Biological Assessment of Stevioside and Sucralose as Sucrose Substitutes for Diabetics on STZ-Induced Diabetes in Rats
Abstract
1. Introduction
2. Results and Discussion
2.1. Glucose Level
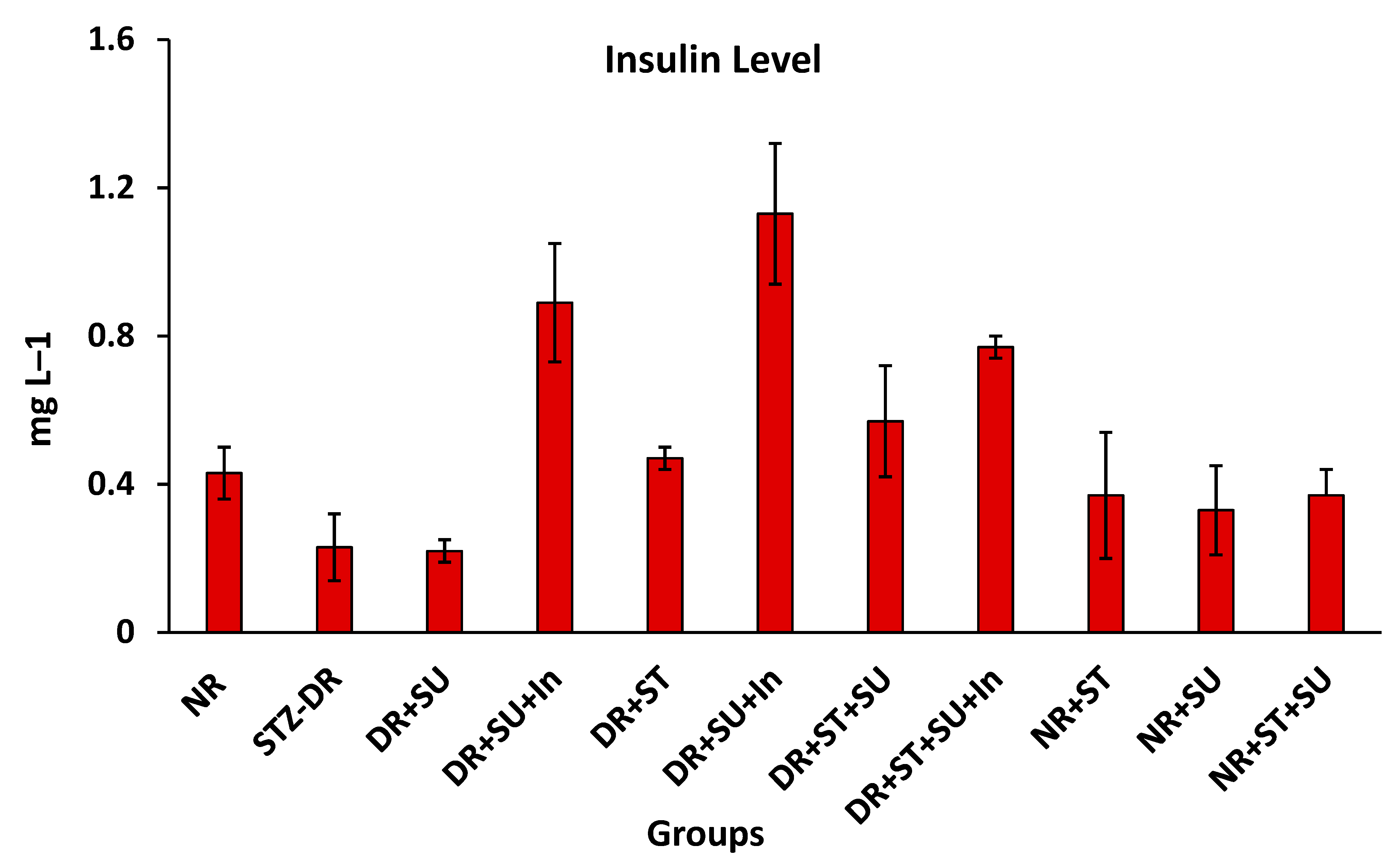
2.2. Serum Insulin Level
2.3. Liver Markers of STZ-Induced Diabetic Rats
2.4. Lipid Profile of STZ-Induced Diabetic Rats
2.5. Histopathological Alterations in Pancreases of STZ-Induced Diabetic Rats
3. Materials and Methods
3.1. Sweeteners
3.2. Animal Model for Diabetics
3.3. Experimental Design
3.4. Biochemical Assessments
3.5. Histological Examination
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dabelea, D.; Bell, R.A.; D’Agostino, R.B., Jr.; Imperatore, G.; Johansen, J.M.; Linder, B.; Liu, L.L.; Loots, B.; Marcovina, S.; Mayer-Davis, E.J.; et al. Incidence of Diabetes in Youth in the United States. JAMA 2007, 297, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of Overweight and Obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Volume 102, pp. 147–148. [Google Scholar]
- Pinheiro, M.V.S.; Oliveira, M.N.; Penna, A.L.B.; Tamime, A.Y. The Effect of Different Sweeteners in Low-Calorie Yogurts—A Review. Int. J. Dairy Technol. 2005, 58, 193–199. [Google Scholar] [CrossRef]
- Wiebe, N.; Padwal, R.; Field, C.; Marks, S.; Jacobs, R.; Tonelli, M. A Systematic Review on the Effect of Sweeteners on Glycemic Response and Clinically Relevant Outcomes. BMC Med. 2011, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Al-Furaydi, A.; Al-Harbi, A.; Al-Shedookhi, A. Nutritional, Chemical and Organoleptical Characteristics of Low-Calorie Fruit Nectars Incorporating Stevioside as A Natural Sweetener. Food Nutr. Sci. 2016, 8, 126–140. [Google Scholar] [CrossRef]
- Mattes, R.D.; Popkin, B.M. Nonnutritive Sweetener Consumption in Humans: Effects on Appetite and Food Intake and their Putative Mechanisms. Am. J. Clin. Nutr. 2009, 89, 1–14. [Google Scholar] [CrossRef]
- Fitch, C.; Keim, K.S. Position of the Academy of Nutrition and Dietetics: Use of Nutritive and Nonnutritive Sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef]
- Garcia, K.; Ferreira, G.; Reis, F.; Viana, S. Impact of Dietary Sugars on Gut Microbiota and Metabolic Health. Diabetology 2022, 3, 549–560. [Google Scholar] [CrossRef]
- Collison, K.S.; Makhoul, N.J.; Zaidi, M.Z.; Al-Rabiah, R.; Inglis, A.; Andres, B.L.; Ubungen, R.; Shoukri, M.; Al-Mohanna, F.A. Interactive Effects of Neonatal Exposure to Monosodium Glutamate and Aspartame on Glucose Homeostasis. Nutr. Metab. 2012, 9, 58. [Google Scholar] [CrossRef]
