The lower limit of detection (LOD) values were approximated using
S/N values of 3:1 (
Table 3); see also
Table 1. The approximated LOD values were 1670 fmol
2H
0-HA and 557 fmol for
2H
4-HA (in EA). The approximated LOD values were 4.6 fmol (in EA) and 7.3 fmol (in TOL) for PUT; 1.1 fmol (in EA) and 1.9 fmol (in TOL) for AGM; 10.4 fmol (in EA) and 22.0 fmol (in TOL) for
13C
0-SPD; 12.6 fmol (in EA) and 21.1 fmol (in TOL) for
13C
4-SPD.
3. Discussion
Histamine (2-(1
H-imidazol-4-yl)ethanamine, C
5H
9N
3, 111.1 g/mol; p
Ka, 6.04 (imidazole NH); p
Ka, 9.75, terminal NH
2) (
Scheme 1) is a biogenic amine produced from L-histidine by enzymatic decarboxylation. Enzymatic decarboxylation of L-arginine leads to agmatine (AGM). Analogous, enzymatic decarboxylation of L-ornithine generates putrescine (PUT), which is further metabolized to spermidine (SPD) and spermine (SPM).
Many different analytical methods have been reported for the measurement of HA and other amines in biological fluids and tissues [
13,
14,
15]. Reported GC-based methods for HA include its derivatization with various derivatization reagents, such as PFPA [
9,
10,
11] and pentafluorobenzyl bromide (PFB-Br) [
4,
16]. PFPA, other perfluorated substances [
17], and PFB-Br [
18] are versatile derivatization reagents in GC-MS. For the measurement of SPD in human serum, we developed a GC-MS method [
9]. We faced considerable difficulties in analyzing reliably and sensitively HA as a PFP derivative by the previously reported GC-MS method which uses toluene (TOL) as the extraction solvent [
9]. The present work shows these difficulties can be overcome by using ethyl acetate (EA) as the extraction and injection solvent in combination with a low starting oven temperature of 40 °C. This is the first demonstration of the derivatization of HA with PFPA to form a HA-(PFP)
2 derivative. Use of TOL for the extraction of PFP derivatives is likely to be misinterpreted as the inability of PFPA to react with HA under standard derivatization conditions (e.g., 30 min at 65 °C).
Base-catalyzed derivatization of HA with PFB-Br in anhydrous acetonitrile results in formation of a tri-PFB derivative, suggesting alkylation of the imidazole NH group and two-fold alkylation of the aliphatic NH
2 group: HA-(PFB)
3 [
4,
16]. In the present work, we did not find formation of a tri-PFP derivative of HA using PFPA in EA (PFPA-EA, 1:4,
v/
v). Our results suggest under the derivatization conditions used in the present study, i.e., derivatization of HA with PFPA at 65 °C for 30 min or 60 min, results in formation of a di-PFP derivative, i.e., HA-(PFP)
2, most likely by acylation of the ring imidazole NH group and mono-acylation of the non-ring aliphatic NH
2 group. Under similar derivatization conditions, PUT, AGM, and SPD react with PFPA in EA to form PUT-(PFP)
2, AGM-(PFP)
3, and SPD-(PFP)
3, respectively. The polyamine cadaverine (CAD, pentane-1,5-diamine; C
5H
14N
2) forms a di-PFP derivative (CAD-(PFP)
2) which elutes at 8.77 min (OTP40), i.e., behind the PUT-(PFP)
2 derivative and in front of the AGM-(PFP)
3 derivative. Obviously, derivatization of HA with PFPA differs from that of PUT, AGM, SPD, and CAD. Other biogenic amines, such as tryptamine and beta-phenyl ethylamine, are expected to be derivatized with PFP, but they are unlikely to interfere with the GC-MS analysis of HA as a PFP derivative.