- Lau, K.; McLean, W.G.; Williams, D.P.; Howard, C.V. Synergistic Interactions between Commonly Used Food Additives in a Developmental Neurotoxicity Test. Toxicol. Sci. 2006, 90, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Tappy, L.; Le, K.A. Dietary Sugars and Health; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Shah, A.B.; Jones, G.P.; Vasiljevic, T. Sucrose-Free Chocolate Sweetened With Stevia Rebaudiana Extract and Containing Different Bulking Agents—Effects on Physicochemical and Sensory Properties. Int. J. Food Sci. Technol. 2010, 45, 1426–1435. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, Source of A High-Potency Natural Sweetener: A Comprehensive Review on the Biochemical, Nutritional and Functional Aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Jahangir Chughtai, M.F.; Pasha, I.; Zahoor, T.; Khaliq, A.; Ahsan, S.; Wu, Z.; Nadeem, M.; Mehmood, T.; Amir, R.M.; Yasmin, I.; et al. Nutritional and Therapeutic Perspectives of Stevia rebaudiana as Emerging Sweetener; A Way Forward for Sweetener Industry. CyTA J. Food 2020, 18, 164–177. [Google Scholar] [CrossRef]
- Ghanta, S.; Banerjee, A.; Poddar, A.; Chattopadhyay, S. Oxidative DNA Damage Preventive Activity and Antioxidant Potential of Stevia rebaudiana (Bertoni) Bertoni, a Natural Sweetener. J. Agric. Food Chem. 2007, 55, 10962–10967. [Google Scholar] [CrossRef]
- Dyrskog, S.E.; Jeppesen, P.B.; Chen, J.; Christensen, L.P.; Hermansen, K. The Diterpene Glycoside, Rebaudioside A, Does Not Improve Glycemic Control or Affect Blood Pressure after Eight Weeks Treatment in the Goto-Kakizaki Rat. Rev. Diabet. Stud. 2005, 2, 84–91. [Google Scholar] [CrossRef]
- Bhasker, S.; Madhav, H.; Chinnamma, M. Molecular Evidence of Insulinomimetic Property Exhibited by Steviol and Stevioside in Diabetes Induced L6 and 3T3L1 Cells. Phytomedicine 2015, 22, 1037–1044. [Google Scholar] [CrossRef]
- Daneshyar, M.; Geuns, J.; Willemsen, H.; Ansari, Z.; Darras, V.; Buyse, J.; Everaert, N. Evaluation of Dietary Stevioside Supplementation on Anti-Human Serum Albumin Immunoglobulin G, Alpha-1-glycoprotein, Body Weight and Thyroid Hormones in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2012, 96, 627–633. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Rother, K.I. Sucralose, A Synthetic Organochlorine Sweetener: Overview Of Biological Issues. J. Toxicol. Env. Health Part B 2013, 16, 399–451. [Google Scholar] [CrossRef]
- Vitetta, L.; Briskey, D.; Alford, H.; Hall, S.; Coulson, S. Probiotics, Prebiotics and the Gastrointestinal Tract in Health and Disease. Inflammopharmacology 2014, 22, 135–154. [Google Scholar] [CrossRef]
- Dlamini, S.N. Effects of Commercially Available Non-Nutritive Sweeteners in an Experimentally Induced Rat Model of Type 2 Diabetes. Master’s Thesis, College of Agriculture, University of KwaZulu-Natal, Durban, South Africa, 2014. [Google Scholar]
- Dhurandhar, D.; Bharihoke, V.; Kalra, S. A histological Assessment of Effects of Sucralose on Liver of Albino Rats. Morphologie 2018, 102, 197–204. [Google Scholar] [CrossRef]
- Rodero, A.B.; de Souza Rodero, L.; Azoubel, R. Toxicity of Sucralose in Humans: A Review. Int. J. Morphol. 2009, 27, 239–244. [Google Scholar] [CrossRef]
- Grotz, V.L.; Henry, R.R.; McGill, J.B.; Prince, M.J.; Shamoon, H.; Trout, J.R.; Pi-Sunyer, F.X. Lack of Effect of Sucralose on Glucose Homeostasis in Subjects with Type 2 Diabetes. J. Am. Diet. Assoc. 2003, 103, 1607–1612. [Google Scholar] [CrossRef]
- Brown, A.W.; Bohan Brown, M.M.; Onken, K.L.; Beitz, D.C. Short-Term Consumption of Sucralose, A Nonnutritive Sweetener, is Similar to Water With Regard to Select Markers of Hunger Signaling and Short-Term Glucose Homeostasis in Women. Nutr. Res. 2011, 31, 882–888. [Google Scholar] [CrossRef]
- Ford, H.E.; Peters, V.; Martin, N.M.; Sleeth, M.L.; Ghatei, M.A.; Frost, G.S.; Bloom, S.R. Effects of Oral Ingestion of Sucralose on Gut Hormone Response and Appetite in Healthy Normal-Weight Subjects. Eur. J. Clin. Nutr. 2011, 65, 508–513. [Google Scholar] [CrossRef]
- Saada, H.N.; Mekky, N.H.; Eldawy, H.A.; Abdelaal, A.F. Biological Effect of Sucralose in Diabetic Rats. Food Nutr. Sci. 2013, 4, 82. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, L.; Yan, Q.; Zeng, J.; Ma, X.; Zhao, Y. A Natural Products Solution to Diabetic Nephropathy Therapy. Pharmacol. Ther. 2023, 241, 108314. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef]
- Hu, Q.; Qu, C.; Xiao, X.; Zhang, W.; Jiang, Y.; Wu, Z.; Song, D.; Peng, X.; Ma, X.; Zhao, Y. Flavonoids on Diabetic Nephropathy: Advances and Therapeutic Opportunities. Chin. Med. 2021, 16, 74. [Google Scholar] [CrossRef]
- Thorens, B. Glucose Transporters in the Regulation of Intestinal, Renal, and Liver Glucose Fluxes. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 270, G541–G553. [Google Scholar] [CrossRef]
- Baird, I.M.; Shephard, N.W.; Merritt, R.J.; Hildick-Smith, G. Repeated Dose Study of Sucralose Tolerance in Human Subjects. Food Chem. Toxicol. 2000, 38, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and Plant-Derived Natural Products: From Ethnopharmacological Approaches to Their Potential for Modern Drug Discovery and Development. Phytother. Res. 2021, 35, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, X.