For the GC-MS analysis of urinary HA, ethyl acetate (EA) was used for the extraction of HA-(PFB)
3 [
16]. In previous work, we used this GC-MS method to identify HA isolated from cultures of
H. pylori patients [
4]. In our group, we commonly use toluene (TOL) for extraction of PFB and PFP derivatives and their subsequent analysis by GC-MS. TOL is entirely immiscible with water and does not require additional treatment with anhydrous Na
2SO
4 to remove the remaining water prior to GC-MA analysis. In addition, PFB and PFP derivatives in TOL are less susceptible to hydrolysis than in EA. For instance, PFP derivatives of methyl esters of amino acids are stable for long periods in TOL extracts [
19]. On the other hand, we observed GC-MS analyses of PFB derivatives, such as that of nitrate (PFB-ONO
2), are associated with considerable carryover effects; we found changing the solvent from TOL to EA eliminates this problem and enables reliable analysis of derivatized nitrate [
12]. For this reason, we investigated whether water-immiscible GC-compatible organic solvents, such as TOL and EA, may influence the GC-MS analysis of HA. To investigate potential effects of the oven temperature, we tested two GC oven temperature programs with different starting temperatures, i.e., 40 °C and 70 °C, yet with the same oven two-step temperature gradient.
To the best of our knowledge, the present study is the first to report that HA reacts with PFPA to form HA-(PFP)2. The results of the present study strongly indicate TOL is not useful for the GC-MS analysis of HA after derivatization with PFPA, although PFP derivatives of PUT, AGM, and SPD can be analyzed even more sensitively in TOL extracts. Changing TOL by EA enables HA analysis as a PFP derivative alongside PUT, AGM, and SPD analysis. The starting GC oven temperature also has an effect, which is considerable for PUT, presumably because of the comparably small molecule and short retention time of its PFP derivative. Among the investigated amines, HA analysis by GC-MS as a PFP derivative is considerably less efficient than the GC-MS analysis of PUT, AGM, and SPD. The PFP derivatives of PUT and AGM are best suitable for sensitive GC-MS analysis. The main reason for the better utility of EA in the GC-MS analysis of HA as a PFP derivative compared to TOL could better solubility and stability of the PFP derivatives in EA. It seems these factors also apply to SPD, albeit to a lower degree. The disadvantage of the use of EA is the requirement of an additional experimental step to remove the remaining water, for instance by using anhydrous Na2SO4. Yet, this is considered neglectable and could be dispensed by using stronger centrifugation forces of the EA extract.