-H.; Mao, S.-L.; Zhao, F. Astragaloside IV Improves Metabolic Syndrome and Endothelium Dysfunction in Fructose-Fed Rats. Molecules 2011, 16, 3896–3907. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, S.; Eskandari, F.; Tangestani, H.; Bagherinejad, S.T.; Bargahi, A.; Bazzi, P.; Daneshi, A.; Sahrapoor, A.; O’Connor, W.J.; Rahbar, A.R. The Effect of Stevia rebaudiana on Serum Omentin and Visfatin Level in STZ-Induced Diabetic Rats. J. Diet. Suppl. 2015, 12, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Vengatash babu, K.; Ramachandran, V. Effect of Rebaudioside A, A Diterpenoid on Glucose Homeostasis in STZ-Induced Diabetic Rats. J. Physiol. Biochem. 2012, 68, 421–431. [Google Scholar] [CrossRef]
- Matikainen, N.; Taskinen, M.-R. Management of Dyslipidemias in the Presence of the Metabolic Syndrome or Type 2 Diabetes. Curr. Cardiol. Rep. 2012, 14, 721–731. [Google Scholar] [CrossRef]
- Latha, S.; Chaudhary, S.; Ray, R.S. Hydroalcoholic Extract of Stevia Rebaudiana Bert. Leaves and Stevioside Ameliorates Lipopolysaccharide Induced Acute Liver Injury in Rats. Biomed. Pharmacother. 2017, 95, 1040–1050. [Google Scholar] [CrossRef]
- Abo Elnaga, N.I.E.; Massoud, M.I.; Yousef, M.I.; Mohamed, H.H.A. Effect of Stevia Sweetener Consumption as Non-Caloric Sweetening on Body Weight Gain and Biochemical’s Parameters in Overweight Female Rats. Ann. Agric. Sci. 2016, 61, 155–163. [Google Scholar] [CrossRef]
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Harris, E.H. Elevated Liver Function Tests in Type 2 Diabetes. Clin. Diabetes 2005, 23, 115–119. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic Steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef]
- Aronson, K.J.; Miller, A.B.; Woolcott, C.G.; Sterns, E.E.; McCready, D.R.; Lickley, L.A.; Fish, E.B.; Hiraki, G.Y.; Holloway, C.; Ross, T.; et al. Breast Adipose Tissue Concentrations of Polychlorinated Biphenyls and Other Organochlorines and Breast Cancer Risk1. Cancer Epidemiol. Biomark. Prev. 2000, 9, 55–63. [Google Scholar]
- Weisbrod, A.V.; Shea, D.; Moore, M.J.; Stegeman, J.J. Species, Tissue and Gender-related Organochlorine Bioaccumulation in White-sided Dolphins, Pilot Whales and Their Common Prey in the Northwest Atlantic. Mar. Environ. Res. 2001, 51, 29–50. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- AbdElwahab, A.H.; Yousuf, A.F.; Ramadan, B.K.; Elimam, H. Comparative Effects of Stevia rebaudiana and Aspartame on Hepatorenal Function of Diabetic Rats: Biochemical and Histological Approaches. J. Appl. Pharm. Sci. 2017, 7, 034–042. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Steel, R.G. Pinciples and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]


| Groups | Period (Week) | |||||
|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W4 | W5 | W6 | |
| NR | 114.0 f ± 2.8 | 106.7 i ± 1.5 | 124.0 f ± 1.4 | 122.7 f ± 1.4 | 125.5 j ± 3.9 | 112.0 f ± 2.6 |
| STZ-DR | 308.7 ab ± 2.8 | 455.7 a ± 4.6 | 422.0 a ± 3.2 | 442.3 a ± 6.4 | 455.4 a ± 5.8 | 464.8 a ± 12.4 |
| DR+ST | 308.0 ab ± 4.9 | 345.0 c ± 16.0 | 392.7 b ± 3.9 | 312.7 cd ± 6.7 | 357.8 d ± 4.3 | 343.0 b ± 7.0 |
| DR+ST+In | 298.7 abc ± 5.3 | 296.5 e ± 1.1 | 281.5 e ± 23.0 | 282.5 cde ± 2.7 | 299.2 ef ± 5.0 | 243.5 de ± 9.3 |
| DR+SU | 298.3 abc ± 5.1 | 369.0 b ± 2.1 | 358.0 c ± 3.8 | 427.0 b ± 5.7 | 412.7 b ± 5.7 | 349.0 b ± 11.5 |
| DR+SU+In | 287.0 d ± 4.0 | 273.0 f ± 1.6 | 263.0 e ± 12.8 | 298.3 cd ± 9.8 | 300.8 ef ± 7.3 | 250.8 d ± 5.0 |
| DR+ST+SU | 318.0 a ± 3.8 | 329.0 c ± 9.6 | 327.7 d ± 4.3 | 314.7 c ± 7.3 | 378.0 c ± 3.3 | 337.8 c ± 6.7 |
| DR+ST+SU+In | 305.7 ab ± 4.5 | 309.0 d ± 9.6 | 307.7 e ± 4.1 | 284.5 cde ± 7.8 | 313.0 e ± 6.5 | 229.5 de ± 13.2 |
| NR+ST | 115.5 f ± 0.4 | 113.0 h ± 0.9 | 128.5 f ± 0.4 | 121.0 f ± 2.8 | 126.0 j ± 3.3 | 110.7 f ± 3.2 |
| NR+SU | 116.0 f ± 1.4 | 120.0 j ± 1.4 | 118.5 f ± 4.6 | 123.0 f ± 2.1 | 124.3 j ± 1.3 | 112.3 f ± 7.6 |
| NR+ST+SU | 124.0 e ± 0.7 | 115.0 h ± 1.4 | 115.0 f ± 2.1 | 125.5 f ± 1.1 | 125.3 j ± 0.8 | 111.3 f ± 2.0 |
| Experimental Group | AST (UL−1) | ALT (UL−1) | TBILI (mg dL−1) |
|---|---|---|---|
| NR | 194.30 ± 2.95 ab | 69.17 ± 2.06 b | 0.08 ± 0.01 ab |
| STZ-DR | 262.63 ± 14.24 a | 220.77 ± 72.03 a | 0.17 ± 0.04 ab |
| DR+SU | 183.47 ± 1.57 b | 127.17 ± 18.31 b | 0.14 ± 0.05 a |
| DR+SU+In | 177.63 ± 14.87 b | 81.70 ± 10.59 b | 0.07 ± 0.01 b |
| DR+ST | 157.27 ± 8.78 b | 108.03 ± 10.2 b | 0.10 ± 0.01 ab |
| DR+ST+In | 173.40 ± 13.89 a | 83.27 ± 16.99 b | 0.07 ± 0.04 b |
| DR+ST+SU | 167.30 ± 16.82 b | 99.43 ± 0.76 b | 0.09 ± 0.00 ab |
| DR+ST+SU+In | 166.60 ± 7.36 b | 82.13 ± 9.74 b | 0.09 ± 0.01 ab |
| NR+ST | 195.53 ± 14.78 ab | 58.57 ± 4.35 b | 0.09 ± 0.01 ab |
| NR+SU | 208.53 ± 21.03 ab | 68.80 ± 6.00 b | 0.08 ± 0.01 ab |
| NR+ST+SU | 215.23 ± 4.67 ab | 86.77 ± 6.83 b | 0.08 ± 0.01 ab |
| Experimental Groups | TG | CHO | HDL-c | LDL-c | VLDL-c |
|---|---|---|---|---|---|
| NR | 46.73 ± 3.79 d | 56.00 ± 3.65 b | 38.62 ± 2.86 a | 8.03 ± 0.75 a | 9.35 ± 0.76 bcd |
| STZ-DR | 180.30 ± 6.83 a | 72.93 ± 4.57 a | 31.99 ± 7.25 a | 8.87 ± 1.33 a | 32.08 ± 4.84 a |
| DR+SU | 160.40 ± 14.21 ab | 70.27 ± 4.13 a | 40.33 ± 3.36 a | 9.00 ± 0.89 a | 20.94 ± 5.03 bc |