Spermine (SPM, C
10H
26N
4) is considerably longer than SPD (C
7H
19N
3) and forms a tetra-PFP derivative (SPM-(PFP)
4) upon derivatization with PFPA under the same derivatization conditions. GC-MS analysis of derivatized SPM showed decomposition, yet not to PUT and SPD [
9]. Increasing length of the polyamine PFP derivatives seems to be associated with increasing thermal instability analogous to the methyl ester PFP derivatives of di- and tripeptides, such as glutathione and ophthalmic acid [
20,
21]. The retention time of the PFP derivatives of the investigated amines is proportional to the molecular weight of the native and derivatized amines (
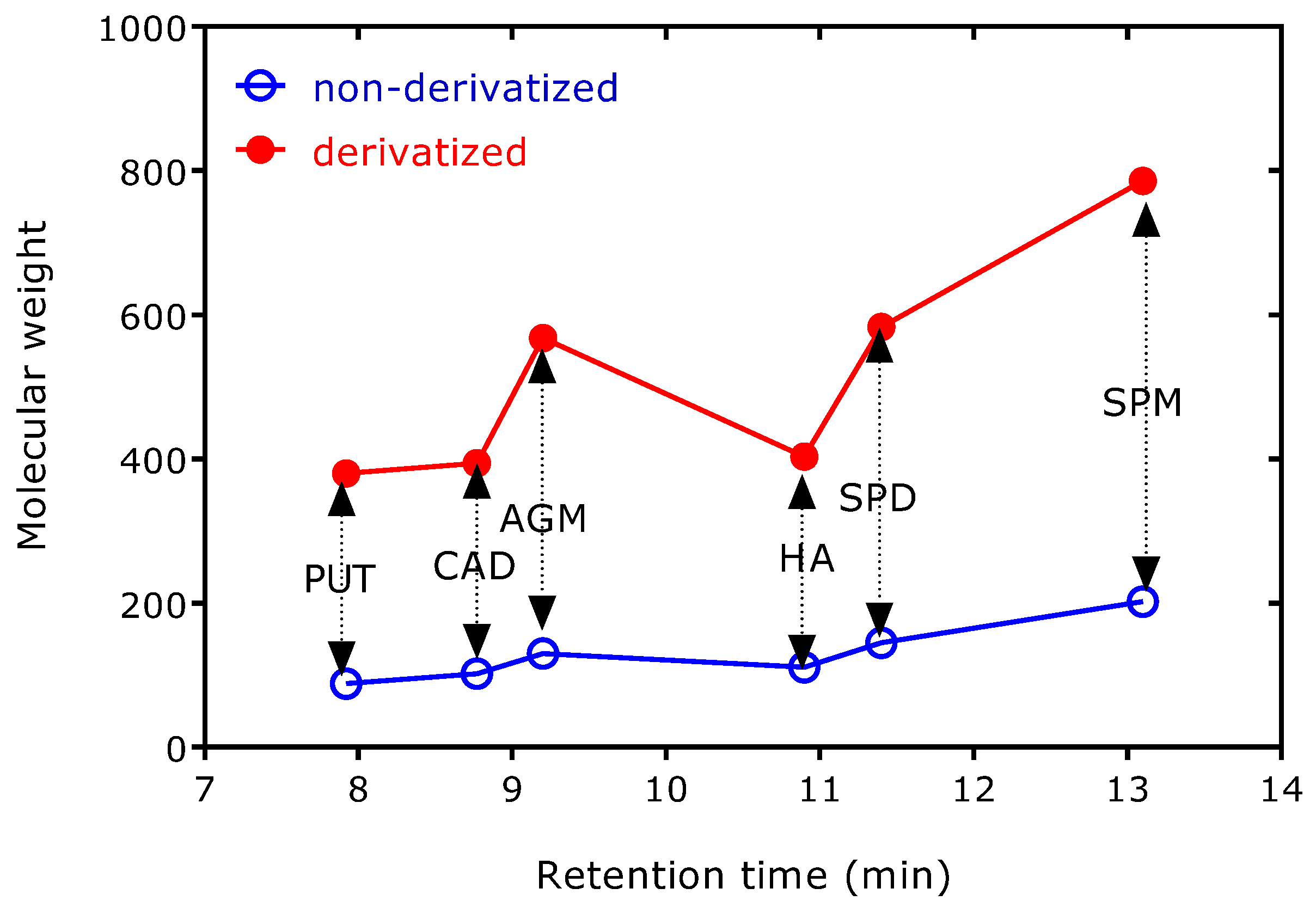
Figure 4), but it is independent of the molar ratio of derivatized to non-derivatized amines (4.01 ± 0.28; CV, 7%).
Figure 4 shows AGM and HA behave differently than the other amines, presumably due their guanidine and imidazole groups, respectively. It seems the PFP residue of the guanidine group of AGM increases the volatility of AGM-(PFP)
3 stronger than the PFP residue of the imidazole group of HA-(PFP)
2. Other polyamines, such as cadaverine and spermine (
Figure 4), and other biogenic amines, such as tryptamine and beta-phenylethylamine (not tested in the present study), are likely not to interfere with the analysis of PUT, AGM, HA, and SPD as their PFP derivatives. They are expected to form PFP derivatives with distinctly different mass spectra and retention times under the same derivatization and GC-MS conditions.
5. Conclusions
The derivatization (30 min, 65 °C) of HA with PFPA in EA (PFPA-EA, 1:4, v/v) yields a HA-(PFP)2 derivative. PFPA acylates the primary aliphatic amine group of HA and the ring imidazole imine group of HA. Under these conditions, the amines, PUT, AGM, and SPD, react with PFPA to form PUT-(PFP)2, AGM-(PFP)3, and SPD-(PFP)3 derivatives, respectively. Simultaneous quantitative analysis of HA, PUT, AGM, and SPD by GC-MS as PFP derivatives is possible when using EA as the extraction and injection solvent for the derivatives and a starting GC column temperature of 40 °C followed by a two-step oven temperature gradient. d4-HA is useful as an internal standard for HA. TOL allows simultaneous analysis of PUT, AGM, and SPD but not alongside HA. The HA-(PFP)2 derivative is stable in EA extracts for at least 30 h at room temperature. Despite the analytical improvements achieved for HA, its analysis by GC-MS as PFP derivative is still challenging and less efficient than that of PUT, AGM, and SPD.