| DR+SU+In | 142.33 ± 15.23 b | 70.33 ± 5.98 a | 35.47 ± 7.25 a | 9.80 ± 0.72 a | 25.79 ± 4.14 ab |
| DR+ST | 145.13 ± 19.14 b | 71.60 ± 10.30 a | 33.27 ± 1.67 a | 9.87 ± 0.78 a | 28.47 ± 10.25 ab |
| DR+ST+In | 104.70 ± 15.15 bc | 74.70 ± 9.92 a | 35.34 ± 5.94 a | 10.33 ± 1.69 a | 29.03 ± 5.83 ab |
| DR+ST+SU | 122.00 ± 6.08 bc | 66.57 ± 6.05 a | 32.17 ± 4.45 a | 10.00 ± 1.72 a | 24.40 ± 12.02 ab |
| DR+ST+SU+In | 83.17 ± 22.80 cd | 67.17 ± 6.50 a | 40.30 ± 2.05 a | 10.23 ± 0.95 a | 16.63 ± 4.56 bc |
| NR+ST | 44.93 ± 6.47 d | 61.87 ± 10.94 ab | 41.87 ± 9.90 a | 11.03 ± 2.14 a | 8.99 ± 1.29 bcd |
| NR+SU | 50.43 ± 13.29 d | 67.57 ± 1.58 a | 45.47 ± 1.94 a | 12.00 ± 0.46 a | 10.09 ± 2.66 bcd |
| NR+ST+SU | 52.73 ± 1.57 d | 52.67 ± 5.73 b | 37.50 ± 3.60 a | 10.00 ± 1.80 a | 5.15 ± 0.31 e |
| Experimental Group | Atrophy of Islands of Langerhans | Absence of Langerhans Cells | Ductal Hyperplasia | Newly Formed Ductules |
|---|---|---|---|---|
| NR | − | − | − | − |
| STZ-DR | +++ | +++ | +++ | +++ |
| DR+SU | +++ | +++ | ++ | ++ |
| DR+SU+In | ++ | + | ++ | ++ |
| DR+ST | + | − | − | − |
| DR+ST+In | − | − | − | − |
| DR+ST+SU | +++ | +++ | − | − |
| DR+ST+SU+In | ++ | − | + | ++ |
| NR+ST | − | − | − | − |
| NR+SU | + | − | − | − |
| NR+ST+SU | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, H.; Al-Roug, K.; Algonaiman, R.; Althwab, S.A.; Alfheeaid, H.A.; Alhomaid, R.M.; Almujaydil, M.S.; Bushnaq, T.; Ebeid, T.A. Biological Assessment of Stevioside and Sucralose as Sucrose Substitutes for Diabetics on STZ-Induced Diabetes in Rats. Molecules 2023, 28, 940. https://doi.org/10.3390/molecules28030940
Barakat H, Al-Roug K, Algonaiman R, Althwab SA, Alfheeaid HA, Alhomaid RM, Almujaydil MS, Bushnaq T, Ebeid TA. Biological Assessment of Stevioside and Sucralose as Sucrose Substitutes for Diabetics on STZ-Induced Diabetes in Rats. Molecules. 2023; 28(3):940. https://doi.org/10.3390/molecules28030940
Chicago/Turabian StyleBarakat, Hassan, Khaled Al-Roug, Raya Algonaiman, Sami A. Althwab, Hani A. Alfheeaid, Raghad M. Alhomaid, Mona S. Almujaydil, Taqwa Bushnaq, and Tarek A. Ebeid. 2023. "Biological Assessment of Stevioside and Sucralose as Sucrose Substitutes for Diabetics on STZ-Induced Diabetes in Rats" Molecules 28, no. 3: 940. https://doi.org/10.3390/molecules28030940
APA StyleBarakat, H., Al-Roug, K., Algonaiman, R., Althwab, S. A., Alfheeaid, H. A., Alhomaid, R. M., Almujaydil, M. S., Bushnaq, T., & Ebeid, T. A. (2023). Biological Assessment of Stevioside and Sucralose as Sucrose Substitutes for Diabetics on STZ-Induced Diabetes in Rats. Molecules, 28(3), 940. https://doi.org/10.3390/molecules28030940







